Abstract
In the evolving discipline of quantitative systems pharmacology (QSP), QSP model (QSPM) applications are expanding. Recently, a QSPM was used by US Food and Drug Administration (FDA) clinical pharmacologists to evaluate the appropriateness of a proposed dosing regimen for a new biologic. This application expands the use-horizon for QSPMs into the regulatory domain. Here we retrace the evolution of the model and suggest a question-based approach to directing model scope, identifying applications, and understanding overall QSPM value.
INITIAL QSP OBJECTIVES
In 2004, denosumab was under development for treating osteoporosis, progressing from proof of concept to registration trials.1 Questions existed that could not be practically addressed with clinical studies due to the protracted dosing interval (q6M) and required trial duration, nor by traditional PKPD models. They included questions about possible effects of drug regimen changes, treatment discontinuation, prior treatments, and sampling schemes. These questions, together with general uncertainty in the scientific community regarding the physiologic links between clinical markers (e.g., serum calcium, parathyroid hormone [PTH], bone turnover markers), clinical endpoints (e.g., bone mineral density [BMD]), and complex linkages between bone and calcium homeostasis, led the authors to embark on creating a model that incorporated much of the known bone physiology and was dependent on maintaining calcium's narrow physiologic range. The representation of calcium homeostatic mechanisms was paramount to ensuring model extensibility to calcium and PTH-related mechanisms.
The initial scientific objective was to arrive at a QSPM connecting bone turnover markers with BMD using known links between organs involved in calcium homeostasis, including bone and relevant cellular mechanisms (Figure 1). Thus, the necessary level of complexity and the nature of datasets mandatorily described simultaneously by the model were crucial questions. Data used included measures of longitudinal system perturbations by drug therapies (e.g., teriparatide) and disease states (e.g., progressive renal failure). Interestingly, while constructing the QSPM, scientific knowledge gaps became vividly clear, and produced an unexpected value. The gaps forced assumptions to be made about a pathway's importance, a diseases' influence, or a drug's potential, and subsequent sensitivity testing of those assumptions. In doing so, knowledge limitations became clearer and true understanding of the system became more transparent.
Figure 1.
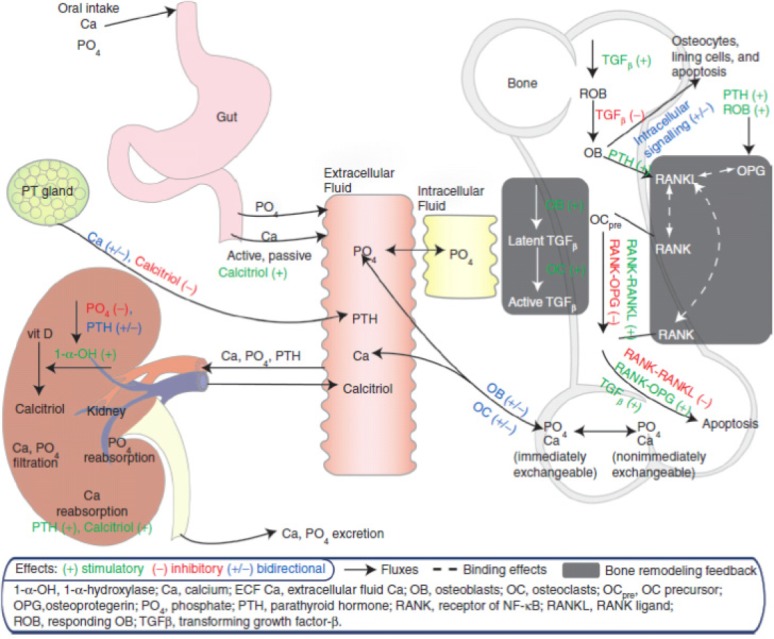
Schematic of calcium homeostasis and bone QSPM (reprinted with permission from Peterson and Riggs, 20102).
EXTENSION OF A QSP
A key value of QSPMs is their extensibility. This arises from a QSPM's foundation in human physiology for understanding a drug's effects (attempting to understand causality), rather than simply drawing a direct line between administration and endpoint observation (sometimes causality, often correlation). This aspiration of QSPMs aligns with the concept of Precision Medicine, and offers a tool by which myriad genomic level data may, in future, be used to understand disease and treatment. In our opinion, since the overall aim of pharmacology is to modify an underlying disease, understanding is increased by first generating a “best estimate” of the (patho)physiology and then adding the drug to that quantitative construct. It seems most relevant to consider investment in QSPM development for drugs impacting physiology with substantial system feedback, forward branching or redundant pathways, or for biologics with long half-lives, to support confidence in target and steering research toward question-based development goals. Additionally, as Precision Medicine generates further mechanistic understanding, a QSPM can be extended, refined, and reapplied with relative rapidity. The bone and calcium homeostasis QSPM provides examples of these extensions, refinements, and adaptable reapplication.
The first bone and calcium homeostasis QSPM publication described Ca, PTH, calcitriol, and bone marker changes associated with denosumab and teriparatide treatment in postmenopausal women, with theoretical changes expected during hypo- and hyperparathyroid disease states.2 Subsequently, the model was expanded to include additional disease states, a range of therapeutic interventions, and “middle-up” extensions describing BMD changes associated with bone marker changes3 and fracture risk associated with BMD changes.4 Qualification has included external validation of bone marker and BMD predictions.5
Disease state expansions and applications have included: 1) secondary hyperparathyroidism associated with chronic kidney disease6 and 2) estrogen-related effects on bone and calcium during menopause transition.7 Notably, this latter work was used to investigate BMD loss associated with treatments of endometriosis-related symptomatic pain and perform target mitigation. GnRH modulation therapies used for this purpose result in an estrogen depravation causing markedly decreased BMD and limiting the duration of approved therapy. Results from this work7 were later stated to direct not just a particular compound in development but Pfizer's entire GnRH modulation research program: “…this work identified target levels for estrogen that would provide symptomatic pain relief with minimal impact on BMD. … targeting the GnRH pathway to achieve the desired range of serum estrogen levels would be difficult to achieve; therefore, the research program was halted before any compound entered the clinic.”8
Another example of extensibility was in assisting the understanding of clinical findings from orally administered calcium-sensing receptor antagonism (CaSRa) programs. CaSRa would conceptually result in a PTH “spike” similar to that resulting from subcutaneously administered teriparatide without exogenous administration. CaSRa programs, however, historically reported BMD increases less than those achieved from teriparatide, often with hypercalcemic events consistent with nontransiently elevated PTH. Using public-source clinical data and preclinical sponsor data, the model was used to describe a capacity-limiting effect in the PT gland that limited the maximum achievable PTH Cmax while describing prolonged PTH elevations with continued dose escalations. These predictions were confirmed in a phase I single-dose study; the results were used to support development criteria with expectations for maximal BMD changes achievable through CaSR antagonism.9
QSP ENTERS THE REGULATORY ARENA
On September 12, 2014, the FDA Endocrine and Metabolic Drugs Advisory Committee met and discussed the biologics license application (BLA) 125511, proposed trade name NATPARA (Recombinant Human Parathyroid Hormone (rDNA) or (rhPTH[1-84]), submitted for the proposed indication of replacement for endogenous parathyroid hormone (1-84) and long-term treatment of hypoparathyroidism.10 As part of the FDA Briefing Document, a clinical pharmacology assessment of the adequacy of NATPARA dosage regimen in the treatment of hypoparathyroidism was rendered; this review used an open-source version of the QSPM2 (available in online Appendix7) to evaluate alternate dosing regimens. The FDA reviewers' recommendation, based on their independent model qualification using phase I data and subsequent simulations representative of the registration trial, was that “the dose regimen should be further optimized to address the safety concerns for hypercalciuria.”10
The significance of this event will take some time to coalesce. This use of a QSPM in a regulatory meeting, however, is an important first demonstration of a QSPM extension beyond the research space, and lends a further element of credibility to the discipline. It is therefore reasonable to consider the event itself as marking a change in how we view the use-horizon for QSPMs and a watershed moment in the maturing QSP discipline. While today we have this first example of regulatory agency QSPM use, there are numerous cases of QSPM uses in early development decisions, trial design discussions, and program advancement decisions. It will be important to the success of QSP as a discipline to make sure these influential QSPM uses are clearly visible to the pharmaceutical community and their relevance understood as we move beyond this landmark event.
At the same time, due in part to the complexity of QSPMs, and the required “fit-for-purpose” status needed to address regulatory questions, we should not expect examples to amass rapidly. Additionally, there are at least three further reasons why examples will likely emerge slowly. First, without question, clinical study and traditional modeling and simulation will continue to provide sufficient data and understanding to support the majority of regulatory discussions/decisions. Second, unlike population pharmacokinetics/pharmacodynamics (PK/PD), there is no regulatory guidance speaking to the application of QSPMs. Third, regulatory acceptance of a QSPM will require comfort by users and consumers with the underlying physiology, mathematics, and mechanistic assumptions supporting the application. Therefore, parameter sourcing and validation needs to be suitably scrutinized to ensure that the model behavior is appropriate. In this example, PTH was an integral part of the QSPM, and so questions asked were well within the model domain. The FDA then used a phase I sponsor study to perform an independent model evaluation. This step undoubtedly promoted confidence in the subsequent simulations that were used to suggest alternative dosing regimens. While this represents a groundbreaking example of QSPM use in a regulatory setting, future QSPM applications will need to continue building case-based evidence to further the scientific community's overall confidence in QSPM application.
REFLECTIONS ON VALUE FROM A QUESTION-BASED PERSPECTIVE
In 2004, two questions motivated construction of the bone and calcium homeostasis QSPM that would, a decade later, be used by the FDA: 1) Can the physiologic link between bone markers and BMD be captured within a model? and 2) Can a physiologically based model be used to explore development questions and guide clinical study? Moreover, the authors believed there was broader potential for a model covering multiple temporal scales, from cellular mechanisms to protracted clinical outcomes, and rooted in understood physiology. For instance, QSPMs can generate hypotheses, frame assumptions about mechanism and physiology, and provide a communication tool for answering questions about why and how a drug might or might not produce certain effects. The QSPM extensions described above clearly demonstrate how a model based on physiology (rather than exclusively program-centered clinical observations) can have applications across therapeutic areas and diseases, addressing unanticipated questions at the time of conception. This sets up a condition of non-forecastable return on investment and underscores the disciplines needed to highlight and communicate the types of questions that can be addressed via QSP modeling. Therefore, it can be useful to cast QSPM uses in terms of addressable questions to help clarify the rationale(s) for QSPM development/investment. A sample list of questions is proposed that a QSP modeler might seek to answer via a QSPM (Table 1). In many ways, our discipline's goals should clearly articulate the value and utility of QSPM investment as we move forward. Published examples, such as the ones provided here, predicated on the triad of physiology-pharmacology-pathology, are crucial tools for such advocacy. Therefore, we are encouraged by the FDA's use of a QSPM to generate scientific deliberation at an advisory meeting and hope that this truly represents a watershed moment for QSP as a discipline.
Table 1.
Quantitative Systems Pharmacology Model example use questions with most likely domain of interest within the pharmaceutical research and development (R&D) process
| QSPM Use Questions | Where in R&D |
|---|---|
| What is the relative therapeutic potential of several competing compounds? | Research/Biology |
| What amount of pathway modulation is needed (aka do we need 90% coverage?)? | Research |
| What is the impact of pharmacologically inhibiting target “x” by “y”% | Research |
| In treating this disease, what combination of targets will optimize response? | Research |
| Which biomarker, of those that can be measured, is most indicative of hitting the target? | Research |
| What experiments should be done to understand the mechanism better and guide target selection (hypothesis generation)? | Research |
| Did the trial design (sampling scheme) allow observation of important changes (safety, efficacy)? | Research/Clinical |
| What clinical trial population should be studied to understand the mechanism better and guide target or dose selection (hypothesis generation)? | Research/Clinical |
| How reasonable are the current mechanism-of-action assumptions (impact of being wrong)? | Research/Clinical |
| What don't we understand about how this drug works? | Research/Clinical |
| What is the impact of treating with mono-therapy versus adding on to standard of care? | Clinical |
| What is the optimal/most efficient sampling scheme for biomarkers/clinical measures we have not previously sampled but are outputs of the QSPM? | Clinical |
| Is there a pharmacologic combination that would increase the therapeutic window of the candidate drug? | Clinical |
| What happens if a patient stops taking the drug? | Clinical |
| How can we answer PKPD and biomarker questions for long t1/2 drugs, where clinical studies are not practical? | Clinical |
| Is the proposed dose/regimen optimized (or what range of doses should be investigated)? | POC onwards |
| Should compound(s) be progressed/entered to new indication? | Clinical lifespan |
Conflict of Interest
The authors declare no conflict of interest.
References
- McClung MR, et al. Denosumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. ) [DOI] [PubMed] [Google Scholar]
- Peterson MC. Riggs MM. A physiologically based mathematical model of integrated calcium homeostasis and bone remodeling. Bone. 2010;46:49–63. doi: 10.1016/j.bone.2009.08.053. [DOI] [PubMed] [Google Scholar]
- Peterson MC. Riggs MM. Predicting nonlinear changes in bone mineral density over time using a multiscale systems pharmacology model. CPT Pharmacometrics Syst. Pharmacol. 2012;1:e14. doi: 10.1038/psp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plan EL, et al. PAGE 21st Meet. 2012. Bayesian joint modeling of bone mineral density and repeated time-to-fracture event for multiscale bone systems model extension. . In: ) [Google Scholar]
- Riggs MM, Baron KT, Plan EL. Gastonguay MR. Qualification of a physiologically-based model for predicted bone marker and bone mineral density changes associated with denosumab treatment. Present. Am. Soc. Bone Miner. Res. Annu. Meet. 2012 [Google Scholar]
- Riggs MM, Peterson MC. Gastonguay MR. Multiscale physiology-based modeling of mineral bone disorder in patients with impaired kidney function. J. Clin. Pharmacol. 2012;52:45S–53S. doi: 10.1177/0091270011412967. [DOI] [PubMed] [Google Scholar]
- Riggs MM, Bennetts M, van der Graaf PH. Martin SW. Integrated pharmacometrics and systems pharmacology model-based analyses to guide {GnRH} receptor modulator development for management of endometriosis. CPT Pharmacometrics Syst. Pharmacol. 2012;1 doi: 10.1038/psp.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan PA, et al. Model-based drug development: a rational approach to efficiently accelerate drug development. Clin. Pharmacol. Ther. 2013;93:502–514. doi: 10.1038/clpt.2013.54. ) [DOI] [PubMed] [Google Scholar]
- Baron K, et al. An evaluation of calcilytic effects on parathyroid hormone and bone mineral density response using a physiologically-based, multiscale systems pharmacology model. J. Bone Miner. Res. 2013;28 ) [Google Scholar]
- Research C. for D. E. and. Endocrinologic and Metabolic Drugs Advisory Committee - 2014 Meeting Materials, Endocrinologic and Metabolic Drugs Advisory Committee < http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm386727.htm>.


