Abstract
Background:
The rapid expansion of trials in emerging regions has raised valid concerns about research subject protection, particularly related to informed consent. The purpose of this study is to assess informed consent form (ICF) compliance with Good Clinical Practice (GCP) guidelines and the readability easeof the ICFs in Abu Dhabi, a potential destination for clinical trials in the UAE.
Materials and Methods:
A multicenter retrospective cross-sectional analysis of 140 ICFs from industry sponsored and non-sponsored studies was conducted by comparing against a local standard ICF. Flesch-Kincaid Reading Scale was used to assess the readability ease of the forms.
Results:
Non-sponsored studies had significantly lower overall GCP compliance of 55.8% when compared to 79.5% for industry sponsored studies. Only 33% of sponsored and 16% of non-sponsored studies included basic information on the participants' rights and responsibilities. Flesch-Kincaid Reading ease score for the informed consent forms from industry sponsored studies was significantly higher 48.9 ± 4.8 as compared to 38.5 ± 8.0 for non-sponsored studies, though both were more complex than recommended. Reading Grade Level score was also higher than expected, but scores for the ICFs from the industry sponsored studies were 9.7 ± 0.7, significantly lower as compared to 12.2 ± 1.3 for non-sponsored studies.
Conclusion:
In spite of the undisputed benefits of conducting research in emerging markets readability, comprehension issues and the lack of basic essential information call for improvements in the ICFs to protect the rights of future research subjects enrolled in clinical trials in the UAE.
Keywords: Clinical trials, GCP, informed consent, readability
INTRODUCTION
In the United States, federal regulations hold Institutional Review Boards (IRBs) responsible for reviewing research protocols involving human subjects and for ensuring the adequacy of subject protection.[1] Globally, the International Conference on Harmonization-Good Clinical Practice (ICH-GCP) protects research participants and assures that research is conducted in a manner that prevents harm.[2] Many countries in the Middle-East follow ICH guidelines for GCP, World Medical Association- Declaration of Helsinki and its elaboration in the Council for International Organizations of Medical Sciences (CIOMS).[3,4,5] GCP mandates that informed consent be obtained from each research subject prior to study enrollment. The informed consent form (ICF) plays an essential role in this process.[6] Despite stringent federal regulations, studies suggest that IRB review may not guarantee inclusion of informed consent requirements, and may not adequately protect subjects.[7] Controversial studies, including the Guatemala syphilis studies and the antiretroviral drug trials in Africa, have led to reassessment of the informed consent process for international research.[8] Both mature and emerging regions report issues with informed consent forms relating to high complexity and poor readability.[9,10]
The subject information document (SID), a component of the ICF that details minimum essential information, often contains complex information regarding the risks and benefits of study participation.[11] Given the increasing technical sophistication of diagnostic and experimental procedures in clinical trials, achieving adequate subject understanding of the ICF has become more challenging. Cultural factors and language barriers pose additional threats to the readability of the already complex ICF.[12,13]
In recent years, both Dubai and Abu Dhabi in the United Arab Emirates (UAE) have become increasingly popular sites for clinical trials. Contributing factors include the country's growing population, state-of-the-art healthcare facilities, treatment naïve subjects, and increasing prevalence of non-communicable and rare genetic diseases.[14] Among the Gulf Cooperation Countries, the UAE, with an increase in clinical trials from 3 in 2000 to 72 in 2013, ranks second only to Saudi Arabia (280), and is followed by Kuwait (43) and Qatar (41).[15] Unlike the US, where the majority (57.4%) of research is sponsored by pharmaceutical and medical devices companies, most (85%) research activities in the UAE are non-industry supported.[16] Although guidelines regulate the ethics of clinical research and subject protection in the UAE,[17] evidence regarding the structure and content of information provided in the ICF and its compliance with standard GCP guidelines is lacking. These concerns prompted the authors to investigate the nature of information provided in the ICF, and to assess compliance with GCP guidelines and readability of informed consent forms used in clinical trials in Abu Dhabi, the capital and largest emirate in the UAE.
MATERIALS AND METHODS
The subject information document (SID) and the consent form together make up the informed consent form (ICF).[11] The globally accepted ICH-GCP guidelines mandate the inclusion of minimum essential items in the SID.[18] UAE Research ethics committees (REC) are required to use ICH-GCP guidelines for ICFs as a standard reference for clinical trials.[17,19]
A multicenter retrospective cross-sectional analysis of 140 informed consent forms was conducted at two Joint Commission-International accredited hospitals in Abu Dhabi, UAE. The study was approved by the local research ethics committee and ICFs accessed following a confidentiality disclosure agreement. Study protocols containing ICF (SID and consent form) as a package from all prospective interventional or observational drug evaluation trials, epidemiologic, or other studies involving human participants submitted for initial review by the Research Ethics Committee (REC) between January 2009 and October 2013 were considered eligible. Both, industry sponsored and non-sponsored trials were included. Altered or amended SID-ICFs undergoing subsequent review were excluded. Out of 156 studies submitted to the REC, 140 met eligibility requirements. Each ICF was compared separately with the standard GCP-ICF and the information was coded by a multilingual physician-investigator blinded to the study hypothesis and verified by a second physician rater. ICF information that was in accordance with the standard GCP requirements, specified in the study protocol and described in non-technical English, was coded as “essential information item present” and scored as + 1.[20,21] If an item was partially or completely absent or did not fulfill all criteria, it was labeled as “essential information item absent” and scored as 0.[20,21] The presence/absence of a certified local language translation, such as English to Arabic, was scored as +1 or -1. A minimum score of 0 points and maximum score of 18 points were possible for each ICF.[20,21] Further, a readability test was applied to all 140 ICFs. The Flesch-Kincaid scale was chosen because of its convenience for computerized use, excellent reproducibility and high correlation with other established readability scales.[22] The Flesch-Kincaid reading ease determines comprehension difficulty of written text on a scale of 0-100, with higher scores indicating that the material is easier to read. The Flesch-Kincaid grade level formula translates the score to a grade level, indicating the number of years of education generally required to understand a given text. Data was statistically analyzed using SPSS, and was expressed as percentage or as mean+/- SD. Inter-rater reliability was determined using Cohen's kappa.[23]
RESULTS
The ICFs reviewed were from several different therapeutic areas, including cardiovascular, endocrine, infectious, renal, and neurologic diseases. ICFs selected were evenly distributed between sponsored and non-sponsored studies. Of the 140 studies, 75% (n = 105) constituted observational studies (sponsored n = 25, non-sponsored n = 80), followed by 21.4% (n = 30) Phase III studies (sponsored n = 9, non-sponsored n = 21). Phase II (3.6%, n = 5) were all sponsored clinical trials. Phase I studies are not permitted by regulatory authorities in the UAE.
Figure 1 shows degree of compliance with GCP mandated information of the informed consent form. Overall, the ICFs from industry sponsored studies were largely compliant (79.5%) when compared with the local standard ICF. Purpose of the study, risk or discomforts, benefits, voluntary nature of participation and ability to withdraw at any time, probability of randomization, and emergency contact information of the investigators and study team were included in almost all (>90%) of the ICFs reviewed for GCP compliance from industry sponsored studies [Figure 1]. Conversely, low compliance was observed for items such as subject rights and responsibilities (33%) and anticipated out of pocket expenses to the participant (24%). Non-sponsored studies had significantly lower overall GCP compliance of 55.8% (P = 0.01). Less than half of the ICFs reviewed included information related to subject risks, benefits, randomization, and right to compensation from study related injuries. Fewer than 20% of ICFs from non-sponsored studies included important information related to subject rights and responsibilities.
Figure 1.
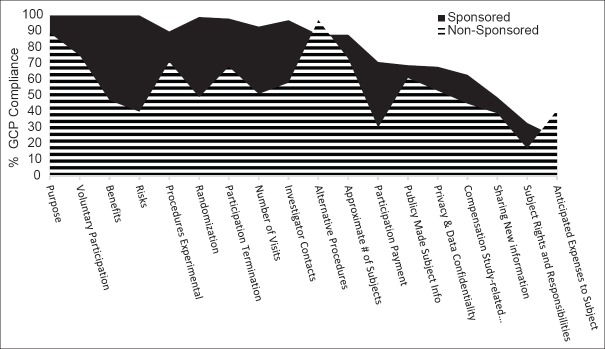
GCP compliance assessment of the ICFs from industry sponsored and non-sponsored studies from two Joint Commission accredited academic medical centers in the UAE. GCP compliance for each of the 18 minimum essential information items required for the ICFs was assessed by comparing the ICFs from industry sponsored (n = 39) and non-sponsored (n = 101) with a local GCP standard ICF and represented as percentage GCP compliance
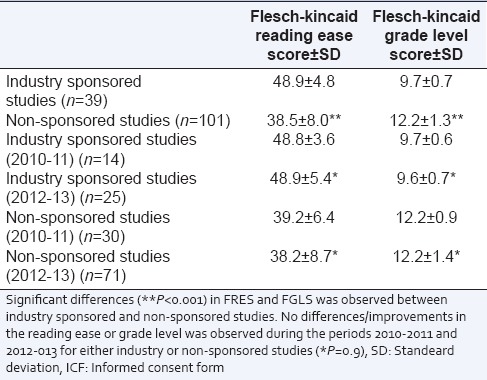
The Flesch-Kincaid readability tests were applied to all 140 ICFs.[22] A reading ease score of more than 60 indicates a “standard” readability score.[22] For the Flesch-Kincaid Grade Level (FGLS), documents, such as the ICFs, should be written at a 7.5-grade level to ensure that patients understand the treatment and procedure options offered.[24] The mean+/-standard deviation (SD) FRES score for the ICFs from industry sponsored studies was 48.9 ± 4.8, significantly higher as compared to 38.5 ± 8.0 (P < 0.001) for non-sponsored studies [Table 1]. Additionally, the mean+/- SD for Flesch-Kincaid Grade Level score for the ICFs from the industry sponsored studies was 9.7+/- 0.7, significantly lower (P < 0.001) as compared to 12.2 ± 1.3 for non-sponsored studies. Readability for the ICFs were not significantly different (P = 0.9) across the periods from 2010 to 11 and 2012 to 13 for both industry sponsored and non-sponsored studies [Table 1].
Table 1.
Flesch-kincaid Reading ease score and Flesch-Kincaid Grade Level Score for the ICFs from industry sponsored and non-sponsored studies
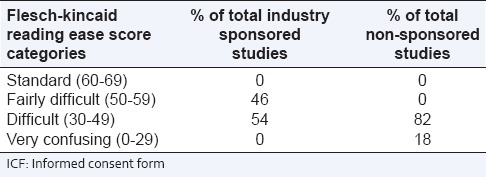
Additionally, based on the FRES, the ICFs were placed into categories ranging from standard readability to very confusing.[21] Almost half (46%) of ICFs from industry sponsored studies were in the fairly difficult category (range 50-59), and the remaining half (51%) in the difficult category (range 30-49). The majority of ICFs from non-sponsored studies were in the difficult range (83%, range 30-49) and the remainder (17%, range 0-29) in the very confusing category [Table 2]. Cohen's kappa coefficient was high (k = 0.81-0.84) for the readability assessment and GCP compliance of the ICFs.
Table 2.
Comparative assessment of the readability ease scores of the ICFs from industry sponsored and non-sponsored studies with respect to standard readability (N=140)
DISCUSSION
The potential for faster subject recruitment, reduced clinical trial development costs, and an already established research infrastructure have made the UAE an attractive location for the conduct of clinical research.[14] As clinical trials increase in the UAE, it becomes critical to ensure an adequate and complete informed consent process. The results of our study highlight deficiencies in the informed consent forms currently used for both industry supported and non-sponsored research involving human subjects. Concerns over exploitation or coercion of vulnerable populations, including the elderly and the large number of laborers in the UAE, are compounded by the finding that less than half of non-industry sponsored trials included adequate information on the risks and benefits of the study. More alarming is the finding that only 33% of sponsored and 16% of investigator-initiated studies listed basic information on the participants' rights and responsibilities. Neither sponsored nor non-sponsored studies provided research participants with appropriate information regarding compensation for study related injuries or disability. Although study sites and ethics committees in Abu Dhabi mandate third-party study insurance to cover expenses related to clinical trial related injuries or disabilities, the ICFs reviewed did not distinguish between medical insurance covered for standard care and insurance for study related injuries. Out-of-pocket expenses incurred by the research participants can clearly impact clinical trial participation. Thus, the inclusion of this information may actually serve to bolster subject recruitment.
In our study, most sponsored projects were part of multi-institutional and multi-national drug trials and utilized standardized ICFs that had considerably higher compliance with GCP mandates than the ICFs used for non-sponsored studies. In fact, a substantial number of ICFs from non-sponsored studies had missing or incomplete information that would preclude true informed consent. Different investigators trained under various international systems have differing concepts of obtaining an appropriate informed consent. Standardizing investigator training is, therefore, critical to improving the informed consent process in the UAE.
Analysis of readability of the ICFs was also a cause for concern. The majority of studies had lower reading ease scores and higher reading grade levels than is recommended for consent forms.[25] None of the ICFs tested had a reading ease score above 60. Also, college-level reading skills (12.1) were required for the ICFs from non-sponsored studies, and grade 9 reading skills were required for ICFs from industry sponsored studies. These findings remained consistent irrespective of the type of study, site oversight and complexity of the study. Studies in the US have highlighted issues with readability of the ICF because almost half of Americans read at or below grade-8 levels.[26] These higher readability level requirements may inappropriately exclude participants, affect study recruitment and create a lack of understanding of the investigational nature of the study.[27] Simplifying the ICF and graphically representing critical informational items, followed by awareness sessions potentially using smart phone technology and visual media, may help research subjects in understanding information related to their well-being.
A limitation of this study is that only 140 ICFs were reviewed and the data presented are from two academic centers. This limitation is partially overcome by the fact that these two institutions contribute the highest number of studies conducted annually in the country. Strengths of our study include the fact that both industry sponsored and non-sponsored studies were selected and ICFs from several therapeutic areas were included. ICF inadequacies highlighted in our study should aid pharmaceutical sponsors, contract research organizations, study site managers, regulators and investigators to develop effective ICFs for clinical research in this multiethnic, multicultural society.
CONCLUSIONS
The expansion of clinical trials in emerging regions has raised valid concerns about research subject protection, particularly related to informed consent. Our study confirms readability and comprehension issues as well as the need to improve basic essential information for the ICFs. Given the undisputed benefits of conducting research in emerging international markets, improvements in ICFs are essential to protect the rights of future research subjects in the UAE.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Classic KL, Porter BL, DiMagno EP. Protection of research subjects with emphasis on protocols involving radiation. Health Phys. 2001;80:S70–6. doi: 10.1097/00004032-200105001-00009. [DOI] [PubMed] [Google Scholar]
- 2.Dixon JR., Jr The International conference on harmonization good clinical practice guidelines. Qual Assur. 1998;6:65–74. doi: 10.1080/105294199277860. [DOI] [PubMed] [Google Scholar]
- 3.Chang JJ, Xu J, Fan D. A comparative method of evaluating quality of international clinical studies in China: Analysis of site visit reports of the clinical research operations and monitoring center. Contemp Clin Trials. 2008;29:654–62. doi: 10.1016/j.cct.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Gupta YK, Padhy BM. India's growing participation in global clinical trials. Trends Pharmacol Sci. 2011:327–9. doi: 10.1016/j.tips.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Alahmad G, Al-Jumah M, Dierickx K. Review of national research ethics regulations and guidelines in Middle Eastern Arab countries. BMC Med Ethics. 2012;13:34. doi: 10.1186/1472-6939-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beecher HK. Ethics and clinical research. N Engl J Med. 1966;274:1354–60. doi: 10.1056/NEJM196606162742405. [DOI] [PubMed] [Google Scholar]
- 7.Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: A new measure of understanding among research subjects. J Natl Cancer Inst. 2001;93:139–47. doi: 10.1093/jnci/93.2.139. [DOI] [PubMed] [Google Scholar]
- 8.Annas GJ, Grodin MA. Human rights and maternal-fetal HIV transmission prevention trials in Africa. Am J Public Health. 1998;88:560–3. doi: 10.2105/ajph.88.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bansal N. The Opportunities and challenges in conducting clinical trials globally. Clin Res Regulatory Affairs. 2012;29:9–14. [Google Scholar]
- 10.Hunt LM, de Voogd KB. Are good intentions good enough. Informed consent without trained interpreters? J Gen Intern Med. 2007;22:598–605. doi: 10.1007/s11606-007-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macklin R. Understanding informed consent. Acta Oncol. 1999;38:83–7. doi: 10.1080/028418699431843. [DOI] [PubMed] [Google Scholar]
- 12.Paasche-Orlow MK, Taylor HA, Brancati FL. Readability standards for informed-consent forms as compared with actual readability. N Engl J Med. 2003;348:721–6. doi: 10.1056/NEJMsa021212. [DOI] [PubMed] [Google Scholar]
- 13.Richardson V. Patient comprehension of informed consent. J Perioper Pract. 2013;23:26–30. doi: 10.1177/1750458913023001-204. [DOI] [PubMed] [Google Scholar]
- 14.Nair SC, Ibrahim H, Celentano DD. Clinical trials in the Middle East and North Africa (MENA) region: Grandstanding or grandeur? Contemp Clin Trials. 2013;36:704–10. doi: 10.1016/j.cct.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Clinicaltrial.gov. [Last accessed on 2014 Jan 14]. Available from: http://www.clinicaltrials.gov .
- 16.Margolin KA, van Besien K, Peace DJ. An introduction to foundation and industry-sponsored research: Practical and ethical considerations. Hematol Am Soc Hematol Educ Program. 2007;7:498–503. doi: 10.1182/asheducation-2007.1.498. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim K, Eada EA, Younis N. Ministry of Health: Drug control-UAE guidance for conducting clinical trials based on drugs/medical products and Good. Clin Pract. 2006;6:1–44. [Google Scholar]
- 18.International conference on harmonization of technical requirements for registration of pharmaceuticals for human use: ICH harmonized tripartite guideline E2-current Step 4 version. 2010:1–26. [Google Scholar]
- 19.HAAD. [Last accessed on 2014 Jan 04]. Available from: http://www.haad.ae .
- 20.Resnik DB, Patrone D, Peddada S. Evaluating the quality of information about alternatives to research participation in oncology consent forms. Contemp Clin Trials. 2010;31:18–21. doi: 10.1016/j.cct.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falagas ME, Korbila IP, Giannopoulou KP, Kondilis BK, Peppas G. Informed consent: How much and what do patients understand? Am J Surg. 2009;198:420–35. doi: 10.1016/j.amjsurg.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Terblanche M, Burgess L. Examining the readability of patient-informed consents. J Clin Trials. 2010;2:157–62. [Google Scholar]
- 23.Viera AJ, Garrett JM. Understanding interobserver agreement: The kappa statistic. Fam Med. 2005;37:360–3. [PubMed] [Google Scholar]
- 24.Landi N. An examination of the relationship between reading comprehension, higher-level and lower-level reading sub-skills in adults. Read Writ. 2010;23:701–17. doi: 10.1007/s11145-009-9180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh TM, Volsko TA. Readability assessment of internet-based consumer health information. Respir Care. 2008;53:1310–5. [PubMed] [Google Scholar]
- 26.Angell M. Industry-sponsored clinical research: A broken system. JAMA. 2008;300:1069–71. doi: 10.1001/jama.300.9.1069. [DOI] [PubMed] [Google Scholar]
- 27.Wolf MS, Williams MV, Parker RM, Parikh NS, Nowlan AW, Baker DW. Patients' shame and attitudes toward discussing the results of literacy screening. J Health Commun. 2007;2:721–3. doi: 10.1080/10810730701672173. [DOI] [PubMed] [Google Scholar]


