Abstract
Context
Negative symptoms of schizophrenia, which respond minimally to antipsychotic medications, have previously been associated with reduced blood folate levels, especially among patients with low-functioning variants in genes that regulate folate metabolism.
Objective
To determine whether folic acid plus B12 supplementation reduces negative symptoms of schizophrenia, and whether functional variants in folate-related genes influence treatment response.
Design
Parallel-group, randomized, double-blind, placebo-controlled clinical trial of 16 weeks of treatment with 2 mg folic acid and 400 mcg B12.
Setting
Three community mental health centers affiliated with academic medical centers in the United States.
Participants
Outpatients with chronic schizophrenia taking stable doses of antipsychotic medications.
Intervention
140 subjects were randomized to receive daily oral folic acid plus B12 or placebo.
Main Outcome Measures
Change in negative symptoms (Scale for Assessment of Negative Symptoms, SANS), as well as positive and total symptoms (Positive and Negative Syndrome Scale).
Results
Folate plus B12 improved negative symptoms significantly compared to placebo (group difference: -0.33 change in SANS per week; 95% CI, -0.62 to -0.05) when genotype was taken into account, but not when genotype was excluded. An interaction of the 484C>T variant of FOLH1 (rs202676) with treatment was observed (p=.02), where only patients homozygous for the 484T allele demonstrated significantly greater benefit with active treatment (-0.59 change in SANS per week, 95% CI, -0.99 to -0.18). In parallel we observed an inverse relationship between red blood cell folate concentration at baseline and 484C allele load (p=.03), which persisted until 8 weeks of treatment. Change in positive and total symptoms did not differ between treatment groups.
Conclusions
Folate plus B12 supplementation can improve negative symptoms of schizophrenia, but treatment response is influenced by genetic variation in folate absorption. These findings support a personalized medicine approach for the treatment of negative symptoms.
Trial Registration
Schizophrenia is a chronic psychiatric illness characterized by positive symptoms (delusions, hallucinations, and disorganization), negative symptoms (apathy, social withdrawal, and loss of emotional expressiveness) and cognitive impairment. Antipsychotic medications control positive symptoms sufficiently to allow most individuals to live safely within the community. However, considerable disability is associated with negative symptoms and cognitive deficits, for which effective treatment is not available1.
Current neurodevelopmental models posit a complex and heterogeneous genetic vulnerability to schizophrenia that interacts with environmental factors2. One specific and well-studied gene-environment interaction, the interplay of dietary folic acid intake with common genetic variants that influence folate metabolism, has potential implications for schizophrenia pathogenesis and treatment3. Folate is a B-vitamin that provides methyl donors for biosynthetic methylation reactions, including the synthesis of neurotransmitters and DNA, and contributes to gene expression through methylation of DNA and histones.
Folate deficiency has been identified as a risk factor for schizophrenia through epidemiologic, biochemical, and gene association studies. The regional incidence of schizophrenia transiently doubled approximately two decades after famines in the Netherlands4 and China5, suggesting that reduced folate intake during early neurodevelopment predisposed to schizophrenia risk. Consistent with this finding, elevated concentrations of homocysteine (which is inversely related to folate) in serum obtained from women during the third trimester of pregnancy were associated with >2-fold increased risk for schizophrenia in offspring6. Cross sectional studies have indicated decreased blood folate levels in patients with schizophrenia7, 8. We previously reported an inverse correlation between serum folate concentrations and negative symptoms among patients with schizophrenia, but positive symptoms were unrelated to folate levels9.
Several common, missense single nucleotide polymorphisms (SNPs) in genes that regulate folate and one-carbon metabolism have been associated with schizophrenia phenotypes. The most widely studied is the 677C>T (222Ala>Val) variant in the methylenetetrahydrofolate reductase (MTHFR) gene, each copy of which reduces MTHFR activity by 35%10. The low-functioning 677T variant is slightly but consistently overrepresented among schizophrenia patients (T allele frequency 0.32 in patients and 0.30 in controls; odds ratio 1.15; 95% CI, 1.04 to 1.26, n=10,202)11. This variant, along with missense variants in three other genes that regulate one-carbon metabolism – folate hydrolase 1 (FOLH1), methionine synthase (MTR), and catechol-O-methyltransferase (COMT) – predict negative symptom severity in schizophrenia without affecting positive symptom scores12.
Two small placebo-controlled trials found therapeutic benefit with folate supplementation in schizophrenia patients under conditions of folate deficiency. In a study conducted in the UK, Godfrey13 reported generalized symptom improvement in 17 patients with low red blood cell (RBC) folate levels treated with methylfolate. Levine and colleagues14 reported improvement of total symptoms in 42 hyperhomocystemic schizophrenia subjects treated with folate and B12 supplementation in a cross-over study conducted in Israel. Because the prevalence of folate deficiency and hyperhomocystemia have dropped markedly in the US since mandatory folate fortification of grain products was implemented in 199815, the benefits of folate supplementation may be less readily detected and may depend upon genotype. We previously conducted a placebo-controlled pilot trial of folate supplementation in 36 schizophrenia patients with prominent negative symptoms and found no treatment effect in the whole sample, but a significant interaction between treatment and MTHFR genotype emerged, reflecting improvement in negative symptoms among subjects with 677C/T or 677T/T genotypes who received folate16.
Here, we report a larger, multi-site study of folate plus B12 supplementation versus placebo in medicated outpatients with schizophrenia. Vitamin B12 (cobalamin), a cofactor for MTR, remethylates homocysteine to methionine, which in turn is converted to the universal methyl donor s-adenosylmethionine (SAM). We included B12 in the active treatment arm to potentiate downstream effects of folate on methylation reactions, and to reduce the risk of masked pernicious anemia17. We hypothesized that folate plus B12 supplementation would improve negative symptoms, but that treatment response would depend on the presence of hypofunctional genetic variants in the folate metabolic pathway.
Methods
Patient characteristics
Subjects were outpatients with schizophrenia who were psychiatrically stable but displayed persistent symptoms despite antipsychotic treatment. Eligible patients were 18-68 years old, treated with an antipsychotic agent for ≥6 months at a stable dose for ≥6 weeks, and scored ≥60 on the Positive and Negative Syndrome Scale (PANSS). Schizophrenia diagnosis was confirmed by a research psychiatrist using a structured clinical diagnostic interview using DSM IV-TR criteria and all available clinical records. Patients were excluded if they exhibited parkinsonism (score of ≥12 on the Simpson Angus Scale), which can mimic negative symptoms. Patients were also excluded if they were taking supplemental folate or B12, were medically unstable, had a history of significant neurological illness, reported abuse of alcohol or illicit substances within 3 months, were pregnant or nursing, tested positive on a baseline urine toxicology screen, or had abnormal serum creatinine. Prior to screening, all subjects provided written informed consent after the study was explained to them by a research physician.
Study design and treatment
This was a randomized, double-blind, placebo-controlled, parallel group, 16 week trial of supplementation with folate and vitamin B12 conducted at three urban community mental health clinics (Boston, MA, Grand Rapids, MI, and Rochester, NY). The study was approved by institutional review boards affiliated with each site.
Following a 2-week single-blind placebo lead-in, subjects who continued to meet entry criteria were randomly allocated in a 2:1 ratio to supplementation with folate 2 mg and B12 400 mcg or placebo in identical capsules and instructed to take one capsule of each daily. The randomization sequence was developed and assigned in the research pharmacy, and was stratified by site. Given the previously reported relationship between serum folate level and negative symptoms16, randomization was also stratified by serum folate concentration at screening (< or ≥14.4 ng/ml, reflecting the mean serum folate value in our previous investigation. This ensured that baseline folate levels would be matched in the active treatment and placebo groups. Subjects returned to the clinic every two weeks to review their medical and psychiatric status. Adherence was monitored by pill counts at each visit. Fasting blood samples were drawn at baseline and weeks 2, 4, 8, 12 and 16 for assay of serum B12, plasma homocysteine, and RBC folate concentrations. Clinical rating scales (Scale for Assessment of Negative Symptoms, SANS; PANSS; Calgary Depression Rating Scale, CDRS) were performed at baseline and weeks 2, 4, 8, 12 and 16. CDRS was included to rule out confounding effects of depression on negative symptoms, given their overlapping phenomenology and previous reports suggesting a therapeutic benefit of folate for depression18. To rule out the possibility that treatment effects could reflect dietary nutrient differences between treatment or genotype groups, dietary folate and B12 intake were determined using the Nutritional Data System for Research (NDSR)19, administered at baseline and weeks 8 and 16. The NDSR is a Windows-based dietary analysis program that provides validated estimates of nutrient intake based on 24-hour recall of food and supplement intake. All assessments were conducted by trained raters blind to treatment and genotype.
DNA extracted from whole blood samples was genotyped for four variants previously associated with negative symptom severity in a different cohort of schizophrenia patients12 (FOLH1 484C>T, rs202676; MTHFR 677C>T, rs1801133; MTR 1298G>A, rs1805087; and COMT 675G>A, rs4680) using the MassArray platform (Sequenom, San Diego, CA).
Several of these variants have previously been shown to affect blood folate and homocysteine levels20, 21. Given the possibility that genotype effects on folate absorption or metabolism could influence treatment response, we evaluated whether these variants affected blood folate levels at baseline and throughout the duration of the trial. Further, to rule out the possibility that any such effects reflected confounds related to schizophrenia diagnosis or medication use, we studied a second cohort of 89 healthy individuals recruited from the Boston community. Participants were without current psychiatric illness, history of prior psychotic illness, or first degree relative with psychosis. Healthy subjects underwent identical testing of RBC folate, genotype, and dietary folate intake as did participants with schizophrenia.
Study end points
The primary objective of the study was to assess the effects of adjunctive folate and B12 on positive and negative symptoms of schizophrenia. Based on pilot results16, we hypothesized that folate and B12 supplementation would specifically improve negative symptoms (SANS total score), and that this pattern would be influenced by genotype. Significant clinical findings were followed up by post hoc analyses of relevant subscales. We also determined relationships among genotype and baseline RBC folate levels, as well as changes in folate levels over time.
For clinical measures with significant treatment effects, we determined whether changes in blood chemistries predicted symptom changes, in conjunction with genotype. Following established methods22, 23, we also examined the relationship between baseline symptoms and symptom change separately based on baseline RBC folate tertiles, given that CSF folate levels reach saturation at moderate blood folate levels23, 24.
Statistical analysis
Effects of FOLH1, MTHFR, MTR, and COMT genotype on baseline blood chemistries were determined using multiple linear regression, based on 0, 1, or 2 copies of the low-functioning alleles10, 12, 20, 25, 26. All randomized subjects with ≥1 post-baseline visit were included in linear mixed model analyses of SANS total, PANSS total, PANSS positive symptoms, and CDRS by treatment, incorporating change from baseline at weeks 2, 4, 8, 12, and 16. Secondary analysis of clinical outcomes included FOLH1, MTHFR, MTR, and COMT genotype, entered simultaneously as between-subject factors into the model. To maximize group size, subjects homozygous for the minor allele were grouped together with heterozygotes. Baseline RBC folate was then entered as a planned covariate given previously reported relationships between folate levels and negative symptoms. Blood chemistries were log-transformed if their distribution deviated significantly from normality. Alpha (two-tailed) was set at 0.05.
Results
Patients
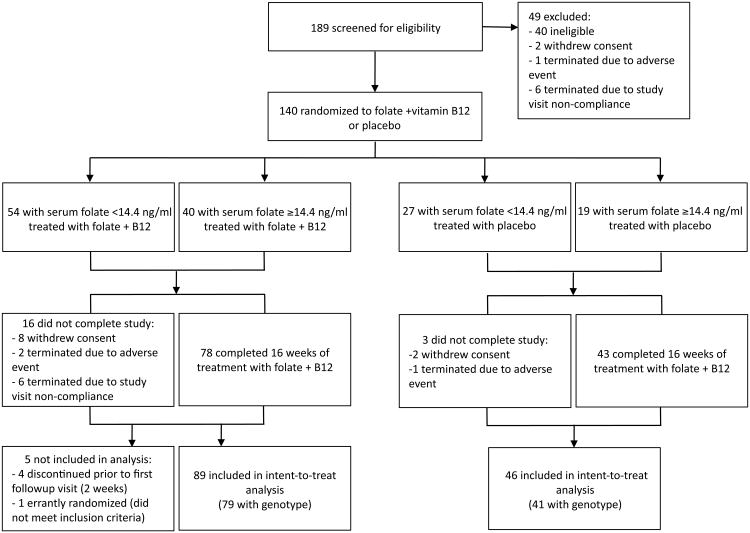
Between December, 2007 and April, 2011, 189 patients were screened, 149 were eligible, and 140 were randomized to folate plus B12 (n=94) or placebo (n=46) (Figure 1). The study continued until the enrollment goal, based on our pilot study16, was reached. A total of 121 subjects (86%) completed 16 weeks of treatment. Completion rates did not vary by site (p=.47). One randomized participant was subsequently found to have an ineligible baseline PANSS score (42) and was dropped from all analyses, although including the subject would not have altered any result. Baseline demographic and clinical characteristics were similar in both groups, as were medication use patterns, and dietary folate and B12 intake (Table 1). Baseline blood chemistries were also similar excepting B12 levels, which were higher in the active treatment group; accordingly, all analyses were covaried by baseline B12 level. Genotype and RBC folate levels were available for 120 randomized patients, the remainder of whom declined to provide blood for genotype analysis, or had missing samples or failed assays. Subjects without genotype did not differ from those with genotype on any baseline demographic variable, baseline clinical ratings, or change in clinical ratings (eTable 1). Genotype distribution did not differ significantly by treatment group (Table 1, eTable 2).
Figure 1.
CONSORT flow diagram.
Table 1. Study participants.
| Folate + B12 n=93 |
Placebo n=46 |
Statistics | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 45.3 ± 1.1 | 45.9 ± 1.6 | t=0.29 | p=.77 |
| Sex (% male) | 71.0% | 71.7% | χ2=0.01 | p=1.0 |
| Race (% Caucasian) | 62.4% | 67.4% | χ2=0.34 | p=.58 |
| Duration of illness (years) | 19.5 ± 1.2 | 19.4 ± 1.6 | t=0.01 | p=1.0 |
| Education level | 4.2 ± 0.1 | 4.1 ±0.2 | t=0.40 | p=.69 |
| Current smoker | 55.3% | 56.5% | χ2=0.02 | p=1.0 |
| Medications | ||||
| Typical antipsychotics | 22.8% | 23.9% | χ2=0.02 | p=1.0 |
| Atypical antipsychotics | 83.7% | 89.1% | χ2=0.73 | p=.45 |
| Antidepressants | 54.3% | 56.5% | χ2=0.06 | p=.86 |
| Anticonvulsants | 38.0% | 28.3% | χ2=1.29 | p=.34 |
| Clinical measures | ||||
| SANS total | 35.2 ± 1.6 | 33.2 ± 2.2 | t=0.72 | p=.47 |
| PANSS positive | 16.8 ± 0.5 | 16.7 ± 0.9 | t=0.06 | p=.95 |
| PANSS total | 71.7 ± 1.0 | 73.6 ± 1.9 | t=0.94 | p=.35 |
| CDRS total | 3.7 ± 0.4 | 3.2 ± 0.5 | t=0.79 | p=.43 |
| Chemistry | ||||
| RBC folate (ng/ml) | 465 ± 16 | 439 ± 22 | t=0.91 | p=.37 |
| Serum B12 (pg/ml) | 631 ± 26 | 511±29 | t=2.81 | p=.01 |
| Plasma homocysteine (μmol/l) | 8.6 ± 0.3 | 8.4 ± 0.3 | t=0.48 | p=.63 |
| Genotype | ||||
| FOLH1 484TT / C carrier | 52% / 48% | 46% / 54% | χ2=0.33 | p=.70 |
| MTHFR 677CC / T carrier | 52% / 48% | 63% / 37% | χ2=1.45 | p=.25 |
| MTR 1298AA / G carrier | 72% / 28% | 54% / 46% | χ2=4.10 | p=.07 |
| COMT 675GG / A carrier | 32% / 68% | 37% / 63% | χ2=0.30 | p=.37 |
Blood chemistries
Baseline RBC folate levels correlated with FOLH1 T (p=.03) and MTHFR T (p=.04) allele loads, despite equivalent dietary folate among genotype groups (eTable 3). Baseline serum B12 and plasma homocysteine levels were not influenced by genotype (p>.17). To validate genotype effects on folate levels, we studied a separate cohort of 89 healthy individuals, confirming the relationship between allele load and folate only for FOLH1 (p=.03) (eTable 3).
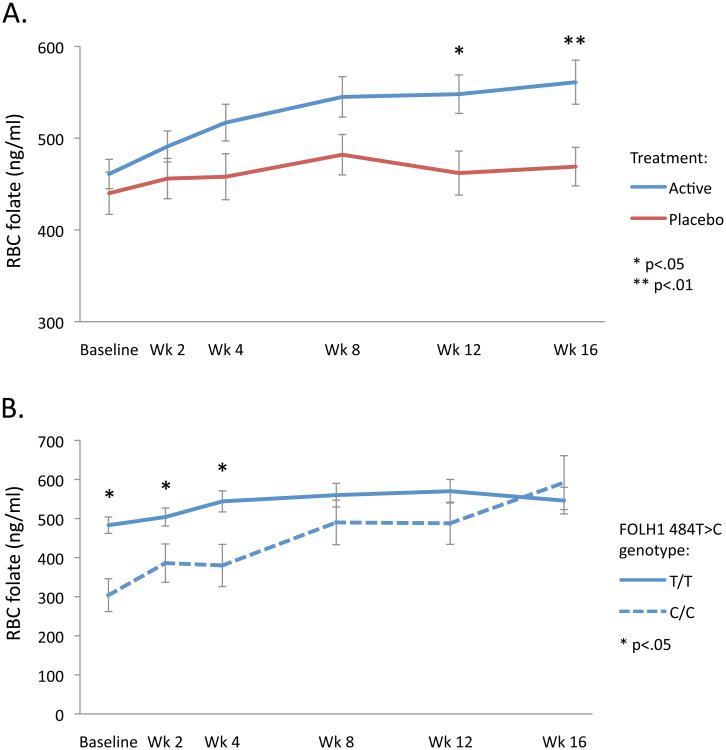
Dietary folate and B12 intake did not differ significantly between treatment groups at baseline, week 8, or week 16 (p>.24) (eTable 4). RBC folate (Figure 2A) and serum B12 increased significantly over time in the active treatment group (p<.001) but not the placebo group (p>.80), yielding significant between-group differences (p≤.001). Further, we observed a significant three-way interaction among treatment, time, and FOLH1 genotype (p=.006), where C allele load predicted RBC folate increases over time in the active treatment group (β=0.29, p=03) but not the placebo group (β=0.06, p=74). Follow-up analyses within the active treatment group indicated that FOLH1 genotype effects on RBC folate levels persisted until week 8 (Figure 2B). There was no effect of anticonvulsant use on RBC folate levels at baseline (t=1.10, p=27), and no effect on change in RBC folate levels over time in either treatment group (p>.29). Homocysteine levels fell significantly only in the active treatment group (p=.006), but not significantly more than in the placebo group (treatment group difference p=.15).
Figure 2.
A. Change in RBC folate over time in active and placebo groups. B. Change in RBC folate over time among patients receiving active treatment, grouped by FOLH1 genotype. Error bars indicate standard error.
Symptom change
Intent-to-treat analysis (Table 2) examined symptom change in patients who completed ≥1 post-baseline visit (n=135). Patients who received folate plus B12 exhibited a significant decline in negative symptoms (-0.19 change in SANS per week; 95% CI, -0.35 to -0.03; p=.02) and those who received placebo did not change (+0.02 per week; 95% CI, -0.21 to 0.24; p=88), although the difference between folate and placebo groups did not reach significance (-0.21 per week; 95% CI, -0.49 to 0.07; p=.15). The same pattern was evident for PANSS positive scores. For PANSS total, both the active and placebo groups improved significantly over time, but again there was no difference in improvement between treatment groups. No significant effects of treatment were seen for depression scores.
Table 2. Intent-to-treat analysis.
| BEFORE INCLUSION OF GENOTYPES IN MODEL | ||||||||
|---|---|---|---|---|---|---|---|---|
| Change in symptoms per week, relative to baseline | ||||||||
| SANS | PANSS positive | PANSS total | CDRS | |||||
| Mean (95%CI) | p value | Mean (95%CI) | p value | Mean (95%CI) | p value | Mean (95%CI) | p value | |
| Active | -0.19 (-0.35 to -0.03) | .02 | -0.06 (-0.12 to -0.01) | .02 | -0.21 (-0.35 to -0.07) | .004 | -0.02 (-0.06 to 0.02) | .25 |
| Placebo | 0.02 (-0.21 to 0.24) | .88 | -0.04 (-0.11 to 0.03) | .28 | -0.22 (-0.42 to -0.03) | .02 | 0.04 (-0.01 to 0.09) | .16 |
| Difference | -0.21 (-0.49 to 0.07) | .15 | -0.02 (-0.12 to 0.07) | .61 | 0.02 (-0.23 to 0.26) | .89 | -0.06 (-0.12 to 0.00) | .07 |
| AFTER INCLUSION OF GENOTYPES IN MODEL | ||||||||
| Change in symptoms per week, relative to baseline | ||||||||
| SANS | PANSS positive | PANSS total | CDRS | |||||
| Mean (95%CI) | p value | Mean (95%CI) | p value | Mean (95%CI) | p value | Mean (95%CI) | p value | |
| Active | -0.17 (-0.34 to -0.01) | .04 | -0.08 (-0.13 to -0.02) | .01 | -0.21 (-0.35 to -0.07) | .004 | -0.00 (-0.04 to 0.04) | .84 |
| Placebo | 0.16 (-0.07 to 0.39) | .18 | -0.01 (-0.10 to 0.07) | .74 | -0.10 (-0.31 to 0.11) | .35 | 0.04 (-0.02 to 0.09) | .20 |
| Difference | -0.33 (-0.62 to -0.05) | .02 | -0.06 (-0.16 to 0.04) | .24 | -0.11 (-0.36 to 0.14) | .39 | -0.04 (-0.11 to 0.03) | .25 |
When including FOLH1, MTHFR, MTR, and COMT genotype simultaneously into a linear mixed model of negative symptoms (n=120), the main effect of treatment over time became significant (group difference -0.33 per week; 95% CI, -0.62 to -0.05; p=.02), again reflecting a significant decline in negative symptoms in the active treatment group (-0.17 per week; 95% CI, -0.34 to -0.01; p=.04) but not the placebo group (+0.16 per week; 95% CI, -0.07 to 0.39; p=.18) (Table 2). To determine whether the between-group differences reflected genotype effects specifically within the active treatment or placebo groups, the groups were then considered separately, and each genotype was tested for an effect on negative symptoms. In the placebo group, no genotype was significantly associated with change in negative symptoms (p<.1). In the active treatment group, only FOLH1 genotype significantly predicted change in negative symptoms (p=.04).
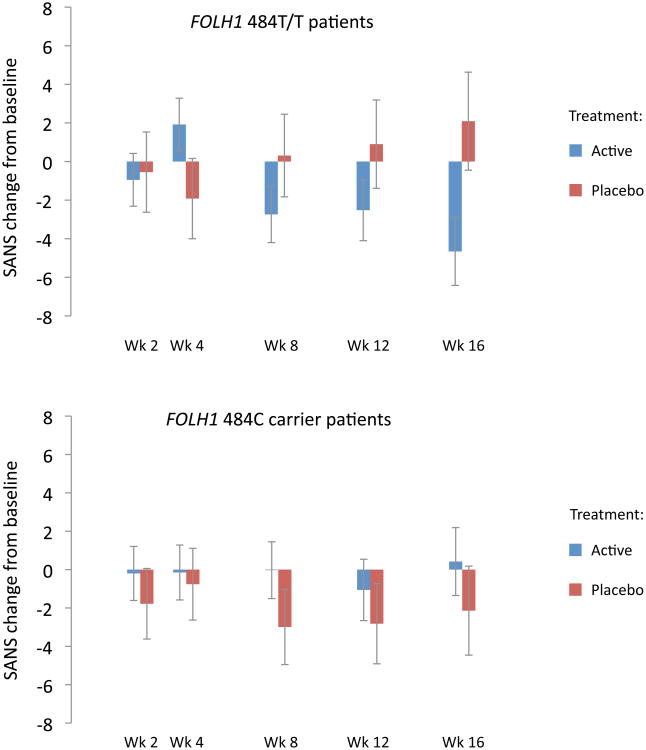
Direct comparison of the treatment groups indicated a significant FOLH1 genotype × treatment interaction on negative symptom change (p=.02), with active treatment conferring a benefit over placebo in the T/T group (treatment group difference -0.59 per week; 95% CI, -0.99 to -0.18; p=.005) but not among C allele carriers (treatment group difference +0.09 per week; 95% CI, -0.29 to 0.47; p=.64) (Figure 3, eTable 5). For MTHFR, benefit of active treatment over placebo was seen among T carrier patients and not C/C patients, although the genotype × treatment × time interaction did not reach significance (p=.17). MTR and COMT genotype did not significantly influence treatment effects on negative symptoms over time (eTable 5). Including baseline RBC folate levels in the model did not influence the significance level of any result.
Figure 3.
Change from baseline negative symptoms (SANS total score) among FOLH1 484T/T and 484C carrier patients, assessed throughout the course of treatment. For T/T patients, change over time was significant among those who received active treatment (p=.003) but not placebo (p=.16), with a significant difference between groups (p=.005). For C carrier patients, change over time was not significant in either the active (p=.93) or placebo (p=.60) groups, with no significant difference between groups (p=.64). Error bars indicate standard error.
Analogous linear mixed models of PANSS positive, PANSS total, and CDRS scores incorporating genotype did not detect significant differences between treatment groups (Table 2, eTable 5), nor were any genotype × treatment interactions observed.
Negative symptom subscales
Post hoc analyses indicated that, among negative symptom subscales, the alogia subscale most strongly contributed to between-group differences in SANS (eTable 6). Similarly, when examining FOLH1 genotype, a significant genotype × treatment interaction was present only for alogia (p=.03). Although alogia was the only subscale that showed a significant treatment effect, changes in alogia scores correlated significantly with changes in affective flattening and anhedonia (r>.30, p<.001) and also correlated at trend level with changes in avolition and attention (r>.15, p<.10).
Relationship between blood chemistry and negative symptoms
Although both RBC folate levels and negative symptoms each improved in the active treatment group, there was no significant overall correlation between change in folate levels and change in negative symptom ratings at any treatment visit. This pattern did not differ by FOLH1 genotype. Changes in negative symptoms were also unrelated to changes in B12 and homocysteine levels.
Secondary analyses considered the relationship between baseline RBC folate levels and negative symptoms according to baseline RBC tertiles. Baseline RBC folate correlated inversely with baseline SANS in the lowest tertile (r= -.39, p=.01), but not in the middle (r= -.27, p=.09) or upper (r= -.16, p=.35) tertiles. In the active treatment group, baseline RBC folate correlated positively with 16-week change in SANS (i.e., greater improvement with lower baseline RBC folate) in the lowest tertile (r=.43, p=.04) but not the middle (r=.29, p=.20) or upper (r=.23, p=.29) tertiles. In the placebo group, baseline RBC folate did not correlate with 16-week change in SANS for any tertile.
Adverse events
Treatment-emergent side effects did not differ substantially between active and placebo groups (eTable 7). Three subjects were hospitalized due to worsening psychosis (2 active, 1 placebo).
Comment
The present results indicate that supplementation of antipsychotic medications with folate and B12 improves negative symptoms of schizophrenia, but only when accounting for common, functional genetic variants in enzymes that regulate folate absorption and metabolism.
Although four such variants have previously been associated with negative symptom severity12, the genotype that contributed most strongly to treatment response was FOLH1 484T>C. FOLH1 is a glutamate carboxypeptidase that is anchored to the intestinal brush border and facilitates transfer of dietary folate into the body27. The 484T>C variant, located in exon 2 of the structural transmembrane region, codes for a substitution of histidine for tyrosine at amino acid position 75 of the protein.
As with all four genetic variants included in the present study, it was the low-functioning variant of FOLH1 (484C) that was associated with increased negative symptoms in a previously studied cohort of patients with schizophrenia12. These effects were mitigated, though, in the presence of higher blood folate levels, leading to the hypothesis that patients with low-functioning variants would tend to show a greater benefit from vitamin supplementation. However, these previous findings were limited by the fact that they were cross-sectional. Protracted exposure to high levels of folate may have been necessary for its protective effects among patients with hypofunctional variants. Moreover, the functionality of the FOLH1 variant had not been as extensively characterized as the others20, 21, 25.
Here, we found that it was the high-functioning variant of FOLH1 (484T) that was required to show a benefit of vitamin supplementation for negative symptoms. While this was counter to our expectations, the effects of the FOLH1 variant on RBC folate levels (both at baseline and throughout the study) may be instructive in understanding its relationship to negative symptoms. Baseline RBC folate levels were inversely related to 484C allele load, a pattern confirmed in a second cohort of healthy individuals. Dietary folate intake did not vary by genotype, suggesting that the 484C allele impairs folate absorption. Thus, the finding that only patients homozygous for the T allele exhibited improvement in negative symptoms after 16 weeks of folate supplementation could reflect diminished folate absorption, and briefer exposure to higher folate levels, among C allele carriers.
The present results are also consistent with previous work involving the hypofunctional MTHFR 677C>T variant, which confers increased schizophrenia risk11, as well as more pronounced negative symptoms12, 28. Here, only T allele carriers exhibited a significant benefit for active treatment over placebo for negative symptoms, reproducing results from our previous, smaller study of folate supplementation in schizophrenia16. However, the interaction of genotype and treatment did not reach statistical significance in the present study, possibly reflecting lower baseline symptom severity than the previous cohort.
It is notable that after genotypes were entered into the model, the slope (SANS change per week) for the placebo group changed more than the slope in the active treatment group, and this pattern contributed to the between-group difference in SANS response. This raises the question of whether gene effects within the placebo group were responsible for the between treatment-group differences that emerged after entering genotype into the model. However, unlike the active treatment group, there was no significant change in negative symptoms among patients in the placebo group, either before or after introduction of genotype; further, followup testing did not identify specific gene effects within the placebo group, as it did in the active treatment group. Still, we cannot rule out that folate-related genes influenced the placebo response, in addition to the response to active treatment.
We did not observe a significant overall relationship between change in RBC folate and change in negative symptoms. This finding may reflect the non-linear relationship between peripheral and central folate levels. Transport of folate across the blood brain barrier is tightly regulated by specialized folate transporters within choroid plexus epithelial cells29. Accordingly, cerebrospinal fluid (CSF) folate levels generally fall within a narrower range than blood folate23, 24. CSF folate levels plateau at moderate blood folate levels23, 24, 30. Specifically, Obeid and colleagues23 reported that blood and CSF folate levels correlated strongly only within the lowest tertile of blood folate in 72 individuals. The lack of an overall relationship between changes in symptoms and RBC folate may reflect the fact that folate supplementation increased blood folate levels over and above the levels required for CSF saturation in most subjects. However, when we divided the cohort by baseline RBC folate tertiles, we observed that blood folate levels correlated inversely with baseline SANS scores, and positively with change in SANS, only for participants who fell within the lowest tertile. This pattern explains the lack of correlation across the entire cohort, and is also very consistent with the known physiology of folate transport into the brain.
For patients with the low-functioning FOLH1 484C variant, response to treatment might have been delayed because it took longer to achieve CSF folate saturation. However, by the midpoint of the study, RBC folate levels among C carriers had reached the same level as T/T patients, and it is possible that C carrier patients would also have shown a benefit had the treatment period been extended. That said, the mechanism underlying the gradual timing of SANS response to vitamin supplementation across the entire cohort remains uncertain. It does not solely reflect the timing of RBC folate increases, since these measures were uncorrelated, potentially due to the saturation kinetics of the blood brain barrier described above. Clinical changes related to vitamin supplementation may lag behind biochemical changes that are directly related to the intervention, as is the case for serotonin reuptake inhibitors and in depression. Rather, clinical improvement may be more directly related to slower downstream mechanisms such as altered gene expression.31 In the case of folate supplementation, gene expression changes may be particularly relevant, given their dependence on DNA methylation status.
Of note, patients with schizophrenia experience differing degrees of negative symptoms, and the present investigation did not include a minimum SANS threshold for study entry. However, all subjects presented with at least some negative symptoms at baseline, and all but 6 participants would have met inclusion criteria for the CONSIST trial32, a recent negative symptom treatment study that required a SANS total score ≥20, or SANS affective flattening or alogia subscale score ≥3. With regard to treatment effects on negative symptom subscales in the present study, while alogia was most strongly affected by vitamin supplementation, the correlation between alogia and other subscale scores suggests that the changes in alogia were part of a broader change in negative symptoms.
Folate and B12 supplementation did not confer a benefit for positive symptoms or depression scores, regardless of genotype. This finding echoes earlier observations that related blood folate levels specifically to negative symptoms9, and genetic variants in folate metabolism specifically to negative symptom severity12, 28. It also suggests that improvement in negative symptoms was not confounded by improvement in depression, a potential concern given their overlapping phenomenology.
Several limitations to the present study should be acknowledged. First, multiple outcomes were studied, although our main hypotheses related to negative symptoms, reflecting the previous findings described above9, 12, 28. The additional ratings in total, positive, and depression symptoms were necessary to establish whether treatment effects were specific to negative symptoms. Four genetic variants were included, although all were again based on previous findings12, 28, and the use of a single regression model incorporating all four genotypes per outcome variable reduced the number of comparisons. Second, the study population included a mixture of races and ethnicities, although the previous work upon which genetic hypotheses were based12 included only subjects of European ancestry. While population stratification artifact remains a possibility, outcomes did not differ by race, and the present results may generalize across a broader population than if only one racial group were studied. Third, the study sample size precluded the analysis of cumulative genotype effects, which have previously been shown to influence negative symptoms and their relationship to blood folate levels12. Finally, treatment effects were modest, with a 15% difference in SANS change between the active treatment and placebo group, and 27% difference between FOLH1 T/T versus C allele carriers. While there remains no gold standard for translating quantitative changes in negative symptom rating scales to qualitative measures of clinical and functional improvement33, the degree of SANS change in the present study is consistent with other studies that have determined clinically detectible thresholds for PANSS total score change through cross-validation with global clinical impression scales34. Even small effects of folate and B12 supplementation could be clinically meaningful, though, given the disability associated with negative symptoms, the lack of available treatments35, and the minimal apparent adverse effects of vitamin supplementation.
With these limitations in mind, the present results have direct treatment implications not only for schizophrenia but also for folate-related interventions in other areas of medicine. Well-replicated associations of reduced folate and elevated homocysteine as risk factors for stroke36, 37, cardiovascular disorders38, 39, and dementia40, 41 have been tempered by large, prospective studies that have failed to find a benefit of folate supplementation on disease progression42-47. The current results suggest that individual differences in folate metabolism related to the presence of common, functional genetic variants may have a bearing on treatment outcomes in these other disorders, as well as negative symptoms of schizophrenia.
Supplementary Material
eTable 1: Nutritional folate and B12 intake
eTable 2: Genotyped versus non-genotyped participants
eTable 3: Genotype frequencies
eTable 4: Effects of genotype on baseline folate levels and nutritional folate intake
eTable 5: Effects of genotype on change in symptoms per week
eTable 6: Effects of genotype on SANS subscale change per week
eTable 7: Adverse events
Acknowledgments
Funding/support: This work was supported by NIMH R01MH070831 (DCG) and the Howard Hughes Medical Institute Early Career Physician-Scientist Award (JLR). This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
Role of the sponsors: None of the sponsors contributed to the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
This work was presented in part at the American College of Neuropsychopharmacology Annual Meeting, Waikoloa, HI, December, 2011.
Author contributions: Drs. Roffman and Goff had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Author contributions are as follows:
Study concept and design: Roffman, Goff
Acquisition of data: Lamberti, Achtyes, Galendez, Raeke, Hill
Analysis and interpretation of data: Roffman, Macklin, Silverstein, Smoller, Goff
Drafting of the manuscript: Roffman, Goff
Critical revision of the manuscript for important intellectual content: Lamberti, Achtyes, Macklin, Galendez, Raeke, Silverstein, Smoller, Hill
Statistical analysis: Roffman, Macklin
Obtained funding: Roffman, Goff
Administrative, technical, or material support: Lamberti, Achtyes, Galendez, Raeke, Silverstein, Hill
Study supervision: Roffman, Smoller, Goff
Conflict of interest disclosures: Dr. Roffman received research support from Pamlab and from the Harvard Medical School Division of Health Sciences and Technology-Massachusetts Institute of Technology Clinical Investigator Training Program, which was supported by an unrestricted educational grant from Merck and Pfizer. Dr. Achtyes received research support from Eli Lilly and Company, Novartis, AssureRx, Otsuka, and Janssen. Dr. Goff has consulted for Indevus Pharmaceuticals, H. Lundbeck, Schering-Plough, Eli Lilly and Company, Takeda, Biovail, Solvay, Hoffman- La Roche, Cypress, Dainippon Sumitomo, Bristol-Meyers Squibb, Abbott Laboratories, Takeda, Genentech, Merck, Endo Pharmaceuticals, Otsuka, Pfizer, Novartis, Janssen, GlaxoSmithKline. Dr. Goff has received research support from Pfizer, Novartis, Janssen, GlaxoSmithKline, and Pamlab. Drs. Roffman and Goff have applied for a US patent, assigned to Massachusetts General Hospital, concerning prediction of treatment response in schizophrenia based on folate-related genes.
Additional contributions: Daniel Tuinstra, BA and Heather Willett, MA, contributed to data acquisition.
References
- 1.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10(1):79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 2.Van Winkel R, Esquivel G, Kenis G, Wichers M, Collip D, Peerbooms O, Rutten B, Myin-Germeys I, Van Os J. REVIEW: Genome-wide findings in schizophrenia and the role of gene-environment interplay. CNS Neurosci Ther. 2010;16(5):e185–192. doi: 10.1111/j.1755-5949.2010.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picker JD, Coyle JT. Do maternal folate and homocysteine levels play a role in neurodevelopmental processes that increase risk for schizophrenia? Harv Rev Psychiatry. 2005;13(4):197–205. doi: 10.1080/10673220500243372. [DOI] [PubMed] [Google Scholar]
- 4.Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, Gorman JM. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53(1):25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 5.St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, Zheng X, Gu N, Feng G, Sham P, He L. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA. 2005;294(5):557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- 6.Brown AS, Bottiglieri T, Schaefer CA, Quesenberry CP, Jr, Liu L, Bresnahan M, Susser ES. Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Arch Gen Psychiatry. 2007;64(1):31–39. doi: 10.1001/archpsyc.64.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Herran A, Garcia-Unzueta MT, Amado JA, Lopez-Cordovilla JJ, Diez-Manrique JF, Vazquez-Barquero JL. Folate levels in psychiatric outpatients. Psychiatry Clin Neurosci. 1999;53(4):531–533. doi: 10.1046/j.1440-1819.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 8.Koren G, Cohn T, Chitayat D, Kapur B, Remington G, Reid DM, Zipursky RB. Use of atypical antipsychotics during pregnancy and the risk of neural tube defects in infants. Am J Psychiatry. 2002;159(1):136–137. doi: 10.1176/appi.ajp.159.1.136. [DOI] [PubMed] [Google Scholar]
- 9.Goff DC, Bottiglieri T, Arning E, Shih V, Freudenreich O, Evins AE, Henderson DC, Baer L, Coyle J. Folate, homocysteine, and negative symptoms in schizophrenia. Am J Psychiatry. 2004;161(9):1705–1708. doi: 10.1176/appi.ajp.161.9.1705. [DOI] [PubMed] [Google Scholar]
- 10.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10(1):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 11.Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40(7):827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 12.Roffman JL, Brohawn DG, Nitenson AZ, Macklin EA, Smoller JW, Goff DC. Genetic Variation Throughout the Folate Metabolic Pathway Influences Negative Symptom Severity in Schizophrenia. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godfrey P, Toone B, Carney M, Flynn T, Bottiglieri T, Laundy M, Chanarin I, Reynolds E. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336:392–395. doi: 10.1016/0140-6736(90)91942-4. [DOI] [PubMed] [Google Scholar]
- 14.Levine J, Stahl Z, Sela BA, Ruderman V, Shumaico O, Babushkin I, Osher Y, Bersudsky Y, Belmaker RH. Homocysteine reducing strategies improve symptoms in chronic schizophrenic patients with hyperhomocysteinemia. Biol Psychiatry. 2006;60:265–269. doi: 10.1016/j.biopsych.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Rader JI. Folic acid fortification, folate status and plasma homocysteine. J Nutr. 2002;132(8 Suppl):2466S–2470S. doi: 10.1093/jn/132.8.2466S. [DOI] [PubMed] [Google Scholar]
- 16.Hill M, Shannahan K, Jasinski S, Mackin EA, Raeke L, Roffman JL, Goff DC. Folate supplementation in schizophrenia: a possible role for MTHFR genotype. Schizophr Res. 2011;127(1-3):41–45. doi: 10.1016/j.schres.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Tucker KL, Mahnken B, Wilson PW, Jacques P, Selhub J. Folic acid fortification of the food supply. Potential benefits and risks for the elderly population. JAMA. 1996;276(23):1879–1885. doi: 10.1001/jama.1996.03540230029031. [DOI] [PubMed] [Google Scholar]
- 18.Fava M, Mischoulon D. Folate in depression: efficacy, safety, differences in formulations, and clinical issues. J Clin Psychiatry. 2009;70(Suppl 5):12–17. doi: 10.4088/JCP.8157su1c.03. [DOI] [PubMed] [Google Scholar]
- 19.Harnack L, Stevens M, Van Heel N, Schakel S, Dwyer JT, Himes J. A computer-based approach for assessing dietary supplement use in conjunction with dietary recalls. J Food Compost Anal. 2008;21(Suppliment 1):S78–S82. doi: 10.1016/j.jfca.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharp L, Little J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: a HuGE review. Am J Epidemiol. 2004;159(5):423–443. doi: 10.1093/aje/kwh066. [DOI] [PubMed] [Google Scholar]
- 21.Tunbridge EM, Harrison PJ, Warden DR, Johnston C, Refsum H, Smith AD. Polymorphisms in the catechol-O-methyltransferase (COMT) gene influence plasma total homocysteine levels. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):996–999. doi: 10.1002/ajmg.b.30700. [DOI] [PubMed] [Google Scholar]
- 22.Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, Olivieri O, Jacques PF, Rosenberg IH, Corrocher R, Selhub J. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A. 2002;99(8):5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obeid R, Kostopoulos P, Knapp JP, Kasoha M, Becker G, Fassbender K, Herrmann W. Biomarkers of folate and vitamin B12 are related in blood and cerebrospinal fluid. Clin Chem. 2007;53(2):326–333. doi: 10.1373/clinchem.2006.076448. [DOI] [PubMed] [Google Scholar]
- 24.Serot JM, Christmann D, Dubost T, Bene MC, Faure GC. CSF-folate levels are decreased in late-onset AD patients. J Neural Transm. 2001;108(1):93–99. doi: 10.1007/s007020170100. [DOI] [PubMed] [Google Scholar]
- 25.Halsted CH, Wong DH, Peerson JM, Warden CH, Refsum H, Smith AD, Nygard OK, Ueland PM, Vollset SE, Tell GS. Relations of glutamate carboxypeptidase II (GCPII) polymorphisms to folate and homocysteine concentrations and to scores of cognition, anxiety, and depression in a homogeneous Norwegian population: the Hordaland Homocysteine Study. Am J Clin Nutr. 2007;86(2):514–521. doi: 10.1093/ajcn/86.2.514. [DOI] [PubMed] [Google Scholar]
- 26.Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34(13):4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 27.Halsted CH. The intestinal absorption of dietary folates in health and disease. J Am Coll Nutr. 1989;8(6):650–658. doi: 10.1080/07315724.1989.10720340. [DOI] [PubMed] [Google Scholar]
- 28.Roffman JL, Weiss AP, Purcell S, Caffalette CA, Freudenreich O, Henderson DC, Bottiglieri T, Wong DH, Halsted CH, Goff DC. Contribution of methylenetetrahyrdofolate reductase (MTHFR) polymorphisms to negative symptoms in schizophrenia. Biol Psychiatry. 2008;63(1):42–48. doi: 10.1016/j.biopsych.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Wollack JB, Makori B, Ahlawat S, Koneru R, Picinich SC, Smith A, Goldman ID, Qiu A, Cole PD, Glod J, Kamen B. Characterization of folate uptake by choroid plexus epithelial cells in a rat primary culture model. J Neurochem. 2008;104(6):1494–1503. doi: 10.1111/j.1471-4159.2007.05095.x. [DOI] [PubMed] [Google Scholar]
- 30.Alperin JB, Haggard ME. Cerebrospinal fluid folate (CFA) and the blood brain barrier. Clin Res. 1970;18:40. [Google Scholar]
- 31.Hyman SE, Nestler EJ. Initiation and adaptation: a paradigm for understanding psychotropic drug action. Am J Psychiatry. 1996;153(2):151–162. doi: 10.1176/ajp.153.2.151. [DOI] [PubMed] [Google Scholar]
- 32.Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164(10):1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- 33.Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermes ED, Sokoloff D, Stroup TS, Rosenheck RA. Minimum clinically important difference in the Positive and Negative Syndrome Scale with data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) J Clin Psychiatry. 2012;73(4):526–532. doi: 10.4088/JCP.11m07162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stroup TS, Lieberman JA, McEvoy JP, Swartz MS, Davis SM, Rosenheck RA, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry. 2006;163(4):611–622. doi: 10.1176/ajp.2006.163.4.611. [DOI] [PubMed] [Google Scholar]
- 36.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, Sides EG, Wang CH, Stampfer M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291(5):565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 37.Casas JP, Bautista LE, Smeeth L, Sharma P, Hingorani AD. Homocysteine and stroke: evidence on a causal link from mendelian randomisation. Lancet. 2005;365(9455):224–232. doi: 10.1016/S0140-6736(05)17742-3. [DOI] [PubMed] [Google Scholar]
- 38.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324(17):1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 39.Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337(4):230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 40.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346(7):476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 41.Van Dam F, Van Gool WA. Hyperhomocysteinemia and Alzheimer's disease: A systematic review. Arch Gerontol Geriatr. 2009;48(3):425–430. doi: 10.1016/j.archger.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 43.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J., Jr Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 44.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354(26):2764–2772. doi: 10.1056/NEJMoa054025. [DOI] [PubMed] [Google Scholar]
- 45.Zhou YH, Tang JY, Wu MJ, Lu J, Wei X, Qin YY, Wang C, Xu JF, He J. Effect of folic acid supplementation on cardiovascular outcomes: a systematic review and meta-analysis. PLoS One. 2011;6(9):e25142. doi: 10.1371/journal.pone.0025142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Sleight P, Peto R, Collins R. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303(24):2486–2494. doi: 10.1001/jama.2010.840. [DOI] [PubMed] [Google Scholar]
- 47.Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, Bottiglieri T, Jin S, Stokes KT, Thomas RG, Thal LJ. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300(15):1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1: Nutritional folate and B12 intake
eTable 2: Genotyped versus non-genotyped participants
eTable 3: Genotype frequencies
eTable 4: Effects of genotype on baseline folate levels and nutritional folate intake
eTable 5: Effects of genotype on change in symptoms per week
eTable 6: Effects of genotype on SANS subscale change per week
eTable 7: Adverse events