Abstract
Complex pathways exist in mammalian cells to regulate the expression and activity of oncogenes and tumor suppressor genes. Defining these regulatory pathways is an important step towards being able to interfere with tumorigenesis. Here we discuss our recent study indicating that activation of the phosphoinositide 3-kinase (PI3K) signaling pathway through inactivating mutations in PTEN or activating mutations in PIK3CA causes functional activation of p53 signaling in human cells.1 Our data suggest that activation of p53 is a fail-safe mechanism triggered by loss of PTEN or oncogenic activation of PI3K, and furthermore, that these events provide selective pressure to mutate p53.
Keywords: p53, PTEN, PIK3CA, gene targeting, microarray
INTRODUCTION
It is of course well-established that the p53 tumor suppressor gene plays a major role in tumorigenesis. Mutations in the p53 gene are present in about half of all human cancers, and several DNA tumor viruses encode proteins that inactivate p53.2,3 Germ line p53 mutations cause the Li-Fraumeni cancer predisposition syndrome.4 Furthermore, targeted disruption of p53 in murine models results in the rapid development of tumors of various histological origins.5
The p53 protein is a transcription factor capable of inducing the expression of genes necessary for cell cycle arrest and/or apoptosis in response to various extracellular and intracellular stimuli. In normal cells, p53 is short-lived and present in barely detectable levels. These low levels of p53 protein are primarily the handiwork of the Hdm2 protein, which binds to p53 and physically separates it from its target genes.6 Furthermore, the ubiquitin ligase function of Hdm2 enables it to earmark both p53 and itself for proteasomal degradation.7,8 Since Hdm2 is also a p53-regulated gene, an autoregulatory feedback loop is formed that maintains low basal levels of p53 within the cell. Thus, p53 induces Hdm2 expression, and Hdm2 decreases p53 protein levels. This feedback loop is further modulated by the Hdm2 binding partner p14ARF, which is activated by mitogenic stimuli and sequesters Hdm2 from p53.9 A number of other post-translational modifications, including phosphorylation, acetylation, and sumoylation, also help protect p53 from degradation.10 Stimuli that activate p53 generally do so by enhancing p53 stability, thereby promoting the transcriptional activation of genes that induce G1 arrest or apoptosis.
PI3K functions as a lipid kinase that catalyzes the formation of the second messenger, phosphoinositide-3,4,5-trisphosphate (PIP3), from phosphoinositide-4,5-bisphosphate (PIP2). Following membrane recruitment by PIP3 and phosphorylation, the serine/threonine kinase Akt is activated and able to regulate the activity of numerous target proteins involved in cellular growth, survival, and proliferation.11 The PTEN tumor suppressor functions primarily as a lipid phosphatase that reverses the phosphorylation reaction catalyzed by PI3K. By depleting cellular levels of PIP3, PTEN acts as a brake on Akt activation. Both inactivating mutations in PTEN and activating mutations in PIK3CA, which encodes the PI3K catalytic subunit, occur frequently in human cancers.12–14
Previous work has shown that the p53 and PI3K pathways intersect at several points. First, a functional p53 binding site was identified within the PTEN promoter, suggesting that p53 is able to regulate PTEN expression.15 Subsequent studies revealed a second point of intersection at Hdm2 that may enable PTEN to regulate p53 protein stability. In particular, Akt was shown to phosphorylate and enhance Hdm2 function, suggesting that PTEN could prevent p53 degradation by suppressing Akt activation.16 PTEN was also shown to negatively regulate Hdm2 expression through its P1 promoter.17 Finally, a third point of intersection was revealed when a physical association between the p53 and PTEN proteins was shown to protect the former from degradation.18 Altogether, these observations suggested that p53 and PTEN formed a positive feedback loop, where p53 induced PTEN expression and PTEN reduced p53 degradation.
Given the ability of PTEN to stabilize p53 in these studies, it is arguably somewhat surprising that activation of PI3K signaling via PTEN deletion or oncogenic mutation of PIK3CA can also lead to p53 activation. By performing expression profiling and qRT-PCR on human HCT116 cell lines that are genetically identical except for the presence or absence of their endogenous, wild-type PTEN genes, we found that a number of well-known p53-regulated genes were upregulated in PTEN−/− cells. To demonstrate that these expression differences were indeed p53-dependent, we used RNAi to deplete p53 in otherwise isogenic PTEN+/+ and PTEN−/− cells. The expression of each of five p53-responsive genes was preferentially reduced in PTEN−/− cells following p53 depletion, strongly suggesting that PTEN deletion results in activation of a p53-dependent gene expression program in human cells.
As we further characterized the effect of PTEN deletion on p53, we observed that normally-proliferating PTEN−/− cells contained significantly higher levels of p53 protein than their PTEN+/+ counterparts. Using etoposide, a robust inducer of p53, we determined that though PTEN deletion led to an increase in the basal level of p53 during normal growth, it did not similarly elevate the maximum inducible level of p53. Pharmacologic inhibition of PI3K with LY294002 restored low basal p53 protein levels to PTEN−/− cells, indicating that the lipid phosphatase function of PTEN regulates p53. These results were recapitulated in PTEN-deficient A172 glioblastoma cells, but not PTEN-proficient normal human astrocytes (NHA), suggesting that our findings may be extended to include genetically unmodified human cells.
Half-life experiments revealed that PTEN deletion resulted in a fourfold prolongation in the half-life of p53. Furthermore, Western blots showed that p53 stabilization in PTEN-deleted HCT116 cells was independent of p14ARF and not secondary to a reduction in Hdm2 protein levels. RNAi experiments demonstrated that depletion of Akt1 in HCT116 PTEN−/− cells partially restored low basal p53 protein levels and reduced the expression of two p53-regulated genes. However, overexpression of a myristolated, activated form of Akt in HCT116 PTEN+/+ cells did not increase p53 levels. These findings are consistent with partial dependence of PTEN-dependent p53 activation on Akt, but suggest that other, as-yet unidentified effectors are also involved.
In an effort to generalize our data to untransformed human cells, we used RNAi to stably deplete PTEN in telomerase-immortalized retinal pigment epithelial cells and telomerase-immortalized BJ fibroblasts. As predicted, PTEN depletion led to an increase in p53 protein levels, but we were more intrigued by the morphological changes that occurred concomitantly in these cells. Following PTEN reduction, untransformed cells became flattened and enlarged—two phenotypic features highly reminiscent of cellular senescence. Subsequent studies showed that these cells contained γ-H2AX foci and had stopped dividing, consistent with senescence. To prove that these changes were p53-dependent, we used RNAi to stably deplete both PTEN and p53 in untransformed human cells. The reduction in p53 levels restored normal size, proliferation, and cell cycle control to PTEN-depleted cells. Our results demonstrate that PTEN inactivation leads to a p53-dependent, senescent-like state in untransformed human cells. These data are consistent with previous work by Miyauchi et al. showing that overexpression of activated Akt in primary cultured human endothelial cells can reduce proliferation and result in senescence- like morphological changes.19 Similarly, Chen et al. recently demonstrated that PTEN inactivation can lead to p53-induced senescence during tumorigenesis in the mouse prostate.20
We then wished to determine whether activating mutations in PIK3CA would similarly lead to p53 activation. Previous work has shown that overexpression of certain mutant PIK3CA proteins can result in increased PI3K activity and oncogenic transformation. To test the effect of mutationally activated PIK3CA on p53, we infected human MCF10A premalignant breast epithelial cells with amphotrophic retroviruses encoding mutant or wild-type PIK3CA. Cells expressing oncogenic, but not wild-type, PIK3CA had higher p53 protein levels and increased expression of two p53-regulated genes. When RNAi was used to deplete p53, the expression of these genes returned to normal levels, demonstrating p53-dependence.
An important caveat when interpreting overexpression studies is the possibility that the high level of expression may generate artifactual phenotypes. To exclude the possibility that our data in MCF10A cells was such an artifact, we once again turned to a gene targeting approach. HCT116 cells are diploid at the PIK3CA locus and naturally contain one wild-type allele and one activated mutant allele. Isogenic cell lines were created in which either the wild-type or the mutant allele of PIK3CA was disrupted. Only deletion of the mutant allele reduced p53 protein levels and lowered the expression of two p53-regulated genes. These findings showed that endogenous activating mutations in PIK3CA can similarly regulate p53 protein levels and activity in human cells. Importantly, soft agar growth assays demonstrated that activated PIK3CA in premalignant MCF10A cells produced anchorage-independent growth, a well-known indicator of oncogenic transformation. Our data is in agreement with the previous work of Isakoff et al.,21 though we further show that p53 depletion by RNAi enhances transformation by oncogenic PIK3CA, as evidenced by an increase in both colony number and size. Interestingly, p53 depletion alone was insufficient to confer anchorage-independent growth. These results suggest that p53 activation functions as a fail-safe mechanism against malignant transformation by oncogenic mutations in PIK3CA. As such, abrogation of the fail-safe by p53 depletion may reveal the actual extent of PIK3CA-induced transformation.
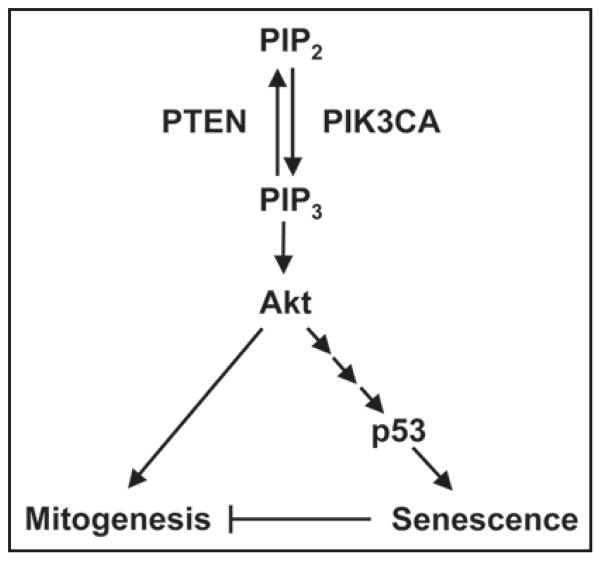
Our study demonstrates that activation of the PI3K signaling pathway via mutations in PTEN or PIK3CA can activate p53 signaling in human cells. Furthermore, we show that p53 stabilization by mutational activation of PI3K signaling can lead to senescence in human cells (Fig. 1). This data is in agreement with a recent study from Chen et al. indicating that PTEN loss leads to p53 activation and cellular senescence in the mouse prostate.20 Taken together, these findings support the idea that senescence is the phenotypic manifestation of a fail-safe mechanism activated by oncogenic mutation and intended to oppose neoplastic transformation. As such, the inability of cells lacking p53 to divert away from the proliferating pool and enter senescence may confer a selective survival advantage.
Figure 1.
Model of PI3K-induced, p53-dependent cellular senescence.
Since aberrant activation of PI3K signaling occurs frequently in human cancers, it has been suggested that compounds designed to inhibit this pathway may be useful therapeutically. Our data suggest it may be important to take into consideration the p53 status of tumors prior to initiating such treatment, since PI3K inhibitors might have the undesired effect of opposing p53-dependent tumor suppression in tumors with functional p53.
Our data also indicate that increased PI3K signaling may be an important and natural inducer of p53. This raises an interesting point regarding p53 activation—that while an extensive list of stimuli can induce p53 experimentally, the relative contributions of these stimuli to p53 induction during human tumorigenesis under physiologic conditions are largely unknown. Our study demonstrates that deletion of endogenous PTEN or oncogenic PIK3CA can activate p53 in both transformed and untransformed human cells. It is also interesting to note that several major p53 inducers, including DNA damage and oncogene activation, are known to activate the PI3K signaling pathway. As such, it is tempting to speculate that the PI3K signaling pathway is a central hub in the regulation of p53. In fact, Bar et al. have recently demonstrated that PI3K activation is required for p53 activation by DNA damage.22 Future studies will hopefully provide additional insights into the genetic and biochemical processes that regulate PI3K-activated, p53-dependent growth suppression.
References
- 1.Kim JS, Lee C, Bonifant CL, Ressom H, Waldman T. Activation of p53-dependent growth suppression in human cells by mutations in PTEN or PIK3CA. Mol Cell Biol. 2007;27:662–77. doi: 10.1128/MCB.00537-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein G. Perspectives in studies of human tumor viruses. Front Biosci. 2002;7:d268–74. doi: 10.2741/A726. [DOI] [PubMed] [Google Scholar]
- 3.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 4.Malkin D, Li FP, Strong LC, Fraumeni JF, Jr, Nelson CE, Kim DH, Kassel J, Gryka MA, Bischoff FZ, Tainsky MA, Friend SH. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–8. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 5.Attardi LD, Jacks T. The role of p53 in tumour suppression: lessons from mouse models. Cell Mol Life Sci. 1999;55:48–63. doi: 10.1007/s000180050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin J, Chen J, Elenbaas B, Levine AJ. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994;8:1235–46. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- 7.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 8.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 9.Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden KH, Peters G. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001–14. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meek DW, Knippschild U. Posttranslational modification of MDM2. Mol Cancer Res. 2003;1:1017–26. [PubMed] [Google Scholar]
- 11.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 12.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23. 3 that is mutated in multiple advanced cancers. Nat Genet. 1997;4:356–62. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 14.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 15.Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW. Regulation of PTEN transcription by p53. Mol Cell. 2001;8:317–25. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 16.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci. 2001;98:11598–603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CJ, Freeman DJ, Wu H. PTEN regulates Mdm2 expression through the P1 promoter. J Biol Chem. 2004;279:29841–8. doi: 10.1074/jbc.M401488200. [DOI] [PubMed] [Google Scholar]
- 18.Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, Liu X, Wu H. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell. 2003;3:117–30. doi: 10.1016/s1535-6108(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 19.Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23:212–20. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isakoff SJ, Engelman JA, Irie HY, Luo J, Brachmann SM, Pearline RV, Cantley LC, Brugge JS. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–1000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 22.Bar J, Lukaschuk N, Zalcenstein A, Wilder S, Seger R, Oren M. The PI3K inhibitor LY294002 prevents p53 induction by DNA damage and attenuates chemotherapy-induced apoptosis. Cell Death Differ. 2005;12:1578–87. doi: 10.1038/sj.cdd.4401677. [DOI] [PubMed] [Google Scholar]