Abstract
The circumstance of how sensitive therapeutic success is under imperfect adherence is driven by the property known as forgiveness. To date, no studies have considered variability in the pharmacokinetic-pharmacodynamic process in conjunction with imperfect adherence patterns in order to develop a comparative criterion to determine the forgiveness of a drug. In this study, we have proposed a criterion to quantify forgiveness; illustrated the criterion for a theoretical example and evaluated the forgiveness of a motivating example, namely warfarin. A forgiveness criterion, relative forgiveness, is defined as the number of times more likely that a target is successfully attained under perfect adherence compared to imperfect adherence; or when comparing two drugs under a standard setting of imperfect adherence. The relative forgiveness criterion may have important implications for both drug development and clinical practice since the choice of drug can account for the likely influence of its forgiveness.
It is known that increased adherence to appropriately prescribed drugs is associated with better therapeutic outcomes1 and contributes to lower mortality.2 Adherence is not simply whether or not a patient takes a dose at the times prescribed. Although often described simply as the percentage of doses taken, it involves a much broader composite of factors and considers initiation, implementation of a dosing regimen, and discontinuation.3 Adherence linked with implementation represents the level of agreement between a patient's actual dosing regimen and the prescribed dosing regimen between initiation and discontinuation. It has been suggested that factors influencing adherence may be grouped into five dimensions which are related to the patient, condition, therapy, health care team and system, and socioeconomics.4 To better understand adherence, it is important to consider the extent of drug taking behavior and the pattern that patients deviate from the nominal prescribed schedule. In this work, we concentrate on adherence with respect to implementation. Suboptimal implementation may be divided into (i) timing variability, (ii) random missed doses which are denoted here as nonconsecutive missed doses or by chance two consecutive missed doses, and (iii) a drug holiday, i.e., three or more consecutive missed doses.5
The circumstance of how sensitive therapeutic success is under imperfect adherence is driven by the property known as forgiveness.6 A forgiving drug would be one in which therapeutic outcomes are robust to common patterns of imperfect adherence. Forgiveness is a function of the duration of action and the dose interval of the drug, conceptually shown as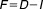 . Here,
. Here, is forgiveness,
is forgiveness, is duration of action and
is duration of action and is dose interval.6 When the duration of action greatly exceeds the dose interval, then the drug is considered forgiving.5,7 The number of sequentially missed doses that can be missed with a minimal loss of drug effect, i.e., a forgiveness index (
is dose interval.6 When the duration of action greatly exceeds the dose interval, then the drug is considered forgiving.5,7 The number of sequentially missed doses that can be missed with a minimal loss of drug effect, i.e., a forgiveness index (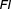 ) can be conceptualized as
) can be conceptualized as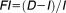 .5–8 Here, the duration of effect (
.5–8 Here, the duration of effect ( ) relates to the pharmacokinetic (PK) and pharmacodynamic (PD) properties of the drug. Since in practice, patients may not take drugs exactly as prescribed,
) relates to the pharmacokinetic (PK) and pharmacodynamic (PD) properties of the drug. Since in practice, patients may not take drugs exactly as prescribed, which represents the actual dose interval relates to drug taking behavior. The PK and PD properties can themselves be further subdivided whereby for PK there are intrinsic and extrinsic drug properties and for PD there are drug and system related properties. For example, an intrinsic PK property is a drug with a long half-life in relation to the dosing interval which leads to persistent plasma concentrations, and an extrinsic PK property is an extended-release formulation which provides an apparently longer half-life. A drug related PD property arises when a given dose yields concentrations that are much higher than the concentration resulting in half maximal effect such that the effect is prolonged for longer than would be expected given the declining plasma drug concentrations. A system related PD property pertains to an effect of the drug on the turn-over of a substrate in the system for which the half-life of turn-over exceeds the dose interval.
which represents the actual dose interval relates to drug taking behavior. The PK and PD properties can themselves be further subdivided whereby for PK there are intrinsic and extrinsic drug properties and for PD there are drug and system related properties. For example, an intrinsic PK property is a drug with a long half-life in relation to the dosing interval which leads to persistent plasma concentrations, and an extrinsic PK property is an extended-release formulation which provides an apparently longer half-life. A drug related PD property arises when a given dose yields concentrations that are much higher than the concentration resulting in half maximal effect such that the effect is prolonged for longer than would be expected given the declining plasma drug concentrations. A system related PD property pertains to an effect of the drug on the turn-over of a substrate in the system for which the half-life of turn-over exceeds the dose interval.
The variability in both the duration of action and adherence behavior should be considered when determining the forgiveness properties of any drug. To date, no studies have considered variability in the PKPD process in conjunction with imperfect adherence patterns in order to develop a comparative criterion to determine the forgiveness of a drug.
In this study, we have developed a criterion to quantify forgiveness and then: (1) illustrate the criterion for a theoretical example and (2) apply the criterion to warfarin as a motivating example. Warfarin was chosen as it is commonly prescribed and a great deal has been studied about its PKPD properties which allows for straightforward interpretation of the clinical importance of forgiveness.
RESULTS
The results are divided into two parts: (1) illustration of the forgiveness criterion with a theoretical example and (2) an application of the criterion to warfarin.
Part 1. Illustration of the forgiveness criterion with a theoretical example
Considering the same individual with the typical values of CL, V, Emax, EC50 (mean values), Figure 1 shows a comparison of the PK and PKPD responses to perfect and imperfect adherence.
Figure 1.
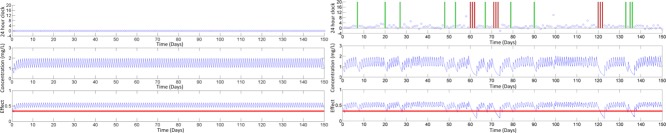
A comparison of a single individual with perfect adherence (left panel) and imperfect adherence (right panel) with the top row representing the adherence profile, the second row the concentration-time profile, and the third row the effect-time profile with the (hypothetical) threshold of a target success.
Quantification of forgiveness as relative forgiveness
For the illustrative example, the probability of target attainment with imperfect adherence (Pip) was 0.38 and the probability of target attainment with perfect adherence (Pp) was 0.62 yielding a relative forgiveness (RF) of 0.38. This means that therapeutic success was 0.38 times as likely (i.e., 62% less likely) with imperfect adherence. When profiles were considered that only contained timing errors the RF was 0.80 and when only considering missed doses (random missed doses and drug holidays) the RF was 0.44. It is clear that missed doses, from any cause, have a quantitatively larger effect on RF than timing errors. Indeed the influence on timing errors resulted in only a 20% reduction in RF, indicating that, in this example, timing is of minimal concern.
The original drug properties are shown in Table 1. Changes in drug properties resulting in different values of RF are presented in Table 2. Here, the relative forgiveness is a comparison of the different drug properties (DRUG B, C, D, A × 2) to the original drug properties (termed DRUG A).
Table 1.
Model
| Parameter/variablea | Mean value | BSV (CV%) |
|---|---|---|
| Dose (mg) | 1 | - |
| CL (L/d) | ln(2) | 30 |
| V (L) | 1 | 30 |
| Emax | 1 | 30 |
| EC50 (mg/L) | 1.3 | 30 |
| Proportional RUV | - | 10 |
BSV, between subject variability; CL, clearance; CV, coefficient of variation; Emax, maximum effect; EC50, drug concentration resulting in half maximal effect; RUV, residual unexplained variability; V, apparent volume of distribution.
Note the units are arbitrary and provided for interpretation of once daily dosing profiles.
Table 2.
Theoretical modifications to the drug
| Drug |
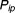
|
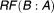
|
|---|---|---|
| DRUG A (original drug) | 0.38 | - |
| DRUG B (twice half-life) | 0.84 | 8.57 |
| DRUG C (twice potency) | 0.69 | 3.63 |
| DRUG D (twice half-life and twice potency) | 0.95 | 31 |
| DRUG A x 2 (double dose) | 0.71 | 3.99 |
The results showed that when compared to the original drug with imperfect adherence that increasing the half-life had the greatest single effect, becoming almost nine-fold more forgiving. Changing half-life and potency together yielded a marked (31-fold) increase in forgiveness. This value from the simulations was also similar to the value from the product of the two independent effects, changing half-life RF = 8.57 and changing potency RF = 3.63. Doubling the dose was similar in effect size to doubling potency (i.e., halving EC50) in this simulated example.
Part 2. Application of the forgiveness criterion to warfarin
Simulations with warfarin included all components of imperfect adherence, i.e., imperfect timing, random missed doses, and drug holidays. The probability of therapeutic success for perfect adherence was 0.58 (Pp) and for imperfect adherence (Pip) was 0.52. The relative forgiveness of warfarin to imperfect adherence was 0.78, which indicates that success was 22% less likely with warfarin for imperfect adherence, suggesting that warfarin is a relatively forgiving drug to nonadherence originating from factors linked to implementation.
DISCUSSION
This study has described a criterion to quantify forgiveness that accounts for variability in both PKPD and adherence. We defined the criterion as relative forgiveness (RF), which has the same interpretation as an odds ratio or relative risk. The RF holds the original concept of forgiveness while incorporating variability in the duration of drug action as well as the dose interval. This criterion can be used to compute the forgiveness of a given drug or to compare the forgiveness between two drugs whose effects can be quantified on the same biomarker of response. This study shows how the proposed criterion can be used to determine forgiveness for a specific drug, to compare between drug entities that have different pharmaceutical and/or pharmacological profiles and how it can be applied to a current therapeutic agent, namely warfarin.
The concept of forgiveness has been considered previously.9–14 In a study of Alzheimer's disease it was reported that, under chronic dosing, one or two consecutive missed dose/s of donepezil 5 mg or 10 mg would be unlikely to affect the attainment of the optimal target of peripheral cholinesterase inhibition.12 Similarly, it has been shown that the administration of either once or twice daily lopinavir/ritonavir resulted in comparable treatment outcomes.15 Later research on lopinavir/ritonavir found that the proportion of patients achieving appropriate virologic suppression over 24 weeks were similar across four quartiles of adherence rates, measured as percentage of prescribed doses taken, ranging from 23.5–53.3% to 92.9–100%.10 In addition, the degree of forgiveness of hypertensive drugs has been established based on “off-rate”, e.g., loss of a decrease in blood pressure in mmHg per day under imperfect adherence circumstances.13 These findings indicate that drugs are forgiving when they have low off-rates, i.e., drugs with a long duration of action. Examples of low off-rate antihypertensive drugs that are administered once daily include amlodipine and aliskiren, and examples of those with high off-rates include enalapril and atenolol.13,16 These measures to date have considered forgiveness informally and attempted to find drug related characteristics that are associated with forgiveness.
Recently the work of Boissel and Nony has provided a theoretical framework for quantification of forgiveness.17 Their work considered alteration of PKPD parameters of direct and delayed models of hypothetical drugs to explore impact on drug effects.17 Our study, and criterion, builds on this approach and also incorporates variability in the PKPD parameters. Nony and Boissel further proposed the use of sensitivity functions to compare forgiveness.18 Methods for comparing forgiveness across drugs were also investigated by Gohore et al.19 In this study, the most sensitive PKPD parameters in relation to the number of subtherapeutic days and smoothness index were determined and forgiveness was based on a sensitivity analysis and a comparison of four calcium channel blocker drugs.19 This work did not consider forgiveness in the context of concurrent variability in adherence and PKPD parameter values.
With respect to the influence of different types of imperfect adherence on forgiveness, we found that the influence of only missed doses was stronger than that of only timing variability with RF values of 0.44 and 0.80, respectively (in our theoretical example). This finding is not surprising since most drugs have a duration of action that spans more than a few hours either side of the nominal dose interval and hence timing variability is likely to be quantitatively less important. This effect will be drug specific and drug candidates that have poor forgiveness to timing variability are likely to fare much worse in RF values. It may be expected that the influence of timing variability will be more influential if the distribution of timing variability deviates to a much larger extent compared to the timing variability used in this study. However, it is believed that the greatest impact of timing variability would be seen in the most extreme timing variability, i.e., random missed doses, which is categorized separately as in this case not only is timing delayed but the dose is also not taken. In general, it, therefore, seems that the number of random missed doses is a more influential adherence pattern than the timing of any given dose. It is also proposed that the influence of missed doses depends also on the dosing schedule. For instance, it has been proposed that drug actions may persist longer in patients prescribed with twice daily compared to once daily dosing since the probability of missing two or three consecutive doses in the former is proposed to be half of that of missing a daily dose in the latter.20 To illustrate, in terms of the maintenance of drug concentrations within a therapeutic range, which may well result in the maintenance of drug actions, twice daily lopinavir/ritonavir was reported to be superior to once daily lopinavir/ritonavir.20 A corresponding result has been shown with saquinavir/ritonavir11 and again similarly for twice daily ticagrelor compared to once daily clopidogrel.21
Our work has shown how theoretical modifications to the properties of a drug (either by chemical alteration or pharmaceutical modification) can be quantified in terms of an alteration in forgiveness. A drug with twice the potency or given at twice the dose shared similar degrees of relative forgiveness (both had RF values around 4 in the theoretical example). By comparison, a drug with twice the half-life was considerably more forgiving (an RF of approximately 8 in the theoretical example). A combination of longer half-life and greater potency combined linearly to provide a relative forgiveness of approximately 30 (the product of the influence of either modification alone). Choice of drug, either in drug development to consider which lead molecule to take forward, or in therapeutics to consider which medicine to use in a patient with known poor adherence, can therefore account a priori for the likely influence of its forgiveness. All things being equal, then a drug with the best relative forgiveness should be considered.
The RF criterion was illustrated by consideration of warfarin. The therapeutic range was chosen to be an INR between 2 and 3.5 and therapeutic success defined when at least 55% of a dosing profile achieved INR values in that range. For simplicity, this example did not consider dose individualization of warfarin dose to achieve the INR target since this example is to show an application rather than a definitive review of warfarin forgiveness. Under imperfect adherence, the value of RF was not diminished greatly from 1 (RF = 0.78). In theory, a confidence interval could be applied to this quantity but this would have natural statistical limitations on its interpretation since the interval (for the same drug) cannot include the null value. The high value of RF for warfarin indicates that the likelihood of patients with suboptimal adherence (comprising timing variability, random missed doses, and random drug holidays) successfully attaining the target was 0.78 times of those with perfect adherence. Hence, the impact of imperfect adherence on achieving a desirable INR was considered minimal. It seems likely, therefore, that under profiles of imperfect adherence that are within the range of plausible profiles explored here that warfarin would remain forgiving. A plausible poor adherence scenario that would have been included in these simulations would have had 3 drug holidays and 25 missed doses over the 150 treatment day period.
It should be noted that the index adherence pattern chosen for this study was not related specifically to anticoagulants and it is possible that adherence patterns may differ in patients with atrial fibrillation or coagulation disorders due to the influence of disease or other patient characteristics (e.g., age). Although it is possible that adherence patterns, and hence forgiveness values, may differ depending on the subpopulation being studied, we have compared our index adherence profile to other adherence profiles and feel that the adherence implementation features that were seen in the index profile are representative of other profiles. In this work, we assumed that the adherence profile of a patient was independent of the PKPD parameters. This assumption is not a requirement of determining RF but was rather a simplification used here in these examples. It is plausible that an association between adherence patterns and PKPD profiles may exist where an association between patient covariates, e.g., age and adherence patterns has been identified previously (as per22). If it were the case that either a PK parameter, e.g., CL, or a PD parameter, e.g., Emax, were correlated with age, then there may be an association between adherence patterns and PKPD parameters. However, as mentioned in the methods section: Parametric simulation of imperfect adherence patterns, the purpose of this current work, was not to learn how patterns of nonadherence arise but rather provide a forgiveness criterion. The influence of covariates would be an important and interesting further exploration. We also note that this study focused on the process of suboptimal implementation rather than failure to initiate or early discontinuation. Here, the concept of forgiveness is articulated in the case where implementation and its issues predominates. However, patients who fail to initiate, while an important population for consideration, are not amenable to choice of forgiving regimens in circumstances when patient autonomy is maintained. Finally, this work illustrated the case of once-daily drug administration. Therefore, it should be noted that for other dosing regimens, profiles of imperfect adherence would differ. However, the RF criterion described in this paper is generalizable to these settings.
In conclusion, this study shows that relative forgiveness can be used as an index to quantify the forgiveness properties of a drug given its dosing regimen. This may have important implications for both drug development and clinical practice. Further work is needed to examine the properties of RF as a measure of forgiveness behavior to determine its clinical utility.
METHODS
Initially, we introduce a criterion for quantifying forgiveness then we describe the methods for exploring the illustrative example and then the motivating example for warfarin. All simulations in this study were conducted in MATLAB® R2012a (The MathWorks™, Natick, MA). For both Parts 1 and 2 of the methods, all simulations included 1,000 individuals each with an individual profile and individual set of PKPD parameters. Note that the adherence profiles were considered to be independent of the PKPD parameter values, such that and for example high or low CL values were as likely to accompany a highly adherent profile as a profile representing poor adherence. Relaxation of this assumption will provide opportunities for further exploration of relative forgiveness. The relationship between adherence profiles and PKPD parameter values would be an interested area for future study.
Quantification of forgiveness as relative forgiveness
A criterion to quantify forgiveness was developed. Its concept is in line with relative forgiveness (RF) which has the same interpretation as an odds ratio or relative risk. Calculation of RF was based on the probability of therapeutic success given imperfect adherence (Pip) and the probability of therapeutic success given perfect adherence (Pp). RF is defined as the number of times more likely that target success is attained under perfect adherence compared to imperfect adherence; or when comparing two drugs under a standard setting of imperfect adherence.
A general form of a relative forgiveness is given by,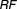 :
:
| 1 |
where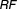 is the relative forgiveness,
is the relative forgiveness,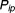 is the probability of successful attainment of a treatment target under imperfect adherence, and
is the probability of successful attainment of a treatment target under imperfect adherence, and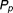 is the probability of successful attainment of a treatment target under perfect adherence. Values of RF close to one indicate that a drug is forgiving to imperfect adherence and values close to zero indicate that the drug is particularly sensitive to imperfect adherence behavior (i.e., not forgiving).
is the probability of successful attainment of a treatment target under perfect adherence. Values of RF close to one indicate that a drug is forgiving to imperfect adherence and values close to zero indicate that the drug is particularly sensitive to imperfect adherence behavior (i.e., not forgiving).
When comparing the RF of two drugs (Drug A and Drug B), then the relative forgiveness of Drug B compared to Drug A can be determined as:
| 2 |
In this setting, Drug A and Drug B could be two formulations of the same drug or could be different drugs. When comparing drugs then values of RF can exceed 1 and, in this circumstance, indicate how many times more likely that Drug B is forgiving compared to Drug A (i.e., how many times more likely therapeutic success will be achieved with Drug B compared to Drug A) given some pattern of imperfect adherence.
Part 1. Illustration of the forgiveness criterion with a theoretical example
PKPD model
A one-compartment instantaneous unit input PK model linked to an immediate effects Emax PD model was used as an illustrative example (as per, for example23,24). Two scenarios were considered: (i) perfect adherence and (ii) imperfect adherence, which consisted of several subtypes of imperfect adherence patterns. In these scenarios, a 1-unit dose was administered every half-life for 150 half-lives. Steady state was assumed at 10 half-lives. The model is shown in Table 1. Proportional residual variability was incorporated to the effect.
Parametric simulation of imperfect adherence patterns
In this work, adherence patterns were simulated from parametric distributions. It is noted in the work of others that various approaches have been used to estimate imperfect adherence including Bayesian approaches and Markov models.13,19,22,25–27 These techniques were not considered in this study since the goal was to simulate acceptable patterns of nonadherence rather than to learn about how patterns of nonadherence arise. Simulated patterns were used to assess the relative forgiveness criterion and its performance.
The three types of imperfect adherence patterns were considered that when layered together would provide an overall imperfect adherence pattern. These were (i) timing variability, (ii) random missed doses, and (iii) drug holidays.
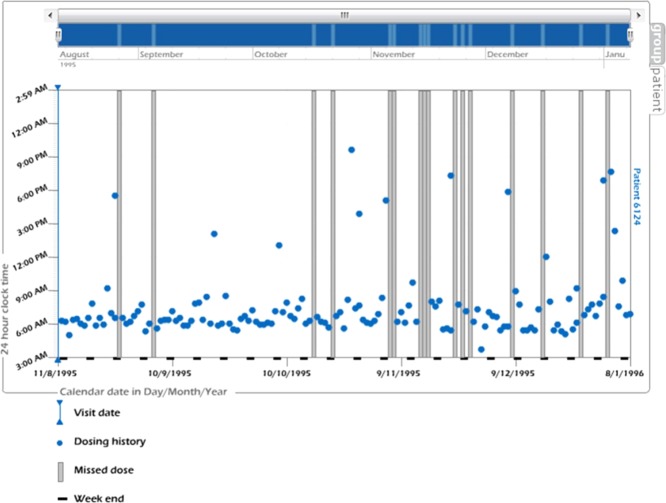
An index adherence profile that included the imperfect adherence patterns described above was identified from an online resource, www.iAdherence.org. The index adherence profile was identified that had once daily dosing prescribed for 150 days (see Figure 2). The profile was compared to other once daily profiles and was determined to contain typical features and importantly contained all the three key patterns of interest but did not contain initiation or discontinuation aspects of nonadherence.
Figure 2.
An index adherence profile. The x-axis is calendar date in day/month/year. The y-axis is 24-h clock time. Each dot represents timing of each dose taken. Each vertical bar depicts each missed dose. (Figure taken from www.iAdherence.org.)
Figure 4.
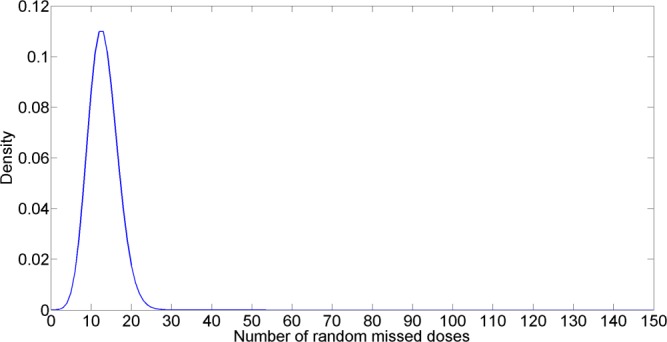
Poisson distribution of random missed doses. The x-axis is number of random missed doses. The y-axis is density.
After identifying the index adherence profile, this index profile was then used to quantify important features of each imperfect adherence pattern. The important features were used to determine reasonable parametric distributions of the three sources of imperfect adherence (timing variability, random missed doses, and drug holidays). Figures 3-5 show the parametric distribution of timing variability, random missed doses, and drug holidays, respectively. Full details are included in Supplementary Materials S01–S15, which are available online.
Figure 3.
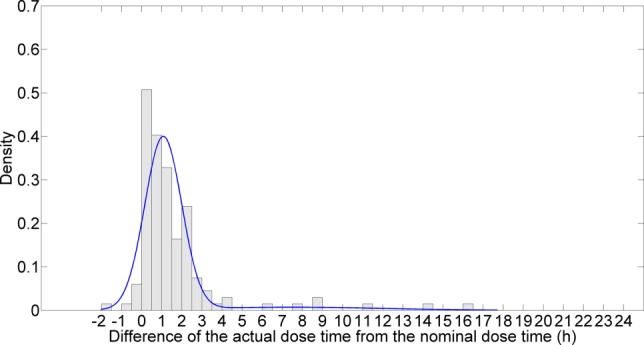
Parametric distribution of timing variability. This distribution is provided by a mixture of 2 normals. The x-axis represents the difference of the actual dose time from the nominal dose time (hours). The y-axis is density.
Figure 5.
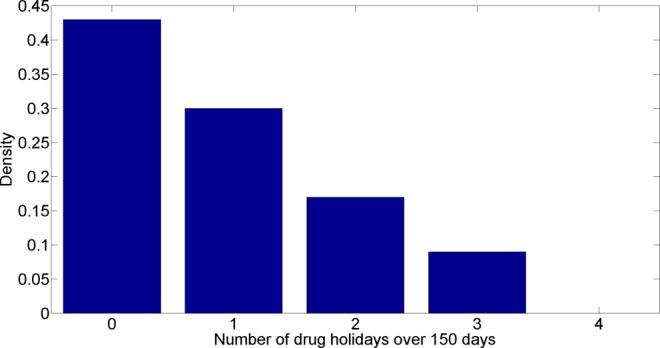
An empirical distribution of drug holidays. The x-axis is number of drug holidays over 150 days. The y-axis is density.
Successful attainment of a treatment target
The profile of the drug effect for each individual was assessed for successful attainment of a target treatment. Only steady state profiles were considered and the first 10 dosing profiles were discarded (corresponding to 10 half-lives of maintenance doses) over the period of 150 days. For the purposes of these simulations, successful attainment of a target was defined as:
Condition 1. The effect level at the trough was greater than a defined lower treatment target. The (hypothetical) threshold of a target success is illustrated in Figure 1 using an individual with the mean values of CL, V, Emax, EC50.
Condition 2. The number of doses where criterion 1 is true meets a defined fraction of doses over the 140 treatment doses.
It should be noted that Condition 1 is sensitive to variability in both PKPD and adherence profiles, whereas Condition 2 is most sensitive to variability in adherence. For the theoretical example, the value for Condition 1 was set to a minimum trough effect value of 0.35 (units) and the value for Condition 2 was 0.9 such that at least 90% of doses (from dose 11) within an individual must have an effect at the trough greater than 0.35 (units).
To evaluate the forgiveness criterion, the success of target attainment for each simulated patient was evaluated with a perfect adherence profile and an imperfect adherence profile. Summing the success values and expressing as a fraction of the 1,000 patients provides the probability of success for either perfect adherence (Pp) or imperfect adherence (Pip). This is shown in the following four steps using warfarin as an example:
Step 1. Success for an individual patient at a particular dosing interval.
For the th patient receiving warfarin, the successful (
th patient receiving warfarin, the successful ( ) attainment of an international normalization ratio (INR) for the
) attainment of an international normalization ratio (INR) for the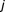 th dose is given by,
th dose is given by,
| 3 |
where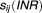 is an indicator variable that takes the value of 1 if the observed INR is in the therapeutic range and 0 otherwise. In this example, the first 20 doses are discarded as these were not considered to be within 90% of steady state.
is an indicator variable that takes the value of 1 if the observed INR is in the therapeutic range and 0 otherwise. In this example, the first 20 doses are discarded as these were not considered to be within 90% of steady state.
Step 2. Time in the therapeutic range.
The proportion of dose intervals within the therapeutic range (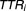 ) for the
) for the th individual is determined by the number of successful doses that arise from Step 1 above as a fraction of the total number of doses under consideration. Each patient receiving 130 doses was considered.
th individual is determined by the number of successful doses that arise from Step 1 above as a fraction of the total number of doses under consideration. Each patient receiving 130 doses was considered.
| 4 |
Step 3. Overall success for a patient's dosing regimen.
The dosing regimen with associated PKPD and adherence variability was considered to be a success (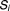 ) when the number of successful dose intervals exceeded the predefined criteria (for warfarin this was
) when the number of successful dose intervals exceeded the predefined criteria (for warfarin this was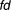 = 0.55). The value of 1 indicates success and 0 otherwise.
= 0.55). The value of 1 indicates success and 0 otherwise.
| 5 |
| 6 |
Step 4. Probability of success for 1,000 patients.
The probability of success is the fraction of successful patient profiles out of the total number of simulations.
| 7 |
where is the probability of success for either perfect adherence (Pp) or imperfect adherence (Pip).
is the probability of success for either perfect adherence (Pp) or imperfect adherence (Pip).
Influence of different types of imperfect adherence on RF
The influence of imperfect timing and missed doses (random missed doses + drug holidays) were considered separately and when combined into the overall composite pattern. In addition, a series of what-if scenarios were investigated in which hypothetical drugs were considered that had the following characteristics: (i) a longer half-life (the value of CL halved) [DRUG B], (ii) greater potency (EC50 halved) [DRUG C], (iii) a combination of longer half-life and greater potency (both CL and EC50 halved) [DRUG D], and (iv) where the dose was doubled [DRUG A × 2].
Part 2. Application of the forgiveness criterion to warfarin
Adherence patterns were simulated using the same methods as described for the illustrative example. For this warfarin example, 1,000 individuals were simulated with perfect and imperfect adherence profiles.
The model used for warfarin in this study was a population kinetic-pharmacodynamic (KPD) model developed by Hamberg et al.28 The model and parameter values are taken from28 and are provided in Supplementary Information S2. The dose was 3.5 mg given once daily for 150 days. The dose was chosen such that the population average patient with perfect adherence would achieve a steady state average INR midway in the therapeutic range. Successful attainment of a treatment target was considered as time in the therapeutic range. The time to steady state was assumed to be 20 days. The therapeutic range was defined as an INR within the range of 2 to 3.5. Successful treatment was defined as where at least 55% of steady state trough values were within the therapeutic INR range (which is similar to the success reported by Wright & Duffull29 when INR monitoring was not performed). Note that, in this example, dose individualization to target INR was not considered. Covariates were not considered and it was assumed that food had no impact on INR.
Acknowledgments
P.A. was supported by a University of Otago PhD Scholarship.
Author Contributions
P.A., R.B., and S.B.D. wrote the manuscript; P.A., R.B., and S.B.D. designed the research; P.A. and S.B.D. performed the research; and P.A. and S.B.D. analyzed the data.
Conflict of Interest
The authors declared no conflict of interest. As an Associate Editor for CPT:PSP, S.B.D. was not involved in the review or decision process for this paper.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THIS TOPIC?
✓A drug is forgiving when its duration of action greatly exceeds the dose interval. Previous research has considered this forgiveness concept and attempted to find drug related characteristics that are associated with forgiveness.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓This study addresses a method to quantify forgiveness that accounts for variability in both PKPD and adherence patterns.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
✓This study shows how a proposed forgiveness criterion can be used to determine forgiveness for a specific drug, to compare between drug entities that have different pharmaceutical and/or pharmacological profiles and how it can be applied to a current therapeutic agent, namely warfarin.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS
✓The relative forgiveness criterion may have important implications for both drug development and clinical practice. Choice of drug, either in drug development to consider which lead molecule to take forward, or in therapeutics to consider which medicine to use in a patient with known poor adherence, can account for the likely influence of its forgiveness.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
References
- DiMatteo MR, Giordani PJ, Lepper HS. Croghan TW. Patient adherence and medical treatment outcomes a meta-analysis. Med. Care. 2002;40:811–794. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- Simpson SH, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijens B, et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012;73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x. ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Adherence to Long-term Therapies: Evidence for Action. Geneva: WHO; 2003. ) [Google Scholar]
- Urquhart J. Pharmacodynamics of variable patient compliance: implications for pharmaceutical value. Adv. Drug Deliv. Rev. 1998;33:207–219. doi: 10.1016/s0169-409x(98)00029-5. ) [DOI] [PubMed] [Google Scholar]
- Urquhart J. The electronic medication event monitor. Lessons for pharmacotherapy. Clin. Pharmacokinet. 1997;32:345–356. doi: 10.2165/00003088-199732050-00001. ) [DOI] [PubMed] [Google Scholar]
- Osterberg LG, Urquhart J. Blaschke TF. Understanding forgiveness: minding and mining the gaps between pharmacokinetics and therapeutics. Clin. Pharmacol. Ther. 2010;88:457–459. doi: 10.1038/clpt.2010.171. [DOI] [PubMed] [Google Scholar]
- Blaschke TF, Osterberg L, Vrijens B. Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu. Rev. Pharmacol. Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- Hughes DA. Walley T. Predicting “real world” effectiveness by integrating adherence with pharmacodynamic modeling. Clin. Pharmacol. Ther. 2003;74:1–8. doi: 10.1016/S0009-9236(03)00091-2. [DOI] [PubMed] [Google Scholar]
- Shuter J, Sarlo JA, Kanmaz TJ, Rode RA. Zingman BS. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95% J. Acquir. Immune Defic. Syndr. 2007;45:4–8. doi: 10.1097/QAI.0b013e318050d8c2. [DOI] [PubMed] [Google Scholar]
- Dickinson L, et al. Pharmacokinetic analysis to assess forgiveness of boosted saquinavir regimens for missed or late dosing. J. Antimicrob. Chemother. 2008;62:161–167. doi: 10.1093/jac/dkn187. ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalbe O, Scheerans C, Freiberg I, Schmidt-Pokrzywniak A, Stang A. Kloft C. Compliance assessment of ambulatory Alzheimer patients to aid therapeutic decisions by healthcare professionals. BMC Health Serv. Res. 2010;10:232. doi: 10.1186/1472-6963-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy A, et al. Effects on blood pressure and cardiovascular risk of variations in patients' adherence to prescribed antihypertensive drugs: role of duration of drug action. Int. J. Clin. Pract. 2011;65:41–53. doi: 10.1111/j.1742-1241.2010.02569.x. ) [DOI] [PubMed] [Google Scholar]
- Srivastava S, Pasipanodya JG, Meek C, Leff R. Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J. Infect. Dis. 2011;204:1951–1959. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode R, Vrijens B, Niemi K, Wikstrom K, Heuser R. Podsadecki T. 2005. pp. 16–19. Differences in treatment compliance between lopinavir/ritonavir (LPV/r) given once (QD) versus twice (BID) daily do not affect virologic or immunologic outcomes. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, December.
- Burnier M, Brede Y. Lowy A. Impact of prolonged antihypertensive duration of action on predicted clinical outcomes in imperfectly adherent patients: comparison of aliskiren, irbesartan and ramipril. Int. J. Clin. Pract. 2011;65:127–133. doi: 10.1111/j.1742-1241.2010.02616.x. [DOI] [PubMed] [Google Scholar]
- Boissel JP. Nony P. Using pharmacokinetic-pharmacodynamic relationships to predict the effect of poor compliance. Clin. Pharmacokinet. 2002;41:1–6. doi: 10.2165/00003088-200241010-00001. [DOI] [PubMed] [Google Scholar]
- Nony P. Boissel JP. Use of sensitivity functions to characterise and compare the forgiveness of drugs. Clin. Pharmacokinet. 2002;41:371–380. doi: 10.2165/00003088-200241050-00004. [DOI] [PubMed] [Google Scholar]
- Gohore DG, Fenneteau F, Barrière O, Li J. Nekka F. Rational drug delineation: a global sensitivity approach based on therapeutic tolerability to deviations in execution. Pharmacol. Pharm. 2010;1:42–52. [Google Scholar]
- Comte L, Vrijens B, Tousset E, Gerard P. Urquhart J. Estimation of the comparative therapeutic superiority of QD and BID dosing regimens, based on integrated analysis of dosing history data and pharmacokinetics. J. Pharmacokinet. Pharmacodyn. 2007;34:549–558. doi: 10.1007/s10928-007-9058-0. [DOI] [PubMed] [Google Scholar]
- Vrijens B, Claeys MJ, Legrand V, Vandendriessche E. Van de Werf F. Projected inhibition of platelet aggregation with ticagrelor BID versus clopidogrel QD based on patient adherence data (the TWICE project) Br. J. Clin. Pharmacol. 2013;77:746–755. doi: 10.1111/bcp.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard P, Blaschke TF, Kastrissios H. Sheiner LB. A Markov mixed effect regression model for drug compliance. Stat. Med. 1998;17:2313–2333. doi: 10.1002/(sici)1097-0258(19981030)17:20<2313::aid-sim935>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Ribbing J. Jonsson EN. Power, selection bias and predictive performance of the Population Pharmacokinetic Covariate Model. J. Pharmacokinet. Pharmacodyn. 2004;31:109–134. doi: 10.1023/b:jopa.0000034404.86036.72. [DOI] [PubMed] [Google Scholar]
- Lagishetty CV, Coulter CV. Duffull SB. Design of pharmacokinetic studies for latent covariates. J. Pharmacokinet. Pharmacodyn. 2012;39:87–97. doi: 10.1007/s10928-011-9231-3. [DOI] [PubMed] [Google Scholar]
- Wang W, Husan F. Chow SC. The impact of patient compliance on drug concentration profile in multiple doses. Stat. Med. 1996;15:659–669. doi: 10.1002/(SICI)1097-0258(19960330)15:6<659::AID-SIM207>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Wong D, Modi R. Ramanathan M. Assessment of Markov-dependent stochastic models for drug administration compliance. Clin. Pharmacokinet. 2003;42:193–204. doi: 10.2165/00003088-200342020-00006. [DOI] [PubMed] [Google Scholar]
- Blesius A, Chabaud S, Cucherat M, Mismetti P, Boissel JP. Nony P. Compliance-guided therapy: a new insight into the potential role of clinical pharmacologists. Clin. Pharmacokinet. 2006;45:95–104. doi: 10.2165/00003088-200645010-00007. [DOI] [PubMed] [Google Scholar]
- Hamberg AK, et al. A pharmacometric model describing the relationship between warfarin dose and INR response with respect to variations in CYP2C9, VKORC1, and age. Clin. Pharmacol. Ther. 2010;87:727–734. doi: 10.1038/clpt.2010.37. ) [DOI] [PubMed] [Google Scholar]
- Wright DF. Duffull SB. A Bayesian dose-individualization method for warfarin. Clin. Pharmacokinet. 2013;52:59–68. doi: 10.1007/s40262-012-0017-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information