Abstract
Objective:
The objective of the study was to assess the incidence and pattern of drug-drug interactions (DDIs) in hypertensive patients by using Micromedex and Medscape databases.
Materials and Methods:
A prospective observational study was carried out in a superspeciality hospital setting in South India for period of 9 months. Hypertensive patients who admitted into the hospital with the age more than 18 years, received more than 3 drugs per prescription and length of hospital stay for more than 24 hours were included in the study. An appropriate data was collected and assessed for DDIs with the help of Micromedex and Medscape databases.
Results:
A total of 227 patients were enrolled during the study period. Among the 227 patients, 48 of them developed 53 clinically significant DDIs. Out of 48 patients, most of them were in the age-group of 50–60 years [18 (37.49%)]. The percentage of DDIs were higher in males [30 (62.5%)] compared to females [18 (37.5%)]. The most common drugs responsible for DDIs in the present study were Insulin [18 (33.96%)] followed by Metoprolol [10 (18.86%)], Torsemide [8 (15.09%)], and Hydrochlorothiazide [8 (15.09%)]. The most commonly interacting pairs were Ciprofloxacin-Insulin [6 (11.32%)], followed by Metoprolol-Insulin [4 (7.54%)] and Atenolol-Insulin [4 (7.54%)]. The most common consequences of interacting pairs were reduced serum potassium levels and hyperglycemia.
Conclusion:
The overall incidence rate of DDIs was found to be 21.14% and the increasing number of co-morbidities (P ≤ 0.003) and polypharmacy (P ≤ 0.002) were the risk factor for the development of significant number of DDIs.
Keywords: Drug-drug interactions, hypertension, incidence, micromedex, medscape
INTRODUCTION
Presently, drug therapy is growing more complex; as a result, making an appropriate decision on drug therapy is increasingly challenging. Drug-drug interactions (DDIs) are defined as two or more drugs interacting in such a manner that the effectiveness or toxicity of one or more drugs is altered on administration of the other. A drug interaction is the quantitative or qualitative modification of the effect of a drug by the simultaneous or successive administration of a different one.[1] Cardiovascular diseases account major part of all morbidities and mortalities worldwide. It has been predicted that by the year 2020, the worldwide cardiovascular diseases burden will be amplified by almost 75%.[2] Hypertension is directly accountable for 57% of all stroke and 24% of all coronary heart disease mortalities in India.[3]
Every year, a proportionate number of anti-hypertensive drugs are being introduced, thus new possible interactions between medications are increasing day by day, leading to the increased risk of hospitalization, healthcare cost and use. International studies have estimated that between 1% and 21% of adverse drug events related hospital admissions are due to drug interactions.[4,5] Moreover, most pharmacists and physicians depend on their own experience to detect or to avoid drug interactions.
Patients with hypertension are particularly vulnerable to DDIs due to their advanced age, gender, polypharmacy, increasing length of hospital stay, and the influence of heart disease on drug metabolism. The DDIs potential for a particular anti-hypertensive drug varies with the individual, the disease being treated, and the extent of exposure to other drugs. There were less number of studies reported DDIs among hypertensive patients in the Indian setting.[6] Hence, the present study was designed to assess the incidence and pattern of clinically significant DDIs in hospitalized hypertensive patients at a superspeciality hospital, with the assessment of reaction characteristics, outcome, and management.
MATERIALS AND METHODS
Study design and population
A prospective observational study was carried out for a period of 9 months (January–September 2012) in a superspeciality hospital. This hospital is envisaged to be a 400-bedded hospital in Hanamkonda, Telangana State, South India with a view to provide superspeciality level healthcare to people. Ethical approval was obtained from institutional ethics committee prior to initiation of the study according to the institution regulations. Patients admitted to the hospital with hypertension were screened for DDIs. Patients of either sex, age more than 18 years, more than 24 hours hospital stay, and the patients received more than 3 drugs per prescription were included in the study. Patients who were not interested to participate in the study and who visited the hospital on out-patient basis were excluded from the study. A brief flow chart of the study method was represented in Figure 1.
Figure 1.
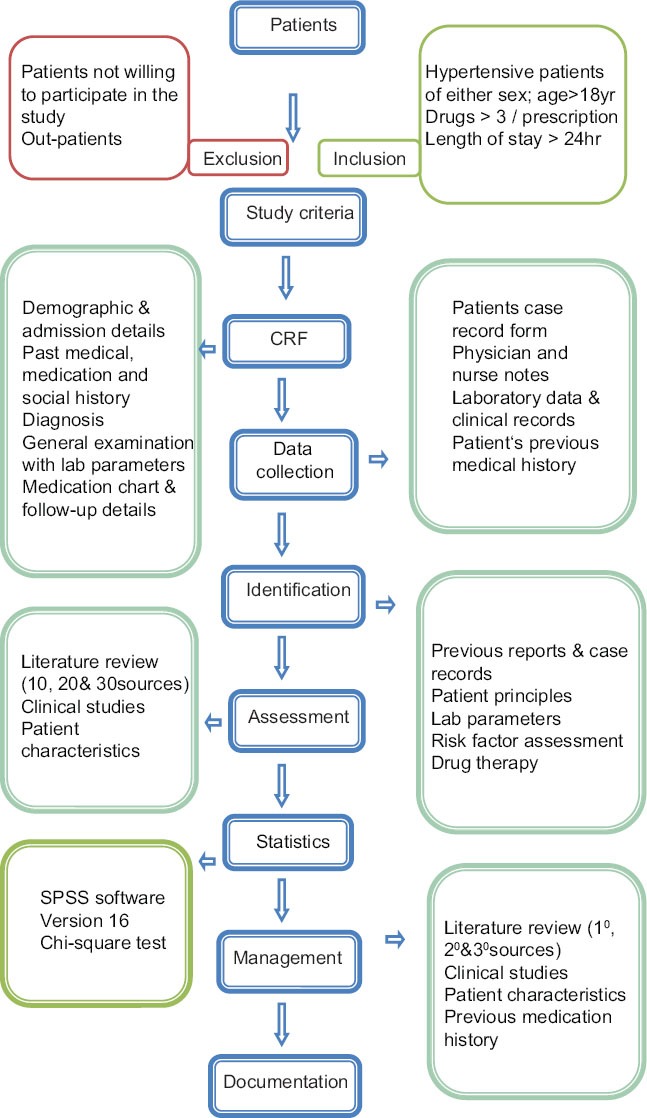
Flow chart of the study method
Data collection
Demographic details, length of hospital stay, habit history (alcohol and tobacco), diagnosis, general examination with lab parameters, medication chart, and follow-up details were collected from patient's case record form, physician, and nurse notes. Laboratory data reports and clinical records of patient's previous medical history were collected from patient/caretaker through an interview. All drugs were classified as per Anatomical Therapeutic Chemical Classification code (ATC).[7,8]
Certain demographic characteristics were considered to identify the predictors of DDIs, such as patient characteristics [age, gender, co-morbidities, and length of hospital stay], and number of drugs per prescription. Risk factor and drug therapy assessment were also performed to identify DDIs by using some previous and case records.
All the identified interactions were assessed for clinical occurrence by considering patient parameters (lab reports follow-up and review details), literature review like primary (journals and reports), secondary (data bases like Micromedex and Medscape), and tertiary (text books) sources. Clinically significant interactions are those DDIs observed in the patients and where as the potential drug-drug interactions (pDDIs) are those not observed in the patients but it gives signal for the detection of interactions.[9] The required guidance in order to manage particular DDIs were provided to physician by referring information provided in tools used for DDI identification as a primary source and patient characteristics and previous medication history as a secondary source.
Tools for identifying DDIs
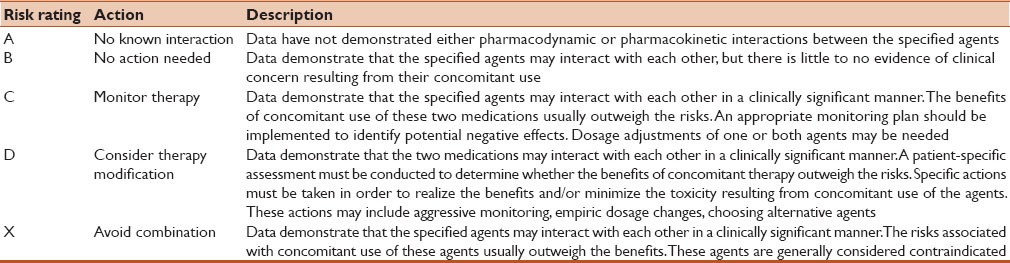
Micromedex electronic database system and Medscape multidrug interaction checker tool were used to identify and analyse the pattern of DDIs.[10,11] Micromedex contains a separate section on DDIs known as the Drug-REAX System. On entering the drugs one by one, the program lists the possible DDIs and categorizes DDIs according to their interaction effect, severity (contra-indicated, serious, moderate, mild, and unknown), onset (rapid, delayed, and unspecified), management, documentation status (excellent, good, fair, poor, unlikely, and unknown), and literature reports. Medscape contains a separate tool for detecting DDIs known as the multidrug interaction checker tool. On entering the drugs one by one, the program lists the possible DDIs and categorizes DDIs according to their interaction effect, severity (contra-indicated, serious, significant, and minor), and management. Risk rating of identified interactions were categorized in Table 1.[12]
Table 1.
Criteria for risk rating drug-drug interactions (DDIs)[11]
Statistical analysis
Chi-square test was used to find the association between age, gender, social history, length of hospital stay, number of co-morbidities, and number of drugs per prescription. A P value of < 0.05 was considered statistically significant. All analyses were performed using Statistical Package for the Social Sciences (SPSS) version 16.
RESULTS
A total of 227 patients were enrolled during the study period and assessed for DDIs. The overall incidence rate of DDIs in the present study was found to be 21.14%.
Patient characteristics
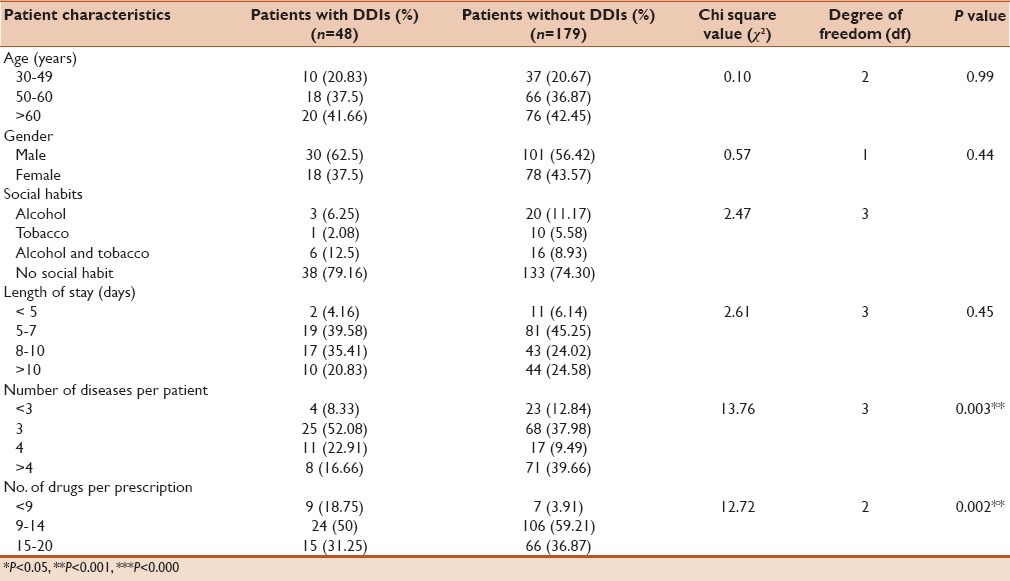
Among the 227 patients, 48 of them developed 53 clinically significant interactions. Out of 227 patients, 73 (32.15%) patients were found without any DDIs and patients with DDIs expected but not observed clinically were 111 (48.89%). Among 53, 23 interactions in 21 patients from Micromedex database and 30 interactions were developed in 27 patients from Medscape database. Out of 53 DDIs, two common interactions were observed. Most of the patients were in the age-group of 50–60 years [18 (37.5%)] happened followed by other age-groups. The percentage of DDIs were higher in males [30 (62.5%)] compared to females [18 (37.5%)]. The patients, who stayed for 5–7 days in the hospital [19 (39.55%)] developed DDIs were more frequently than other groups. On an average, each patient had 3 coded diagnosis/co-morbidities [25 (52.08%)] in which stroke was the most common condition [76 (33.92%)], followed by diabetes mellitus [24 (10.57%)], chronic renal failure [15 (6.6%)] and others [111 (48.89%)]. Most of the patients were prescribed with 9–14 drugs per prescription [24 (50%)]. Patient characteristics and Chi-square and P values of the DDIs were summarized in Table 2.
Table 2.
Statistical analysis for patient characteristics
Characteristics of drugs and DDIs
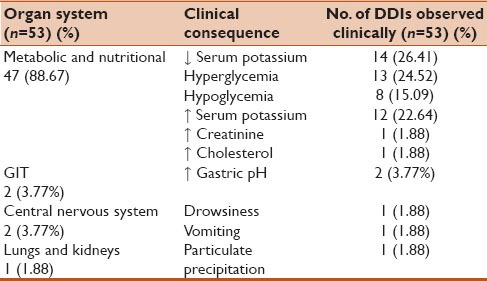
Out of 53 DDIs, the most common drugs responsible for DDIs in the present study were Insulin [18 (33.96%)] followed by Metoprolol [10 (18.86%)], Torsemide [8 (15.09%)] Hydrochlorothiazide [8 (15.09%)], and others. The most commonly interacting pairs were Ciprofloxacin-Insulin [6 (11.32%)], Metoprolol-Insulin [4 (7.54%)], followed by Atenolol-Insulin [4 (7.54%)] and others. Clinically important DDIs among the prescribed drugs from the Medscape and Micromedex data bases were summarized in Tables 3 and 4. The most common clinical consequences of DDIs were reduced serum potassium levels [14 (26.41%)] followed by hyperglycaemia [13 (24.52%)] and the organ systems affected were metabolic and nutritional [47 (88.67%)] followed by gastrointestinal tract (GIT) [2 (3.77%)] and others were summarized in Table 5.
Table 3.
Clinically important drug-drug interactions (DDIs) among the prescribed drugs from Medscape database
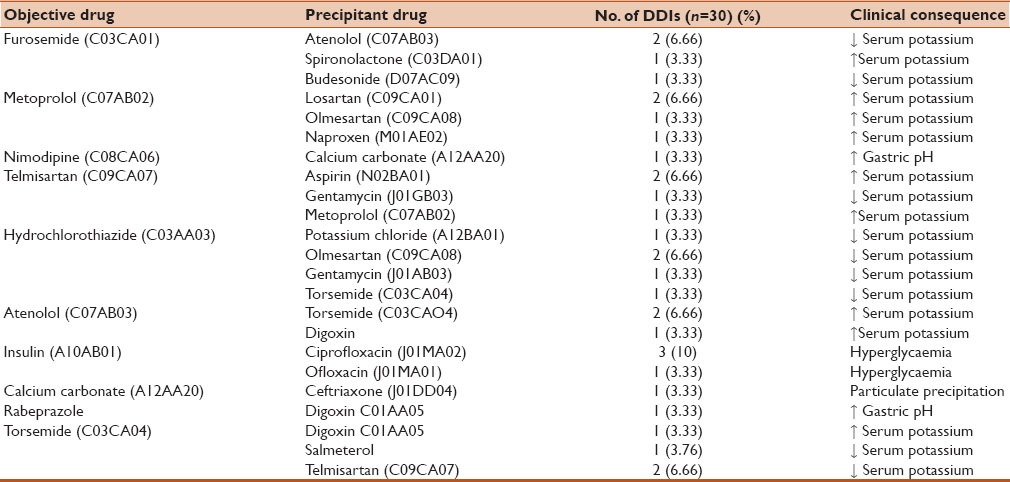
Table 4.
Clinically important drug-drug interactions (DDIs) among the prescribed drugs from Micromedex database
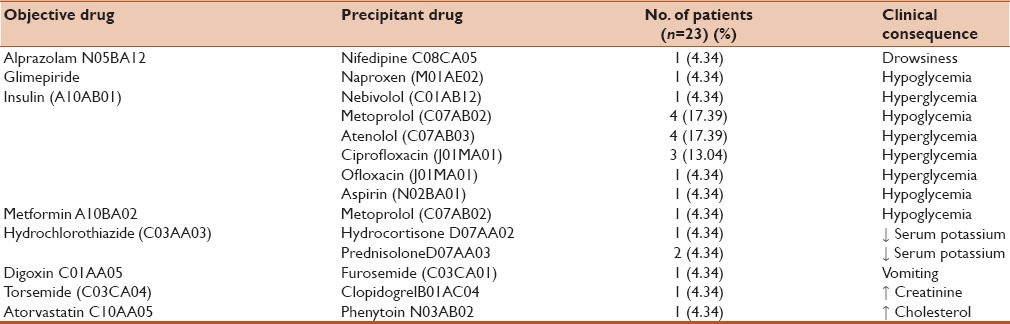
Table 5.
Clinical consequences of drug-drug interactions (DDIs)
In terms of severity, most of the DDIs were moderate/significant in nature [45 (84.90%)] followed by major/serious [5 (9.43%)], contraindicated [2 (3.77%)], and minor [1 (1.88%)]. Pharmacokinetic interactions [33 (62.26%)] presented a predominance in relation to pharmacodynamic interactions [20 (37.73%)]. Among the pharmacokinetic mechanisms of the interactions, the most frequent process was metabolism, which corresponded to up to 86.14% of the interactions. In terms of risk rating, category C was most prevalent [45 (84.9%)] followed by category D [5 (9.43%)], category X [2 (3.77%)], and category B [1 (1.88%)]. With respect to onset, delayed interactions were the most prevalent [17 (32.07%)], with frequencies of greater than 50% observed at the different times of hospitalization. The data of last characteristic “onset” was applicable only to the interactions observed from Micromedex tool, since the information on this specific characteristic was not mentioned in the multidrug interaction tool of Medscape database.
DISCUSSION
In the present study, DDIs were assessed with the help of Drug REAX-Micromedex system and Medscape multidrug interaction checker tool. In most of the previous studies, DDIs were assessed with the help of resources like Drug REAX-Micromedex system (Dinesh KU et al.,) Drug REAX-Micromedex system 2008 (Virendra KP et al.,) and Vidal, British National Formulary, Drug Interaction Facts and Drug REAX (Elizabeth ER et al.,).[6,13,14]
The overall incidence rate of DDIs in the present study was found to be 21.14% which was lower than incidence rate of pDDIs study conducted by Virendra KP et al., (30.67%) and more than the study conducted by Mateti UV et al., (14.66%).[6,15]
In the present study, most of the patients were in the age-group of 50–60 years (37.5%) when compared to a study conducted by Aravind NC et al., (46%). In general, elderly patients are at higher risk for DDIs because they are likely to have multiple diseases and polypharmacy that usually occur with an increased duration of disease condition and altered physiology. In many of the reported studies, age more than 60 was reported as an independent risk factor for DDIs.[12]
In the present study, males (62.5%) were at a higher risk than females (37.5%) of experiencing DDIs. In general, hypertension was more numerous in men, which may increase their vulnerability to polypharmacy and may bring about a higher incidence rate of DDIs. Our findings were similar in the studies conducted by Shobha C et al., (males 62%), Dinesh KU et al., (males 58%), and Virendra KP et al., (males 66%).[6,12,13]
In our study, the patients who consumed both alcohol and tobacco were more [6 (12.5%)] when compared to patients who consumed either alcohol or tobacco. Patients with length of hospital stay 5–7 days were found more in number [19 (39.55%)]. This was correlated with the study conducted by Virendra KP et al., the length of hospital stay was 12 ± 3 days (range 2–31) days. A linear correlation was found between length of stay and percentage of drug interactions as the chance of getting multiple drugs increases with longer stays in the hospital.[6]
In our study, 50% of the patients were with three co-morbidities and patients with 9–14 drugs per prescription were also found to be 50%. It was seen that there was a linear increase in the percentage incidence of drug interactions with an increase in the number of co-morbidities and drugs prescribed to the patient. This has been correlated with the previous reports published by other authors.[15]
Out of 53 identified significant DDIs from the Micromedex and Medscape interaction tools, there were only two common interacting pairs such as Ciprofloxacin-Insulin and Ofloxacin-Insulin. The concomitant administration of ciprofloxacin and insulin were led to the symptoms of hyperglycemia (includes increased thirst, hunger, and increased urination) and also increased blood glucose levels in the patients. This indicates that there will be a chance of developing diabetic co-morbidities. Careful monitoring of blood glucose levels was recommended for safe use of both medications. In the study conducted by Shobha C et al., 67% of drug interacting pairs were between Paracetamol-Pantoprazole and requires monitoring of therapy.[12]
Study conducted by Virendra KP et al., Torsemide is more commonly involved drug [diuretic] responsible for DDIs.[6] Whereas in the present study, Insulin [18 (33.96%)] followed by Metoprolol [10 (18.86%)], Torsemide [8 (15.09%)], and Hydrochlorothiazide [8 (15.09%)] were the most common drugs responsible for DDIs. In our study, out of 53 interactions observed, 45 (84.9%) of the DDIs were moderate and significant (Micromedex and Medscape findings, respectively) in severity. These DDIs suggest that there is a need for monitoring therapy and/or dosage adjustment. These were also correlated with the studies conducted by Herrlinger (90%), Shobha C et al., (60%), Dinesh KU et al., (92%), and Virendra KP et al., (60%).[6,12,13,16]
In our study, most of the drug interactions were pharmacokinetic [33 (62.26%)] in nature followed by pharmacodynamic interactions [20 (37.37%)]. This data varies in the study conducted by Vonbach and Aparasu were 76% were pharmacokinetic and 22% were pharmacodynamic interactions.[17,18] In this study, most [17 (32.07%)] of the DDIs were delayed type. For example, in case of Atenolol and Insulin, the interaction effect is delayed. This suggests that the need for counselling patients who are at a risk for experiencing these DDIs. These findings were slightly lower than those of the study conducted by Dinesh KU et al., 71% and Virendra KP et al., 52%.[6,13]
Among all the DDIs observed, 45 (84.9%) of the DDIs were of the risk rating C and 6 (11.32%) DDIs were with risk rating D, these findings were in contrary to the study conducted by Aravind NC et al., where interactions of risk C were 30% and risk D were of 67%.[7] Age [23 (43.39%)], pharmacokinetic and pharmacodynamic [30 (56.6%)] factors were the predisposing factors responsible for all the interactions observed clinically.[12]
Anti-hypertensives, anti-diabetics, Nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, calcium supplements, anti-asthmatics and cardiac glycosides were most commonly prescribed classes of drugs in the present study. This was also correlated with the study conducted by Dinesh KU et al., showed that anti-hypertensives, NSAIDs, anti-diabetics, anti-histamines, anti-depressants, and proton pump inhibitors were prescribed commonly.[13] Out of 42 drugs prescribed, 15 were anti-hypertensives (38.09%) and among them beta-blockers were predominant (50.72%). A study conducted by Dinesh KU et al., shows that 50% of interacting drugs were anti-hypertensives.[13]
Limitations
DDIs were identified based on the information obtained from the Micromedex and Medscape databases only. Some of the drugs were not found in Micromedex and Medscape databases. The study was limited to in-patients and the outpatients were excluded from the study. Coumadins are the one major potential source of significant DDIs, however the significant DDIs were not found in this study. Certainly, the level, location, populations of patients, and the settings of the hospital play an important role in the type of end results for significant DDIs. The close monitoring and long-term follow-up is required to identify the significant DDIs. Controlled studies were needed in the future discipline to evaluate whether good clinical management of DDIs can reduce medication-related morbidity and mortality.
CONCLUSION
The overall incidence rate of DDIs was found to be 21.14% and the most commonly interacting pairs were Ciprofloxacin-Insulin followed by Atenolol-Insulin, Metoprolol-Insulin. The DDIs were found to be more common in males, elders, patients with more than 3 co-morbidities, and polypharmacy. The considerable numbers of DDIs were moderate in severity, risk rating C category, and delay in onset. Both the Micromedex and Medscape interaction tools are considered for identification and assessment of DDIs and only two common interacting pairs were found in present study. Thorough medication review programs should be focussed and implemented in hospitals to avoid life threatening DDIs and ensuring better patient care.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Morales-Olivas FJ, Estañ L. Antihypertensive drug-drug interactions. Med Clin. 2005;124:782–9. doi: 10.1157/13075851. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R. Burden of coronary heart disease in India. Indian Heart J. 2005;57:632–8. [PubMed] [Google Scholar]
- 3.Gupta R. Trends in hypertension epidemiology in India. J Hum Hypertens. 2004;18:73–8. doi: 10.1038/sj.jhh.1001633. [DOI] [PubMed] [Google Scholar]
- 4.Jankel C, Fitterman LK. Epidemiology of drug-drug interactions as a cause of hospital admissions. Drug Saf. 1993;9:51–9. doi: 10.2165/00002018-199309010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as a cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ. 2004;329:15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel VK, Acharya LD, Rajakannan T, Surulivelrajan M, Guddattu V, Padmakumar R. Potential drug interactions in patients admitted to cardiology wards of a South Indian teaching hospital. Australas Med J. 2011;4:9–14. doi: 10.4066/AMJ.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization ATC/DDD Index 2012. WHO collaborating centre for drug statistics methodology. [Last accessed on 2012 July]. Available from: http://en.wikipedia.org/wiki/Anatomical_Therapeutic_Chemical_Classification_System .
- 8.World Health Organization ATCvet Index 2012. WHO collaborating centre for drug statistics methodology. [Last accessed on 2012 Aug]. Available from: http://en.wikipedia.org/wiki/Anatomical_Therapeutic_Chemical_Classification_System .
- 9.Chauhan N, Moin S, Pandey A, Mittal A, Bajaj U. Indian aspects of drug information resources and impact of drug information centre on community. J Adv Pharm Technol Res. 2013;4:84–93. doi: 10.4103/2231-4040.111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DRUGDEX® System [database on CD-ROM]. Version 5.1. Greenwood Village, Colorado: Thomson Reuters (Healthcare); [Google Scholar]
- 11.WebMD LLC Copyright© in 1994-2012. [Last accessed on 2012 Mar]. Available from: http://reference.medscape.com/drug-interactionchecker .
- 12.Arvind K, Umesh, Shobha M. Assessment of drug-drug interactions in hospitalised patients in India. Asian J Pharm Clin Res. 2011;4:62–5. [Google Scholar]
- 13.Dinesh K, Subish P, Pranaya M, Ravi Shankar P, Anil S, Durga B. Pattern of potential drug-drug interactions in diabetic out-patients in a tertiary care teaching hospital in Nepal. Med J Malaysia. 2007;62:294–8. [PubMed] [Google Scholar]
- 14.Roughead EE, Kalisch LM, Barratt JD, Gilbert AL. Prevalence of potentially hazardous drug interactions amongst Australian veterans. Br J Clin Pharmacol. 2010;70:252–7. doi: 10.1111/j.1365-2125.2010.03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mateti U, Rajakannan T, Nekkanti H, Rajesh V, Mallaysamy S. Drug-drug interactions in hospitalized cardiac patients. J Young Pharm. 2011;3:329–33. doi: 10.4103/0975-1483.90246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrlinger C, Klotz U. Drug metabolism and drug interaction in the elderly. Best Pract Res Clin Gastroenterol. 2001;15:897–918. doi: 10.1053/bega.2001.0249. [DOI] [PubMed] [Google Scholar]
- 17.Vonbach P, Dubied A, Krahenbuhl S, Beer JH. Prevalence of drug-drug interactions at hospital entry and during hospital stay of patients in internal medicine. Eur J Intern Med. 2008;19:413–20. doi: 10.1016/j.ejim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426–37. doi: 10.1016/j.sapharm.2006.12.002. [DOI] [PubMed] [Google Scholar]


