Abstract
Cyclic stretch has been shown to alter cell physiology, cytoskeletal structure, signal transduction, and gene expression in a variety of cell types. To determine the effects of stretch on the gene transfer process, we compared the transfection efficiencies of human A549 cells grown either statically or exposed to 10% cyclic stretch (Δ surface area) at 60 cycles/min (1 Hz) for 24 hours prior to, and/or after transfection with pEGFP-N1 and pCMV-lux-DTS using lipoplex or electroporation. Stretching the cells prior to transfection had no effect on gene transfer. By contrast, cyclic, but not continuous, stretch applied immediately after transfection for as little as 30 minutes resulted in a 10-fold increase in gene transfer and expression by either transfection technique. These stretch conditions did not result in rupture of the plasma membrane based on the fact that DNA was unable to enter stretched cells unless either an electric field was applied or the DNA was complexed with liposomes. Taken together with the timing of the stretch response and the known effects of stretch on transcription, these findings suggest that cyclic stretch may be altering the intracellular transport of plasmids to increase gene expression.
Keywords: gene therapy, gene delivery, gene expression, nonviral vectors, plasmid DNA, transfection, lipoplex, electroporation, cyclic stretch
Introduction
Numerous studies have demonstrated that a variety of cells and tissues respond to mechanical signals. Such signals, including cyclic stretch and shear stress, are especially important in the lung where during the course of respiration and mechanical ventilation, cells of the epithelium are distorted and stress responses are initiated. Of the numerous biological and physiological responses to cyclic stretch, the majority can be classified into four broad areas: alterations in the cytoskeleton and extracellular matrix, activation of cell signaling pathways, alteration of transcription factor function and gene regulation, and changes in cell proliferation [1,2]. It is rather intuitive that by stretching cells, changes in their cytoskeletal structure will occur. When smooth muscle cells are exposed to cyclic stretch (10 to 20% area strain at 30 to 60 cycles/min), stress fibers reorient within 15 minutes, suggesting that stretch can induce rapid reorganization of actin filaments [3]. Similarly, the application of similar short term stretch to smooth muscle cells reorganizes microtubules [4,5]. Such changes in cytoskeletal organization are bound to have physiological consequences. Further, based on the model of tensegrity, Ingber and colleagues have demonstrated that alterations in cytoskeletal organization have a profound effect on integrin-mediated signal- and mechano-transduction in the cell and much of this effect is dependent on the intermediate filament network [6–8].
Mechanical strain can also alter signal transduction pathways within the cell [2]. In osteoblasts, cardiac myocytes, smooth muscle cells, and alveolar epithelial cells the activities of multiple protein kinases and phosphatases, including Ras, JNK, MAPK, PKC, ERK, and src-kinases are altered by cyclic stretch or shear stress [9–16]. Similarly, cyclic stretch of airway and alveolar epithelial cells has been shown to increase intracellular Ca2+ release, and cAMP and inositol 1,4,5-triphosphate levels [17–19]. One consequence of these activated kinase cascades and intracellular second messengers is the potential modulation of the cytoskeleton. Indeed, the stretch-induced activation of certain PKC isoforms and ERK have been shown by several groups to be regulated by cytoskeletal elements [14,20]. A second consequence of these activated kinase cascades and intracellular second messengers is the activation of a number of transcription factors. Transient cyclic stretch at low strain causes kinases to activate the transcription factor NF-κB by promoting its nuclear localization in osteoblasts and smooth muscle cells [9,21]. Both components of the AP-1 transcription factor, fos and jun, are also activated upon cyclic stretch of cells [22–25]. In all of these cases, strain alters the cellular localization and/or activity of these factors, as opposed to up-regulating their expression. This stimulation of transcription factor activity most likely accounts for the increases in gene expression in cells exposed to cyclic stretch. Finally, activation of transcription factors may also account for the increased cellular proliferation seen with mechanical stress [2,10,22].
Intriguingly, these cyclic stretch-induced cellular responses are directly related to the process of gene delivery to cells and tissues. Exogenous DNA, either viral or non-viral, must cross the plasma membrane into the cell, travel through the cytoplasm and the cytoskeletal networks, enter the nucleus, and be transcribed in order for gene therapy to be successful. Based on the similarities in the pathways used for gene transfer and those affected by cyclic stretch, the present studies were undertaken to determine whether cyclic stretch can modulate the gene transfer process.
Results
Cyclic Stretch Increases Transfection Efficiency and Gene Expression
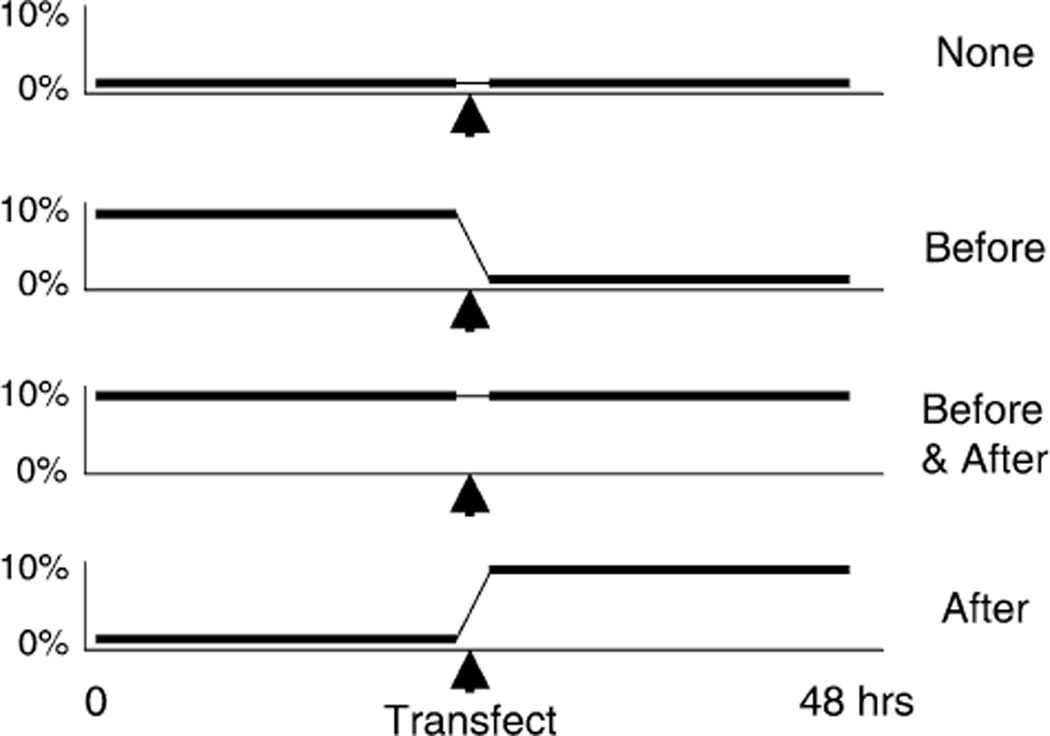
To test whether cyclic stretch had any impact on the transfection efficiency of cells, we transfected A549 cells derived from a human lung epithelial carcinoma and exposed them to cyclic stretch. The cells were grown on laminin-coated BioFlex stretch plates to 60% confluency and transfected with two plasmids, one expressing luciferase and the other expressing green fluorescent protein (GFP) [26]. The cells were subjected to one of four stretching regimens (Fig. 1). The cells were either unstretched before and after transfection, stretched for 24 hours prior to transfection and left unstretched afterwards, unstretched before transfection and stretched immediately following DNA addition, or stretched both prior to and after transfection. All cells were stretched at 1 Hz (60 cycles/min) at 10% change in surface area (10% area strain) using the Flexercell 3000FX with loading posts in place for equibiaxial strain. These conditions were chosen based on the experiments of Tschumperlin and Margulies [27–29] and Sznajder, Glucksberg and colleagues [16,30–32]. The normal respiratory rate of rats is 60 breaths/min (hence 1 Hz) and a change in alveolar basement membrane surface area of 10–20% corresponds to between 60 and 80% total lung capacity (TLC), based on morphometric analysis [27].
FIG. 1.
Stretching regimen. Pulmonary epithelial cells were plated on laminin-coated BioFlex plates and grown to 60% confluency. Cells were either subjected to stretch or grown under static conditions for 24 hours before and/or after transfection as described in Materials and Methods. Cyclic stretch of 10% area strain (equivalent to a 10% change in basement membrane surface area) was used at 60 cycles/min (1Hz).
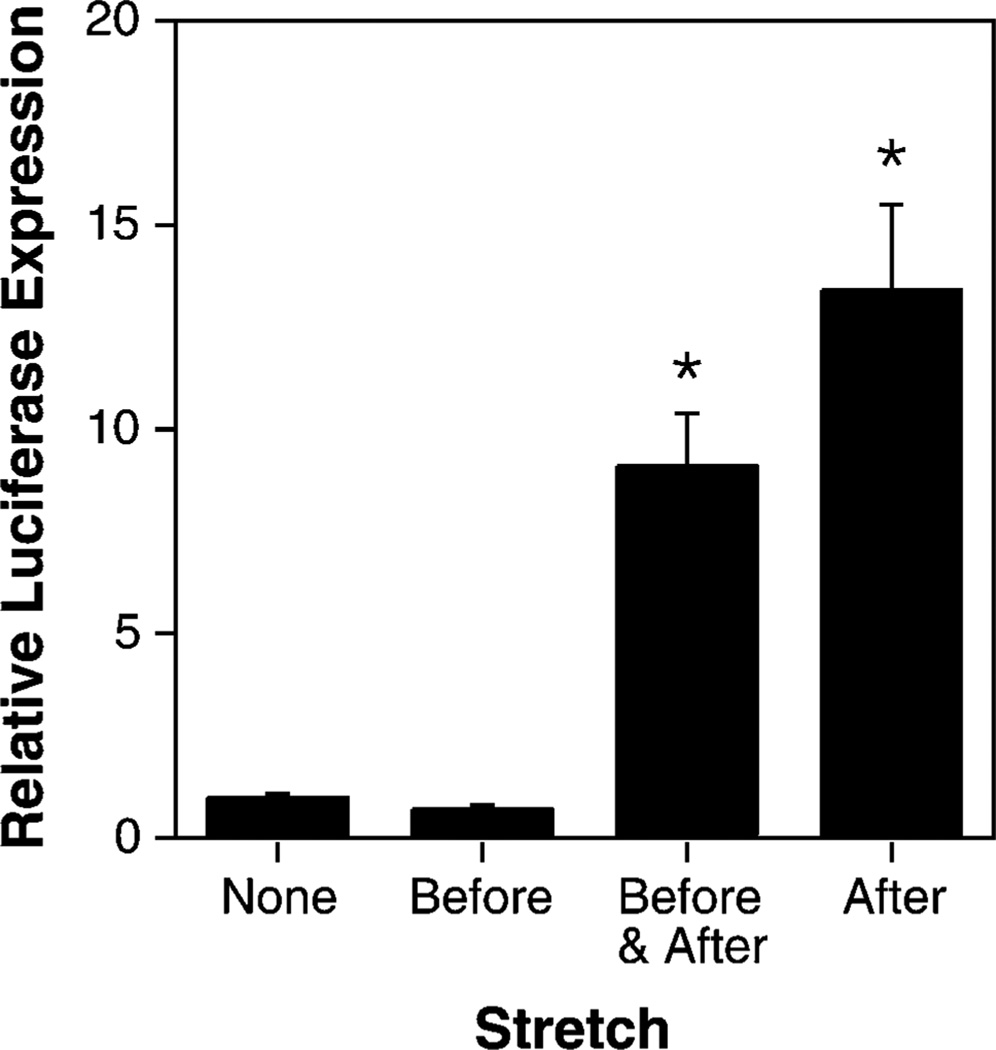
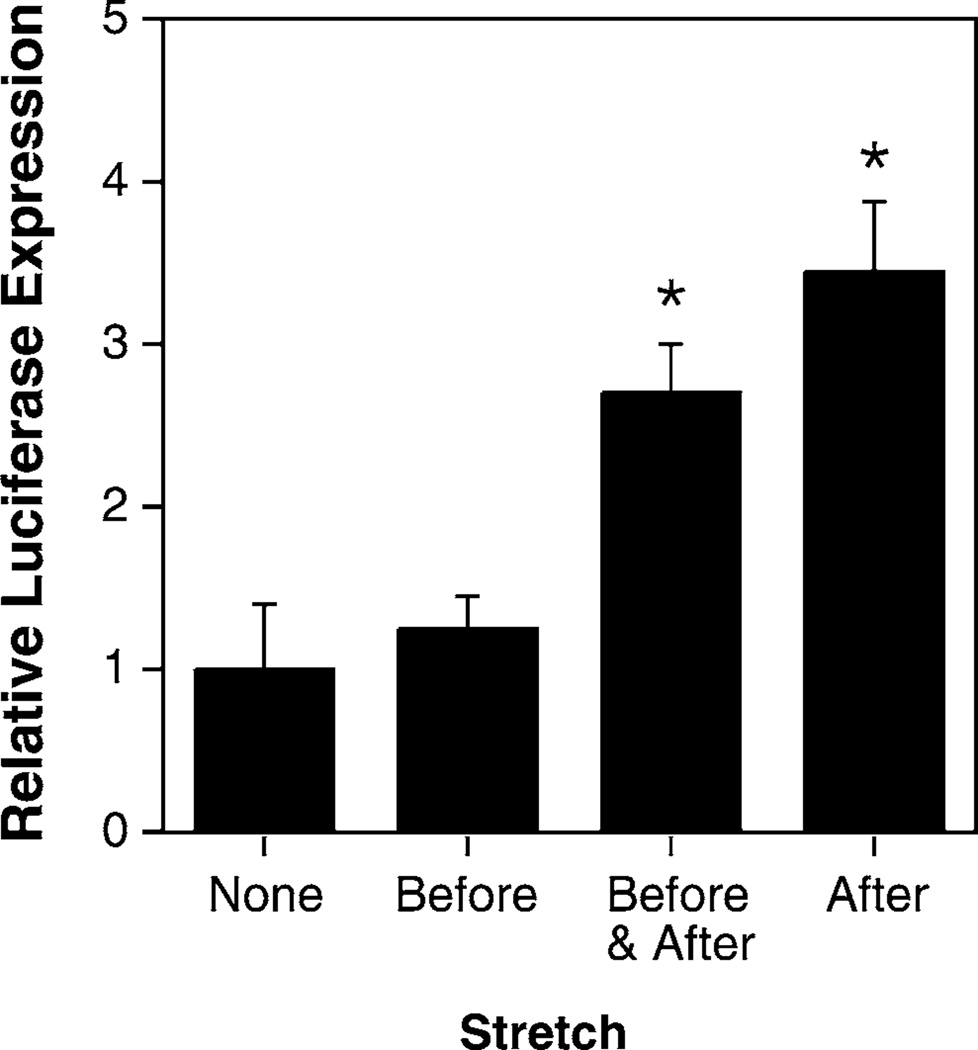
Cells were transfected using lipoplex (Lipofectin-complexed plasmid) or by electroporation of the adherent cells using a Petri-pulser electrode (BTX, San Diego, CA). In order to carry out the transfections, cells were removed from the stretch device for no more than 5 minutes. Twenty-four hours post-transfection, the cells were viewed by fluorescence microscopy for GFP expression, and subsequently lysed for measurement of luciferase activity. Using this approach, we are able to obtain both qualitative data, including the number of cells that have productively received the transgene and the relative levels of gene expression within these cells, as well as quantitative data, including the total level of gene expression for the population at large. In lipoplex transfected cells, reasonable luciferase gene expression was obtained in cells that were grown under static conditions prior to and following transfection, as is typically seen in standard transfections (Fig. 2; relative transfection efficiency of 1.0). Similar levels of expression were seen in cells that were stretched for 24 hours prior to DNA addition. In contrast, when the cells were stretched either prior to and after DNA transfection, or only after transfection, an almost 10-fold increase in gene expression was detected over that found in cells grown under static conditions. There was no statistical difference between cells stretched before and after transfection or just after DNA addition. When the results from 8 independent experiments using A549 cells were combined, the mean increase in gene expression seen in cells stretched after DNA administration vs. static cells was 9.5 ± 4.8 fold. Similar results were obtained when the DNA was delivered to the cells by electroporation (Fig. 3). In this case, the absolute level of gene expression was much lower in electroporated cells than in lipoplex-transfected cells, but the application of cyclic stretch after electroporation (with or without stretch prior to DNA addition) increased gene expression between 2 and 15-fold compared to that seen in static cells. As seen in lipoplex-transfected cells, the stretchinduced increase in gene expression was statistically significant compared to cells grown under static conditions or with stretch applied only before electroporation; there was no statistical difference between cells stretched after transfection only or both before and after. Thus, the application of cyclic stretch to cells after the addition of DNA results in increased transfection efficiency.
FIG. 2.
Cyclic stretch applied immediately following DNA-liposome addition greatly increases gene transfer. A549 cells were grown for 24 hours prior to and after transfection with pCMV-lux-DTS and Lipofectin. Cyclic stretch (10% area strain at 1 Hz) was applied as indicated with respect to DNA addition. Transfections were performed in triplicate. Luciferase expression was normalized relative to expression seen in statically grown transfected cells. Non-parametric Mann-Whitney U test gave a p < 0.01 (*) compared to cells grown under static conditions (“none”).
FIG. 3.
Cyclic stretch applied immediately following DNA electroporation greatly increases gene transfer. A549 cells were grown and treated as in Fig. 2, but DNA was electroporated into the adherent cells using a Petri Pulser electrode. Luciferase expression was normalized relative to expression seen in statically grown electroporated cells. *, p < 0.02 compared to cells grown under static conditions (“none”).
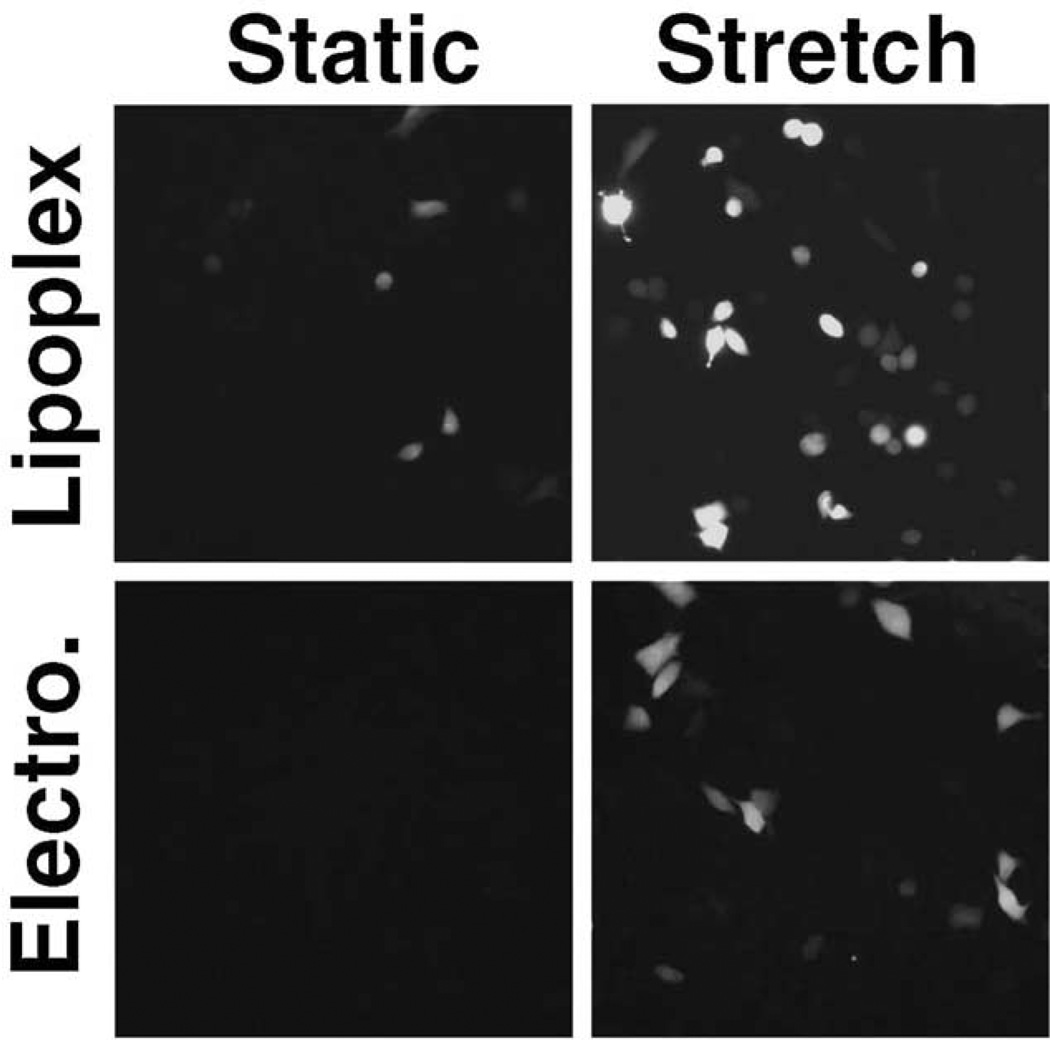
To determine whether the stretch-induced increase in gene expression was due to increased numbers of cells expressing product or increased gene expression in a limited number of individual cells, we visualized the expression of GFP in cells that had been co-transfected with plasmids expressing GFP and luciferase (Fig. 4). As seen with luciferase expression, the application of cyclic stretch to cells after transfection resulted in increased gene expression in both lipoplex-transfected and electroporated cells. It appears that cyclic stretch increases both the number of cells expressing gene product as well as the absolute level of gene expression within individual cells. Similar to the results obtained in Figs. 2 and 3, the application of stretch prior to transfection had no effect on gene expression compared to cells grown under static conditions (not shown).
FIG. 4.
Cyclic stretch increases the number of cells transfected. A549 cells were grown under static conditions for 24 hours before and 24 hours after transfection with pEGFP-N1 (“static”) or exposed to cyclic stretch for 24 hours before and after transfection with pEGFP-N1 (“stretch”). Transfections were performed using lipoplex or electroporation as described in Materials and Methods. Twenty-four hours after transfection, cells were fixed and GFP expression was visualized and photographed using the same exposure times for all conditions.
Short Durations of Cyclic Stretch are Sufficient to Cause Increased Gene Transfer
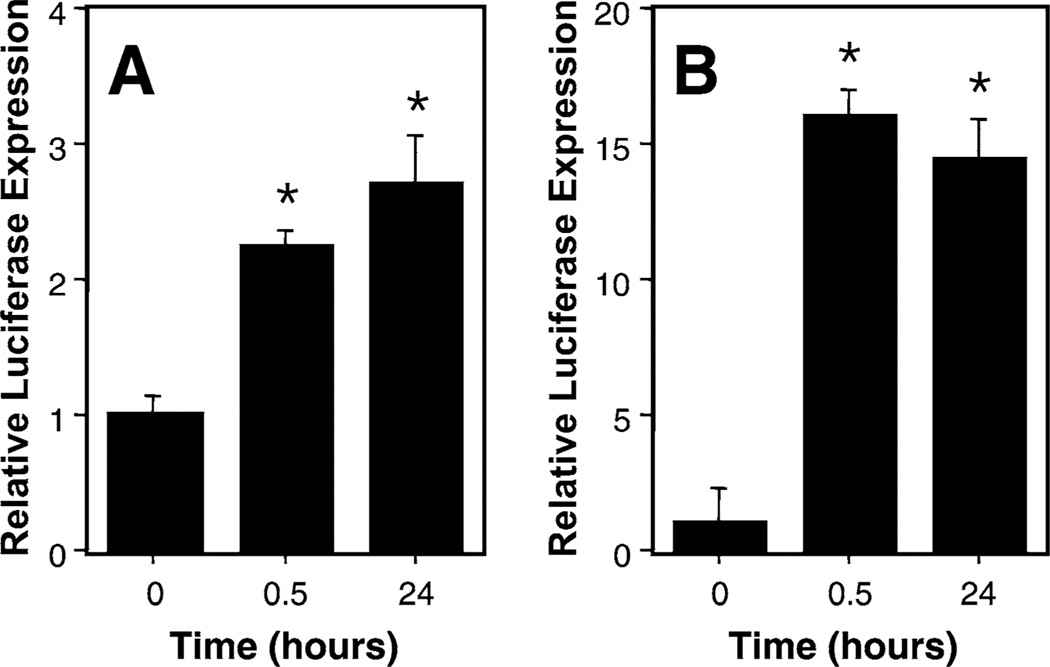
Figures 2 to 4 show that the application of cyclic stretch to cells for 24 hours following DNA transfection resulted in increased gene transfer and/or expression in pulmonary epithelial cells. To determine whether cyclic stretch was required for the entire 24 hour period following transfection, cells were exposed to cyclic stretch for varying amounts of time immediately following DNA transfection (Fig. 5). When cells were exposed to as little as 30 minutes of cyclic stretch (1 Hz, 10% area stretch) and then grown under static conditions for another 23 hours, the levels of expression obtained were essentially the same as those seen in cells exposed to cyclic stretch for the entire 24 hour period. Similar results were obtained in cells transfected with lipoplex (Fig. 5A) and by electroporation (Fig. 5B). These results demonstrate that the effects of cyclic stretch are initiated within the first 30 minutes following transfection.
FIG. 5.
Cyclic stretch for 30 min post-transfection is sufficient for maximal enhancement of gene transfer. A549 cells were transfected with DNA using liposomes (A) or electroporation (B) and then stretched for 30 min and grown statically for 23.5 hours or stretched for 24 hours. Luciferase expression was normalized relative to expression seen in statically grown transfected cells. In these experiments, the absolute level of transfection was 10-fold greater in lipoplex-transfected cells. *, p < 0.01 versus 0 time. No statistical difference was observed between 0.5 and 24 hours for either method.
Cyclic and Continuous Stretch Elicit Different Responses on Gene Transfer
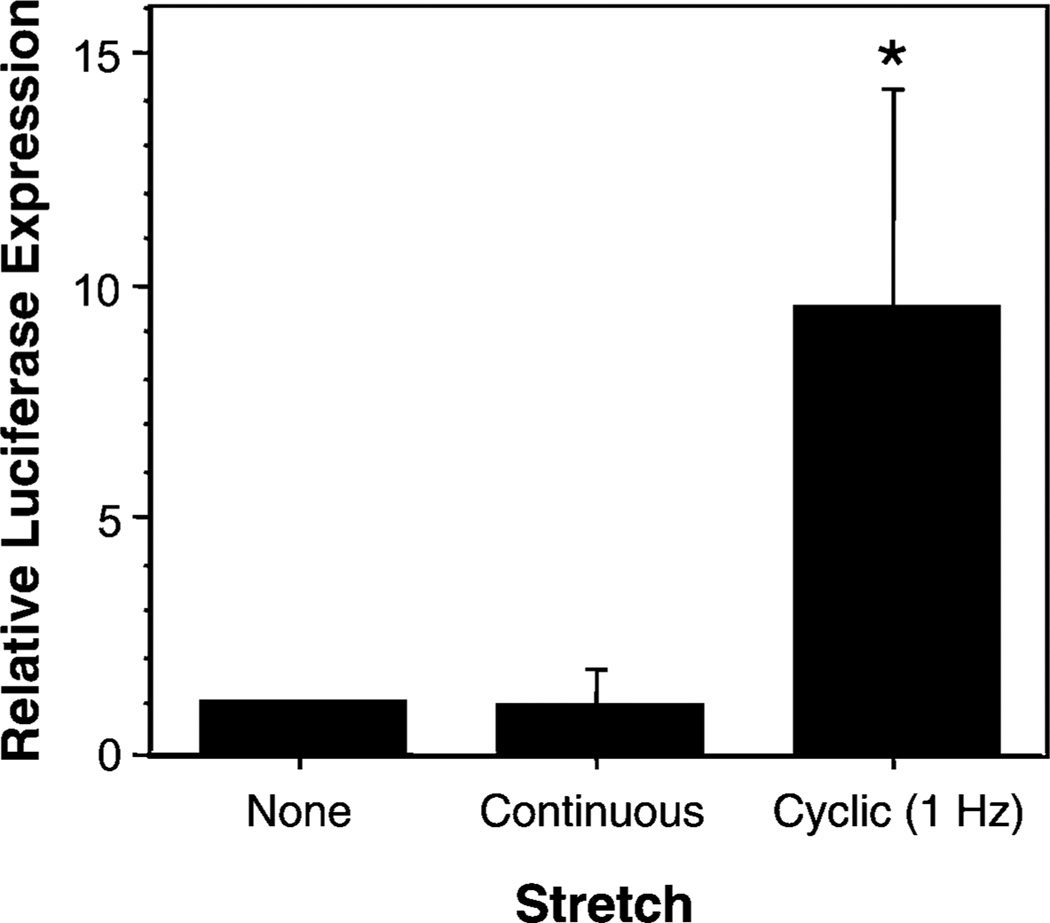
Static deformation of cells often results in responses that are different from those obtained under dynamic conditions. To test whether the application of a continuous 10% surface area deformation increased gene transfer and expression to the same levels as cyclic stretch of the same intensity, cells were grown on Bioflex plates, transfected with lipoplex, and then either subjected to our standard cyclic stretch regimen (10% area stretch, 1 Hz for 24 hours) or to static deformation (stretched to a 10% area change over 1 second and then left at this strain without cycling for 24 hours). Figure 6 shows that in contrast to the effects of cyclic stretch on transfection efficiency, the application of continuous stretch for 24 hours did not increase gene transfer when compared to cells grown without stretch. Similar results were obtained when cells were electroporated (not shown). Thus, stretch-enhanced gene transfer requires cyclic stretch.
FIG. 6.
Cyclic stretch, but not continuous stretch increases gene transfer efficiency. A549 cells were transfected with DNA using liposomes and then grown for 24 hours with or without stretch. Stretch (10% area change) was either cyclic at 1 Hz or continuous (one 24 hour stretch). *, p < 0.001 for cyclic stretch vs continuous or no stretch.
Rupture of the Plasma Membrane is Not Responsible for Stretch-Enhanced Gene Delivery
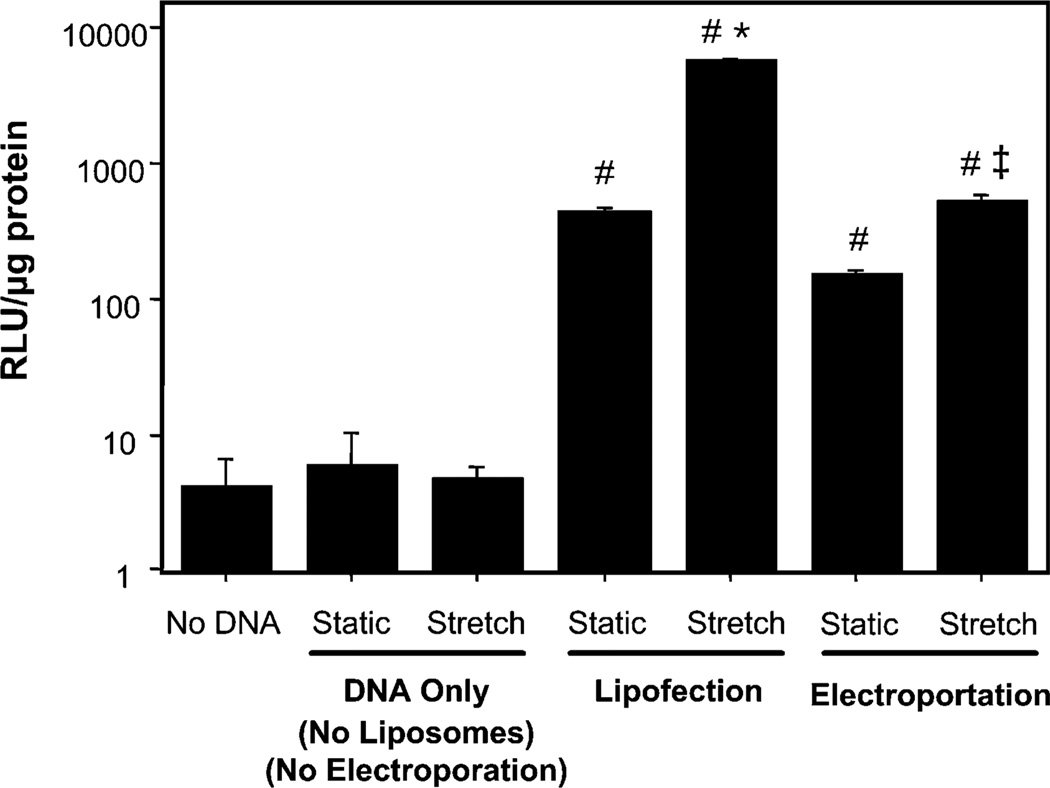
One explanation for the stretch-induced increase in gene transfer is that the plasma membrane of the cells is being damaged during application of cyclic stretch, thus allowing DNA in the medium to enter the cytoplasm via these transient tears in the membrane. This could account for the fact that transfection enhancement is observed only when stretch is applied after DNA addition. To determine whether this was the case, DNA alone in the absence of liposomes or electroporation was added to cells that were then grown under static conditions or stretched (1 Hz, 10% area strain) for 24 hours after DNA addition (Fig. 7). If tears in the membrane occur with stretch, as has been suggested [33], DNA in the absence of transfection reagent or an electric field should be capable of entering the cells and directing transgene expression. Addition of DNA in the absence of liposomes to cells that were subsequently stretched resulted in no gene expression above background (no DNA), although transfection of cells grown statically with DNA complexed with liposomes gave high level gene expression that could be further increased upon stretch. This suggests that cyclic stretch is not promoting membrane tears large enough to allow the entry of plasmids. However, it does not rule out the possibility that the stretch applied after lipoplex addition increases expression by facilitating lipoplex uptake. To address this, we effectively bypassed the membrane uptake event by using electroporation. Cells were electroporated with DNA and immediately washed with fresh medium to remove extracellular DNA and stretch was, or was not, applied 10 minutes later. Because the membrane pores formed by electroporation close when, or very shortly after, the electric field is removed (t1/2 on the order of msec to sec) [34], the enhancement of gene expression obtained in electroporated cells after stretch is due to effects on DNA already in the cell.
FIG. 7.
Rupture of the plasma membrane is not responsible for stretch-enhanced gene delivery. A549 cells were grown with or without cyclic stretch prior to and after addition of DNA in the medium (no liposomes, no electroporation, “DNA Only”) as in Fig. 2, transfected with liposomes and stretched or grown statically with the same regimen (“lipofection”), or electroporated and stretched or grown statically with the same regimen (“electroporation”). No statistical differences were observed within the no DNA or no liposome group. *, p < 0.001 for stretched liposomes vs static liposomes, #, p < 0.001 vs DNA only, ‡, p < 0.01 for stretched electroporated vs static electroporated.
Discussion
Mechanical stretch induces multiple biological responses in cells, including alterations in the cytoskeleton, activation of cell signaling pathways, and upregulation of transcription factors. These responses are directly related to the process of gene delivery. Exogenous DNA, either viral or non-viral, must cross the plasma membrane into the cell, travel through the cytoplasm either utilizing or circumnavigating the cytoskeletal networks, form complexes with transcription factors and/or other nuclear proteins in order to enter the nucleus, and be transcribed in order for gene therapy to be successful. Because mechanical stress affects the plasma membrane, the cytoskeleton, and the levels and subcellular localization of many transcription factors, it is likely that mechanical strain could play a large role in gene delivery. By exposing pulmonary epithelial cells to a 10% area strain at 60 cycles/min prior to and/or after transfection of reporter plasmids, we found that cyclic stretch applied after DNA exposure resulted in increased gene transfer and expression. Statistically significant increases of 2- to 15-fold were observed in cells that were stretched after transfection using either lipoplex or electroporation of naked DNA. As little as 30 minutes of cyclic stretch immediately after transfection followed by 23 hours of static growth elicited the same enhancement of gene transfer and expression as did 24 hours of stretch following transfection. This enhancement was only observed when cyclic strain was applied; application of non-cycling, continuous stretch of the same magnitude caused no increase in transfection efficiency. These results demonstrate that there is likely a link between the biological responses induced by cyclic stretch and the mechanisms of gene transfer.
The mechanism(s) by which cyclic stretch enhances gene transfer are unknown. Based on our data, we can eliminate several possible mechanisms and steps in the gene transfer process. First, it is unlikely that cyclic stretch is exerting its effects at the plasma membrane because the enhancement of gene transfer is seen when cells are transfected by lipoplex as well as by electroporation (Figs. 2–4). DNA complexed with cationic lipids interacts with the plasma membrane and is internalized into endosomes prior to release into the cytoplasm [35]. By contrast, electroporation opens transient pores in the plasma membrane to allow the external DNA to enter the cell during the pore lifetime [34]. Because stretch increases gene expression obtained with both of these mechanistically distinct transfection methods, the step at which stretch acts must be downstream of plasma membrane entry. Our data also suggest that it is unlikely that cyclic stretch is rupturing the plasma membrane thereby allowing access of exogenous DNA into the cell (Fig. 7). If such rupture occurs with cyclic stretch under the conditions employed in these experiments, uncomplexed DNA in the medium should have had access to the cytoplasm and lead to increased gene expression. This was not the case. Although these results do not preclude the possibility of stretch-induced plasma membrane tearing, if such tears do result from stretch, they appear not to be of sufficient size to allow the entry of substantial amounts of plasmid into the cell. These results suggest that the effects of stretch are downstream of DNA uptake by the cell, although in the case of lipoplex-transfected cells, membrane effects may play an additional role.
Second, it is unlikely that the increases in transfection efficiency are due to increased transcription in the cells. It is well documented that cyclic stretch can increase expression of a variety of endogenous genes, primarily through the activation of a number of transcription factors, including AP1 and NF-ΚB, that in turn stimulate expression [9,15,21,24,36,37]. In most cases, the increases in transcription factor activation and subsequent gene expression occur rapidly within minutes of stretching and can last for up to 48 to 72 hours post-stretch. Thus, if the stretch-induced enhancement of gene transfer is due to increased transcription via transcription factor activation, when cells were stretched prior to transcription, we should have detected an increase in gene expression versus static controls. However, stretch-induced increases in expression occurred only when stretch was applied after addition of DNA (Figs. 2 and 3). Taken together, these results suggest that stretch may be exerting its effect either on the movement of DNA through the cytoplasm (e.g., altered interactions with the cytoskeletal network) or on the nuclear import of the DNA.
Although it is clear that DNA-protein complexes must enter the nucleus, it is unclear how DNA moves through the cytoplasm to arrive at the nuclear envelope. It has been shown that macromolecules over 500 kDal are highly restricted in their cytoplasmic diffusion [38–40]. Thus, if movement were strictly by diffusion, viral particles and plasmids should never reach the nucleus, even though they do. It has been shown that certain viral particles utilize motors such as kinesin or dynein and the microtubule network (adenovirus, HIV, and herpesvirus), or motors associated with actin filaments (baculovirus) for their directed movement to the nucleus [41–45]. Thus, it is possible that cytoplasmically delivered DNA-protein complexes use similar mechanisms to traverse the cytoplasm. Because cyclic stretch has been demonstrated in a number of cell types to alter cytoskeletal networks [3–5], any re-organization of the structural components will affect the ability of their motors as well. Further, stretch-induced reorganization of any of the filament or tubule networks in the cytoplasm will greatly affect the “meshwork” structure of the cytoplasm and alter the ability of molecules to move by diffusion [46]. If DNA simply diffuses through the cytoplasm, the lack of any one network should alter the ability of the DNA to move. Consequently, the stretch-induced reorganization of microtubules, microfilaments, or intermediate filaments could alter the viscosity and ability of DNA to move toward the nucleus.
A second mechanism by which cyclic stretch could be increasing gene transfer is at the level of nuclear import. The nuclear envelope is one of the major barriers to gene transfer [47–50]. Our laboratory has demonstrated that nuclear import of plasmids in non-dividing cells is sequence-specific [47,48]. Plasmids containing one of these DNA nuclear import sequences are able to enter the nucleus, while those lacking such a sequence remain in the cytoplasm until cell division or until they are degraded [47,48]. The common feature of these DNA import sequences is that they contain binding sites for transcription factors that in turn harbor protein nuclear localization signals that interact with the cell’s machinery for nuclear protein import. Thus, the DNA sequence acts as a scaffold for transcription factors and the plasmid becomes coated with protein NLSs which enables it to enter the nucleus. Because cyclic stretch has been widely shown to upregulate transcription factors, either by increased transcription [24,37], activation [22], or by promoting the nuclear import of existing transcription factors in the cytoplasm [9,21], it is possible that under conditions of cyclic stretch, more DNA-transcription factor complexes could form in the cytoplasm and be transported into the nucleus than in static cells. Using DNA microarrays, we have found that cyclic stretch upregulates the expression of a number of transcription factors known to bind to the SV40 DNA nuclear import sequence, including AP1 family members, GATA factors, and NF-ΚB (Machado-Aranda and Dean, unpublished data). It is also possible the cyclic stretch alters the transport properties of the nuclear pore complex itself, to allow more DNA to enter the nucleus. Feldherr and colleagues have reported that the functional diameter of nuclear pore complexes is dependent on cell shape and is larger in flattened, extended cells than in rounded ones [51–53], supporting the possibility of a role for increased nuclear transport as a mechanism for stretch-enhanced gene transfer. More recently, it was demonstrated that the shape of the nucleus can also affect rates of transcription and even which genes are turned on in cells, perhaps through a mechanism involving nuclear import or nuclear organization [54]. This supports the model of tensegrity which has also been used to explain how mechanical stimuli are translated into transcriptional responses [6–8].
Our results demonstrate that cyclic stretch can increase gene transfer and expression in pulmonary epithelial cells. These results are important not only for gaining insight into the cell biology of gene transfer and the mechanisms of gene delivery, but may also have implications for the development of gene therapy strategies in the injured lung. Current nonviral technologies to deliver genes are not sufficient to make gene therapy a reality in patients under even optimal conditions, let alone under stresses associated with many disease states [55]. Based on our results, we can speculate that short-term exposure to the cyclic mechanical force induced by ventilators may lead to increased gene transfer to the injured lung. Thus, while the effects caused by high levels of mechanical strain are presumed to be deleterious to cells and the lungs [56,57], our results on stretch-enhanced gene transfer suggest that mild stretch may be beneficial in terms of gene delivery.
Materials and Methods
Plasmids
The plasmids pEGFP-N1 (Clontech, Palo Alto, CA) and pCMV-lux-DTS express green fluorescent protein (GFP) and firefly luciferase, respectively, from the CMV immediate early promoter/enhancer [26]. Plasmids were propagated in Escherichia coli and purified using Qiagen Giga-prep kits, as described by the manufacturer (Qiagen, Chatsworth, CA). Agarose gel electrophoretic analysis demonstrated that greater than 80% of the purified DNA was present in the supercoiled form, and no RNA was detected.
Cell culture
A549 cells were purchased from ATCC (Rockville, MD). Cells were maintained in DMEM containing 10% fetal bovine serum, kanamycin, and antibiotic/antimycotic solution (Invitrogen, Carlsbad, CA). For transfection experiments, cells were plated to 50% confluency on laminin coated 6-well BioFlex plates (Flexcell International, McKeesport, PA).
Application of cyclic or continuous stretch
Cells were grown on laminin coated 6-well BioFlex plates and exposed to 10% equibiaxial cyclic stretch (Δ surface area) at 60 cycles/min (1 Hz) using the Flexercell baseplate with loading posts in place, unless otherwise stated. Stretch was applied to the plates using the Flexercell FX3000 (Flexcell International, McKeesport, PA). Cells were also continuously stretched using the same device, where indicated. For continuous stretch, the membrane was stretched to increase area by 10% over a 1 second period and left at this strain for the remainder of the experiment.
Transfections and quantification of gene expression
Cells were transfected at approximately 60% confluency using either lipoplex or electroporation. Under conditions where the cells were stretched prior to and/or after transfection, the plates of cells were removed from the Flexercell FX3000 for less than 10 minutes to perform the washes and addition of DNA. Stretch was reapplied within 5 minutes of DNA addition. For lipofection, plasmids (5 µg/well) were complexed with Lipofectin (10 µg/well; Invitrogen) and added in serum-free DMEM to cells that had been washed with phosphate-buffered saline, as described by the manufacturer. Four hours later, DMEM containing 10% fetal bovine serum was added to the cells and the stretch was continued. For electroporation studies, plasmids (10 µg/well) were suspended in serum-free medium (1 ml/well) and added to washed cells. One 10 msec pulse of 100V was delivered to the adherent cells using the PetriPulser electrode (BTX, San Deigo, CA) that was rested on the monolayer of cells. Five minutes after electroporation, serumcontaining DMEM was added to the cells and the plates were returned to the stretching device. Twenty-four hours after addition of DNA by either method, the cells were removed from the apparatus, rinsed with cold phosphate-buffered saline, and GFP fluorescence was visualized in individual cells using a Leica DM RA upright microscope equipped with water immersible objectives. Images were captured and analyzed with a Hamamatsu ORCA 2 cooled CCD camera and Openlab 3.0 software (Improvision, Lexington, MA). Immediately following visualization of GFP expression (typically within 30 minutes for a 6 well plate), the cells were lysed in Promega lysis buffer and luciferase activity was measured using the Promega Luciferase Assay System, as previously described [26]. All transfections and electroporations were done in triplicate wells in each experiment, and experiments were repeated at least three times. All luciferase activities were normalized to protein levels in the cells and are reported as fold-increase over cells that were transfected but never exposed to cyclic stretch. For statistical analyses, non-parametric Mann-Whitney U tests were performed.
Acknowledgments
We would like to thank Jacob I. Sznajder and Jennifer L. Young for stimulating discussions. This work was supported in part by a grant from the Institute for Bioengineering and Nanoscience in Advanced Medicine of Northwestern University, the Crane Asthma Foundation, and NIH grants HL59956 and EY12962 (DAD).
References
- 1.Liu M, Post M. Mechanochemical signal transduction in the fetal lung. J. Appl. Physiol. 2000;89:2078–2084. doi: 10.1152/jappl.2000.89.5.2078. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Xu Q. Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cell Signal. 2000;12:435–445. doi: 10.1016/s0898-6568(00)00096-6. [DOI] [PubMed] [Google Scholar]
- 3.Hayakawa K, Sato N, Obinata T. Dynamic reorientation of cultured cells and stress fibers under mechanical stress from periodic stretching. Exp. Cell Res. 2001;268:104–114. doi: 10.1006/excr.2001.5270. [DOI] [PubMed] [Google Scholar]
- 4.Putnam AJ, Cunningham JJ, Dennis RG, Linderman JJ, Mooney DJ. Microtubule assembly is regulated by externally applied strain in cultured smooth muscle cells. J. Cell Sci. 1998;111:3379–3387. doi: 10.1242/jcs.111.22.3379. [DOI] [PubMed] [Google Scholar]
- 5.Putnam AJ, Schultz K, Mooney DJ. Control of microtubule assembly by extracellular matrix and externally applied strain. Am. J. Physiol. Cell Physiol. 2001;280:C556–C564. doi: 10.1152/ajpcell.2001.280.3.C556. [DOI] [PubMed] [Google Scholar]
- 6.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 7.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 8.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granet C, Boutahar N, Vico L, Alexandre C, Lafage-Proust MH. MAPK and SRC-kinases control EGR-1 and NF-kappa B inductions by changes in mechanical environment in osteoblasts. Biochem. Biophys. Res. Commun. 2001;284:622–631. doi: 10.1006/bbrc.2001.5023. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki H, Eguchi S, Ueno H, Marumo F, Hirata Y. Mechanical stretch stimulates growth of vascular smooth muscle cells via epidermal growth factor receptor. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H521–H529. doi: 10.1152/ajpheart.2000.278.2.H521. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen HT, Adam RM, Bride SH, Park JM, Peters CA, Freeman MR. Cyclic stretch activates p38 SAPK2-, ErbB2-, and AT1-dependent signaling in bladder smooth muscle cells. Am. J. Physiol. Cell Physiol. 2000;279:C1155–C1167. doi: 10.1152/ajpcell.2000.279.4.C1155. [DOI] [PubMed] [Google Scholar]
- 12.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J. Appl. Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 13.Numaguchi K, Eguchi S, Yamakawa T, Motley TD, Inagami T. Mechanotransduction of rat aortic vascular smooth muscle cells requires RhoA and intact actin filaments. Circ. Res. 1999;85:5–11. doi: 10.1161/01.res.85.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Ingram AJ, James L, Cai L, Thai K, Ly H, Scholey JW. NO inhibits stretch-induced MAPK activity by cytoskeletal disruption. J. Biol. Chem. 2000;275:40301–40306. doi: 10.1074/jbc.M007018200. [DOI] [PubMed] [Google Scholar]
- 15.Gruden G, Zonca S, Hayward S, Thomas S, Maestrini S, Gnudi L, Viberti GC. Mechanical stretch-induced fibronectin and transforming growth factorbeta1 production in human mesangial cells in p38 mitogen-activated protein kinase-dependent. Diabetes. 2000;49:655–661. doi: 10.2337/diabetes.49.4.655. [DOI] [PubMed] [Google Scholar]
- 16.Correa-Meyer E, Pesce L, Guerrero C, Sznajder JI. Cyclic stretch activates ERK1/2 via G proteins and EGFR in alveolar epithelial cells. Am. J. Physiol. 2002;282:L883–L891. doi: 10.1152/ajplung.00203.2001. [DOI] [PubMed] [Google Scholar]
- 17.Felix JA, Woodruff ML, Dirksen ER. Stretch increases inositol 1,4,5-triphosphate concentration in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 1996;14:296–301. doi: 10.1165/ajrcmb.14.3.8845181. [DOI] [PubMed] [Google Scholar]
- 18.Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol triphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 19.Scott JE, Yang S-Y, Stanik E, Anderson JE. Influence of strain on [3H]-thymidine incorporation, surfactant-related phospholipid synthesis, and cAMP levels in fetal type II alveolar cells. Am. J. Respir. Cell Mol. Biol. 1993;8:258–265. doi: 10.1165/ajrcmb/8.3.258. [DOI] [PubMed] [Google Scholar]
- 20.Geng WD, Boskovic G, Fultz ME, Li C, Niles RM, Ohno S, Wright GL. Regulation of expression and activity of four PKC isozymes in confluent and mechanically stimulated UMR-108 osteoblastic cells. J. Cell Physiol. 2001;189:216–228. doi: 10.1002/jcp.10019. [DOI] [PubMed] [Google Scholar]
- 21.Chaqour B, Howard PS, Richards CF, Macarak EJ. Mechanical stretch induces platelet-activating factor receptor gene expression through the NF-kappaB transcription factor. J. Mol. Cell Cardiol. 1999;31:1345–1355. doi: 10.1006/jmcc.1999.0967. [DOI] [PubMed] [Google Scholar]
- 22.Park JM, Adam RM, Peters CA, Guthrie PD, Sun Z, Klagsbrun M, Freeman MR. AP-1 mediates stretch-induced expression of HB-EGF in bladder smooth muscle cells. Am. J. Physiol. 1999;277:C294–C301. doi: 10.1152/ajpcell.1999.277.2.C294. [DOI] [PubMed] [Google Scholar]
- 23.Peake MA, Cooling LM, Magnay JL, Thomas PB, El Haj AJ. Selected contribution: regulatory pathways involved in mechanical induction of c-fos gene expression in bone cells. J. Appl. Physiol. 2000;89:2498–2507. doi: 10.1152/jappl.2000.89.6.2498. [DOI] [PubMed] [Google Scholar]
- 24.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J. Clin. Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura K, Chen YE, Lopez-Ilasaca M, Daviet L, Tamura N, Ishigami T, Akishita M, Takasaki I, Tokita Y, Pratt RE, Horiuchi M, Dzau VJ, Umemura S. Molecular mechanism of fibronectin gene activation by cyclic stretch in vascular smooth muscle cells. J. Biol. Chem. 2000;275:34619–34627. doi: 10.1074/jbc.M004421200. [DOI] [PubMed] [Google Scholar]
- 26.Vacik J, Dean BS, Zimmer WE, Dean DA. Cell-specific nuclear import of plasmid DNA. Gene Therapy. 1999;6:1006–1014. doi: 10.1038/sj.gt.3300924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tschumperlin DJ, Margulies AS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J. Appl. Physiol. 1999;86:2026–2033. doi: 10.1152/jappl.1999.86.6.2026. [DOI] [PubMed] [Google Scholar]
- 28.Tschumperlin DJ, Margulies AS. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am. J. Physiol. Lung Cell Mol. Physiol. 1998;275:L1173–L1183. doi: 10.1152/ajplung.1998.275.6.L1173. [DOI] [PubMed] [Google Scholar]
- 29.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am. J. Respir. Crit. Care Med. 2000;162:357–362. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- 30.Waters CM, Ridge KM, Sunio G, Venetsanou K, Sznajder JI. Mechanical stretching of alveolar epithelial cells increases Na(+)-K(+)- ATPase activity. J. Appl. Physiol. 1999;87:715–721. doi: 10.1152/jappl.1999.87.2.715. [DOI] [PubMed] [Google Scholar]
- 31.Waters CM, Glucksberg MR, Lautenschlager EP, Lee CW, Van Matre RM, Warp RJ, Savla U, Healy KE, Moran B, Castner DG, Bearinger JP. A system to impose prescribed homogenous strains on cultured cells. J. Appl. Physiol. 2001;91:1600–1610. doi: 10.1152/jappl.2001.91.4.1600. [DOI] [PubMed] [Google Scholar]
- 32.Savla U, Sporn PH, Waters CM. Cyclic stretch of airway epithelium inhibits prostanoid synthesis. Am. J. Physiol. 1997;273:L1013–L1019. doi: 10.1152/ajplung.1997.273.5.L1013. [DOI] [PubMed] [Google Scholar]
- 33.Ko KS, McCulloch CA. Partners in protection: interdependence of cytoskeleton and plasma membrane in adaptations to applied forces. J. Membr. Biol. 2000;174:85–95. doi: 10.1007/s002320001034. [DOI] [PubMed] [Google Scholar]
- 34.Somiari S, Glasspool-Malone J, Drabick JJ, Gilbert RA, Heller R, Jaroszeski MJ, Malone RW. Theory and in vivo application of electroporative gene delivery. Mol. Ther. 2000;2:178–187. doi: 10.1006/mthe.2000.0124. [DOI] [PubMed] [Google Scholar]
- 35.Barron LG, Szoka FC. The perplexing delivery mechanism of lipoplexes. In: Huang L, Hung M-C, Wagner E, editors. Nonviral vectors for gene therapy. San Diego: Academic Press; 1999. pp. 230–266. [Google Scholar]
- 36.Chen J, Fabry B, Schiffrin EL, Wang N. Twisting integrin receptors increases endothelin-1 gene expression in endothelial cells. Am. J. Physiol. Cell Physiol. 2001;280:C1475–C1484. doi: 10.1152/ajpcell.2001.280.6.C1475. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Beqaj S, Kemp P, Ariel I, Schuger L. Stretch-induced alternative splicing of serum response factor promotes bronchial myogenesis and is defective in lung hypoplasia. J. Clin. Invest. 2000;106:1321–1330. doi: 10.1172/JCI8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luby-Phelps K, Castle PE, Taylor DL, Lanni F. Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc. Natl. Acad. Sci. USA. 1987;84:4910–4913. doi: 10.1073/pnas.84.14.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Provance DW, Jr, McDowall A, Marko M, Luby-Phelps K. Cytoarchitecture of size-excluding compartments in living cells. J. Cell Sci. 1993;106:565–577. doi: 10.1242/jcs.106.2.565. [DOI] [PubMed] [Google Scholar]
- 40.Seksek O, Biwersi J, Verkman AS. Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J. Cell Biol. 1997;138:131–142. doi: 10.1083/jcb.138.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suomalainen M, Nakano MY, Keller S, Boucke K, Stidwill RP, Greber UF. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 1999;144:657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leopold PL, Kreitzer G, Miyazawa N, Rempel S, Pfister KK, Rodriguez-Boulan E, Crystal RG. Dynein- and microtubule-mediated translocation of adenovirus serotypes 5 occurs after endosomal lysis. Hum. Gene Ther. 2000;11:151–165. doi: 10.1089/10430340050016238. [DOI] [PubMed] [Google Scholar]
- 43.Glotzer JB, Michou A-I, Baker A, Saltik M, Cotten M. Microtubule-independent motility and nuclear targeting of adenoviruses with fluorescently labeled genomes. J. Virol. 2001;75:2421–2434. doi: 10.1128/JVI.75.5.2421-2434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Loo ND, Fortunati E, Ehlert E, Rabelink M, Grosveld F, Scholte BJ. Baculovirus infection of nondividing mammalian cells: mechanisms of entry and nuclear transport of capsids. J. Virol. 2001;75:961–970. doi: 10.1128/JVI.75.2.961-970.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye G-J, Vaughan KT, Vallee RB, Roizman B. The herpes simplex virus 1 UL34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J. Virol. 2000;74:1355–1363. doi: 10.1128/jvi.74.3.1355-1363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuchs E, Yang E. Crossroads on cytoskeletal highways. Cell. 1999;98:547–550. doi: 10.1016/s0092-8674(00)80041-0. [DOI] [PubMed] [Google Scholar]
- 47.Dean DA. Import of plasmid DNA into the nucleus is sequence specific. Exp. Cell Res. 1997;230:293–302. doi: 10.1006/excr.1996.3427. [DOI] [PubMed] [Google Scholar]
- 48.Dean DA, Dean BS, Muller S, Smith LC. Sequence requirements for plasmid nuclear entry. Exp. Cell Res. 1999;253:713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sebestyen MG, Wolff JA. Nuclear Transport of Exogenous DNA. In: Huang L, Hung M-C, Wagner E, editors. Nonviral vectors for Gene Therapy. San Diego: Academic Press; 1999. pp. 140–169. [Google Scholar]
- 50.Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J. Biol. Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- 51.Jiang LW, Schindler M. Nuclear transport in 3T3 fibroblasts: effects of growth factors, transformation, and cell shape. J. Cell Biol. 1988;106:13–19. doi: 10.1083/jcb.106.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feldherr CM, Akin D. Regulation of nuclear transport in proliferating and quiescent cells. Exp. Cell Res. 1993;205:179–186. doi: 10.1006/excr.1993.1073. [DOI] [PubMed] [Google Scholar]
- 53.Feldherr CM, Akin D. The permeability of the nuclear envelope in dividing and nondividing cell cultures. J. Cell Biol. 1990;111:1–8. doi: 10.1083/jcb.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas CH, Collier JH, Sfeir CS, Healy KE. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc. Natl. Acad. Sci. USA. 2002;99:1972–1977. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West J, Rodman DM. Gene therapy for pulmonary diseases. Chest. 2001;119:613–617. doi: 10.1378/chest.119.2.613. [DOI] [PubMed] [Google Scholar]
- 56.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end- expiratory pressure. Am. Rev. Respir Dis. 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 57.Dreyfuss D, Basset G, Soler P, Saumon G. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am. Rev. Respir. Dis. 1985;132:880–884. doi: 10.1164/arrd.1985.132.4.880. [DOI] [PubMed] [Google Scholar]