Abstract
Background and Objectives:
Randomized controlled trials (RCTs) are considered as the gold standard evidence for determining efficacy of interventions. Physiotherapeutic interventions are essential in the management of various conditions. However, information on the quantity and quality of RCTs published by Indian physiotherapists is largely unknown. Therefore, the primary objective of this study was to review the RCTs published by Indian physiotherapists for analyzing publication trend and its quality.
Materials and Methods:
Medline database was searched for eligible RCTs published by Indian physiotherapists between the years 2000 and 2013. We performed quantitative analysis of RCTs including type of participants, area of focus in physiotherapy, clinical condition and geographical location of first author's affiliation and analyzed the methodological quality and reporting of RCTs using Physiotherapy Evidence Database (PEDro) scale and consolidated standards of reporting trials (CONSORTs) key criterion statement, respectively.
Results:
A total of 45 RCTs have been published by Indian physiotherapists. The common conditions investigated in the trials were low back pain (16.3%), followed by diabetes (6.7%) and chronic obstructive pulmonary disease (6.7%). The mean score of PEDro is 5.5 (standard deviation: 1.2). Trial registration (3 [7%]) and sample size calculation (28.9%) are the most common CONSORT items not reported in the trials.
Interpretation and Conclusions:
RCTs published by Indian physiotherapists is gradually increasing in numbers and the methodological qualities of studies are fair. However, there is substantial scope for improvement in conducting and reporting trials. In the future, Indian physiotherapists should focus more on conditions such as stroke, asthma, and others, which have a larger burden of illness among Indian population.
Keywords: Clinical trials, consolidated standards of reporting trials, evidence-based practice, Physiotherapy Evidence Database
INTRODUCTION
Evidence-based practice is recommended as an important and necessary step for improving the quality of health care.[1] Within the paradigm of evidence-based practice, randomized controlled trials (RCTs), and systematic reviews of RCTs are considered as the gold standard evidence for determining efficacy of interventions.[2] Clinicians are encouraged to make treatment decisions based upon RCTs.[3] Knowledge obtained from high quality RCTs are recommended as essential for patients, clinicians, and the policymakers for making sound evidence-informed health care decisions. Physiotherapeutic interventions are essential in the management of various conditions. Hence, knowledge about evidence regarding the effectiveness of physiotherapy interventions is critical.
Randomized controlled trials specific to each country is vital. Researches conducted in other countries pose a serious challenge because of the intricacies involved in the generalizability of its results. The trials might not take into account the important local challenges that often occur when implementing medical interventions in developing countries.[4,5] For example, trials on exercise interventions for improving deep knee flexion activities such as squatting or sitting on the floor which are of significance in Asian context are often neglected in the literature.[6] To address this discrepancy, research conducted by Indian researchers that takes into account the culture and diversity of Indian population when developing research ideas, conducting research, and exploring the applicability of research findings are needed.
Worldwide, there is rapid growth in physiotherapy research reflecting the growth of physiotherapy in terms of both as a profession and science. Indian physiotherapist's contribution to the growth of research production, however is not clear. Scientific research production taking into consideration of the local context can contribute to significant improvements in the standard of practice and development of country specific clinical practice guidelines. Moreover, for those researches to be considered credible and noteworthy, it needs to be published in respectable, peer-reviewed, and indexed journals.
Though RCTs are considered as the gold standard evidence for effectiveness of treatment, validity of the trial results and its applicability are significantly determined by the methodological quality of the published RCTs.[7] Quality gives us an estimate of the likelihood that the results are a valid estimate of the truth.[8] Hence, high quality RCTs are necessary to develop evidence-informed practice guidelines and promote efficient practice in physiotherapy. A description of the quantity and quality of these RCTs might increase awareness of current evidence, and thereby facilitate an evidence-based approach to clinical decision making.[9] However; information on the quantity and quality of RCTs by Indian therapists is largely unknown. Therefore, the primary objective of this study was to review the RCTs published by Indian physiotherapists for analyzing its publication trend and quality.
MATERIALS AND METHODS
This study involved three major steps: (1) Identify RCTs published by Indian physiotherapy researchers from Medline. (2) Perform quantitative analysis including type of participants, area of focus in physiotherapy, clinical condition and geographical location of first author's affiliation. (3) Analyze the methodological quality and reporting of RCTs using Physiotherapy Evidence Database (PEDro) scale and consolidated standards of reporting trials (CONSORT) key criterion statement respectively.
Data extraction
Selection of trials
We chose Medline database for selecting relevant RCTs for the following reasons: (1) Medline is considered to cover the most important quality scientific journals in the world.[10] (2) Medline covers all journals identified as core journals that publish clinical trials of physiotherapy interventions.[11] (3) Medline provide institutional affiliation and its location for the first author.
We searched Medline for papers published by Indian physiotherapists from January 2000 to May 2013, using the key terms India OR Indian AND physiotherapy OR physical therapy.
To be included in the review, a study had to meet the following eligible criteria:
-
The study should be a RCT:
- The trial compares at least two interventions
- There is random allocation or intended-to-be- random allocation of subjects to interventions
- At least one of the interventions is currently part of physiotherapy practice, or could become part of physiotherapy practice
- The interventions are applied to human subjects who are representative of those to whom the intervention might be applied in the course of physiotherapy practice (i.e., people with or at risk of developing a health condition or disability).
First author affiliation with an Indian institution and one of the authors being a physiotherapist. Studies with animal subjects, articles that are not related to physiotherapy intervention and non RCTs were excluded.
First and second author independently reviewed the abstracts in an unblinded standardized manner, identified eligible studies and removed duplicates. Of 934 records identified 885 records were excluded by reading the title, abstract, and author's affiliation. Of the 885, 772 articles are excluded as they are not related to physiotherapy and/or not having physiotherapist as author. One hundred and thirteen articles excluded due to one of the following reasons: (1) Not a clinical trial (2) not related to treatment [Figure 1]. Of the remaining 47 studies, one was excluded for not having human subjects as study participants and another for no random allocation of subjects to study groups. Finally, a total of 45 studies were identified as eligible for analysis [Figure 1]. Disagreements were resolved by discussion between the two review authors; if no agreement could be reached, it was planned the third author would decide.
Figure 1.
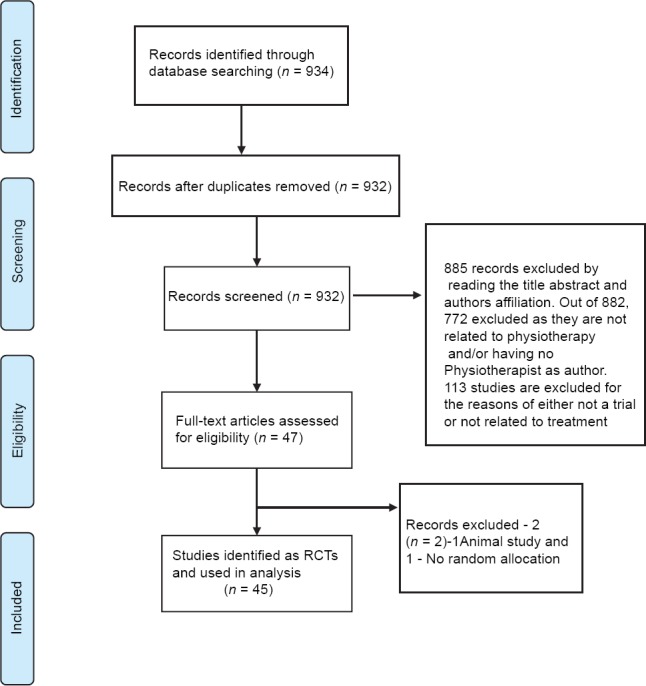
Flow diagram of the process of identifying and including articles for the review
Data synthesis
Randomized controlled trial quantity
A full-text copy of each included trial report was obtained. We were unable to retrieve full text of two trials, even after contacting the authors. Of the two RCTs without full text one was indexed in PEDro and other was not. Each trial was independently reviewed and coded by first and second author. For quantitative analysis, both reviewers coded each article for variables viz. type of participants, area of focus in physiotherapy, clinical condition studied, and geographical location of first author's affiliation as appropriate for quantitative analysis. We believed that these variables can reveal the trend of the RCTs published by Indian physiotherapists. Coding methods used in this study was adapted from those reported by Coronado et al.[12]
Randomized controlled trial quality
Physiotherapy evidence database scale
To assess RCTs' methodological quality, we used the PEDro scale. PEDro scale is demonstrated as a valid and reliable measure of methodological quality of RCTs.[13,14] The PEDro scale is an 11-item scale designed for rating methodological quality of RCTs. Each satisfied item (except for item 1, which, unlike other scale items, pertains to external validity) contributes one point to the total PEDro score (range = 0-10 points). The scale has been used to rate the quality of over 3,000 RCTs in the PEDro database[15] and in several systematic reviews.[16,17,18] Physiotherapy Evidence Database scale permits to classify high or low quality papers based on a cut-off score. Studies scoring 9-10 on the PEDro scale were considered methodologically to be of “excellent” quality. Scores ranging from 6 to 8 were considered to be of “good” quality, while studies scoring 4 or 5 were of “fair” quality and studies scoring below 4 were felt to be of “poor” quality.[18,19]
Each article's PEDro score was extracted directly from the PEDro database (accessed on January 5, 2014). Of 45 trials, 14 trials were not listed in the PEDro database and due to unavailability of full text of one trial; its PEDro rating was not done. Manual rating of these trials were done by two independent raters (first and second author), blinded to each other, reviewed each article with a final consensus provided by a PEDro trained independent rater, whenever necessary.
Consolidated standards of reporting trial statement checklist
The consolidated standards of reporting trial statement are a set of recommendations developed to improve the reporting of trials. The CONSORT statement has 25 items related to reporting of trials (e.g. sample size, statement of primary and secondary outcomes, trial registration). There are several items in CONSORT statement that overlaps with the items covered by PEDro scale. For this study, we used only the items derived from the CONSORT statement checklist: (1) RCT in title. (2) Patient flow chart. (3) Statement of primary outcomes. (4) Sample size calculation. (5) Reporting of adverse effect. (6) Trial registration. (7) Source of funding for the trial. We believed that these key items can be of value in understanding the methodological and reporting quality.
Two independent reviewers (first and second author), coded each studies for the aforementioned CONSORT checklist items as present or absent (1 for present and 0 for absent). Scoring discrepancies were resolved by a third reviewer, who reached a compromise score that constituted the final score.
RESULTS
A total of 45 RCTs have been published by Indian physiotherapists between January 2000 and May 2013. First RCT with a physiotherapist as an author was published in the year 2004[20] and by an Indian physiotherapist as a first author was published in the year 2006.[21] There is a small, but consistent upward trend in a number of RCTs published between the years 2004 and 2013 [Figure 2].
Figure 2.
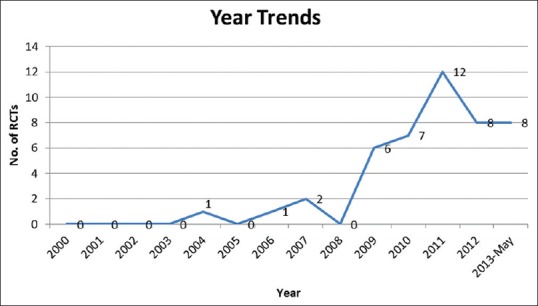
Number of randomized controlled trials published in each year between the years 2000 and 2013
Majority of the studies (37 [82.2%]) included symptomatic humans as participants, among those studies most were done on adult participants (35 [77.7%]), with children being participants only in two studies (4.4%). Common clinical conditions investigated in trials were low back pain 7 (16.3%), followed by diabetes (3 [6.7%]), and COPD (3 [6.7%]).
Area of focus of the published RCTs is evenly divided among three core specialties within physiotherapy with a slight upward trend in musculoskeletal physiotherapy viz. musculoskeletal 16 (35.6%), neurological 8 (17.8%), and cardio respiratory physiotherapy 8 [17.8%]. Other than these three core areas, RCTs are also published in specialties such as women's health 2 4.4%, sports 5 11.6%, diabetes 2 4.4%, palliative care 1 [2.2%], and integumentary 3 [6.7%].
Most of the trials (26 [57.8%]) were conducted from institutions located in the southern region of India followed by Northern region (16 [35.6%]). The number of trials from other regions is very few with East producing no trials, (0) West 2 (4.4%) and North East 1 (2.2%).
Quality rating of randomized controlled trial
The mean score of PEDro is 5.5 (SD: 1.2, range: 4-8) for 44 studies out of 45. Classification of the RCTs according to the total PEDro score revealed 20 studies were classified as good and 24 as fair. We were unable to retrieve the full text of one trial (which was also not listed in the PEDro database) even after contacting the author through E-mail.
The least fulfilled criteria in the PEDro scale are: (1) Blinding of all therapists who administered the therapy (0%), (2) intention-to-treat analysis (11 [24.9%]) (3) blinding of participants 6 (13.3%). (4) Blinding of assessors (12 [26.7%]). Reporting of between-group statistical comparisons (42 [93.3%]), reporting of point measures and measures of variability (39 [86.7%]) and more than 85% follow-up (37 [82.2%]) were the most common criterion, which was fulfilled [Figure 3].
Figure 3.
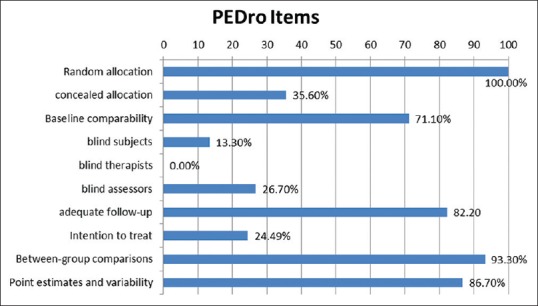
Proportions of individual Physiotherapy Evidence Database scale items satisfied by 45 trials
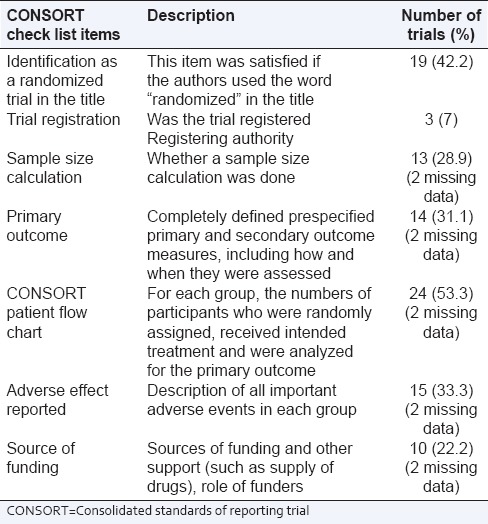
Consolidated standards of reporting trials checklist items like sample size calculation was done only in 13 (28.9%) trials, identification as an RCT in the title 19 (42.2%), specifying the primary outcome 14 (32%), patient flow chart 24 (53.3%), 10 (22.2%) RCTs were funded studies and description of all important adverse events in each group 15 (33.3%) (two missing data) were also not fulfilled in many studies. Trial registration was uncommon with only 3 (7%) trials were registered with a competent authority [Table 1].
Table 1.
Proportions of individual CONSORT checklist items satisfied by 45 trials
DISCUSSION
From 2004 when the first trial was published by an Indian physiotherapist, the number of trials has been steadily increasing [Figure 1]. Quality of the published studies both in terms of methodological quality and reporting standards were found to be fair; however, there few areas such as blinding outcome assessors, priori sample size calculation requires further improvement.
With the first RCT published only 10 years ago the total number of RCTs published by Indian physiotherapists can be considered satisfactory. Though it is considerably less when compared with other countries[22] including developing countries like Brazil;[23] it would be worthy of noting that the environment for research in India is fairly different. Only 10 (22%) studies in the review were found to be funded, which may reflect either poor availability or awareness about funding. Along with poor funding, few physiotherapists with a doctoral degree,[24] which have been identified as limiting factor to research productivity, can explain in part the low numbers of trials. More than half of the studies are published by researchers affiliated to an institution located in the Southern region of India followed by Northern region. The increased production from the Southern region may be due to increase in a number of physiotherapy educational institutions and the introduction of doctoral degree decade before. There is a significant gap in the production of research output from Western and Eastern regions of India. India is a subcontinent with divergent culture and people with varying rehabilitation needs; hence, studies done in one region may not automatically lead to generalization of study results in other regions.
Although the number of trials showing positive trend, it does not adequately reflect the public health emphasis given to a disease of the Indian population. Significant proportions of the studies published by Indian physiotherapists are done on conditions pertaining to the musculoskeletal problem. Notably very few studies were done on conditions identified as contributing to significant burden to Indian population such as stroke, low back pain, and asthma.[25] Fraser[26] argues that clinical trials must focus on research that is most likely to improve health and added that in judging the most promising areas for research, problems that cause the greatest burden and interventions that can be applied cost-effectively should clearly be favored. We believe that in the future, physiotherapists conducting RCTs in India should focus on conditions that contribute to a significant burden to the public health of the Indian population. However, number of studies done on diabetes, another condition with significant illness burden indicates an optimistic trend.
Physiotherapy Evidence Database scale rating of the RCTs indicates that the studies are of fair quality from the perspective of methodological quality. Similar trial quality ratings are reported for the researches published by physiotherapists from other countries.[27] It is worthy of mentioning that certain criteria such as blinding the patients and therapists are almost impossible in many areas of physiotherapy practice. Hence, pragmatic view of quality rating of physiotherapy RCTs is necessary. There is empirical evidence that sample size calculation, random allocation and blinding assessors can reduce the bias and overestimation of results.[28,29] These criteria which are not satisfied in many RCTs, is practically feasible to implement; hence, researchers should include these strategies in future trials to improve the trial quality.
It has been suggested, even though good reporting is not a direct measure of the quality of a study, it allows a reader to assess the validity and applicability of the study's findings.[30] Our analysis of the trial reporting using CONSORT checklist suggests that there is significant scope for improvement in this area. Very few studies have reported sample size calculation, identified primary outcome measure, and reported trial registration information. Similar trend in methodological quality was observed in clinical trials published in a few Indian medical journals.[31] Stern implementation of reporting guidelines such as CONSORT and trial registration with a competent authority like Clinical Trial Registry of India (www.ctri.in) by journal editors can significantly improve the quality of reporting of clinical trials.[32]
CONCLUSION
Randomized controlled trials published by Indian physiotherapists is gradually increasing in numbers, and the methodological quality of the studies is fair. However, there is substantial scope for improvement in conducting and reporting of the trials. In Indian context conditions like stroke, asthma and others, which have a larger burden of illness should be given adequate focus in future research. Clinical Trial Registry, guidelines for reporting, better editorial policy, and maturation of physiotherapy researchers may help in improving the quality and reporting of the trials.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Lugtenberg M, Burgers JS, Westert GP. Effects of evidence-based clinical practice guidelines on quality of care: A systematic review. Qual Saf Health Care. 2009;18:385–92. doi: 10.1136/qshc.2008.028043. [DOI] [PubMed] [Google Scholar]
- 2.Sibbald B, Roland M. Understanding controlled trials. Why are randomised controlled trials important? BMJ. 1998;316:201. doi: 10.1136/bmj.316.7126.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: What it is and what it isn't. BMJ. 1996;13(312):71–2. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ofori-Adjei D, Antes G, Tharyan P, Slade E, Tamber PS. Have online international medical journals made local journals obsolete? PLoS Med. 2006;3:e359. doi: 10.1371/journal.pmed.0030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isaakidis P, Swingler GH, Pienaar E, Volmink J, Ioannidis JP. Relation between burden of disease and randomised evidence in sub-Saharan Africa: Survey of research. BMJ. 2002;324:702. doi: 10.1136/bmj.324.7339.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hariohm K, Prakash V. Deep flexion activity training in a patient with stroke using task-oriented exercise: A case report. Physiother Theory Pract. 2014;30:196–201. doi: 10.3109/09593985.2013.850564. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–13. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S. Assessing the quality of randomized controlled trials: An annotated bibliography of scales and checklists. Control Clin Trials. 1995;16:62–73. doi: 10.1016/0197-2456(94)00031-w. [DOI] [PubMed] [Google Scholar]
- 9.Moseley A, Sherrington C, Herbert R, Maher C. The extent and quality of evidence in neurological physiotherapy: An analysis of the physiotherapy evidence database (PEDro) Brain Impair. 2000;1:130–40. [Google Scholar]
- 10.Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews. Empirical study? Health Technol Assess. 2003;7:1–76. [PubMed] [Google Scholar]
- 11.Costa LO, Moseley AM, Sherrington C, Maher CG, Herbert RD, Elkins MR. Core journals that publish clinical trials of physical therapy interventions. Phys Ther. 2010;90:1631–40. doi: 10.2522/ptj.20090419. [DOI] [PubMed] [Google Scholar]
- 12.Coronado RA, Riddle DL, Wurtzel WA, George SZ. Bibliometric analysis of articles published from 1980 to 2009 in Physical Therapy, journal of the American Physical Therapy Association. Phys Ther. 2011;91:642–55. doi: 10.2522/ptj.20100267. [DOI] [PubMed] [Google Scholar]
- 13.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust J Physiother. 2009;55:129–33. doi: 10.1016/s0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 14.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–21. [PubMed] [Google Scholar]
- 15.Sherrington C, Herbert RD, Maher CG, Moseley AM. PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man Ther. 2000;5:223–6. doi: 10.1054/math.2000.0372. [DOI] [PubMed] [Google Scholar]
- 16.Herbert RD, Gabriel M. Effects of stretching before and after exercising on muscle soreness and risk of injury: Systematic review. BMJ. 2002;325:468. doi: 10.1136/bmj.325.7362.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira ML, Ferreira PH, Latimer J, Herbert R, Maher CG. Does spinal manipulative therapy help people with chronic low back pain? Aust J Physiother. 2002;48:277–84. doi: 10.1016/s0004-9514(14)60167-7. [DOI] [PubMed] [Google Scholar]
- 18.Maher CG. A systematic review of workplace interventions to prevent low back pain. Aust J Physiother. 2000;46:259–69. doi: 10.1016/s0004-9514(14)60287-7. [DOI] [PubMed] [Google Scholar]
- 19.Foley NC, Teasell RW, Bhogal SK, Speechley MR. Stroke Rehabilitation Evidence-Based Review: Methodology. Top Stroke Rehabil. 2003;10:1–7. [PubMed] [Google Scholar]
- 20.Taly AB, Sivaraman Nair KP, Murali T, John A. Efficacy of multiwavelength light therapy in the treatment of pressure ulcers in subjects with disorders of the spinal cord: A randomized double-blind controlled trial. Arch Phys Med Rehabil. 2004;85:1657–61. doi: 10.1016/j.apmr.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Arun Maiya G, Sagar MS, Fernandes D. Effect of low level helium-neon (He-Ne) laser therapy in the prevention and treatment of radiation induced mucositis in head and neck cancer patients. Indian J Med Res. 2006;124:399–402. [PubMed] [Google Scholar]
- 22.Richter RR, Schlomer SL, Krieger MM, Siler WL. Journal publication productivity in academic physical therapy programs in the United States and Puerto Rico from 1998 to 2002. Phys Ther. 2008;88:376–86. doi: 10.2522/ptj.20060266. [DOI] [PubMed] [Google Scholar]
- 23.Sturmer G, Viero CC, Silveira MN, Lukrafka JL, Plentz RD. Profile and scientific output analysis of physical therapy researchers with research productivity fellowship from the Brazilian National Council for Scientific and Technological Development. Braz J Phys Ther. 2013;17:41–8. doi: 10.1590/s1413-35552012005000068. [DOI] [PubMed] [Google Scholar]
- 24.Dundar H, Lewis DR. Determinants of research productivity in higher education. Res High Educ. 1998;39:607–31. [Google Scholar]
- 25.Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370:1929–38. doi: 10.1016/S0140-6736(07)61696-1. [DOI] [PubMed] [Google Scholar]
- 26.Fraser DW. Overlooked opportunities for investing in health research and development. Bull World Health Organ. 2000;78:1054–61. [PMC free article] [PubMed] [Google Scholar]
- 27.Geha NN, Moseley AM, Elkins MR, Chiavegato LD, Shiwa SR, Costa LO. The quality and reporting of randomized trials in cardiothoracic physical therapy could be substantially improved. Respir Care. 2013;58:1899–906. doi: 10.4187/respcare.02379. [DOI] [PubMed] [Google Scholar]
- 28.Chalmers TC, Celano P, Sacks HS, Smith H., Jr Bias in treatment assignment in controlled clinical trials. N Engl J Med. 1983;309:1358–61. doi: 10.1056/NEJM198312013092204. [DOI] [PubMed] [Google Scholar]
- 29.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 30.Fung AE, Palanki R, Bakri SJ, Depperschmidt E, Gibson A. Applying the CONSORT and STROBE statements to evaluate the reporting quality of neovascular age-related macular degeneration studies. Ophthalmology. 2009;116:286–96. doi: 10.1016/j.ophtha.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Jaykaran, Kantharia ND, Yadav P, Deoghare S. Reporting of the methodological quality and ethical aspects in clinical trials published in Indian journals: A survey. J Postgrad Med. 2011;57:82–3. doi: 10.4103/0022-3859.74300. [DOI] [PubMed] [Google Scholar]
- 32.Tharyan P, Ghersi D. Registering clinical trials in India: A scientific and ethical imperative. Natl Med J India. 2008;21:31–4. [PubMed] [Google Scholar]


