Abstract
Salt sensitivity is probably caused by either a hereditary or acquired defect of salt excretion by the kidney, and it is reasonable to consider that this is the basis for differences in hypertension between black and white people. Dopamine acts in an autocrine/paracrine fashion to promote natriuresis in the proximal tubule and thick ascending loop of Henle. G-protein receptor kinases (or GRKs) are serine and threonine kinases that phosphorylate G protein-coupled receptors in response to agonist stimulation and uncouple the dopamine receptor from its G protein. This results in a desensitisation process that protects the cell from repeated agonist exposure. GRK4 activity is increased in spontaneously hypertensive rats, and infusion of GRK4 antisense oligonucleotides attenuates the increase in blood pressure (BP). This functional defect is replicated in the proximal tubule by expression of GRK4 variants namely p.Arg65Leu, p.Ala142Val and p.Val486Ala, in cell lines, with the p.Ala142Val showing the most activity. In humans, GRK4 polymorphisms were shown to be associated with essential hypertension in Australia, BP regulation in young adults, low renin hypertension in Japan and impaired stress-induced Na excretion in normotensive black men. In South Africa, GRK4 polymorphisms are more common in people of African descent, associated with impaired Na excretion in normotensive African people, and predict blood pressure response to Na restriction in African patients with mild to moderate essential hypertension. The therapeutic importance of the GRK4 single nucleotide polymorphisms (SNPs) was emphasised in the African American Study of Kidney Disease (AASK) where African-Americans with hypertensive nephrosclerosis were randomised to receive amlodipine, ramipril or metoprolol. Men with the p.Ala142Val genotype were less likely to respond to metoprolol, especially if they also had the p.Arg65Leu variant. Furthermore, in the analysis of response to treatment in two major hypertension studies, the 65Leu/142Val heterozygote predicted a significantly decreased response to atenolol treatment, and the 65Leu/142Val heterozygote and 486Val homozygote were associated in an additive fashion with adverse cardiovascular outcomes, independent of BP. In conclusion, there is considerable evidence that GRK4 variants are linked to impaired Na excretion, hypertension in animal models and humans, therapeutic response to dietary Na restriction and response to antihypertensive drugs. It may also underlie the difference in hypertension between different geographically derived population groups, and form a basis for pharmacogenomic approaches to treatment of hypertension.
Keywords: salt sensitivity, GRK4, ethnicity
1. Introduction
Hypertension is a complex, poorly understood disorder with strong environmental and genetic influences. Salt sensitivity is a term designated to define a group of individuals with a greater rise in blood pressure (BP) after salt loading or a greater fall in blood pressure after salt restriction [1]. People of African descent (hereafter referred to as Blacks) appear to be more salt sensitive than people of Caucasian/European origins (hereafter referred to as Whites) [2]. Greater sodium retention is thought to underlie the BP-determining physiology of Blacks, but specific mechanisms have not been identified. Additionally, African Americans have a higher prevalence of hypertension and morbidity from hypertension for comparable levels of BP, when compared to Whites [3,4].
In a recent prospective observational study in the United States, involving children and adults, plasma renin activity (PRA) and plasma aldosterone concentration were significantly lower in Blacks compared to Whites, and there was a greater rise in 24 h ambulatory BP, brain natriuretic peptide and body weight after treatment with 9-α fludrocortisone indicating greater sodium (Na) retention by the kidney [5]. Similarly, Rayner et al. [6] showed that PRA and plasma aldosterone concentrations were significantly lower in South African Blacks compared to Whites for comparable intake of Na, possibly suggesting an underlying genetic predisposition to Na retention.
Regulation of Na balance is critical for the survival of land-based animals. Every day the kidney filters approximately 25,000 mmol of Na, with 65% reabsorbed in the proximal tubule, 27% in ascending loop of Henle, 5% in the distal convoluted tubule and 3% in the collecting duct [7]. As dietary intake ranges from as little as 20 mmols/day (ultra-low, hunter-gatherer diet) to as high as 300 mmols/day (in a typical Western diet), it is essential for the kidney to maintain Na homeostasis by reabsorbing over 99% of the filtered Na. Any defect in Na reabsorption (such as occurs in Barter’s syndrome) results in Na wasting, hypotension, and massive activation of the renin-angiotensin-aldosterone system, often incompatible with long term survival [8]. Barter’s syndrome is due to a “loss of function” mutation in the Na-K-2Cl transporter located in the thick ascending loop of Henle [8]. Conversely, increased function of Na transporters in the kidney, e.g., the epithelial Na channel, results in Liddle’s syndrome characterised by hypertension, hypokalaemia, and suppressed PRA and aldosterone [8].
Bochud et al. [9] showed that fractional excretion of Na in the proximal convoluted tubule is highly hereditable, and fractional excretion of Na was significantly higher in Black South Africans when compared to White Belgians. Dopamine is an important regulator of Na balance in the proximal convoluted tubule, and GRK4 is a serine and threonine kinase that phosphorylates G protein-coupled receptors in response to agonist stimulation. It uncouples the dopamine receptor from its G protein. In this review, the importance of GRK-4 single nucleotide polymorphisms (SNPs) in determining salt sensitivity and salt sensitive hypertension response to dietary and antihypertensive therapy in people of African descent will be explored.
2. Dopamine, G-Protein-Coupled Receptors and GRK4
The catecholamines, dopamine and norepinephrine are synthesized from the same precursors; namely, the amino acid tyrosine and its hydroxylated product L-DOPA. (Figure 1) In the kidney, dopamine is mainly synthesised in the proximal tubules by decarboxylation of L-DOPA, which is transported to the tubules from the circulation as the renal tubules are unable to synthesise L-DOPA due to lack of tyrosine hydroxylase. The proximal tubules also lack dopamine β-hydroxylase, and there is no conversion to norepinephrine. Dopamine receptors are present on both the basolateral and apical membranes [10].
Figure 1.
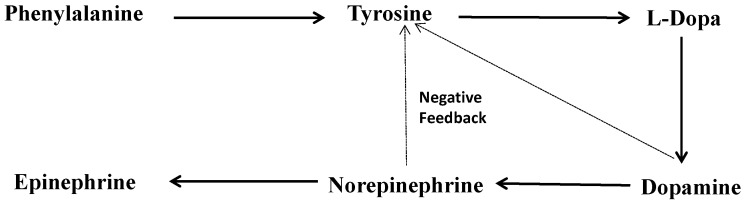
Biosynthesis of catecholamines.
Dopamine secreted by the tubules acts in an autocrine/paracrine fashion to regulate Na transport mainly in the renal proximal tubule and thick ascending loop of Henle [10]. The dopamine receptors are distributed widely in the kidney, and the D1 receptors (D1R) belong to a super family of G protein-coupled receptors, which stimulate adenyl cyclase and protein kinase A. Under conditions of Na excess, locally generated dopamine acts on D1R of the renal tubular cells to reduce Na transport and promote natriuresis. Dopamine inhibits renal Na transport by inhibiting the Na-H exchanger, and Na/phosphate co-transporter in apical membranes, and Na/HCO3− co-transporter and Na K-ATPase in basolateral membranes in the proximal tubule and thick ascending loop of Henle. Renal dopamine is responsible for over 50% of the incremental Na excretion in response to increased intake [11].
Dopamine interacts with atrial natriuretic peptide (ANP), angiotensin II and α adrenergic receptors in the proximal tubule to maintain Na homeostasis [11]. The model for bidirectional regulation of tubular Na transport is shown in Figure 2. There is evidence that dopamine opposes the anti-natriuretic effect of ANG II, both in the short and long term. It stimulates the activity of proximal tubular Na/K ATPase, which is abolished by dopamine or its messenger cAMP [11]. In fish, dopamine is the predominant catecholamine, but in the terrestrial environment, where Na is a relatively scarce resource, norepinephrine predominates and is essential in the regulation of the anti-natriuretic forces by stimulating the Na/K ATPase in the proximal tubule. Dopamine opposes the action of norepinephrine. Atrial natriuretic peptide (ANP) is an important natriuretic hormone but it requires the presence of dopamine receptors for its full effect. The inhibitory effect of dopamine on the Na/K ATPase is potentiated by ANP.
Figure 2.
Bidirectional regulation of proximal tubular Na [10]. ANP = atrial natriuretic peptide receptor, D1R = dopamine receptor, αAR = α adrenergic receptor, AT1R = angiotensin 1 receptor.
The effects of dopamine in the kidney provide a physiological model for salt-sensitive hypertension. Any defect in the dopaminergic system will result in unopposed action of ANG II and norepinephrine, and impaired action of ANP on the proximal tubule, resulting in a pronounced antinatriuretic effect [11].
Current research suggests that a defect in the D1R is not a likely candidate for salt-sensitive hypertension in humans. However, the uncoupling of the D1R from its G protein effector complex is similar to a desensitisation process [12]. This is a mechanism which protects the cell from repeated agonist exposure. Desensitisation involves several processes including phosphorylation, sequestration and degradation of receptor protein. The initial step in this process (phosphorylation) is mediated by a member of the G protein-coupled receptor kinase (GRK). GRKs are serine and threonine kinases that phosphorylate G protein-coupled receptors in response to agonist stimulation [12]. The phosphorylation of G protein-coupled receptor kinases, including D1R, leads to binding with members of the arrestin family of proteins that uncouple the receptor from its G protein, and reduction in the functional response [12].
3. The Role of GRK4 in the Pathogenesis of Salt Sensitivity in Animal Models
In hypertension, there appears to be constitutive desensitisation of the renal D1R [11]. This appears to be caused by GRK as decreasing its activity or expression in the proximal tubular cells in hypertensives normalises the ability of D1R to increase cAMP. In the proximal tubule, GRK4 is the most important component, compared to other GRKs. GRK4 activity is increased in spontaneously hypertensive rats, and infusion of GRK4 antisense oligonucleotides attenuates the increase in BP [13]. This functional defect is replicated in the proximal tubule by expression of GRK4 variants in cell lines, and this is rectified by prevention of GRK4 expression [14]. These three tested variants were the p.Arg65Leu, p.Ala142Val, and p.Val486Ala, with the p.Ala142Val showing the most activity [14]. This impairment of dopaminergic function, as evidenced by the GRK4 variants in animal models, would allow the antinatriuretic function of ANG II and norepinephrine to be unopposed, which may then be responsible for salt sensitivity and rise in BP. It is therefore an attractive candidate gene for human salt sensitivity and hypertension.
In addition, GRK4 polymorphisms may affect BP through enhanced activity of AT1 receptors. GRK4 is expressed in vascular smooth muscle cells of the aorta and heterologous expression of the GRK4γ variant p.Ala142Val in A10 cells increased AT1 receptor protein expression and increase in intracellular calcium concentration [15]. Angiotensin II-mediated vasoconstriction of the aorta was also higher in GRK4γ p.Ala142Val than in wild-type transgenic mice.
4. The Role of GRK4 Variants on Na Excretion in Humans
Several lines of evidence point to a role for GRK4 variants in the pathogenesis of salt sensitivity and salt sensitive hypertension in humans, especially in African populations. The p.Arg65Leu allele was also associated with impaired stress-induced Na excretion in normotensive Black men [16]. In a study from South Africa, salt excretion was examined in healthy normotensive and lean black and white men after an acute saline load [17]. The GRK4 p.Ala142Val polymorphism, which was significantly more frequent in black men, was associated with significantly lower plasma aldosterone concentrations and impaired incremental urinary Na excretion. Hypertension-related polymorphisms and cardiovascular indices were also analyzed in 97 normotensive, healthy Japanese adults. NT-proBNP levels were significantly higher in subjects with two or more GRK4 polymorphic alleles strongly suggesting enhanced Na retention [18]. Sodium excretion was inversely related to the number of GRK4 variants in hypertensive persons, and the natriuretic response to dopaminergic stimulation was impaired in normotensive persons having GRK4 gene variants [19].
5. GRK4 Variants and Hypertension
In an Australian study, involving 168 unrelated white subjects/patients and 312 normotensive controls, GRK4 polymorphisms were associated with essential hypertension [20]. Furthermore, a recent study linked the GRK4 variants, in particular p.Arg65Leu, with BP regulation in adolescents and young adults [21]. A Japanese study of hypertensive subjects developed a multi-variant genetic model based on the p.Arg65Leu, p.Ala142Val, and p.Val486Ala changes in GRK4 which was 94.4% predictive of salt-sensitive hypertension, while a single-variant model with a single p.Ala142Val variant (in GRK4) was only 78.4% predictive [19].
The putative pathogenesis of salt sensitive hypertension due to GRK4 SNPs is shown in Figure 3 demonstrating the interaction of genes and environment. Environmental factors interplay with the underlying genetic factors to develop the “perfect storm” for the development of hypertension.
Figure 3.
Putative pathogenesis of salt sensitive hypertension related to GRK-4 SNPs. SNS = sympathetic nervous system, RAAS = renin-angiotensin-aldosterone system.
6. GRK4 Variants and Response to Dietary and Pharmacological Intervention
In South Africa, GRK4 polymorphisms are more common in people of African descent, are associated with impaired Na excretion in normotensive people, and predict blood pressure response to Na restriction in African patients with mild to moderate essential hypertension [22]. The therapeutic importance of the GRK4 SNPs was emphasised in the African American Study of Kidney Disease (AASK) where African-Americans with hypertensive nephrosclerosis were randomised to receive amlodipine, ramipril or metoprolol [23]. Men with the p.142Ala genotype were less likely to respond to metoprolol, especially if they had co-inherited the p.65Leu variant. Furthermore, in the analysis of response to treatment in two major hypertension studies, the 65Leu/142Val haplotype predicted a significantly decreased response to atenolol treatment, and the 65Leu/142Val heterozygote and the 486Val/486Val homozygote were associated in an additive fashion with adverse CV outcomes independent of BP [24]. The effects of GRK-4 variants are summarized in Table 1.
Table 1.
Summary of the associations of GRK variants with Na excretion and hypertension.
| GRK Variant | Parameter | Comment |
|---|---|---|
| p.Arg65Leu, p.Ala142Val, and p.Val486Ala | Hypertension in rats | p.Ala142Val shows greatest activity |
| p.Ala142Val | Enhanced activity of AT1 in smooth muscle | – |
| p.Arg65Leu | Impaired stress related Na excretion in normotensive blacks | – |
| p.Ala142Val | Impaired incremental Na excretion in healthy men, and lower aldosterone levels | p.Ala142Val significantly more common in Blacks compared to Whites |
| p.Arg65Leu, p.Ala142Val, and p.Val486Ala | Impaired Na excretion and higher BNP levels in healthy Japanese with two or more variants | – |
| p.Val486Ala | Association with hypertension in Australian population | – |
| p.Arg65Leu, p.Ala142Val, and p.Val486Ala | 94.4% predictive of salt sensitive renin hypertension in Japanese population | p.Ala142Val 78.4% predictive |
| p.Arg65Leu, p.Ala142Val | BP response to Na restriction in black South Africans with hypertension | – |
| p.142Ala genotype | In AASK males less likely to respond to metoprolol | Especially if co-inherited the p.65Leu variant |
| 65Leu/142Val haplotype | In two major hypertension studies predicted a reduced response to atenolol and increased cardiovascular outcomes | – |
7. Conclusions
There is now mounting evidence that GRK4 variants are linked to impaired Na excretion, hypertension in animal models and humans, therapeutic response to dietary Na restriction and response to antihypertensive drugs. It may also underlie the difference in hypertension between individuals of African and European origin, and form a basis to a pharmacogenomics approach to the understanding and treatment of hypertension.
Author Contributions
Brian Rayner, Raj Ramesar contributed to the research, writing and reviewing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Weinberger M.H. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.HYP.27.3.481. [DOI] [PubMed] [Google Scholar]
- 2.Luft F.C., Grim C.E., Higgins J.T., Jr., Weinberger M.H. Differences in response to sodium administration in normotensive white and black subjects. J. Lab. Clin. Med. 1977;90:555–562. [PubMed] [Google Scholar]
- 3.Mensah G.A., Mokdad A.H., Ford E.S., Greenlund K.J., Croft J.B. State of disparities in cardiovascular health in the United States. Circulation. 2005;1111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 4.Redmond N., Baer H.J., Hicks L.S. Health behaviors and racial disparity in blood pressure control in the National Health and Nutrition Examination Survey. Hypertension. 2011;57:383–389. doi: 10.1161/HYPERTENSIONAHA.110.161950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tu W., Eckert G.J., Hannon T.S., Liu H., Pratt L.M., Wagner M.A., DiMeglio L.A., Jung J., Pratt J.H. Racial differences in sensitivity of blood pressure to aldosterone. Hypertension. 2014;63:1212–1218. doi: 10.1161/HYPERTENSIONAHA.113.02989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rayner B.L., Meyers J.E., Opie L.H., Trinder Y.A., Davidson J.S. Screening for primary aldosteronism—Normal ranges for aldosterone and renin in three South African groups. S. Afr. Med. J. 2001;91:594–599. [PubMed] [Google Scholar]
- 7.Bailey M.A., Shirley D.G., Unwin R.J. Renal physiology. In: Johnson R.J., Feehally J., Floege J., editors. Comprehensive Clinical Nephrology. 5th ed. Chapter 2. Elsevier, Sanders; Philadelphia, PA, USA: 2014. pp. 14–27. [Google Scholar]
- 8.Gross P., Heduschka P. Inherited disorders of sodium and water metabolism. In: Johnson R.J., Feehally J., Floege J., editors. Comprehensive Clinical Nephrology. 5th ed. Chapter 49. Elsevier, Sanders; Philadelphia, PA, USA: 2014. pp. 579–589. [Google Scholar]
- 9.Bochud M., Staessen J.A., Maillard M., Mazeko M.J., Kuznetsova T., Woodiwiss A., Richart T., Norton G., Thijs L., Elston R., et al. Ethnic differences in proximal and distal tubular sodium reabsorbtion are heritable in black and white populations. J. Hypertens. 2009;27:606–612. doi: 10.1097/HJH.0b013e32832104b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aperia A.C. Intrarenal dopamine: A key signal in the interactive regulation of sodium metabolism. Annu. Rev. Physiol. 2000;62:621–647. doi: 10.1146/annurev.physiol.62.1.621. [DOI] [PubMed] [Google Scholar]
- 11.Jose P.A., Eisner G.M., Felder R.A. Dopamine and the kidney: A role in hypertension? Curr. Opin. Nephrol. Hypertens. 2003;12:189–194. doi: 10.1097/00041552-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Jose P.A., Eisner G.M., Felder R.A. Role of dopamine receptors in the kidney in the regulation of blood pressure. Curr. Opin. Nephrol. Hypertens. 2002;11:87–92. doi: 10.1097/00041552-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Sanada H., Yatabe J., Midorikawa S., Katoh T., Hashimoto S., Watanabe T., Xu J., Luo Y., Wang X., Zeng C., et al. Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension. 2006;47:1131–1139. doi: 10.1161/01.HYP.0000222004.74872.17. [DOI] [PubMed] [Google Scholar]
- 14.Felder R., Sanada H., Xu J., Wang Z., Watanabe H., Asico L.D., Wang W., Zheng S., Yamaguchi I., Williams S.M., et al. G protein-coupled receptor kinase 4 variants in human essential hypertension. Proc. Natl. Acad. Sci. USA. 2002;99:3872–3877. doi: 10.1073/pnas.062694599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K., Fu C., Chen C., Liu L., Ren H., Han Y., Yang J., He D., Zhou L., Yang Z., et al. Role of GRK4 in the Regulation of Arterial AT1 Receptor in Hypertension. Hypertension. 2014;63:289–296. doi: 10.1161/HYPERTENSIONAHA.113.01766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu H., Lu Y., Wang X., Snieder H., Treiber F.A., Harshfield G.A., Dong Y. The G protein-coupled receptor kinase 4 gene modulates stress-induced sodium excretion in black normotensive adolescents. Pediatr. Res. 2006;60:440–442. doi: 10.1203/01.pdr.0000238250.64591.44. [DOI] [PubMed] [Google Scholar]
- 17.Rayner B., Musekiwa A., Lombard C., Ramesar R. The A142V polymorphism of the G protein-coupled receptor kinase 4 gene predicts natriuretic response to saline challenge in young normotensive lean Black and White South African men. Nephrol. Rev. 2011;3:e9. doi: 10.4081/nr.2011.e9. [DOI] [Google Scholar]
- 18.Yatabe Y., Yatabe M.S., Yoneda M., Felder R.A., Jose P.A., Sanada H. Hypertension-related gene polymorphisms of G-protein-coupled receptor kinase 4 are associated with NT-proBNP concentration in normotensive healthy adults. Int. J. Hypertens. 2012;2012 doi: 10.1155/2012/806810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanada H., Yatabe J., Midorikawa S., Hashimoto S., Watanabe T., Moore J.H., Ritchie M.D., Williams S.M., Pezzullo J.C., Sasaki M., et al. Single-nucleotide polymorphisms for diagnosis of salt-sensitive hypertension. Clin. Chem. 2006;52:352–360. doi: 10.1373/clinchem.2005.059139. [DOI] [PubMed] [Google Scholar]
- 20.Speirs H.J.L., Katyk K., Kumar N.N., Benjafield A.V., Wang W.Y.S., Morris B.J. Association of G-protein-coupled receptor kinase 4 haplotypes, but not HSD3B1 or PTP1B polymorphisms, with essential hypertension. J. Hypertens. 2004;22:931–936. doi: 10.1097/00004872-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H., Lu Y., Wang X., Treiber F.A., Harshfield G.A., Snieder H., Dong Y. The G protein-coupled receptor kinase 4 gene affects blood pressure in young normotensive twins. Am. J. Hypertens. 2006;19:61–66. doi: 10.1016/j.amjhyper.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Rayner B., Ramesar R., Steyn K., Levitt N., Lombard C., Charlton K. G-protein-coupled receptor kinase 4 (GRK-4) polymorphisms predict blood pressure response to dietary modification in black patients with mild to moderate hypertension. J. Hum. Hypertens. 2011;26:334–239. doi: 10.1038/jhh.2011.33. [DOI] [PubMed] [Google Scholar]
- 23.Bhatnagar V., O’Connor D.T., Brophy V.H., Schork N.J., Richard E., Salem R.M., Nievergelt C.M., Bakris G.L., Middleton J.P., Norris K.C., et al. G-Protein-coupled receptor kinase 4 polymorphisms and blood pressure response to metoprolol among african americans: Sex-specificity and interactions. Am. J. Hypertens. 2009;22:332–338. doi: 10.1038/ajh.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandell A.G., Lobmeyer M.T., Gawronski B.E., Langaee T.Y., Gong Y., Gums J.G., Beitelshees A.L., Turner S.T., Chapman A.B., Cooper-Dehoff R.M., et al. G protein receptor kinase 4 polymorphisms: β-Blocker Pharmacogenetics and treatment-related outcomes in Hypertension. Hypertension. 2012;60:957–964. doi: 10.1161/HYPERTENSIONAHA.112.198721. [DOI] [PMC free article] [PubMed] [Google Scholar]




