Abstract
Simian varicella virus (SVV) infection of non-human primates models human varicella zoster virus (VZV) infection. Assessment of cell signaling immune responses in monkeys after primary SVV infection, after immunosuppression and during reactivation revealed strong pro-inflammatory responses and lesser anti-inflammatory components during varicella and reactivation. Pro-inflammatory mediators elevated during varicella included interferon-gamma (IFN-γ), interleukin (IL)-6, mono-cyte chemoattractant protein (MCP-1), interferon inducible T-cell α chemoattractant protein (I-TAC), interferon processing protein (IP-10), and anti-inflammatory interleukin-1 Receptor antagonist (IL-1Ra). After immunosuppression and at reactivation, levels of pro-inflammatory mediators MCP-1, eotaxin, IL-6, IL-8, MIF, RANTES (regulated-on-activation normal T-cell expressed and secreted), and HGF (hepatocyte growth factor) were elevated, as was the anti-inflammatory mediator IL-1Ra. Characterization of cytokine, chemokine and growth factor responses during different stages of varicella virus infection will facilitate immunotherapeutic and vaccine strategies.
Keywords: Varicella zoster virus, Simian varicella virus, Cytokines, Chemokines, Immune response
Introduction
The mechanisms by which the immune system regulates latency and reactivation after varicella zoster virus (VZV) infection in humans are unknown. Simian varicella virus (SVV) infection of nonhuman primates is a useful animal model of VZV pathogenesis in humans (Mahalingam et al. 2001, 2007, 2010a, b; Messaoudi et al. 2009). Expression of CXCL10 correlates with T-cell infiltration in ganglia of macaques containing reactivated SVV (Ouwendijk et al. 2013), and increases in circulating CD4+ and CD8+ T lymphocytes before and during SVV reactivation correlate with increases in cellular PD-1 expression (James et al. 2014). Because the time of primary VZV infection and reactivation in humans cannot be predicted, in this work we identified and quantified expression of cytokines, chemokines, and growth factors in the peripheral circulation of SVV-infected rhesus macaques during acute varicella, latency and reactivation.
Materials and methods
Animals
SVV-seronegative male rhesus macaques (2 to 5 years old), housed in the Tulane National Primate Research Center in Covington, LA, were used in all experiments. All procedures were performed following guidelines and protocols approved by the Tulane University Institutional Animal Care and Use Committee and Institutional Biosafety Committee.
Establishment of latent SVV infection and immunosuppressive regimens
Experimental inoculation of the five rhesus macaques with SVV and immunosuppressive treatment have been described earlier (James et al. 2014). Briefly, five rhesus macaques (HB62, HI83 HF39, HC44 and HA95) were each inoculated intrabronchially with 104 plaque-forming units (pfu) of SVV (n=3) or SVV-GFP (SVV expressing green fluorescent protein) (n=2). All monkeys developed acute varicella at 9–14 days post-inoculation (dpi). Five months later (142 dpi), four monkeys (HB62, HI83 HF39 and HC44) were transported to the Tulane Cancer Center and exposed to onetime total body irradiation (200 cGy) followed by oral treatment with tacrolimus (80 µg/kg/day) and prednisone (2 mg/kg/day) for the duration of the experiment, as described (Mahalingam et al. 2007, 2010b). One monkey (HA95) was all not treated but as a control, was similarly transported. Zoster rash developed in all five monkeys 48–84 days later.
Quantification of cytokine, chemokine and growth factors
Plasma obtained weekly was centrifuged (14,000×g for 5 min) and aliquots were frozen at −80 °C. Before analysis, once-thawed plasma samples were filtered using Ultra-free Centrifugal Filters (Millipore, Billerica, MA, USA). The Monkey Cytokine Magnetic 28-Plex Panel (Life Technologies, Carlsbad, CA, USA) was used according to the manufacturer's instructions to analyze expression of a total of 28 cytokines, chemokines and growth factors before and after primary infection, during latency, after immunosuppression and at reactivation. Cytokines included: granulocyte colony-stimulating factor (G-CSF), granulocyte/macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFN-γ), interleukin-1 Receptor antagonist (IL-1Ra), interleukin 1beta (IL-1β), interleukin 2 (IL-2), IL-4, IL-5,IL-6, IL-10, IL-12, IL-15, IL-17, macrophage migration inhibitory factor (MIF), and tumor necrosis factor-alpha (TNF-α). Chemokines included CC family members such as: eosinophil chemotactic protein (eotaxin), monocyte chemoattractant protein (MCP-1), macrophage-derived chemokine (MDC), macrophage inflammatory protein alpha (MIP-1α), macrophage inflammatory protein beta (MIP-1β), and regulated-on-activation normal T-cell expressed and secreted (RANTES), as well as CXC family members, such as: interleukin 8 (IL-8), interferon inducible T-cell α chemoattractant protein (I-TAC), and monokine induced by interferon-γ (MIG); one additional CXC chemokine, the interferon processing protein (IP-10), was tested by single-assay (Life Technologies) according to the manufacturer’s instructions. The growth factors included epidermal growth factor (EGF), basic fibroblast growth factor (FGF basic), hepatocyte growth factor (HCF) and vascular endothelial growth factor (VEGF). All reactions were performed in microtiter plates, analyzed using the Bioplex-200, and results were calculated using BioPlex software version 6 (Bio-Rad, Hercules, CA, USA).
Results
Five rhesus macaques (HA95, HI83, HB62, HC44, and HF39) were inoculated intrabronchially with 104 pfu of SVV. All animals developed acute varicella with peak titers and vesicular rash at 10–14 dpi. Three to four months after primary infection when latency was established, four monkeys (HI83, HB62, HC44, and HF39) were immunosuppressed with total body irradiation, oral tacrolimus, and prednisone (142 dpi). Virus reactivation (zoster rash) developed in all monkeys at 204 dpi (HB62), 224 dpi (HF39), 197 dpi (HI83), 239 dpi (HC44) and 183 dpi (HA95), respectively.
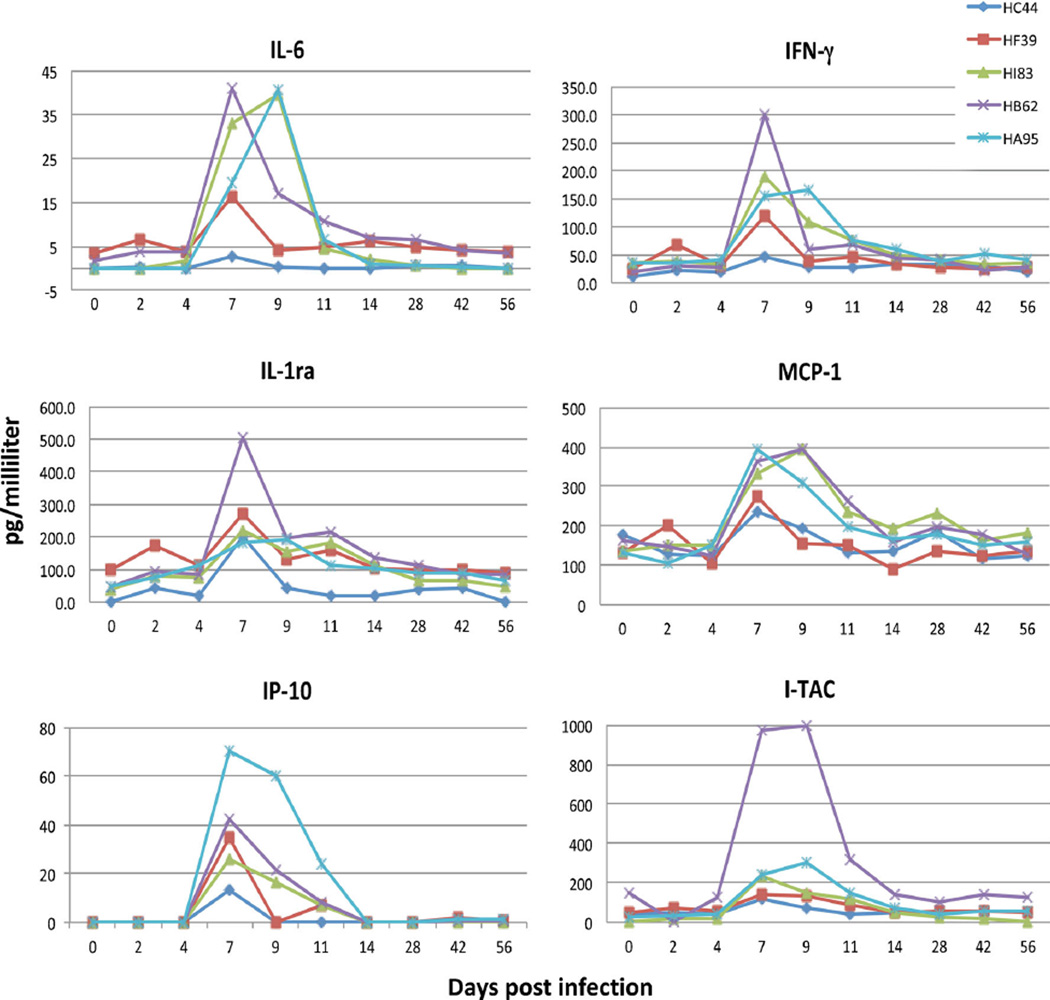
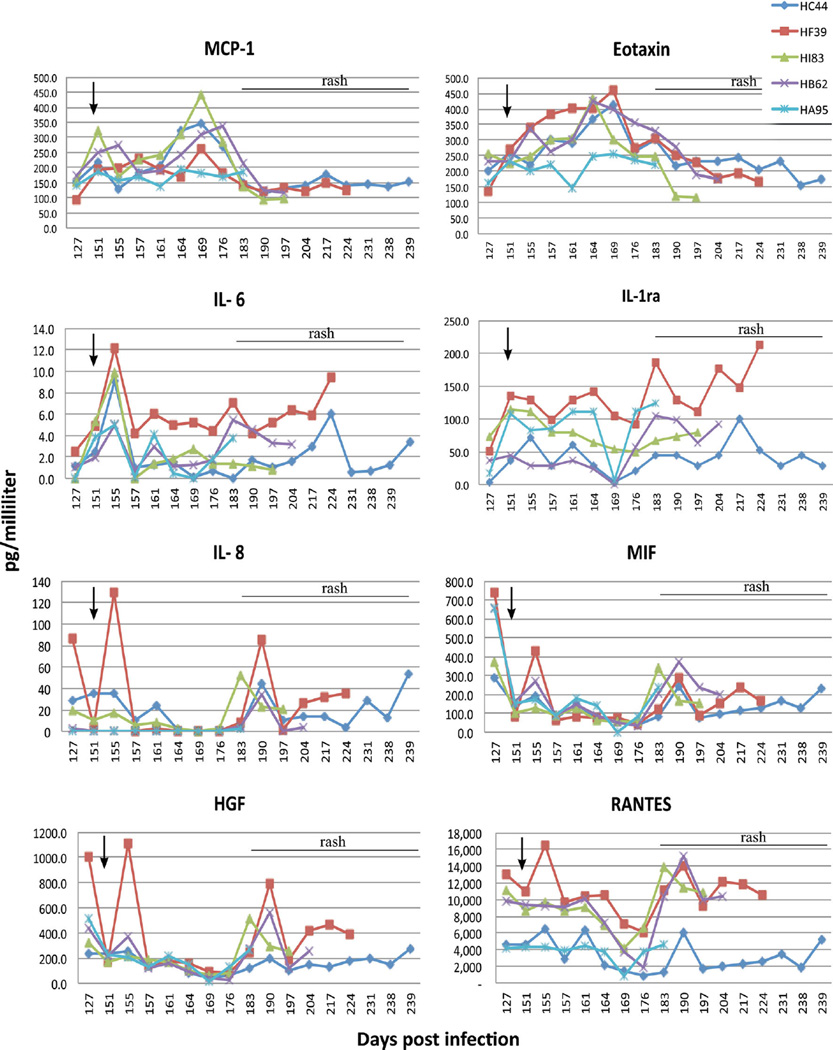
Six cytokines/chemokines were upregulated in plasma of all five rhesus macaques after experimental infection with SVV and development of varicella at 10–14 dpi: increased expression of IL-1Ra, IFN-γ, IL-6, MCP-1, I-TAC and IP-10 was seen at 7–11 dpi and returned to baseline at 14–56 dpi (Fig. 1). Eight cytokines, chemokines and growth factors were upregulated after immunosuppression in four latently infected monkeys (HB62, HC44, HF39 and HI83) and in one monkey (HA95) that was not immunosuppressed; upregulated mediators included eotaxin, MCP-1, IL-6, IL-8, HGF, MIF, RANTES and IL-1Ra (Fig. 2). MCP-1 levels increased 2- to 4-fold in all immunosuppressed monkeys at 9 days (151 dpi) after the start of immunosuppression (142 dpi) and gradually decreased by 161 dpi. A second larger increase in MCP-1 was found at 164–176 dpi (22 to 34 days after immunosuppression [dpx]) that returned to baseline by 183 dpi (39 dpx); these changes were not seen in the non-immunosuppressed monkey (HA95). Eotaxin levels increased in all four immunosup-pressed monkeys at 151–183 dpi (9–39 dpx), but not in the non-immunosuppressed monkey (HA95), after which all levels returned to baseline. IL-6 levels increased in all monkeys, including the non-immunosuppressed monkey, at 151–155 dpi (9–13 dpx). IL-6 levels remained high in one monkey (HF39), with minor variations at 161 (monkeys HA95 and HB62), 169 (monkey HI83), and 183 (monkeys HA95 and HB62) dpi (19, 27, and 39 dpx, respectively). All monkeys had elevated IL-6 levels at reactivation. IL-1Ra levels increased in all but one immunosuppressed monkey (HB62) at 151–164 dpi (9–22 dpx) and in the non-immunosuppressed monkey (HA95). IL-1Ra levels remained high at 169–239 dpi in all five monkeys. IL-8 levels increased at 155 dpi in two immunosuppressed monkeys (HC44, HF39) and did not change significantly in the other immunosuppressed monkeys; at 190 dpi, IL-8 increased in the four immunosuppressed monkeys, but not the non-immunosuppressed monkey (HA95). MIF levels increased in all monkeys including the non-immunosuppressed monkey at 155 and 183–190 dpi (13 and 41–48 dpx, respectively). HGF levels increased at 155 dpi (13 dpx) in two immunosuppressed monkeys (HB62, HF39) and at 190 dpi (48 dpx) in all four immunosuppressed monkeys, but not in the non-immunosuppressed monkey (HA95). RANTES levels were increased in two immunosuppressed monkeys (HC44, HF39) at 155 dpi (13 dpx), whereas at 190 dpi (48dpx), a second, marked increase in RANTES levels was seen in all immunosuppressed monkeys and at reactivation.
Fig. 1.
Six cytokines and chemokines upregulated in five rhesus macaques after experimental infection with simian varicella virus. Levels of IL-6, IL-1Ra, IFN-γ, MCP-1, I-TAC and IP-10 measured longitudinally from 0 to 56 dpi are shown.
Fig. 2.
Eight cytokines, chemokines and growth factors upregulated after immunosuppression of four rhesus macaques latently infected with simian varicella virus and immunosuppressed beginning at 142 dpi (arrow) (HB62, HC44, HF39, HI83) and in one latently infected, non- immunosuppressed macaque (HA95). Levels of MCP-1, eotaxin, IL-6, IL-1Ra, IL-8, MIF, HGF and RANTES measured longitudinally from 127 to 239 dpi are shown
Discussion
Analysis of cytokine, chemokine and growth factor levels in plasma of rhesus macaques revealed a robust pro-inflammatory response with a lesser anti-inflammatory component during primary infection as well as during reactivation. High levels of pro-inflammatory mediators IFN-γ, IL-6, MCP-1, IP-10 and I-TAC and anti-inflammatory cytokine IL-1Ra were seen at 4–14 dpi in all five monkeys. Our results confirm and extend a recent report of increased levels of IL-6, IFN-γ and IL-1Ra during primary SVV infection in rhesus macaques (Meyer et al. 2013), findings consistent with a strong pulmonary innate immune response to SVV after respiratory exposure. IFN-γ is produced by lymphocytes during primary varicella zoster virus infection (Arvin et al. 1986) and suppresses VZV replication in vitro (Rabalais et al. 1989). Chemokines IP-10 and MCP-1 likely recruit and trigger differentiation of monocytes, macrophages, lymphocytes, NK and dendritic cells, as well as stimulate their migration across the vascular endothelium, while I-TAC is responsible for intradermal infiltration (Dufour et al. 2002; Li et al. 2010; Deshmane et al. 2009) and may also be involved in intrader-mal infiltration of SVV-infected T lymphocytes and development of rash. Activated SVV-specific CD4 and CD8 lymphocytes drive the adaptive immune response needed for resolution of varicella and establishment of latency. Continued expression of MCP-1 beyond 28 dpi suggests involvement of macrophage chemotaxis into the nervous system (Selenica et al. 2013; Deshmane et al. 2009). High levels of the anti-inflammatory and regulatory cytokine IL1-Ra were also found during acute infection and persisted until 28 dpi in four of the five monkeys. IL-1Ra functions to block IL-1 binding to its receptor, preventing signal transduction by the transcription factors NFkB and AP-1 (Dinarello 1991, Arend 1991), which regulate cytokine expression and cell signaling necessary for resolution of varicella and establishment of SVV latency in ganglionic neurons (Zerboni et al. 2014).
After immunosuppression, levels of the pro-inflammatory chemokines MCP-1 and eotaxin peaked at 151 and 169 dpi (Fig. 2). These chemokines recruit monocytes, macrophages, dendritic cells (MCP-1) and eosinophils (eotaxin) to sites of inflammation induced by infection. Levels of both modulators returned to baseline at 6 to 7 weeks after immunosuppression (197 dpi) and before reactivation, suggesting their role in triggering viral reactivation. MCP-1 also recruits macro-phages into the nervous system and activates microglia (Selenica et al. 2013; Deshmane et al. 2009); thus, its expression could also indicate subclinical SVV reactivation (James et al. 2014). Additional robust pro-inflammatory immune responses were revealed by induction of IL-6, IL-8, MIF, RANTES and HGF, while an anti-inflammatory response was represented by increased cytokine IL-1Ra levels after immunosuppression (Fig. 2). The ability of IL-6 and RANTES to stimulate cytokine production, migration of lymphocytes, macrophages, neutrophils and dendritic cells into inflammatory sites, as well as the action of IL-8, MIF and HGF to stimulate epithelial, keratinocytes and endothelial cells with resultant trans-migration of lymphocytes into tissues, suggest their potential roles in viral reactivation. Increased levels of IL-6 and IL-8 have been reported in zoster patients as early as 9 days after reactivation (Zak-Prelich et al. 2003). Increased expression of specific immune mediators herein also correlates directly with the increase in peripheral T cells before zoster in the same monkeys, strongly supporting their role in T cell recruitment (James et al. 2014).
Acknowledgments
This work was supported in part by Public Health Service grants AG032958 (R.M, D.G., V.T-D., L.D-M., E.D., M.W., R.S.) and NS0007321 (S.J.) from the National Institutes of Health and federal funds from the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) through grant P51 RR001464 (V.T-D., L.D-M., E.D., R.S.) at the Tulane National Primate Research Center (TNPRC).We thank members of the Pathogen Detection and Quantification Core Laboratory at TNPRC for technical assistance and Ms. Marina Hoffman for editorial assistance.
Footnotes
Conflict of interest The authors declare that they do not have any conflict of interest.
Contributor Information
Vicki Traina-Dorge, Email: vtraina@tulane.edu, Division of Microbiology, Tulane National Primate Research Center, Tulane University, 18703 Three Rivers Road, Covington, LA 70433, USA.
Robert Sanford, Radiation Oncology, Tulane University Cancer Center, Tulane School of Medicine, 1415 Tulane Avenue, Box HC-82, New Orleans, LA 70112, USA.
Stephanie James, Department of Neurology, University of Colorado School of Medicine, 12700 E. 19th Avenue, Box B-182, Aurora, CO 80045, USA.
Lara A. Doyle-Meyers, Division of Veterinary Medicine, Tulane National Primate Research Center, Tulane University, 18703 Three Rivers Road, Covington, LA 70433, USA
Eileen de Haro, Division of Microbiology, Tulane National Primate Research Center, Tulane University, 18703 Three Rivers Road, Covington, LA 70433, USA.
Mary Wellish, Department of Neurology, University of Colorado School of Medicine, 12700 E. 19th Avenue, Box B-182, Aurora, CO 80045, USA.
Don Gilden, Department of Neurology, University of Colorado School of Medicine, 12700 E. 19th Avenue, Box B-182, Aurora, CO 80045, USA; Department of Microbiology, University of Colorado School of Medicine, 12700 E. 19th Avenue, Box B-182, Aurora, CO 80045, USA.
Ravi Mahalingam, Department of Neurology, University of Colorado School of Medicine, 12700 E. 19th Avenue, Box B-182, Aurora, CO 80045, USA.
References
- Arend WP. Interleukin 1 receptor antagonist. Anewmember of the interleukin 1 family. J Clin Invest. 1991;88:1445–1451. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin AM, Koropchak CM, Williams BR, Grumet FC, Foung SK. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986;154:422–429. doi: 10.1093/infdis/154.3.422. [DOI] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- James SF, Traina-Dorge V, Deharo E, Wellish M, Palmer BE, Gilden D, Mahalingam R. T cells increase before zoster and PD- 1 expression increases at the time of zoster in immunosuppressed nonhuman primates latently infected with simian varicella virus. J Neurovirol. 2014;20:309–313. doi: 10.1007/s13365-014-0237-7. (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Xu W, Xu L, Jiang Z, Wen Z, Li K, Xiong S. I-TAC is a dominant chemokine in controlling skin intragraft inflammation via recruiting CXCR3+ cells into the graft. Cell Immunol. 2010;260:83–91. doi: 10.1016/j.cellimm.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Soike K, White T, Kleinschmidt-DeMasters BK, Gilden DH. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology. 2001;279:339–342. doi: 10.1006/viro.2000.0700. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Traina-Dorge V, Wellish M, Lorino R, Sanford R, Ribka EP, Alleman SJ, Brazeau E, Gilden DH. Simian varicella virus reactivation in cynomolgus monkeys. Virology. 2007;368:50–59. doi: 10.1016/j.virol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Messaoudi I, Gilden D. Simian varicella virus pathogenesis. Curr Top Microbiol Immunol. 2010a;342:309–321. doi: 10.1007/82_2009_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam R, Traina-Dorge V, Wellish M, Deharo E, Singletary ML, Ribka EP, Sanford R, Gilden D. Latent simian varicella virus reactivates in monkeys treated with tacrolimus with or without exposure to irradiation. J Neurovirol. 2010b;16:342–354. doi: 10.3109/13550284.2010.513031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I, Barron A, Wellish M, Engelmann F, Legasse A, Planer S, Gilden D, Nikolich-Zugich J, Mahalingam R. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLoS Pathog. 2009;5:e1000657. doi: 10.1371/journal.ppat.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Kerns A, Haberthur K, Dewane J, Walker J, Gray W, Messaoudi I. Attenuation of the adaptive immune response in rhesusmacaques infected with simian varicella virus lacking open reading frame 61. J Virol. 2013;87:2151–2163. doi: 10.1128/JVI.02369-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwendijk WJ, Abendroth A, Traina-Dorge V, Getu S, Steain M, Wellish M, Andeweg AC, Osterhaus AD, Gilden D, Verjans GM, Mahalingam R. T-cell infiltration correlates with CXCL10 expression in ganglia of cynomolgus macaques with reactivated simian varicella virus. J Virol. 2013;87:2979–2982. doi: 10.1128/JVI.03181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabalais GP, Berkowitz FE, Hayward AR, Levin MJ. Inhibition of varicella-zoster virus in vitro by human peripheral blood mononuclear cells. Clin Exp Immunol. 1989;75:381–386. [PMC free article] [PubMed] [Google Scholar]
- Selenica ML, Alvarez JA, Nash KR, Lee DC, Cao C, Lin X, Reid P, Mouton PR, Morgan D, Gordon MN. Diverse activation of microglia by chemokine (C-C motif) ligand 2 overexpression in brain. J Neuroinflammation. 2013;10:86. doi: 10.1186/1742-2094-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak-Prelich M, McKenzie RC, Sysa-Jedrzejowska A, Norval M. Local immune responses and systemic cytokine responses in zoster: relationship to the development of postherpetic neuralgia. Clin Exp Immunol. 2003;131:318–323. doi: 10.1046/j.1365-2249.2003.02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12:197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]