Abstract
BACKGROUND
A wide range of false-negative rates has been reported for sentinel lymph node (SLN) biopsy after preoperative chemotherapy. The purpose of this study was to determine whether histologic findings in negative SLNs after preoperative chemotherapy are helpful in assessing the accuracy of SLN biopsy in patients with confirmed lymph node-positive disease before treatment.
METHODS
Eighty-six patients with confirmed lymph node-positive disease at presentation underwent successful SLN biopsy and axillary dissection after preoperative chemotherapy at a single institution between 1994 and 2007. Available hematoxylin and eosin-stained sections from patients with negative SLNs were reviewed, and associations between histologic findings in the negative SLNs and SLN status (true negative vs false negative) were evaluated.
RESULTS
Forty-seven (55%) patients had at least 1 positive SLN, and 39 (45%) patients had negative SLNs. The false-negative rate was 22%, and the negative predictive value was 67%. The negative SLNs from 17 of 34 patients with available slides had focal areas of fibrosis, some with associated foamy parenchymal histiocytes, fat necrosis, or calcification. These histologic findings occurred in 15 (65%) of 23 patients with true-negative SLNs and in only 2 (18%) of 11 patients with false-negative SLNs (P =.03, Fisher exact test, 2-tailed). The lack of these histologic changes had a sensitivity and specificity for identifying a false-negative SLN of 82% and 65%, respectively.
CONCLUSIONS
Absence of treatment effect in SLNs after chemotherapy in patients with lymph node-positive disease at initial presentation has good sensitivity but low specificity for identifying a false-negative SLN.
Keywords: sentinel lymph node, breast cancer, lymph node positive, preoperative chemotherapy, treatment effect
Lymphatic mapping and sentinel lymph node (SLN) biopsy have become routine procedures for patients with early stage breast cancer, as they provide a minimally invasive method of assessing axillary lymph node status.1–5 Patients with negative SLNs have a low likelihood of having metastases in nonsentinel axillary lymph nodes, so such patients can be spared the morbidity of an axillary lymph node dissection. Even in patients who receive preoperative chemotherapy, also known as neoadjuvant or induction chemotherapy, SLN biopsy has been reported to be an accurate method for staging the axilla.6,7 However, there has also been concern that lymphatic fibrosis or tumor debris could alter lymphatic draining patterns and decrease the accuracy of SLN biopsy in these patients.
The false-negative rates for SLN biopsy after preoperative chemotherapy have been reported to range from 0% to 33%.6,8–14 Although the number of patients in each series is relatively small, a recent meta-analysis concluded that the SLN biopsy is accurate after neoadjuvant chemotherapy.7 Most investigators have focused on patients who present with clinically lymph node-negative disease before chemotherapy, and have largely excluded patients with confirmed lymph node-positive disease at initial presentation.8–14 At our institution, we have noted a higher false-negative rate for SLN biopsy after chemotherapy in patients with confirmed lymph node-positive disease before treatment.15 We hypothesized that particular histologic features in negative SLNs after preoperative chemotherapy in this patient population might help to predict the likelihood of a false-negative SLN.
Histologic findings indicative of treatment effect, such as fibrosis, characteristically occur at the site of a tumor that undergoes a partial or complete pathologic response to chemotherapy.16,17 Positive SLNs that undergo a complete pathologic response to chemotherapy would be expected to have similar histologic findings. In patients with a fine-needle aspiration (FNA)-proven positive lymph node before chemotherapy, the SLN might not be the same as the FNA-proven positive lymph node. However, non-SLNs are expected to be positive only when the SLN is also positive. Therefore, all patients with an FNA-proven positive lymph node are expected to have had positive SLNs. A positive SLN after treatment is expected to have fibrosis or other treatment-related changes. If fibrosis or other changes associated with tumor response are not observed in the SLN after chemotherapy in a patient with confirmed lymph node-positive disease before treatment (ie, in a patient expected to have had a positive sentinel lymph node before treatment), the apparent SLN might not be the true SLN. Treatment-induced lymphatic fibrosis or tumor debris could have altered the normal lymphatic draining pattern. Therefore, the absence of such histologic changes in a negative SLN after chemotherapy in a patient with confirmed lymph node-positive disease before treatment should raise the possibility of a false-negative SLN.
The purpose of this study was to determine whether histologic findings in negative SLNs after chemotherapy can predict the accuracy of SLN biopsy in patients who present initially with confirmed lymph node-positive disease.
MATERIALS AND METHODS
Eighty-six patients with operable breast cancer (T1-T3) and lymph node-positive disease (N1-N3) confirmed by ultrasound-guided FNA were treated on prospective pre-operative chemotherapy protocols, and underwent successful SLN biopsy and concomitant axillary dissection as components of their surgical treatment at The University of Texas M. D. Anderson Cancer Center between 1994 and 2007. Intraoperative lymphatic mapping was performed after peritumoral injection (4 injections around the primary tumor site) of isosulfan blue dye alone, technetium-labeled sulfur colloid alone, or a combination of both. Nonpalpable tumors were injected under mammographic or ultrasound guidance. Clinicopathologic features of a subset of these patients were reported previously.15
The sentinel lymph nodes were serially sectioned at 2-mm intervals, and all of the lymph node sections were routinely processed and embedded in paraffin blocks for histologic evaluation. A single hematoxylin and eosin (H & E) section from each block was prepared. Immunohistochemical stains for keratin were not evaluated in this study, as patients with isolated tumor cells in SLNs are currently staged as N0(i+) and treated as lymph node negative at our institution. Moreover, in a recent analysis from our institution, the presence of occult keratin-positive tumor cells in axillary lymph nodes after chemotherapy in patients with positive lymph nodes at initial presentation did not impact disease-free or overall survival.18
For this retrospective study, the available H & E sections of both the true- and false-negative SLNs were retrieved from The University of Texas M. D. Anderson Cancer Center surgical pathology archives for review. A true-negative SLN was defined as a negative SLN after chemotherapy in a patient with negative non-SLNs. A false-negative SLN was defined as a negative SLN after chemotherapy in a patient with metastatic carcinoma in 1 or more of the non-SLNs. In this setting, a false-negative SLN could have resulted either from misidentification of the SLN or from complete response in the SLN but less than complete response in 1 or more non-SLNs. Histologic evidence of treatment effect, including fibrosis with or without calcification, a parenchymal foamy histiocytic infiltrate with partial obliteration of lymph node architecture, or fat necrosis was recorded for both the true- and false-negative SLNs. Associations between histologic findings and SLN status (true negative vs false negative) were evaluated using 2-tailed Fisher exact test.
RESULTS
Of the 86 lymph node-positive patients in this study who underwent successful SLN biopsy and concomitant axillary dissection after preoperative chemotherapy, the median number of SLNs identified was 2 (range, 1–10). Forty-seven (55%) patients had at least 1 positive SLN. Thirty-nine (45%) patients had negative SLNs, of which 13 were found to be false negatives. This resulted in a false-negative rate of 22% and a negative predictive value of 67% (Table 1). The false-negative rate appeared to be related to the number of SLNs removed. In the 56 patients with ≤3 SLNs identified, the false-negative rate was 33%, and in the 30 patients with ≥4 SLNs identified, the false-negative rate was 5%.
Table 1.
Status of SLNs and Non-SLNs in Lymph Node-Positive Patients After Preoperative Chemotherapy
| Lymph Nodes | Negative Non-SLNs | Positive Non-SLNs | Total |
|---|---|---|---|
| Negative SLNs | 26 | 13 | 39 |
| Positive SLNs | 14 | 33 | 47 |
| Total | 40 | 46 | 86 |
SLN indicates sentinel lymph node; TP, true positive; TN, true negative; FN, false negative.
False negative: FN/(TP + FN) =13/60 =22%; negative predictive value: TN/(TN + FN) =26/39 =67%.
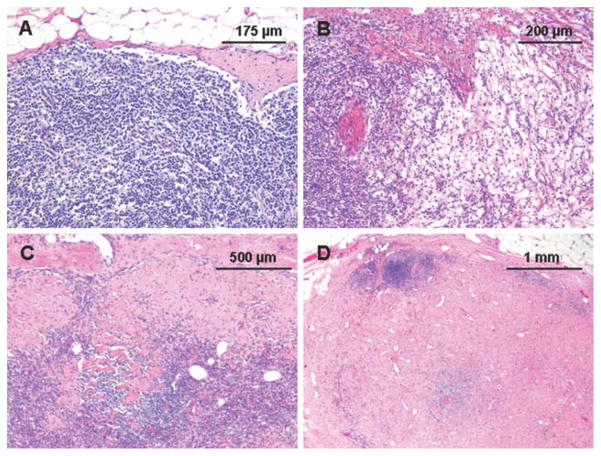
The H & E sections were available for 34 (87%) of the 39 patients with negative SLNs. Seventeen (50%) of the 34 patients with negative SLNs available for review had histologic evidence of treatment effect in at least 1 of the SLNs. These included 7 patients with focal areas of fibrosis (≤5% of the SLN), including 1 with associated calcification, 4 with moderate amounts of fibrosis (>5% but <50% of the SLN), including 1 with associated fat necrosis, 4 with diffuse fibrosis (≥50%), and 2 with parenchymal infiltration by foamy histiocytes with partial obliteration of lymph node architecture (Fig. 1).
Figure 1.
Representative photomicrographs of sentinel lymph nodes in patients with invasive breast cancer after preoperative chemotherapy show (A) no detectable histologic change, (B) a parenchymal histiocytic infiltrate, (C) focal subcapsular fibrosis, and (D) diffuse parenchymal fibrosis.
Of the 17 patients with histologic findings suggestive of treatment effect, 15 were patients with true-negative SLNs, and 2 were patients with false-negative SLNs (Table 2). Histologic changes suggestive of treatment effect were observed in 15 (65%) of 23 patients with true-negative SLNs and 2 (18%) of 11 patients with false-negative SLNs (Table 3). The association between histologic findings suggestive of treatment effect and SLN status (true negative vs false negative) was statistically significant (P =.03, Fisher exact test, 2-tailed). The absence of histologic changes suggestive of treatment effect resulted in a sensitivity for identifying a false-negative SLN of 82% and a specificity of 65%.
Table 2.
Histologic Findings Suggestive of Treatment Effect in True-Negative and False-Negative SLNs
| Treatment Effect | True-Negative SLNs | False-Negative SLNs |
|---|---|---|
| Fibrosis (≤5%) | 5 | 1 |
| Fibrosis (≤5%) & calcification | 1 | 0 |
| Fibrosis (>5% but <50%) | 3 | 0 |
| Fibrosis (>5 but <50%) & fat necrosis | 1 | 0 |
| Fibrosis (≥50%) | 3 | 1 |
| Parenchymal histiocytes | 2 | 0 |
| None | 8 | 9 |
| Total | 23 | 11 |
SLN indicates sentinel lymph node.
Table 3.
Association Between Histologic Evidence of Treatment Effect and SLN Status
| SLNs | SLNs | ||
|---|---|---|---|
| Treatment effect | 15 | 2 | 17 |
| No treatment effect | 8 | 9 | 17 |
| Total | 23 | 11 | 34 |
SLN indicates sentinel lymph node.
P =.03, Fisher exact test, 2-tailed.
DISCUSSION
Chemotherapy is known to induce characteristic histologic changes at the site of a primary breast carcinoma when the tumor undergoes either a partial or complete response to therapy. There is generally marked fibrosis, often accompanied by a foamy histiocytic infiltrate and hemosiderin deposition.16,17 Similar changes also occur in the regional lymph nodes at the site of metastasis when the metastatic tumor undergoes a partial or complete response to chemotherapy.17,19,20 The patients in this study had confirmed lymph node-positive disease before treatment. For some patients, the SLN might not have been the same as the FNA-proven positive lymph node. However, non-SLNs are expected to be positive only when the SLN is also positive. Therefore, as all patients in this study had an FNA-proven positive lymph node, all are expected to have had positive SLNs. A positive SLN after treatment is expected to have fibrosis or other treatment-related changes. If fibrosis or other changes associated with tumor response are not observed in the SLN after chemotherapy in a patient with confirmed lymph node-positive disease before treatment (ie, in a patient expected to have had a positive SLN before treatment), the apparent SLN might not be the true SLN. Treatment-induced lymphatic fibrosis or tumor debris could have altered the normal lymphatic draining pattern. Our findings in patients with confirmed lymph node-positive disease at presentation support this concept, as absence of treatment effect in the SLNs after chemotherapy had increased sensitivity for identifying a false-negative SLN.
A recent meta-analysis included 21 studies involving a total of 1273 patients who received preoperative chemotherapy followed by SLN biopsy and completion axillary dissection. The analysis found that the average SLN identification rate and false-negative rate were 91% and 12%, respectively.7 The false-negative rate was only slightly higher than that reported in meta-analyses of SLN biopsy without preoperative chemotherapy, which ranged from 5.1% to 9%.21–23 A report on a subgroup of patients from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-27 trial, 1 of the largest studies to date on SLN biopsy after chemotherapy, recently reported similar findings.6 The purpose of NSABP B-27 was to compare 3 chemotherapy regimens: neoadjuvant doxorubicin/cyclophosphamide versus neoadjuvant doxorubicin/cyclophosphamide followed by neoadjuvant docetaxel versus neoadjuvant doxorubicin/cyclophosphamide followed by adjuvant docetaxel. Level I and II axillary dissection was required. Although SLN biopsy was not required, some surgeons performed lymphatic mapping and SLN biopsy as part of the surgical procedure. A total of 343 patients in the NSABP B-27 trial underwent SLN biopsy with concomitant axillary dissection after neoadjuvant chemotherapy, and the SLN identification rate and false-negative rate were 84.8% and 10.7%, respectively.6 Although the NSABP investigators concluded that SLN biopsy is applicable after preoperative chemotherapy, the investigators did not distinguish between patients with lymph node-positive disease and lymph node-negative disease at presentation.
We have noted a higher false-negative rate for SLN biopsy after chemotherapy in patients with confirmed lymph node-positive disease at initial presentation at our institution.15 A false-negative SLN would provide inaccurate information about residual nodal disease and thus might inappropriately impact the decision for additional regional therapy in these patients. However, the clinical impact of a false-negative SLN in this clinical setting is not entirely clear. A false-negative SLN does not affect the decision to give chemotherapy to these patients, who by definition have undergone preoperative chemotherapy after confirmation of axillary lymph node positivity. The 25-year follow-up data from the NSABP B-04 trial failed to show a significant survival advantage from removing clinically occult positive nodes at the time of initial surgery, as long as axillary dissection was performed later for patients who subsequently developed axillary nodal disease.24 Omitting additional regional therapy because of a false-negative SLN might not impact survival, but the standard of care is to perform an axillary lymph node dissection if an SLN is found to be positive, before or after chemotherapy.
The false-negative SLN rate in our study appeared to be related to the number of SLNs removed. The overall false-negative rate of 22% that we observed is at the upper end of the range reported in other studies of SLN biopsy after preoperative chemotherapy. Our study included only patients with documented regional lymph node involvement before chemotherapy, whereas the lower false-negative rates cited in most studies are for patients who underwent SLN biopsy after preoperative chemotherapy, irrespective of the initial lymph node status.6,8–14 Although the NSABP B-27 subgroup analysis found that the false-negative rate was independent of clinical lymph node status, some patients classified with clinically positive lymph nodes based on physical examination alone may not have confirmed lymph node metastases by FNA or core biopsy. The inclusion of only patients with documented FNA-positive axillary lymph nodes in our study should provide a more accurate assessment of this specific patient population. In contrast to our study, some patients in the NSABP B-27 trial underwent lymphatic mapping before and after chemotherapy, and there was no standardized method for the histologic evaluation of the SLNs in the NSABP study.
Our data suggest that in biopsy-proven lymph node-positive breast cancer patients who undergo preoperative chemotherapy and subsequent axillary SLN biopsy, a negative SLN with no histologic changes to suggest treatment effect, such as fibrosis or a parenchymal histiocytic infiltrate, appears to have a higher likelihood of being a false-negative SLN compared with a negative SLN with these histologic changes. If a patient or clinician were reluctant to pursue a SLN biopsy in this clinical setting because of concern that the false-negative rate may be unacceptably high, the presence of treatment effect in the SLN might be reassuring, whereas the absence of treatment effect would raise concern about a false-negative SLN. If future studies confirm our findings, a lymph node-positive patient could undergo lymphatic mapping and SLN biopsy after chemotherapy, and a decision to perform axillary dissection could be based on the presence of residual metastatic disease or the absence of treatment effect in the SLN, either of which would indicate an increased likelihood of residual metastatic disease in the axilla.
Although the absence of treatment effect appears to have good sensitivity for identifying a false-negative SLN, it has a low specificity. If a negative SLN were required to have treatment effect as a necessary component of a successful SLN biopsy in lymph node-positive patients after preoperative chemotherapy, our study suggests that approximately half of the biopsies with negative SLNs would be regarded as unsuccessful, and these patients (in addition to patients with positive SLNs) would require completion axillary dissection. Extrapolating from the data in our study, although almost a quarter of all lymph node-positive patients after chemotherapy would have unsuccessful SLN biopsy procedures, the false-negative rate would be reduced from 22% (13 false negatives per 60 positives, including 47 true positives and 13 false negatives) to a more acceptable rate of 4.1% (2 false negatives per 49 positives, including 47 true positives and 2 false negatives).
Despite the absence of randomized clinical trials evaluating SLN biopsy after neoadjuvant chemotherapy, it appears likely that SLN biopsy after preoperative chemotherapy will become routine based on retrospective analyses from single institutions and multicenter series of patients who underwent SLN biopsy followed by completion axillary dissection. Clinicians and patients who pursue SLN biopsy in this clinical setting will need to accept a somewhat higher false-negative SLN rate or find a method to predict the likelihood of a false-negative SLN in individual patients. Assessing treatment effect in a negative SLN after chemotherapy from a patient with lymph node-positive disease at initial presentation might be 1 such method, but future studies are needed to confirm its potential clinical utility.
Acknowledgments
FUNDING STATEMENT:
This article was supported by NIH. The grant number is P30 CA016672.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Albertini JJ, Lyman GH, Cox C, et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA. 1996;276:1818–1822. [PubMed] [Google Scholar]
- 2.Giuliano AE, Jones RC, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15:2345–2350. doi: 10.1200/JCO.1997.15.6.2345. [DOI] [PubMed] [Google Scholar]
- 3.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer—a multicenter validation study. N Engl J Med. 1998;339:941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymphnodes. Lancet. 1997;349:1864–1867. doi: 10.1016/S0140-6736(97)01004-0. [DOI] [PubMed] [Google Scholar]
- 5.Yi M, Meric-Bernstam F, Ross MI, et al. How many sentinel lymph nodes are enough during sentinel lymph node dissection for breast cancer? Cancer. 2008;113:30–37. doi: 10.1002/cncr.23514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23:2694–2702. doi: 10.1200/JCO.2005.05.188. [DOI] [PubMed] [Google Scholar]
- 7.Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN. Meta-analysis of sentinel lymph node biopsy after pre-operative chemotherapy in patients with breast cancer. Br J Surg. 2006;93:539–546. doi: 10.1002/bjs.5209. [DOI] [PubMed] [Google Scholar]
- 8.Nason KS, Anderson BO, Byrd DR, et al. Increased false negative sentinel node biopsy rates after preoperative chemotherapy for invasive breast carcinoma. Cancer. 2000;89:2187–2194. [PubMed] [Google Scholar]
- 9.Breslin TM, Cohen L, Sahin A, et al. Sentinel lymph node biopsy is accurate after neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2000;18:3480–3486. doi: 10.1200/JCO.2000.18.20.3480. [DOI] [PubMed] [Google Scholar]
- 10.Julian TB, Patel N, Dusi D, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for breast cancer. Am J Surg. 2001;182:407–410. doi: 10.1016/s0002-9610(01)00736-x. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez A, Cortes M, Benito E, et al. Gamma probe sentinel node localization and biopsy in breast cancer patients treated with a neoadjuvant chemotherapy scheme. Nucl Med Commun. 2001;22:361–366. doi: 10.1097/00006231-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Stearns V, Ewing CA, Slack R, Penannen MF, Hayes DF, Tsangaris TN. Sentinel lymphadenectomy after neoadjuvant chemotherapy for breast cancer may reliably represent the axilla except for inflammatory breast cancer. Ann Surg Oncol. 2002;9:235–242. doi: 10.1007/BF02573060. [DOI] [PubMed] [Google Scholar]
- 13.Haid A, Tausch C, Lang A, et al. Is sentinel lymph node biopsy reliable and indicated after preoperative chemotherapy in patients with breast carcinoma? Cancer. 2001;92:1080–1084. [PubMed] [Google Scholar]
- 14.Miller AR, Thomason VE, Yeh IT, et al. Analysis of sentinel lymph node mapping with immediate pathologic review in patients receiving preoperative chemotherapy for breast carcinoma. Ann Surg Oncol. 2002;9:243–247. doi: 10.1007/BF02573061. [DOI] [PubMed] [Google Scholar]
- 15.Shen J, Gilcrease MZ, Babiera GV, et al. Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer. 2007;109:1255–1263. doi: 10.1002/cncr.22540. [DOI] [PubMed] [Google Scholar]
- 16.Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681–695. doi: 10.1002/cncr.10741. [DOI] [PubMed] [Google Scholar]
- 17.Sharkey FE, Addington SL, Fowler LJ, Page CP, Cruz AB. Effects of preoperative chemotherapy on the morphology of resectable breast carcinoma. Mod Pathol. 1996;9:893–900. [PubMed] [Google Scholar]
- 18.Loya A, Guray M, Hennessy BT, et al. Prognostic significance of occult axillary lymph node metastases after chemotherapy-induced pathologic complete response of cytologically proven axillary lymph node metastases from breast cancer. Cancer. 2009;115:1605–1612. doi: 10.1002/cncr.24173. [DOI] [PubMed] [Google Scholar]
- 19.Aktepe F, Kapucuoglu N, Pak I. The effects of chemotherapy on breast cancer tissue in locally advanced breast cancer. Histopathology. 1996;29:63–67. doi: 10.1046/j.1365-2559.1996.d01-485.x. [DOI] [PubMed] [Google Scholar]
- 20.Cohen LF, Breslin TM, Kuerer HM, Ross MI, Hunt KK, Sahin AA. Identification and evaluation of axillary sentinel lymph nodes in patients with breast carcinoma treated with neoadjuvant chemotherapy. Am J Surg Pathol. 2000;24:1266–1272. doi: 10.1097/00000478-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Fraile M, Rull M, Julian FJ, et al. Sentinel node biopsy as a practical alternative to axillary lymph node dissection in breast cancer patients: an approach to its validity. Ann Oncol. 2000;11:701–705. doi: 10.1023/a:1008377910967. [DOI] [PubMed] [Google Scholar]
- 22.Miltenburg DM, Miller C, Karamlou TB, Brunicardi FC. Meta-analysis of sentinel lymph node biopsy in breast cancer. J Surg Res. 1999;84:138–142. doi: 10.1006/jsre.1999.5629. [DOI] [PubMed] [Google Scholar]
- 23.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006;106:4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]