Abstract
Certain benzo[f]indole-4,9-dione derivatives were synthesized and evaluated for their inhibitory effects on superoxide anion generation and neutrophil elastase (NE) release in formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLF)-activated human neutrophils. Results indicated that (Z)-1-benzyl-4-(hydroxyimino)-1H-benzo[f]indol-9(4H)-one (10) showed a potent dual inhibitory effect on NE release and superoxide anion generation with IC50 value of 2.78 and 2.74 μM respectively. The action mechanisms of 10 in human neutrophils were further investigated. Our results showed that compound 10 did not alter fMLF-induced phosphorylation of Src (Src family Y416). Notably, phosphorylation of Akt (S473) and mobilization of [Ca2+]i caused by fMLF was inhibited by compound 10. Further structural optimization of 10 is ongoing.
Keywords: benzo[f]indole-4,9-dione derivatives; superoxide anion generation; elastase release; anti-inflammatory agents
1. Introduction
Human neutrophils play an important role in the defense system against invasion by microorganisms and in the pathogenesis of various diseases such as rheumatoid arthritis, ischemia-reperfusion injury, chronic obstructive pulmonary disease, and asthma [1,2,3,4,5]. In response to diverse stimuli, activated neutrophils secrete a series of cytotoxins, such as superoxide anion, a precursor of other reactive oxygen species (ROS), granule proteases, and bioactive lipids [2,6,7]. Of these, neutrophil elastase (NE) is stored in the azurophil granules of neutrophils and is released following neutrophil exposure to inflammatory stimuli. High concentrations of ROS and NE have been implicated in the pathogenesis of many acute and chronic pulmonary diseases including asthma, chronic obstructive pulmonary disease, cystic fibrosis, and acute respiratory distress syndrome [2,8,9,10]. Therefore, inhibition of neutrophils activation and the following release of inflammatory mediators provide a promising strategy for the development of potential anti-inflammatory agents.
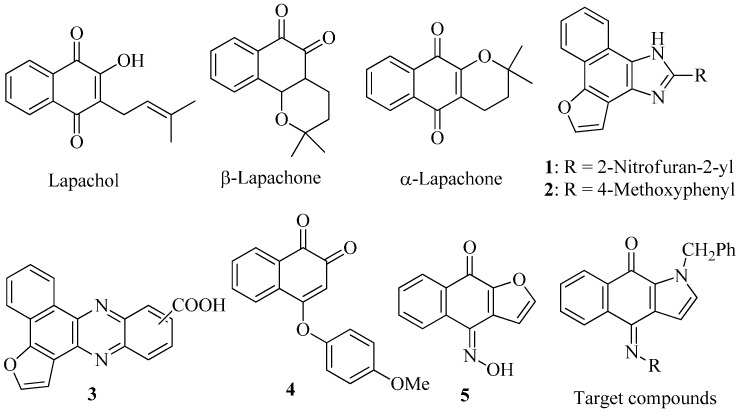
Many efforts have been devoted to the discovery of novel anti-inflammatory agents for the past few years [11,12,13,14,15,16]. The natural quinones including lapachol, α-lapachone, and β-lapachone (β-LAPA) (Figure 1) were isolated from the heartwood of the Bignoniaceae family (Tabebuia sp.) and evaluated for their biological activities. Among them, β-LAPA was found to be able to inhibit the expression of nitric oxide (NO) and PGE2 in alveolar macrophages [17]. In order to discover novel drug candidates, we have synthesized certain furo[3',2':3,4]naphtho[1,2-d]imidazole derivatives and evaluated for their anti-inflammatory activities. Our results indicated that (E)-2-[2-(5-nitrofuran-2-yl)vinyl)]-furo[3',2':3,4]naphtho[1,2-d]imidazole (1) [18] was capable of inhibiting iNOS expression, with an IC50 value of 0.52 μM while 2-(4-methoxyphenyl)furo[3',2':3,4]naphtho[1,2-d]imidazole (2) [19] exhibited a strongly inhibitory activity on LPS-induced PGE2 production, with an IC50 value of 0.047 μM. We have also demonstrated that benzo[a]furo[2,3-c]phenazinecarboxylic acid (3) [20] strongly inhibited superoxide anion generation while 4-(4-methoxyphenoxy)naphthalene-1,2-dione (4) [21] was able to inhibit NO and TNF-α released in LPS-induced Raw 264.7 cells. In continuation of our search for novel type of anti-inflammatory agents, the present study describes preparation and biological evaluation of certain benzo[f]indole-4,9-dione derivatives which belong to a new structural type possessing versatile iminoquinone moiety. Their cytotoxicities were also evaluated due to the structural similarity of these tricyclic compounds to the cytotoxic (Z)-4-(hydroxyimino)naphtho[2,3-b]furan-9(4H)-one (5) [22] which exhibited an IC50 value of 0.82 μM against the growth of K562 cell.
Figure 1.
Structures of lapachol, β-lapachone, α-lapachone, and compounds 1–5.
2. Results and Discussion
2.1. Chemistry
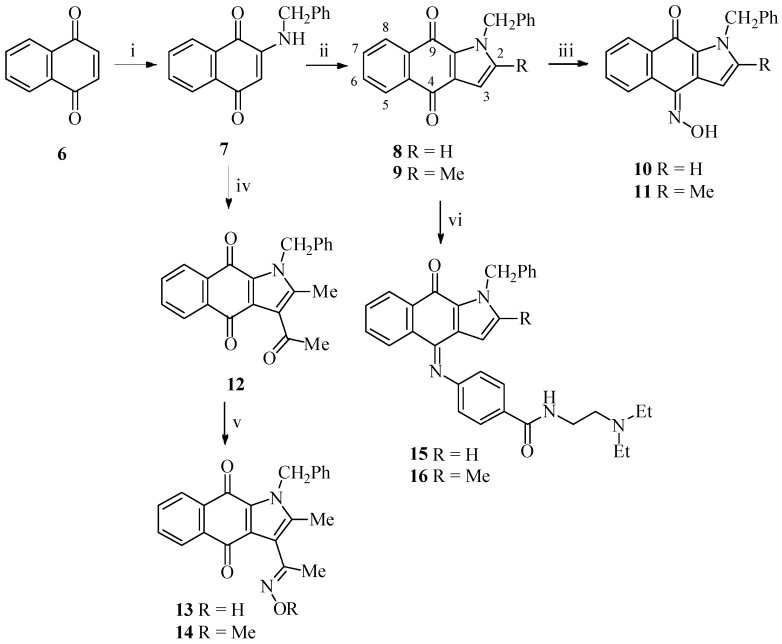
Treatment of naphthalene-1,4-dione (6) with benzylamine afforded 2-(benzylamino)naphthalene-1,4-dione (7) [23] in 78% yield as described in Scheme 1. Condensation of 7 with acetaldehyde or acetone afforded 1-benzyl-1H-benzo[f]indole-4,9-dione (8) and 1-benzyl-2-methyl-1H-benzo[f]indole-4,9-dione (9) respectively in a moderate yield. Treatment of 8 with NH2OH proceeds in the regiospecific and the stereospecific manners to give (Z)-1-benzyl-4-(hydroxyimino)-1H-benzo[f]indol-9(4H)-one (10) as a sole product. The regiospecific oximination occurred at C-4 rather than C-9 carbonyl was established based on the 13C-NMR in which the more downfield C-4 carbonyl shifted from 180.98 to 141.42 ppm while the more upfield C-9 carbonyl shifted from 176.23 to 174.12 ppm [22,24]. The stereospecific oximination to give Z-form product rather than the E-isomer can be realized in which the hydroxyl group proximate the pyrrole ring is less sterically hindered [25]. Accordingly, compound 11 was prepared from 9 with NH2OH.
Scheme 1.
Synthesis of benzo[f]indole-4,9-dione derivatives 8–16.
Reagents and conditions: i) benzylamine, EtOH, reflux; ii) MeCHO or Me2CO, Mn(Ac)3, iii) NH2OHHCl, K2CO3, EtOH; iv) acetylacetone, CAN; v) NH2OR-HCl (R = H or Me), K2CO3, EtOH; vi) procainamide, TiCl4, CH2Cl2.
The known 3-acetyl-1-benzyl-2-methyl-1H-benzo[f]indole-4,9-dione (12) [26] was obtained by the reaction of 7 with acetylacetone. Treatment of 12 with NH2OH or NH2OMe gave 1-benzyl-3-[1-(hydroxyimino)ethyl]-2-methyl-1H-benzo[f]indole-4,9-dione (13) and its methoxyimino analog 14 respectively. Reaction of 8 and 9 with procainamide proceeds in the regiospecific and the stereospecific manners to afford (Z)-4-(1-benzyl-9-oxo-1H-benzo[f]indol-4(9H)-ylideneamino)-N-[2-(diethylamino)ethyl]benzamide (15) and (Z)-4-(1-benzyl-2-methyl-9-oxo-1H-benzo[f]indol-4(9H)-ylideneamino)-N-[2-(diethylamino)ethyl]benzamide (16) respectively in a fairly good overall yield. The formation of Z-form product rather than the E-isomer can be realized in which the 4-substituted phenyl group proximate the pyrrole ring is less sterically hindered [22,25].
2.2. Biological Results and Discussion
Certain benzo[f]indole-4,9-dione derivatives were synthesized and evaluated for their inhibitory effects on superoxide anion generation and neutrophil elastase (NE) release in formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLF)-activated human neutrophils and results are shown in Table 1. 1-Benzyl-1H-benzo[f]indole-4,9-dione (8) and its 2-methyl derivative 9 exhibited weak inhibitory effects on superoxide anion generation and were inactive on the inhibition of NE release. (Z)-1-Benzyl-4-(hydroxyimino)-1H-benzo[f]indol-9(4H)-one (10) showed a potent dual inhibitory effect on NE release and superoxide anion generation with IC50 value of 2.78 and 2.74 μM respectively. In contrast, its 2-methyl derivative 11 exhibited only marginal activity on superoxide anion generation and was inactive on the inhibition of NE release. These results indicated that the oxime moiety enhanced anti-inflammatory activities while methyl substituent at C-2 position was unfavorable especially in the inhibition of NE release. The same structure-activity relationships were observed in which 3-acetyl-1-benzyl-2-methyl-1H-benzo[f]indole-4,9-dione (12) was inactive while its oxime derivative 13 exhibited a weak dual inhibitory effect on NE release and superoxide anion generation. Compound 14, the methyl derivative of 13, was inactive. Among these benzo[f]indole-4,9-dione derivatives, (Z)-4-(1-benzyl-9-oxo-1H-benzo[f]indol-4(9H)-ylideneamino)-N-[2-(diethylamino)ethyl]benzamide (15) was the most potent dual inhibitor on NE release and superoxide anion generation with IC50 value of 0.51 and 2.05 μM respectively. Although (Z)-4-(1-benzyl-2-methyl-9-oxo-1H-benzo[f]indol-4(9H)-ylideneamino)-N-[2-(diethylamino)ethyl]benzamide (16) exhibited a strong inhibitory effect on superoxide anion generation with an IC50 value of 0.52 μM, it induced NE release of human neutrophils.
Table 1.
Anti-inflammatory activities of benzo[f]indole-4,9-dione derivatives in formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLF)-activated human neutrophils (IC50 in μM) a.
| Compound | Superoxide Anion | Elastase Release |
|---|---|---|
| 8 | 16.10 ± 1.87 | >30 |
| 9 | 14.04 ± 4.40 | >30 |
| 10 | 2.78 ± 0.89 | 2.74 ± 1.20 |
| 11 | 7.47 ± 1.39 | >30 |
| 12 | >30 | >30 |
| 13 | 10.54 ± 0.52 | 14.65 ± 2.44 |
| 14 | >30 | >30 |
| 15 | 0.51 ± 0.12 | 2.05 ± 0.21 |
| 16 | 0.52 ± 0.11 | – b |
| LY294002 c | 1.36 ± 0.33 | 2.21±0.45 |
a Concentration necessary for 50% inhibition (IC50); Results are presented as mean ± SEM (n = 3); b Alone induced elastase release of human neutrophils; and c LY294002 (a phosphatidylinositol-3-kinase inhibitior) was used as a positive control for superoxide anion generation and elastase release.
These benzo[f]indole-4,9-dione derivatives were evaluated in vitro against a panel of cell lines consisting of MCF7 (breast), NCI-H460 (lung), and SF-268 (CNS) as described previously [27]. Compounds which reduced the growth of any one of the cell lines to 50% or less at the concentration of 4 μg/mL were considered cytotoxic and subjected to further evaluation for their dose–response effects and IC50 measurement. Results from Table 2 indicated compounds 15 and 16 were cytotoxic and their IC50 against three cancer cells and a normal cell, Detroit 551, were shown in Table 3. The IC50 value of compounds 15 and 16 ranged between 3.16 and 27.41 μM and were much less cytotoxic than that of camptothecin (CPT).
Table 2.
Cytotoxicity (% survival rate) of benzo[f]indole-4,9-dione derivatives at 4 μg/mL.
| Cells\Compd | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | CPT a |
|---|---|---|---|---|---|---|---|---|---|---|
| MCF7 | 99 | 105 | 110 | 104 | 78 | 106 | 104 | 82 | 57 | 33 |
| NCI-H460 | 96 | 104 | 110 | 110 | 88 | 111 | 108 | 31 | 18 | 1 |
| SF-268 | 103 | 106 | 106 | 102 | 115 | 109 | 112 | 120 | 106 | 25 |
a CPT: camptothecin.
Table 3.
Inhibition of in vitro cancer cell lines by benzo[f]indole-4,9-dione derivatives [IC50 (μM) ± standard deviation] a.
| Compd\Cells | MCF7 | NCI-H460 | SF-268 | Detroit 551 |
|---|---|---|---|---|
| 15 | 4.98 ± 0.15 | 3.16 ± 0.13 | 27.41 ± 0.39 | 7.68 ± 0.58 |
| 16 | 4.95 ± 0.21 | 3.23 ± 0.34 | 25.76 ± 1.66 | 4.78 ± 0.57 |
| CPT b | 0.57 ± 0.03 | 0.03 ± 0.003 | 0.19 ± 0.006 | 0.99 ± 0.09 |
a Values representative mean ± standard deviation from three experiments; and b CPT: camptothecin.
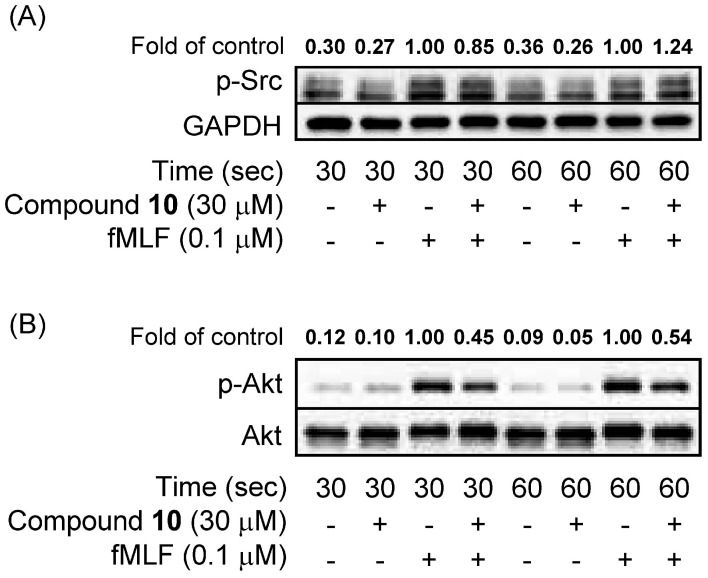
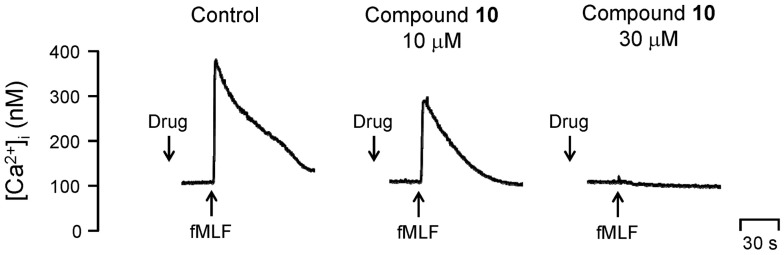
Compound 15 (3, 10 and 30 μM) showed cytotoxicity effects in human neutrophils, as measured by lactate dehydrogenase (LDH) release. In contrast, compound 10, even at high concentration of 30 μM, showed no cytotoxicity effects in human neutrophils (data not shown). The action mechanisms of 10 in human neutrophils were further investigated. Human neutrophil activations, such as respiratory burst and degranulation, are regulated by Akt and calcium signal pathways [28,29]. Therefore, calcuim and Akt are considered as therapeutic target for developing anti-inflammatory agents. Compound 10 did not alter fMLF-induced phosphorylation of Src (Src family Y416) (Figure 2A). Notably, phosphorylation of Akt (S473) and mobilization of [Ca2+]i caused by fMLF was inhibited by compound 10 (Figure 2B and Figure 3).
Figure 2.
Compound 10 inhibits phosphorylation of Akt, but not Src, in fMLF-activated human neutrophils. Quantitation of the p-Scr/GADPH (A) and p-Akt/Akt (B) ratios is shown. Representative images from one of three experiments are shown.
Figure 3.
Compound 10 inhibits fMLF-induced [Ca2+]i increase in human neutrophils. The traces shown are from three different experiments.
3. Experimental Section
3.1. General
TLC: Precoated (0.2 mm) silica gel 60 F254 plates from EM Laboratories, Inc. (Darmstadt, Germany); Detection by UV light (254 nm). All chromatographic separations were performed using silica gel (Merck 60 230–400 mesh, Darmstadt, Germany). M.p.: Yamato MP-21 melting-point apparatus (Yamato Scientific Co., Tokyo, Japan); Uncorrected. 1H and 13C NMR spectra: Varian-Unity-400 spectrometer at 400 and 100 MHz (Varian Inc., Palo Alto, CA, USA), chemical shifts in ppm with SiMe4 as an internal standard (=0 ppm), coupling constants J in Hz. Mass spectra (HRMS) were recorded on Finnigan/Thermo Quest MAT 95XL (ThermoQuest Finnigan, Bremen, Germany). Elemental analyses were carried out on a Heraeus CHN-O-Rapid elemental analyzer (Austin, TX, USA), and results were within ±0.4% of calculated values.
3.1.1. 2-(Benzylamino)naphthalene-1,4-dione (7)
To a stirred solution of naphthalene-1,4-dione (6, 0.16 g, 1.0 mmol) in EtOH (100 mL) was added benzylamine (0.31 g, 3.0 mmol) and refluxed for 12 h (TLC monitoring). The resulting solution was concentrated in vacuo and the residue thus obtained was purified by flash chromatography on silica gel, using hexane/CH2Cl2 (1/1) as eluent and crystallized from MeOH to give 0.21 g (80%) of 7 as a red solid. M.p.: 159–160 °C (lit. 160–161 °C) [23]. 1H-NMR (400 MHz, CDCl3): 4.38 (d, 2H, J = 6.0 Hz), 5.79 (s, 1H), 6.23 (br s, 1H, NH), 7.31–7.40 (m, 5H), 7.62 (ddd, 1H, J = 7.6, 7.2, 1.6 Hz), 7.73 (ddd, 1H, J = 7.6, 7.6, 1.2 Hz), 8.04–8.10 (m, 2H). 13C-NMR (100 MHz, CDCl3): 46.77, 101.71, 126.19, 126.26, 127.61 (2C), 128.11, 128.99 (2C), 130.46, 132.05, 133.50, 134.76, 135.84, 147.69, 181.832, 183.07.
3.1.2. 1-Benzyl-1H-benzo[f]indole-4,9-dione (8)
A mixture of 7 (0.19 g, 1.0 mmol), acetaldehyde (0.22 g, 5.0 mmol) and 1.34 g (5.0 mmol) of Mn(OAc)3 in acetic acid (20 mL) was heated at 80 °C for 16 h (by TLC monitoring). The reaction mixture was diluted with 100 mL of ethyl acetate and washed with H2O (3 × 50 mL), brine (50 mL), dried over anhydrous MgSO4, and evaporated in vacuo. The residue was purified by flash chromatography on silica gel, using a gradient of ethyl acetate/hexane (1/15 to 1/12) as eluent and crystallized from MeOH to give 0.15 g (54%) of 8 as a yellow solid. M.p.: 173–174 °C. 1H-NMR (400 MHz, CDCl3): 5.71 (s, 2H, CH2), 6.79 (d, 1H, J = 2.8 Hz, 3-H), 6.99 (d, 1H, J = 2.8 Hz, 2-H), 7.25–7.37 (m, 5H, Ar-H), 7.65–7.69 (m, 2H, 6- & 7-H), 8.12–8.19 (m, 2H, 5- & 8-H). 13C-NMR (100 MHz, CDCl3): 52.36 (CH2), 108.27 (3-C), 126.49 (2-C), 126.59 (Ar-C), 127.46 (2Ar-C), 128.13 (5-C), 128.91 (2Ar-C), 129.04, 130.37, 130.82 (8-C), 133.06 (6-C), 133.11 (7-C), 133.73, 133.99, 136.39 (Ar-C), 176.23 (9-C), 180.98 (4-C). Anal. calcd for C19H13NO2: C 79.43, H 4.56, N 4.88; found: C 79.49, H 4.55, N 4.88.
3.1.3. 1-Benzyl-2-methyl-1H-benzo[f]indole-4,9-dione (9)
This was prepared from 7 as described in the synthesis of 8 from 6 with acetone (0.29 g, 5 mmol) instead of acetaldehyde, to give 9 as a yellow solid (crystallized from MeOH) in a 58% yield. M.p.: 139–140 °C. 1H-NMR (400 MHz, CDCl3): 2.27 (s, 3H, 2-Me), 5.78 (s, 2H, CH2), 6.60 (s, 1H, 3-H), 7.06–7.08 (m, 2H, Ar-H), 7.23–7.33 (m, 3H, Ar-H), 7.63–7.67 (m, 2H, 6- & 7-H), 8.09–8.17 (m, 2H, 5- & 8-H). 13C-NMR (100 MHz, CDCl3): 12.32 (2-Me), 48.67 (CH2), 107.80 (3-C), 126.19 (2Ar-C), 126.38 (Ar-C), 126.45 (5-C), 127.57 (8-C), 128.57, 128.86 (2Ar-C), 130.21, 132.81 (6-C), 133.02 (7-C), 133.46, 134.21, 136.35 (2-C), 140.23 (Ar-C), 175.48 (9-C), 181.19 (4-C). Anal. calcd for C20H15NO2: C 79.72, H 5.02, N 4.65; found: C 79.71, H 5.03, N 4.64.
3.1.4. 1-Benzyl-4-(hydroxyimino)-1H-benzo[f]indol-9(4H)-one (10)
To a suspension of 8 (0.29 g, 1.0 mmol) in 2-ethoxyethanol (30 mL) was added hydroxylamine hydrochloride (0.20 g, 3.0 mmol). After reflux for 8 h (by TLC monitoring), the cooled mixture was evaporated in vacuo and the residue was poured into H2O (20 mL). The crude product was purified by flash chromatography on silica gel, using MeOH/CH2Cl2 (1/20) as eluent and crystallized from MeOH to give 0.22 g (73%) of 10 as a yellow solid. M.p.: 202–203 °C. 1H-NMR (400 MHz, DMSO-d6): 5.80 (s, 2H, CH2), 7.21–7.34 (m, 6H, 3-H and Ar-H), 7.57–7.69 (m, 3H, 2-, 6- & 7-H), 8.11–8.14 and 8.28–8.31 (m, 2H, 5- & 8-H), 12.76 (s, 1H, NOH). 13C-NMR (100 MHz, DMSO-d6): 51.23 (CH2), 111.54 (3-C), 121.71, 123.22 (2-C), 125.62, 125.76 (Ar-C), 126.93 (2Ar-C), 127.42 (5-C), 128.57 (2Ar-C), 128.91 (8-C), 131.26, 132.00 (6-C), 132.21 (7-C), 133.96, 138.28 (Ar-C), 141.42 (4-C), 174.12 (9-C). Anal. calcd for C19H14N2O2∙0.1H2O: C 75.04, H 4.71, N 9.21; found: C 74.98, H 4.69, N 9.24.
3.1.5. 1-Benzyl-4-(hydroxyimino)-2-methyl-1H-benzo[f]indol-9(4H)-one (11)
This was prepared from 9 as described in the synthesis of 10 from 8, to give 11 as a yellow solid (crystallized from EtOH) in a 73% yield. M.p.: 259–260 °C. 1H-NMR (400 MHz, DMSO-d6): 2.28 (s, 3H, 2-Me), 5.88 (s, 2H, CH2), 7.02–7.04 (m, 2H, Ar-H), 7.16 (s, 1H, 3-H), 7.23–7.34 (m, 3H, ar-H), 7.57–7.68 (m, 2H, 6- & 7-H), 8.10–8.12 and 8.28–8.30 (m, 2H, 5- & 8-H), 12.80 (s, 1H, NOH). 13C-NMR (100 MHz, DMSO-d6): 11.92 (2-Me), 47.80 (CH2), 111.73 (3-C), 121.40, 123.22 (5-C), 125.86 (Ar-C), 126.20 (2Ar-C), 126.26, 127.29 (8-C), 128.83 (2Ar-C), 129.02 (6-C), 131.64, 131.88 (7-C), 133.66, 137.78 (2-C), 140.57 (Ar-C), 141.56 (4-C), 173.48 (9-C). Anal. calcd for C20H16N2O2∙0.6H2O: C 73.43, H 5.30, N 8.56; found: C 73.42, H 5.10, N 8.16.
3.1.6. 3-Acetyl-1-benzyl-2-methyl-1H-benzo[f]indole-4,9-dione (12)
To a solution of 7 (0.26 g, 1.0 mmol), acetylacetone (0.40 g, 4.0 mmol), and CHCl3 (3 mL) in MeOH (20 mL) was added four times with CAN (0.41 g × 4, 3.0 mmol) at 10 min intervals. The result mixture was stirred at room temperature for another 10 min and then diluted with ethyl acetate (100 mL), washed with H2O (50 mL × 3), brine (50 mL), dried over anhydrous MgSO4, and evaporated in vacuo. The residue was purified by flash chromatography on silica gel, using a gradient of MeOH/CH2Cl2 (1/100) as eluent and crystallized from MeOH to give 0.24 g (71%) of 12 as a orange solid. M.p.: 135–136 °C. (lit. 237–238 °C) [26]. 1H-NMR (400 MHz, CDCl3): 2.37 (s, 3H, 2-Me), 2.74 (s, 3H, 3-COMe), 5.84 (s, 2H, 1-NCH2), 7.06–7.09 (m, 2H, Ar-H), 7.25–7.35 (m, 3H, Ar-H), 7.66–7.73 (m, 2H, 6- & 7-H), 8.11–8.13 and 8.15–8.18 (m, 2H, 5- & 8-H). 13C-NMR (100 MHz, CDCl3): 10.92 (2-Me), 31.72 (Me), 48.78 (CH2), 123.12, 125.31, 126.23 (2Ar-C), 126.42 (Ar-C), 126.72 (5-C), 127.83 (8-C), 128.99 (2Ar-C), 129.82, 133.01, 133.25 (6-C), 133.42 (7-C), 133.53, 135.51, 142.03 (Ar-C), 176.23 (9-C), 180.74 (4-C), 199.23 (C=O). Anal. calcd for C22H17NO3: C 76.96, H 4.99, N 4.08; Found: C 77.01, H 4.99, N 4.42.
3.1.7. 1-Benzyl-3-[1-(hydroxyimino)ethyl]-2-methyl-1H-benzo[f]indole-4,9-dione (13)
This was prepared from 12 as described in the synthesis of 10 from 8, to give 13 as a yellow solid (crystallized from MeOH) in a 78% yield. M.p.: 204–205 °C. 1H-NMR (400 MHz, DMSO-d6): 2.12 (s, 3H, 2-Me), 2.21 (s, 3H, 3-C(=N)Me), 5.84 (s, 2H, 1-NCH2), 7.12–7.14 (m, 2H, Ar-H), 7.26–7.37 (m, 3H, Ar-H), 7.77–7.81 (m, 2H, 6- & 7-H), 8.01–8.04 (m, 2H, 5- & 8-H), 11.09 (s, 1H, NOH). 13C-NMR (100 MHz, DMSO-d6): 10.17 (2-Me), 16.03 (Me), 48.29 (CH2), 119.87, 124.85, 125.98 (5-C), 126.03 (Ar-C), 126.33 (2Ar-C), 127.45 (8-C), 128.83 (2Ar-C), 129.21, 132.94, 133.28, 133.46 (6-C), 133.53 (7-C), 136.48, 139.01, 149.88 (C=N), 174.78 (9-C), 179.87 (4-C). Anal. calcd for C22H18N2O3: C 73.73, H 5.06, N 7.82; Found: C 73.56, H 5.04, N 7.76.
3.1.8. 1-Benzyl-3-[1-(methoxyimino)ethyl]-2-methyl-1H-benzo[f]indole-4,9-dione (14)
This was prepared from 12 as described in the synthesis of 10 from 8 and O-methylhydroxylamine hydrochloride instead of hydroxylamine hydrochloride, to give 14 as a yellow solid (crystallized from MeOH) in a 79% yield. M.p.: 168–169 °C. 1H-NMR (400 MHz, DMSO-d6): 2.13 (s, 3H, 2-Me), 2.26 (s, 3H, 3-C(=N)Me), 3.88 (s, 3H, NOMe), 5.84 (s, 2H, 1-NCH2), 7.13–7.15 (m, 2H, Ar-H), 7.27–7.36 (m, 3H, Ar-H), 7.79–7.82 (m, 2H, 6- & 7-H), 8.02–8.05 (m, 2H, 5- & 8-H). 13C-NMR (100 MHz, DMSO-d6): 10.04 (2-Me), 16.68, 48.29, 61.32, 118.66, 124.85, 126.01 (5-C), 126.08 (Ar-C), 126.38 (2Ar-C), 127.47 (8-C), 128.84 (2Ar-C), 129.36, 132.87, 133.25, 133.53 (6-C), 133.61 (7-C), 136.39, 139.09, 151.19 (C=N), 174.86 (9-C), 179.85 (4-C). Anal. calcd for C23H20N2O3: C 74.18, H 5.41, N 7.52; Found: C 74.15, H 5.39, N 7.35.
3.1.9. 4-(1-Benzyl-9-oxo-1H-benzo[f]indol-4(9H)-ylideneamino)-N-[2-(diethylamino)ethyl]benzamide (15)
To a vigorously stirred solution of 8 (0.29 g, 1.0 mmol) in dry dichloromethane (20 mL) at room temperature was added a 1.0 M solution titanium tetrachloride in dichloromethane (1.0 mL, 1.0 mmol). To the resulting violet solution was added a solution of the procainamide (1.2 g, 5.0 mmol) in dichloromethane (10 mL) followed immediately by dry triethylamine (1.78 mL, 12.4 mmol). Two portions of 1.0 M solution of titanium tetrachloride in dichloromethane (2.0 mL, 2.0 mmol each) were added at 30 min intervals, and then the reaction mixture was poured over 100 mL of cold water and extracted with dichloromethane. The organic layer was dried with MgSO4 and evaporated in vacuo. The crude product was chromatographed on a column of silica gel using CH2Cl2/MeOH (10/1) to give 0.34 g (67%) of 15 as a brown solid. M.p.:152–153 °C. 1H-NMR (400 MHz, CDCl3): 1.36 (t, 6H, J = 7.2 Hz, NCH2CH3), 3.07 (q, 4H, J = 7.2 Hz, NCH2CH3), 3.17 (t, 2H, J = 5.2 Hz, NHCH2CH2N), 3.85 (q, 2H, J = 5.2 Hz, NHCH2CH2N), 5.32 (d, 1H, J = 2.8 Hz, 3-H), 5.68 (s, 2H, CH2), 6.67 (d, 1H, J = 2.8 Hz, 2-H), 6.94 (d, 2H, J = 8.8 Hz, Ar-H), 7.17–7.32 (m, 5H, Ar-H), 7.60–7.68 (m, 2H, 7- & 8-H), 8.09 (d, 2H, J = 8.8 Hz, Ar-H), 8.21 (dd, 1H, J = 7.6, 0.8 Hz, 5-H), 8.47 (br s, 1H, NH), 8.50 (dd, 1H, J = 7.2, 0.8 Hz, 8-H). 13C-NMR (100 MHz, CDCl3): 9.36 (2C), 35.88, 48.15 (CH2), 52.26 (2C), 52.94, 110.21 (3-C), 117.72 (2Ar-C), 123.76, 126.07 (5-C), 126.45 (8-C), 127.30 (Ar-C), 127.85, 128.29, 128.53 (2Ar-C), 128.76 (2Ar-C), 129.13 (2Ar-C), 129.81, 130.80 (6-C), 132.39 (7-C), 132.95, 135.80, 136.77, 151.75, 156.24, 167.43 (4-C), 176.07 (9-C). Anal. calcd for C32H32N4O2∙1.9H2O: C 71.33, H 6.70, N 10.40; found: C 71.69, H 6.52, N 10.01.
3.1.10. 4-(1-Benzyl-2-methyl-9-oxo-1H-benzo[f]indol-4(9H)-ylideneamino)-N-[2-(diethylamino)-ethyl]benzamide (16)
This was prepared from 9 as described in the synthesis of 15 from 8, to give 16 as a yellow solid in a 43% yield. M.p.:138–139 °C. 1H-NMR (400 MHz, CDCl3): 1.38 (t, 6H, J = 7.2 Hz, NCH2CH3), 2.02 (s, 3H, 2-Me), 3.08 (q, 4H, J = 7.2 Hz, NCH2CH3), 3.11 (t, 2H, J = 4.8 Hz, , NHCH2CH2N), 3.86 (q, 2H, J = 4.8 Hz, NHCH2CH2N), 5.18 (s, 1H, 3-H), 5.77 (s, 2H, CH2), 6.94–7.01 (m, 4H, Ar-H), 7.20–7.32 (m, 4H, Ar-H), 7.58–7.66 (m, 2H, 6- & 7-H), 8.12 (d, 2H, J = 8.4 Hz, Ar-H), 8.19 (dd, 1H, J = 7.6, 1.2 Hz, 5-H ), 8.48–8.50 (m, 2H, 8-H & NH). 13C-NMR (100 MHz, CDCl3): 9.33 (2C), 12.14 (2-Me), 35.90, 48.40 (CH2), 48.48 (2C), 53.23, 109.80 (3-C), 117.72 (2Ar-C), 123.38, 126.07, 126.10 (2Ar-C), 126.33 (5-C), 127.35 (8-C), 127.91, 128.08, 128.76 (2Ar-C), 129.17 (2Ar-C), 130.76 (6-C), 132.12 (7-C), 133.20, 135.51, 136.77, 138.97, 151.65, 156.35, 167.47 (4-C), 175.31 (9-C). Anal. calcd for C33H34N4O2∙2.3H2O: C 70.77, H 6.95, N 10.00; found: C 70.56, H 6.75, N 9.74.
3.2. Biological Evaluation
3.2.1. Preparation of Human Neutrophils
Blood was taken from healthy human donors (20–30 years old) by venipuncture, using a protocol approved by the institutional review board at Chang Gung Memorial Hospital. Neutrophils were isolated with a standard method of dextran sedimentation prior to centrifugation in a Ficoll Hypaque gradient and hypotonic lysis of erythrocytes [30].
3.2.2. Superoxide Generation and Elastase Release
Superoxide generation and elastase release were carried out according to the procedures described previously [31]. Neutrophils (6 × 105/mL) were equilibrated at 37 °C for 2 min and incubated with compounds for 5 min. Neutrophils were then activated by fMLF (100 nM) in the pretreatment of cytochalasin B (1 μg/mL for superoxide generation and 0.5 μg/mL for elastase release) for 10 min. Superoxide anion production was assayed by monitoring the superoxide dismutase-inhibitable reduction of ferricytochrome c. Elastase release was performed using MeO-Suc-Ala-Ala-Pro-Valp-nitroanilide as the elastase substrate.
3.2.3. Western Analysis
Neutrophils were pretreated with compounds for 5 min before being stimulated with fMLF at 37 °C. The reaction was stopped by adding 5 × Laemmli’s sample buffer [32,33]. Proteins derived from whole-cell lysates were separated by SDS-polyacrylamide gel electrophoresis (PAGE) using polyacrylamide gels and blotted onto nitrocellulose membranes. Immunoblotting was performed using the indicated antibodies and horseradish peroxidase (HRP)-conjugated secondary anti-rabbit antibodies (Cell Signaling Technology, Beverly, MA, USA). The immunoreactive bands were visualized by an enhanced chemiluminescence system (Amersham Biosciences, Bucks, UK) and detected by Ultraviolet Product (UVP) imaging system (Upland, CA, USA).
3.2.4. Measurement of Intracellular Calcium Concentration ([Ca2+]i)
Neutrophils were loaded with fluo-3 AM (2 μM) at 37 °C for 45 min. Cells were preincubated with compounds for 5 min, and then activated by fMLF (100 nM). The change in fluorescence was monitored using a Hitachi F-4500 spectrofluorometer (Tokyo, Japan). The excitation wavelength was 488 nm, and the emission wavelength was 520 nm.
4. Conclusions
We have identified (Z)-1-benzyl-4-(hydroxyimino)-1H-benzo[f]indol-9(4H)-one (10) as a novel structural type of potential anti-inflammatory agent. Compound 10 exhibited dual inhibitory activities on NE release and superoxide anion generation in formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLF)-activated human neutrophils with IC50 value of 2.78 and 2.74 μM respectively. Mechanism studies indicated that compound 10 did not alter fMLF-induced phosphorylation of Src (Src family Y416). Notably, phosphorylation of Akt (S473) and mobilization of [Ca2+]i caused by fMLF was inhibited by compound 10. Further structural optimization of 10 is ongoing.
Acknowledgments
Financial support of this work by the Ministry of Science and Technology of the Republic of China and Chang Gung Memorial Hospital (CMRPD1B0481~3, BMRP450, and EMRPD1E1701 to H-L Hwang) is gratefully acknowledged.
Author Contributions
You-Ren Chen participated in synthesis, purification and characterization of the chemical compounds; Chih-Hua Tseng and Yeh-Long Chen participated in synthesis, the interpretation of the results and in manuscript writing; Tsong-Long Hwang participated in the biological activity, the interpretation of the results and in manuscript writing; Cherng-Chyi Tzeng suggested the research idea, participated in the interpretation of the results and in manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Malech H.L., Gallin J.I. Current concepts: Immunology. Neutrophils in human diseases. N. Eng. J. Med. 1987;317:687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- 2.Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 3.Okajima K., Harada N., Uchiba M. Ranitidine reduces ischemia/reperfusion-induced liver injury in rats by inhibiting neutrophil activation. J. Pharmacol. Exp. Ther. 2002;301:1157–1165. doi: 10.1124/jpet.301.3.1157. [DOI] [PubMed] [Google Scholar]
- 4.Ennis M. Neutrophils in asthma pathophysiology. Curr. Allergy Asthma Rep. 2003;3:159–165. doi: 10.1007/s11882-003-0029-2. [DOI] [PubMed] [Google Scholar]
- 5.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Borregaard N. The human neutrophil. Function and dysfunction. Eur. J. Haematol. 1998;41:401–413. doi: 10.1111/j.1600-0609.1988.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 7.Roos D., van Bruggen R., Meischl C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003;5:1307–1315. doi: 10.1016/j.micinf.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Burgos R.A., Hidalgo M.A., Figueroa C.D., Conejeros I., Hancke J.L. New potential targets to modulate neutrophil function in inflammation. Mini Rev. Med. Chem. 2009;9:153–168. doi: 10.2174/138955709787316092. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh P.W., Hwang T.L., Wu C.C., Chang F.R., Wang T.W., Wu Y.C. The evaluation of 2,8-disubstituted benzoxazinone derivatives as anti-inflammatory and anti-platelet aggregation agents. Bioorg. Med. Chem. Lett. 2005;15:2786–2789. doi: 10.1016/j.bmcl.2005.03.104. [DOI] [PubMed] [Google Scholar]
- 10.Cho H.Y., Kleeberger S.R. Genetic mechanisms of susceptibility to oxidative lung injury in mice. Free Radic. Biol. Med. 2007;42:433–445. doi: 10.1016/j.freeradbiomed.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Meng X.L., Yang J.Y., Chen G.L., Zhang L.J., Wang L.H., Li J., Wu C.F. RV09, a novel resveratrol analogue, inhibits NO and TNF-alpha production by LPS-activated microglia. Int. Immunopharmacol. 2008;8:1074–1082. doi: 10.1016/j.intimp.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Shin H.M., Lee Y.R., Chang Y.S., Lee J.Y., Kim B.H., Min K.R., Kim Y. Suppression of interleukin-6 production in macrophages by furonaphthoquinone NFD-37. Int. Immunopharmacol. 2006;6:916–923. doi: 10.1016/j.intimp.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Meng X.L., Yang J.Y., Chen G.L., Wang L.H., Zhang L.J., Wang S., Li J., Wu C.F. Effects of resveratrol and its derivatives on lipopolysaccharide-induced microglial activation and their structure-activity relationships. Chem. Biol. Interact. 2008;174:51–59. doi: 10.1016/j.cbi.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Shin E.M., Zhou H.Y., Guo L.Y., Kim J.A., Lee S.H., Merfort I., Kang S.S., Kim H.S., Kim S., Kim Y.S. Anti-inflammatory effects of glycyrol isolated from Glycyrrhiza uralensis in LPS-stimulated RAW264.7 macrophages. Int. Immunopharmacol. 2008;8:1524–1532. doi: 10.1016/j.intimp.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Cote B., Boulet L., Brideau C., Claveau D., Ethier D., Frenette R., Gagnon M., Giroux A., Guay J., Guiral S., et al. Substituted phenanthrene imidazoles as potent, selective, and orally active mPGES-1 inhibitors. Bioorg. Med. Chem. Lett. 2007;17:6816–6820. doi: 10.1016/j.bmcl.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Liu F.Z., Fang H., Zhu H.W., Wang Q., Yang Y., Xu W.F. Design, synthesis, and preliminary evaluation of 4-[6-(3-nitroguanidino)hexanamido]pyrrolidine derivatives as potential iNOS inhibitors. Bioorg. Med. Chem. 2008;16:578–585. doi: 10.1016/j.bmc.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Liu S.H., Tzeng H.P., Kuo M.L., Lin-Shiau S.Y. Inhibition of inducible nitric oxide synthase by β-lapachone in rat alveolar macrophages and aorta. Br. J. Pharmacol. 1999;126:746–750. doi: 10.1038/sj.bjp.0702341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng C.H., Lin C.S., Shih P.K., Tsao L.T., Wang J.P., Cheng C.M., Tzeng C.C., Chen Y.L. Furo[3',2':3,4]naphtho[1,2-d]imidazole derivatives as potential inhibitors of inflammatory factors in sepsis. Bioorg. Med. Chem. 2009;17:6773–6779. doi: 10.1016/j.bmc.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 19.Tseng C.H., Tzeng C.C., Shih P.K., Yang C.N., Chuang Y.C., Peng S.I., Lin C.S., Wang J.P., Cheng C.M., Chen Y.L. Identification of furo[3',2':3,4]naphtho[1,2-d]imidazole derivatives as orally active and selective inhibitors of microsomal prostaglandin E(2) synthase-1 (mPGES-1) Mol. Divers. 2012;16:215–229. doi: 10.1007/s11030-011-9347-9. [DOI] [PubMed] [Google Scholar]
- 20.Tsai Y.R., Huang L.J., Lee M.R., Chen Y.L., Kuo S.C., Tzeng C.C., Hsu M.F., Wang J.P. The signaling mechanisms mediating the inhibitory effect of TCH-1116 on formyl peptide-stimulated superoxide anion generation in neutrophils. Eur. J. Pharm. 2012;682:171–180. doi: 10.1016/j.ejphar.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Tseng C.H., Cheng C.M., Tzeng C.C., Peng S.I., Yang C.L., Chen Y.L. Synthesis and anti-inflammatory evaluations of β-lapachone derivatives. Bioorg. Med. Chem. 2013;21:523–531. doi: 10.1016/j.bmc.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 22.Tseng C.H., Chen Y.L., Yang S.H., Peng S.I., Cheng C.M., Han C.H., Lin S.R., Tzeng C.C. Synthesis and antiproliferative evaluation of certain iminonaphtho[2,3-b]furan derivatives. Bioorg. Med. Chem. 2010;18:5172–5182. doi: 10.1016/j.bmc.2010.05.062. [DOI] [PubMed] [Google Scholar]
- 23.Aristoff P.A., Johnson P.D. Synthesis of CBI-PDE-I-dimer, the benzannelated analog of CC-1065. J. Org. Chem. 1992;57:6234–6239. doi: 10.1021/jo00049a035. [DOI] [PubMed] [Google Scholar]
- 24.Urbanek R.A., Suchard S.J., Steelman G.B., Knappenberger K.S., Sygowski L.A., Veale C.A., Chapdelaine M.J. Potent reversible inhibitors of the protein tyrosine phosphatase CD45. J. Med. Chem. 2001;44:1777–1793. doi: 10.1021/jm000447i. [DOI] [PubMed] [Google Scholar]
- 25.Ito C., Katsuno S., Kondo Y., Tan H.T., Furukawa H. Chemical constituents of Avicennia alba. Isolation and structural elucidation of new naphthoquinones and their analogues. Chem. Pharm. Bull. 2000;48:339–343. doi: 10.1248/cpb.48.339. [DOI] [PubMed] [Google Scholar]
- 26.Tseng C.C., Wu Y.L., Chuang C.P. Cerium salts in the oxidative free radical reactions between 2-amino-1,4-naphthoquinones and β-dicarbonyl compounds. Tetrahedron. 2002;58:7625–7633. doi: 10.1016/S0040-4020(02)00864-5. [DOI] [Google Scholar]
- 27.Chen Y.C., Cheng M.J., Lee S.J., Dixit A.K., Ishikawa T., Tsai I.L., Chen I.S. Coumarinolignans from the Root of Formosan Antidesma pentandrum var.barbatum. Helv. Chim. Acta. 2004;87:2805–2811. doi: 10.1002/hlca.200490251. [DOI] [Google Scholar]
- 28.Hwang T.L., Su Y.C., Chang H.L., Leu Y.L., Chung P.J., Kuo L.M., Chang Y.J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res. 2009;50:1395–1408. doi: 10.1194/jlr.M800574-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang T.L., Wang C.C., Kuo Y.H., Huang H.C., Wu Y.C., Kuo L.M., Wu Y.H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010;80:1190–1200. doi: 10.1016/j.bcp.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Boyum A., Lovhaug D., Tresland L., Nordlie E.M. Separation of leucocytes: Improved cell purity by fine adjustments of gradient medium density and osmolality. Scand. J. Immunol. 1991;34:697–712. doi: 10.1111/j.1365-3083.1991.tb01594.x. [DOI] [PubMed] [Google Scholar]
- 31.Hwang T.L., Li G.L., Lan Y.H., Chia Y.C., Hsieh P.W., Wu Y.H., Wu Y.C. Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. Med. 2009;46:520–528. doi: 10.1016/j.freeradbiomed.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert C., Rollet-Labelle E., Caon A.C., Naccache P.H. Immunoblotting and sequential lysis protocols for the analysis of tyrosine phosphorylation-dependent signaling. J. Immunol. Methods. 2002;271:185–201. doi: 10.1016/S0022-1759(02)00347-2. [DOI] [PubMed] [Google Scholar]
- 33.Chan H.H., Hwang T.L., Thang T.D., Leu Y.L., Kuo P.C., Nguyet B., Dai D.N., Wu T.S. Isolation and synthesis of melodamide A, a new anti-inflammatory phenolic amide from the leaves of Melodorum fruticosum. Planta Med. 2013;79:288–294. doi: 10.1055/s-0032-1328131. [DOI] [PubMed] [Google Scholar]