Abstract
Objective(s)
HIV stigma is considered to be a major driver of the HIV/AIDS pandemic, yet there is a limited understanding of its occurrence. We describe the geographic patterns of two forms of HIV stigma in a cross-sectional sample of women of childbearing age from western Kenya: internalized stigma (associated with shame) and externalized stigma (associated with blame).
Design
Geographic studies of HIV stigma provide a first step in generating hypotheses regarding potential community-level causes of stigma and may lead to more effective community-level interventions.
Methods
Spatial regression using generalized additive models and point pattern analyses using K-functions were used to assess the spatial scale(s) at which each form of HIV stigma clusters, and to assess whether the spatial clustering of each stigma indicator was present after adjustment for individual-level characteristics.
Results
There was evidence that externalized stigma (blame) was geographically heterogeneous across the study area, even after controlling for individual-level factors (P=0.01). In contrast, there was less evidence (P=0.70) of spatial trend or clustering of internalized stigma (shame).
Conclusion
Our results may point to differences in the underlying social processes motivating each form of HIV stigma. Externalized stigma may be driven more by cultural beliefs disseminated within communities, whereas internalized stigma may be the result of individual-level characteristics outside the domain of community influence. These data may inform community-level interventions to decrease HIV-related stigma, and thus impact the HIV epidemic.
Keywords: community-level interventions, geographic information systems, HIV stigma, maternal and child health, spatial epidemiology
Introduction
HIV stigma has been a major driver of the HIV/AIDS pandemic and its negative effects on people living with HIV/AIDS (PLWHA) are well documented [1–9]. Among PLWHA, ‘internalized’ HIV stigma, characterized by the acceptance and internalization of public attitudes towards PLWHA [10], acts as a major barrier to uptake of healthcare and undermines the effectiveness of prevention and treatment programs [6,8]. The effects of HIV stigma have been associated with increased risky sexual behavior [11], delay of HIV testing, and deferral of treatment [2,12], often until significant HIV progression has occurred [8]. PLWHA who report experiencing internalized stigma tend to hide their status for fear of being ostracized, which hinders their ability to receive proper treatment and care. One study from Tanzania in 2011, for example, found that the main reason PLWHA did not seek treatment was fear of social exclusion should the community find out their status [1].
For HIV-infected pregnant women, HIV-related stigma may be a barrier to uptake of care, and contributes to the gap between the availability of prevention of mother-to-child transmission (PMTCT) services and the use of those services [13]. One cross-sectional study showed lower uptake of antiretroviral therapy (ART) use among HIV-infected mothers in Kenya who reported feeling ashamed of their HIV status compared to those who did not [14]. Despite the recent global scale-up of PMTCT services, it is estimated that only roughly half of the HIV-infected pregnant women in low and middle-income countries receive ART for PMTCT, with wide country-level disparities in PMTCT coverage ranging from 5 to 10% in Sudan and Chad, to 80 to 90% in South Africa, Botswana, Swaziland, and Namibia [15]. In a study from Kenya in 2011, fear of stigmatization among pregnant women was thought to account for lower rates of uptake of PMTCT services during childbirth compared to rates of uptake of antenatal care (ANC) services, which are often accessed before a mother’s HIV status is known [16] and for which participation does not result in identification as HIV-affected. Studies on the determinants of stigmatizing behaviors (both what drives negative perceptions towards PLWHA and what reinforces PLWHA to internalize stigma) among women of childbearing age in Africa are needed, as this population continues to experience a large burden of HIV/AIDS and is highly vulnerable to the effects of HIV stigma on health-seeking behavior. Furthermore, women living with HIV may be more stigmatized than men due to the higher social and moral expectations on women [17].
Research on HIV stigma has focused primarily on its negative effects, with less attention given to factors that shape stigma, both at the individual and community levels [7]. Qualitative studies on HIV stigma, mostly using focus groups, point to a number of individual-level risk factors that promote internalized HIV stigma among PLWHA, including fear, lack of HIV knowledge, and a lack of social spaces to engage in constructive dialogue on HIV/AIDS [9,18]. It remains unknown whether results from qualitative studies can be translated into effective community-level, antistigma interventions. Government-sponsored, information-based stigma-reduction and awareness campaigns, targeted at dispelling myths and promoting tolerance, have had minimal success [3]. Antistigma interventions remain a low priority for HIV/AIDS programs, mainly because of the difficulty in identifying effective interventions [7]. Population-level research on the structural drivers of HIV stigma will be necessary to inform how and where community-level interventions can be most effective [3,5,19].
Here we explore the geographic distribution of two forms of HIV stigma: internalized stigma, associated with feelings of shame for being HIV-positive; and externalized stigma, sometimes called ‘public stigma’, which is associated with blame towards PLWHA. Because stigma in communities is dependent on the cultural context in which it is manifested [3], we hypothesize that individuals in the same geographic region harbor similar levels of HIV-related stigma due to sharing of information, social networks, influential political and religious leaders, and other shared sociocultural factors. We first explore whether individuals who reside in the same geographic area are more likely to share similar views with respect to internalized and externalized HIV stigma (based on a responses to a questionnaire described in the ‘Methods’ section). Second, we assess the spatial scale(s) at which such clustering of each form of HIV stigma occurs across the study area. This analysis serves as a first step towards understanding the spatial distribution of HIV stigma in an area of high HIV transmission with the goal of identifying specific community-level factors associated with HIV stigma that could motivate targeted intervention strategies.
Methods
Study population
The study was reviewed and approved by the Institutional Review Boards of the University of Washington and Kenya Medical Research Institute (KEMRI). This study was nested in a community-based survey of recently pregnant women in a rural area of western Kenya. A random, cross-sectional sample of 405 women with unknown HIV status was selected from a comprehensive list (n=~8000) of female residents of the KEMRI-Centers for Disease Control and Prevention (CDC) Health and Demographic Surveillance System (KEMRI-CDC HDSS) who were pregnant and delivered within the previous year. All participants were sampled from within the Health and Demographic Surveillance Area (HDSA), a rural region Northeast of Lake Victoria (−0.220 S to 0.230 N latitude, 34.530 W to 34.280 E longitude) that encompasses 385 villages, with a population of approximately 220 000, and an estimated HIV prevalence among women that exceeds 10% [20].
Inclusion criteria were as follows: maternal age of 14 years and older at enrollment, resided in the HDSA catchment area at time of enrollment, delivered a baby within the year prior to the 2011 stigma survey, and willing to give written, informed consent. Participants were asked a series of questions regarding their knowledge and attitudes about HIV. Women were also asked to self-report their HIV status. Latitude and longitude coordinates of participants’ residences were obtained with GPS devices and only individuals with nonmissing GPS coordinates were included in the analysis.
Survey
We categorized responses to a series of questions for each type of stigma into a binary variable to capture whether an individual reported any indicator of stigma (i.e. those who answered ‘agree’ to any of the following questions), or whether an individual did not report any indicator of stigma (i.e. those who answered ‘disagree’ to all of the following four statements), following results from a previously validated HIV stigma survey from sub-Saharan Africa [21]:
- Externalized stigma:
- HIV is a punishment from God
- HIV/AIDS is a punishment for bad behavior
- Women prostitutes spread HIV in the community
- People with HIV are promiscuous
- Internalized stigma:
- I would be ashamed if I were infected with HIV
- I would be ashamed if a person in my family had HIV/AIDS
- People with HIV should be ashamed of themselves
Exploratory spatial data analysis
The residential locations of each of the 373 respondents who provided GPS coordinates were visualized in a geographic information system (GIS) using Arc View 10.0 [22]. Exploratory spatial data analysis (ESDA) methods were used to explore the spatial distribution of both internalized and externalized HIV stigma across the study region. All spatial data analyses were done in the R statistical package version 3.0.2 [23], using the Spatial and Space-Time Point Pattern Analysis Functions (splancs) [24].
The scale(s) of spatial clustering of individuals reporting any stigma relative to individuals reporting no stigma across the study area was assessed using a second-order Ripley’s K-function for case-control data [25]. We first define the K-function for a single set of (unlabeled) points:
where λ is the expected number of points per unit area in a study region; dij is the distance between each pair of residential locations i and j; n is the total number of residential locations; and I(dij ≤ d) is the indicator function, where I(dij ≤ d) = 0 if dij > d and 1 if dij ≤ d. The K-function may be used to assess clustering with respect to a set of points, in particular, to assess whether the distances are consistent with complete spatial randomness (CSR). CSR is of little interest for our purposes, as residential locations are not distributed randomly in space. Out interest, instead, lies in determining the extent of clustering among individuals reporting stigma, as compared to those not reporting stigma. We let K1(d) and K0(d) represent the K-functions for reporting stigma versus not, respectively. Under the hypothesis of no spatial clustering, the locations of individuals reporting stigma versus not are independent random samples from the underlying population at risk, so that K1(d) = K0(d). The difference between K-functions, D(d) = K1(d) − K0(d), is thus a measure of additional clustering among the individuals reporting stigma relative to that among individuals not reporting stigma. We plotted D(d) against d to assess the degree of clustering of each stigma indicator with distance between locations for both individuals reporting stigma and individuals not reporting stigma, and simulated upper and lower 99% bounds, using Monte-Carlo simulations of random labeling of stigma-present and stigma-absent points in our data. Significant clustering occurs in the case (noncase) when the curve D(d) falls above (below) the 99% upper (lower) bounds. For example, if individuals reporting stigma are more spatially clustered relative to individuals not reporting stigma at certain distances, then the difference in K-functions between those groups would be significantly positive at those distances.
Spatial regression was performed to identify clustering of individuals reporting stigma (those who were found to harbor some form of HIV stigma) relative to that of individuals reporting no stigma (those who were found to harbor no form of HIV stigma), adjusting for potential confounding by location of individual-level variables that might account for any observed spatial pattern of stigma. These variables include age, occupation, marital status, education, self-reported HIV status, whether or not the individual knew someone else with HIV, and the number of televisions, cellular phones, and radios in the household. Income was left out of the adjusted model due to large amounts of missing data. Maps of adjusted odds were produced using a locally weighted regression smoother in a generalized additive model (GAM) framework for case-control data [26] using a logistic link function and a nonparametric component for the residual spatial surface:
where P is the probability of reporting any stigma versus reporting no stigma; x1 is the linear combination of demographic variables (age, occupation, marital status, education); x2 is the linear combination of technology variables (number of televisions, phones, and radios); x3 is the linear combination of HIV variables (self-reported HIV status and whether or not the individual knows someone living with HIV); and S is the spatial smoothing term of the log odds of reporting any stigma relative to reporting no stigma over the geographic extent of the study area. We adjusted for individual-level factors in the model to estimate the residual spatial surface and test whether it was significantly different from a flat surface. Individuals who had missing HIV status (n = 39) were automatically excluded from the model. We then plotted the ‘residual’ surface to explore the spatial clustering of individuals reporting stigma relative to individuals not reporting stigma beyond that explained by individual-level covariates.
Results
Among the 405 participants surveyed, 29 had missing geographic coordinates and were dropped from the analysis. Participants were sampled over a 20 km by 13 km region in the district of Gem, encompassing 11 Kenyan sublocations. Among the 376 with nonmissing lat/lon data, the median age was 25 years [interquartile range (IQR) 22–30 years]. Of the 337 who reported an HIV status, 41 (12%) were HIV-positive by self-report. Most of the respondents (77%) reported not knowing someone living with HIV.
Two hundred and five (54.5%) of those surveyed reported some indication of internalized stigma and 336 (89.4%) reported some indication of externalized stigma (Table 1). Individuals who reported any of the internalized stigma indicators tended to have worse socio-demographic status than those who reported none, though the differences were not significant. Specifically, those reporting internalized stigma trended towards being less likely to have completed primary school or higher level of education (41 versus 51%; P=0.06) and more likely to be in the lowest income category (36 versus 27%; P=0.06). There was insufficient evidence of differences in self-reported HIV status or whether or not an individual knew someone else with HIV between those reporting internalized stigma versus those not reporting internalized stigma.
Table 1.
Characteristics of individuals reporting versus not reporting internalized and externalized stigma indicators.
| Internalized stigma (shame) | Externalized stigma (blame) | |||||||
|---|---|---|---|---|---|---|---|---|
| No reported stigma indicator [n=205 (54.5%)] |
≥ 1 reported stigma indicator [n=171 (45.5%)] |
No reported stigma indicator [n=40 (10.6%)] |
≥ 1 reported stigma indicator [n=336 (89.4%)] |
|||||
| (number/ median) |
(percentage/ IQR) |
(number/ median) |
(percentage/ IQR) |
(number/ median) |
(percentage/ IQR) |
(number/ median) |
(percentage/ IQR) |
|
| Age (median/IQR) | 26 (22–31) | 24 (22–30) | 25 (23–30) | 25 (22–30) | ||||
| Monthly household income (KSH) | ||||||||
| 0–2000 | 54 | (26.6) | 60 | (35.5) | 10 | (25.6) | 104 | (31.2) |
| 2001–5000 | 26 | (12.8) | 27 | (16.0) | 4 | (10.3) | 49 | (14.7) |
| 5001–10 000 | 8 | (3.9) | 5 | (3.0) | 3 | (7.7) | 10 | (3.0) |
| 10 0001–20 000 | 0 | (0.0) | 3 | (1.8) | 0 | (0.0) | 3 | (0.9) |
| 21 000–30 000 | 1 | (0.5) | 0 | (0.0) | 0 | (0.0) | 1 | (0.3) |
| Do not know | 114 | (56.2) | 74 | (43.8) | 22 | (56.4) | 166 | (49.8) |
| Highest level of education* | ||||||||
| None | 7 | (3.4) | 3 | (1.8) | 0 | (0.0) | 10 | (3.0) |
| Some primary | 93 | (45.6) | 97 | (57.1) | 16 | (40.0) | 174 | (52.1) |
| Completed primary | 81 | (39.7) | 47 | (27.6) | 14 | (35.0) | 114 | (34.1) |
| Some secondary | 16 | (7.8) | 17 | (10.0) | 6 | (15.0) | 27 | (8.1) |
| Completed secondary | 6 | (2.9) | 4 | (2.4) | 4 | (10.0) | 6 | (1.8) |
| College | 1 | (0.5) | 2 | (1.2) | 0 | (0.0) | 3 | (0.9) |
| Occupation | ||||||||
| Housewife | 89 | (43.6) | 58 | (33.9) | 18 | (45.0) | 129 | (38.5) |
| Salaried job | 4 | (2.0) | 2 | (1.2) | 2 | (5.0) | 4 | (1.2) |
| Self-employed/small business | 79 | (38.7) | 75 | (43.9) | 14 | (35.0) | 140 | (41.8) |
| Unemployed | 32 | (15.7) | 36 | (21.1) | 6 | (15.0) | 62 | (18.5) |
| Marital status | ||||||||
| Married (monogamous) | 160 | (78.0) | 137 | (80.1) | 35 | (87.5) | 262 | (78.0) |
| Married (polygamous) | 31 | (15.1) | 22 | (12.9) | 3 | (7.5) | 50 | (14.9) |
| Single | 9 | (4.4) | 7 | (4.1) | 2 | (5.0) | 14 | (4.2) |
| Widowed | 5 | (2.4) | 5 | (2.9) | 0 | (0.0) | 10 | (3.0) |
| Number of mobile phones in household* | ||||||||
| None | 56 | (27.5) | 51 | (29.8) | 6 | (15.0) | 101 | (30.2) |
| 1 | 91 | (44.6) | 85 | (49.7) | 17 | (42.5) | 159 | (47.5) |
| 2+ | 57 | (27.9) | 35 | (20.5) | 17 | (42.5) | 75 | (22.4) |
| Self-reported HIV status | ||||||||
| HIV-positive | 22 | (11.6) | 19 | (12.8) | 6 | (15.8) | 35 | (11.7) |
| Knows someone with HIV | ||||||||
| Answered ‘yes’ | 53 | (25.9) | 32 | (18.8) | 6 | (15.0) | 79 | (23.6) |
All percentages are calculated out of nonmissing responses for each variable.
P-value=0.01 by test for trend for externalized stigma.
Individuals who reported externalized stigma were comparable to those who reported no externalized stigma with regard to age. Individuals reporting externalized stigma had worse socio-demographic status than individuals not reporting externalized stigma; specifically they had lower levels of education (P=0.01) and had fewer mobile phones in the household (P=0.01). There was insufficient evidence of differences in self-reported HIV status or whether or not an individual knew someone else with HIV between those reporting externalized stigma versus those not reporting externalized stigma. We found a strong association between reporting internalized stigma and reporting externalized stigma [odds ratio (OR) 3.77, 95% confidence interval (CI) 1.63–9.72, P < 0.001). This association depended on HIV status, with a strong positive association among those who reported negative HIV status (OR 4.78, 95% CI 1.74–16.32, P < 0.001) and no evidence of an association among those who reported positive HIV status (OR 0.84, 95% CI 0.10–7.22, P=0.85).
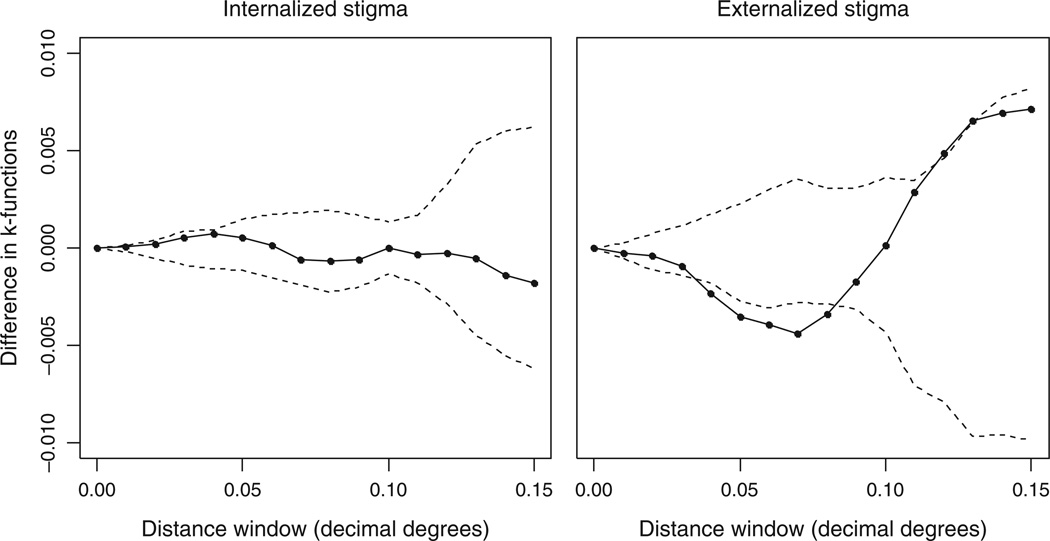
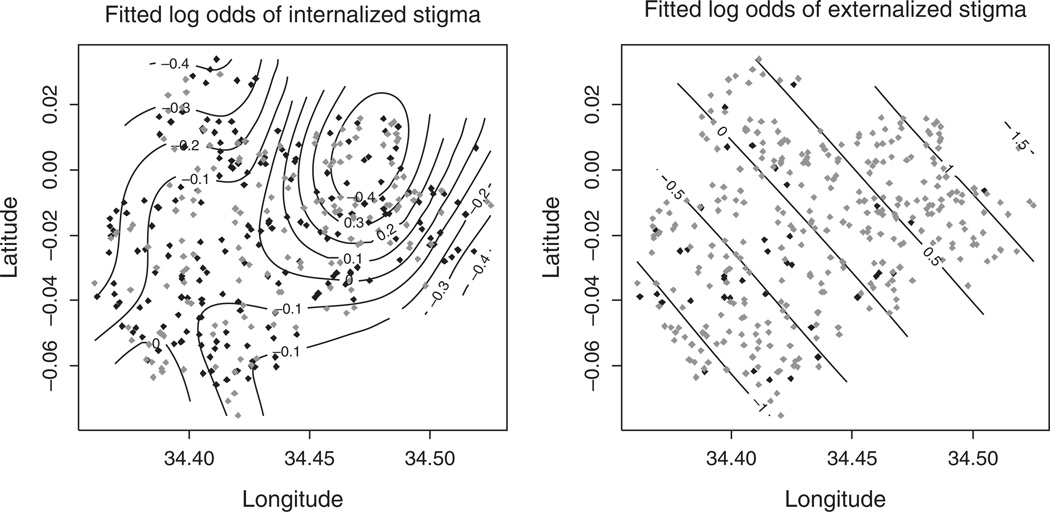
The geographic distribution comparing individuals reporting any stigma to those reporting no stigma, when mapped, showed some evidence of different spatial patterns by stigma type. Results of the K-function indicate significant clustering of those not reporting stigma relative to those reporting any stigma for externalized stigma but not for internalized stigma (Fig. 1). For externalized stigma, a statistically significant difference in clustering between stigma present and stigma absent was observed at a radius of 7 ± 1 km, as can be seen where the K-function is outside of the 99% bounds (Fig. 1). In other words, individuals reporting no indicator of externalized stigma were more spatially clustered relative to those who reported any indicator. The residual spatial surface, derived from the GAM, indicated an association between location and the odds of reporting versus not reporting externalized stigma, which remained even after adjusting for individual level factors that might explain the differences in clustering (P=0.01) (Fig. 2). With respect to the internalized stigma indicator, there was no significant spatial pattern at any distance as measured by the K-function. After adjusting for individual-level covariates, the spatial surface showed no deviation from a flat surface (P=0.70) (Fig. 2). Each of the four survey questions, which reflect a different construct of the externalized stigma indicator, were similar in their spatial structure to the composite indicator used in the primary analysis, though none of the spatial trends were pronounced enough to show significance.
Fig. 1. Differences in K-functions of stigma present and stigma absent for both internalized and externalized stigma with distance [in decimal degrees, with 99% upper and lower bounds (dashed lines) derived from permuting stigma present and stigma absent under complete spatial randomness].
Significant clustering of residential locations of those who did not harbor stigma relative to those who did is indicated where the solid line is negative and outside of the lower bound.
Fig. 2. Fitted log odds surface from the generalized additive model of reporting any stigma (grey points) relative to reporting no stigma (black points) for internalized (left) and externalized (right) stigma, both adjusted for age, education, occupation, marital status, self-reported HIV status, knowing someone with HIV, number of televisions, number of cellular phones, and number of radios.
In contrast to the internalized indicator which is flat (P=0.70), there is a significant deviation from a flat surface for externalized stigma (P=0.01), with a gradient indicating lower rates of externalized stigma than expected in the southwest portion of the study area.
Discussion
Despite the growing recognition that HIV stigma plays an important role in fueling the HIV/AIDS pandemic, there is little knowledge on what drives stigma at the community level. In this geographic analysis of HIV stigma among women who recently gave birth in rural Kenya, we found that respondents overwhelmingly hold a sense of externalized stigma (blame) towards others with HIV, whereas less than half would experience internalized stigma (shame) if they were HIV-infected (this includes those who self-reported HIV). This finding is consistent with the results of an USAID report that tested the validity of the questionnaire used here in another context [4].
We found distinct spatial patterns in respondents’ attitudes and values towards others with HIV for each type of HIV stigma, after controlling for individual-level factors. The clustering in the spatial distribution of individuals reporting any indicator of internalized stigma showed no evidence of being different from the clustering of the spatial distribution of those with no indicator of internalized stigma. On the contrary, the spatial distribution of those reporting any indicator of externalized stigma relative to none showed a distinct spatial pattern, with higher-than-expected rates of stigma in the north-east and lower than expected rates in the south-west of the study area. Although we originally hypothesized that rates of externalized stigma would be highly clustered at small spatial scales – that of the size of neighborhoods – we found more of a large-scale geographic trend across the study area. The spatial patterns for each of the four survey questions used to generate the composite externalized stigma indicator were similar to that of the composite indicator. None of the individual components alone appears to drive the overall spatial effect.
To our knowledge, this is the first population-based study that takes a geographic approach to explore the distribution and spatial structure of HIV stigma. Spatial analysis in health research employs unique statistical approaches that can be used to quantitatively explore how individuals are distributed geographically with respect to important epidemiological attributes [27]. The geographic areas where individuals are more likely than not to share similar attributes may point to underlying place-based or cultural phenomena that drive those patterns.
Results of this analysis suggest that the two forms of HIV stigma explored here do not follow similar geographic distributions in this population. Whereas further investigation will be needed to confirm this observation in other populations and at different spatial scales, the differences in geographic distributions of each form of HIV stigma have certain implications for community-level interventions. Though we were unable to assess specific community-level drivers of HIV stigma, based on our findings, we hypothesize that externalized stigma may be influenced more by dominant cultural beliefs disseminated within communities (i.e. via messages from churches, health facilities, influential leaders, as well as any ethnic differences by geographic region). Internalized stigma, on the contrary, may be the result of individual-level characteristics outside the domain of community influence. Religious institutions are thought to heavily influence community-level attitudes towards PLWHA [5], though no formal evaluation of the influence of religious institutions on HIV stigma has been done. As this analysis was exploratory in nature, the spatial structure and scale of clustering observed for the blame indicator should be confirmed in further study through hypothesis-driven analysis. Further study could also explore whether lower levels of externalized stigma tend to be concentrated in communities known to promote tolerance towards PLWHA and/or in geographic areas where stigma-reduction interventions are currently being implemented at the community level.
Finally, it will be important for further study to consider the geographic variability in HIV prevalence when investigating what drives differences in HIV stigma across geographic regions. Though we lacked statistical power to demonstrate an effect, we observed that individuals who reported externalized stigma were slightly more likely to know someone with HIV than those not reporting stigma. Similarly, the prevalence of HIV among our study participants also tended to be greater in the areas with higher-than-expected rates of HIV stigma. In small areas like our study region, however, there may be insufficient variability in HIV prevalence to observe its effect on HIV stigma.
There are several limitations to using the methods presented here. First, factors like social desirability may have affected participants’ self-reports on the stigma scales, and as such, the standardized questionnaires used in the study may not capture an individual’s actual attitudes and values towards PLWHA. Second, this study only measures HIV stigma among women who recently gave birth, ignoring other demographic groups, namely men and women of nonchildbearing age, who may play an important role in perpetuating (or working against) stigmatizing attitudes. Finally, some of the questions may be considered ambiguous as to what underlying cause of the stigmatizing behavior is being measured, or whether answering ‘no’ to a question in the stigma survey necessarily indicates the absence of stigma [5]. We attempted to overcome this limitation by conservatively categorizing individuals into the ‘no stigma’ category only if they answered ‘no’ to every question, following previous study [4]. In light of the difficulty in accurately measuring levels of stigma in individuals [5], which has resulted in a limited literature on the causes of stigma [7], it will be important to refine the stigma measurement tools we already have and to test those tools in a variety of settings.
For PLWHA, the benefits of seeking HIV services are often weighed against the social costs of accessing care [28]. This can be especially true for women living with HIV, who are both highly vulnerable to being stigmatized and are more at risk to the negative sequelae associated with stigma. The extent to which communities reduce levels of stigma while also increasing the availability and accessibility of HIV services may improve the effectiveness of HIV programs in treating PLWHA and preventing new infections. In order to do this, more research is needed to identify attributes of communities that promote high (and low) levels of stigma (and tolerance) towards PLWHA, and to turn those lessons into effective community-level interventions.
In conclusion, we observed spatial heterogeneity in the reporting of externalized (blame) HIV stigma among recently pregnant women in a rural area of Kenya. This result begs further study into what community-level risk factors might drive high versus low rates of externalized stigma in a population. This information will be crucial to inform community-wide stigma-reduction campaigns.
Acknowledgements
The material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program (Grant No. DGE-0718124), ‘A Kenya Free of AIDS Project 2’ (Grant No. HD056799), the University of Washington Center for AIDS Research (Grant No. AI027757), and the NIH K24 Grant: ‘Pediatric HIV-1 in Africa: Pathogenesis and Management’ (Grant No. HD054314–06). The authors would also like to thank the Kenya Medical Research Institute (KEMRI) research community and the KEMRI director for their support.
Conflicts of interest
The findings and conclusions in this report are those of the authors, and do not necessarily represent the views of their institutions, including the Centers for Diseases Control and Prevention and Kenya Medical Research Institute.
Footnotes
A.A., G.J-S., and P.K. contributed to study concept and design. P.K., J.K., G.O., K.L., L.M., J.O., M.O., and F.O. contributed to acquisition of data. A.A. contributed to drafting of the manuscript. J.W., G.J-S., F.O., and D.R. contributed to critical revision of the manuscript for important intellectual content. A.A. and J.W. contributed to statistical analysis. A.A., P.K., G.J-S., D.R., and J.W. contributed to analysis and interpretation of data. G.J-S., P.K., J.K., G.O., K.L., L.M., and J.O. obtained funding.
Disclaimer: Published with the approval of the Director, Kenya Medical Research Institute.
References
- 1.Theilgaard Z, Katzenstein T, Chiduo M, Pahl C, Bygbjerg I, Gerstoft J, et al. Addressing the fear and consequences of stigmatization: a necessary step towards making HAART accessible to women in Tanzania: a qualitative study. AIDS Res Ther. 2011;8:28. doi: 10.1186/1742-6405-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steward WT, Bharat S, Ramakrishna J, Heylen E, Ekstrand ML. Stigma is associated with delays in seeking care among HIV-infected people in India. J Int Assoc Phys AIDS Care. 2012 doi: 10.1177/1545109711432315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Soc Sci Med. 2003;57:13–24. doi: 10.1016/s0277-9536(02)00304-0. [DOI] [PubMed] [Google Scholar]
- 4.Nyblade LCea. Working report measuring HIV stigma: results of a field test in Tanzania. USAID; 2005. [Google Scholar]
- 5.Nyblade LC. Measuring HIV stigma: existing knowledge and gaps. Psychol Health Med. 2006;11:335–345. doi: 10.1080/13548500600595178. [DOI] [PubMed] [Google Scholar]
- 6.Mbonye AK, Hansen KS, Wamono F, Magnussen P. Barriers to prevention of mother-to-child transmission of HIV services in Uganda. J Biosoc Sci. 2010;42:271–283. doi: 10.1017/S002193200999040X. [DOI] [PubMed] [Google Scholar]
- 7.Mahajan AP, Sayles JN, Patel VA, Remien RH, Sawires SR, Ortiz DJ, et al. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS. 2008;22(Suppl 2):S67–S79. doi: 10.1097/01.aids.0000327438.13291.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duff P, Kipp W, Wild TC, Rubaale T, Okech-Ojony J. Barriers to accessing highly active antiretroviral therapy by HIV-positive women attending an antenatal clinic in a regional hospital in western Uganda. J Int AIDS Soc. 2010;13:37. doi: 10.1186/1758-2652-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell C, Nair Y, Maimane S, Nicholson J. ’Dying twice’: a multilevel model of the roots of AIDS stigma in two South African communities. J Health Psychol. 2007;12:403–416. doi: 10.1177/1359105307076229. [DOI] [PubMed] [Google Scholar]
- 10.Corrigan PW, Watson AC. The paradox of self-stigma and mental illness. Clin Psychol Sci Pract. 2002;9:35–53. [Google Scholar]
- 11.Preston DB, D’Augelli AR, Kassab CD, Starks MT. The relationship of stigma to the sexual risk behavior of rural men who have sex with men. AIDS Educ Prev. 2007;19:218–230. doi: 10.1521/aeap.2007.19.3.218. [DOI] [PubMed] [Google Scholar]
- 12.Rao D, Feldman BJ, Fredericksen RJ, Crane PK, Simoni JM, Kitahata MM, et al. A structural equation model of HIV-related stigma, depressive symptoms, and medication adherence. AIDS Behav. 2012;16:711–716. doi: 10.1007/s10461-011-9915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turan JM, Miller S, Bukusi EA, Sande J, Cohen CR. HIV/AIDS and maternity care in Kenya: how fears of stigma and discrimination affect uptake and provision of labor and delivery services. AIDS Care. 2008;20:938–945. doi: 10.1080/09540120701767224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinuthia J, Kiarie JN, Farquhar C, Richardson BA, Nduati R, Mbori-Ngacha D, et al. Uptake of prevention of mother to child transmission interventions in Kenya: health systems are more influential than stigma. J Int AIDS Soc. 2011;14:61. doi: 10.1186/1758-2652-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report. 2010
- 16.Birungi H, Obare F, van der Kwaak A, Namwebya JH. Maternal healthcare utilization among HIV-positive female adolescents in Kenya. Int Perspect Sex Reprod Health. 2011;37:143–149. doi: 10.1363/3714311. [DOI] [PubMed] [Google Scholar]
- 17.Hong KT, Van Anh NT, Ogden J. ‘Because this is the disease of the century’. Understanding HIV and AIDS-related stigma and discrimination. ICRW; 2004. [Google Scholar]
- 18.Kalichman SC, Simbayi LC, Cain D, Jooste S, Skinner D, Cherry C. Generalizing a model of health behaviour change and AIDS stigma for use with sexually transmitted infection clinic patients in Cape Town. South Africa AIDS Care. 2006;18:178–182. doi: 10.1080/09540120500456292. [DOI] [PubMed] [Google Scholar]
- 19.Hayes RA, Han CVU, MedeffiOs T, Dubuque E. Stigma directed toward chronic illness is resistant to change through education and exposure. Psychol Rep. 2002;90:1161–1173. doi: 10.2466/pr0.2002.90.3c.1161. [DOI] [PubMed] [Google Scholar]
- 20.Odhiambo FO, Laserson KF, Sewe M, Hamel MJ, Feikin DR, Adazu K, et al. Profile: the KEMRI/CDC Health and Demographic Surveillance System: western Kenya. Int J Epidemiol. 2012;41:977–987. doi: 10.1093/ije/dys108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.USAID. Working report measuring HIV stigma: results of a field test in Tanzania. 2005 [Google Scholar]
- 22.ESRI. ArcGIS. 10.0 ed. Redlands, California: 2010. [Google Scholar]
- 23.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 24.Rowlingson B, Diggle PJ. Spatial and space-time point pattern analysis functions. 2013 [Google Scholar]
- 25.Chetwynd AG, Diggle PJ, Marshall A, Parslow R. Investigation of spatial clustering from individually matched case-control studies. Biostatistics. 2001;2:277–293. doi: 10.1093/biostatistics/2.3.277. [DOI] [PubMed] [Google Scholar]
- 26.Webster T, Vieira V, Weinberg J, Aschengrau A. Method for mapping population-based case-control studies: an application using generalized additive models. Int J Health Geographics. 2006;5:26. doi: 10.1186/1476-072X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auchincloss AH, Gebreab SY, Mair C, Diez Roux AV. A review of spatial methods in epidemiology, 2000–2010. Annu Rev Public Health. 2012;33:107–122. doi: 10.1146/annurev-publhealth-031811-124655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chivonivoni C, Ehlers VJ, Roos JH. Mothers’ attitudes towards using services preventing mother-to-child HIV/AIDS transmission in Zimbabwe: an interview survey. Int J Nurs Stud. 2008;45:1618–1624. doi: 10.1016/j.ijnurstu.2008.04.002. [DOI] [PubMed] [Google Scholar]