Abstract
Type 2 diabetes involves aberrant misfolding of human islet amyloid polypeptide (h-IAPP) and resultant pancreatic amyloid deposits. Curcumin, a biphenolic small molecule, has offered potential benefits in other protein misfolding diseases, such as Alzheimer’s disease. Our aim was to investigate whether curcumin alters h-IAPP misfolding and protects from cellular toxicity at physiologically relevant concentrations. The effect of curcumin on h-IAPP misfolding in vitro was investigated by electron paramagnetic resonance spectroscopy, ThT fluorescence and electron microscopy. Our in vitro studies revealed that curcumin significantly reduces h-IAPP fibril formation and aggregates formed in the presence of curcumin display alternative morphology and structure. We then tested a potential protective effect of curcumin against h-IAPP toxicity on β-cells. Micromolar concentrations of curcumin partially protect INS cells from exogenous IAPP toxicity. This protective effect, however, is limited to a narrow concentration range, as curcumin becomes cytotoxic at micromolar concentrations. In different models of endogenous over-expression of h-IAPP (INS cells and h-IAPP transgenic rat islets), curcumin failed to protect β-cells from h-IAPP-induced apoptosis. While curcumin has the ability to inhibit amyloid formation, the present data suggest that, without further modification, it is unlikely to be therapeutically useful in protection of β-cells in type 2 diabetes.
Keywords: h-IAPP, curcumin, type 2 diabetes mellitus, amyloid, protein misfolding, amyloid inhibitor, β-cell
Introduction
Several degenerative diseases including Alzheimer’s disease, Parkinson’s disease, type 2 diabetes mellitus (T2DM) and spongiform encephalopathies are characterised by gradual cellular attrition associated with aggregation of locally expressed misfolded proteins [1]. As a result of the apparently shared proteotoxicity as a consequence of protein misfolding, these diseases have been collectively referred to as protein misfolding diseases. The islet of Langerhans in T2DM is characterised by islet amyloid derived from human islet amyloid polypeptide (hIAPP), a 37 amino acid peptide co-expressed and secreted with insulin by pancreatic β-cells [2,3]. The islet in T2DM is also characterised by a deficit in β-cells and increased β-cell apoptosis that are due at least in part to endoplasmic reticulum stress [4–7].
The islet and metabolic phenotype of T2DM can be recapitulated in rodents by over-expressing h-IAPP in β-cells. Rodent IAPP (r-IAPP) differs from h-IAPP in that it is not amyloidogenic. This is thought to be due to the proline substitutions in the hydrophobic region between residues 20 and 29 [8]. Moreover, the over-expression of rodent IAPP does not lead to diabetes. These data imply that β-cell toxicity due to high expression rates of h-IAPP is dependent on the aggregation property of h-IAPP, although this is not necessarily in the form of amyloid fibrils. Accumulating evidence suggests that the toxic forms of amyloidogenic proteins are small membrane permeant oligomers that may form intracellularly [9]. Intriguingly, an antibody raised against toxic oligomers of Alzheimer’s b protein, also binds to toxic oligomers of other amyloidogenic proteins, including IAPP, implying that the toxic oligomers of amyloidogenic proteins share a close structural relationship [10].
If cellular dysfunction and toxicity in protein misfolding diseases are mediated by oligomers, then by implication, inhibition of the formation of these oligomers might beneficially modify the disease progression. A candidate molecule to achieve such benefit is curcumin (diferuloylmethane), a biphenolic small molecule and the main constituent of the rhizome C. longa (turmeric). Curcumin has been reported to have several potentially beneficial properties including anti-inflammatory, anti-oxidant and anti-HIV effects [11–13]. Recent studies have also shown that curcumin can inhibit formation of amyloid fibrils from monomers of Abeta [14,15], α-synuclein [16] and prion [17].
While these studies have shown the anti-amyloidogenic effects of curcumin, it is still poorly understood how curcumin interacts with amyloid proteins and by what mechanism it inhibits misfolding. Additionally, it is important to know at what physiological concentrations curcumin accomplishes this effect, and whether it is protective against cytotoxicity. Moreover, since there is still some uncertainty whether the proteotoxicity mediated by misfolded proteins is mediated intraor extracellularly, it is important to examine the ability of curcumin to suppress cytoxicity of misfolded proteins expressed in, or applied to, cells.
This prompted us to first investigate the molecular interaction of curcumin with h-IAPP. In order to understand whether curcumin inhibits or modulates the misfolding process of h-IAPP in vitro, we employed a combination of biophysical tools, including site-directed spin labelling with electron paramagnetic resonance (EPR), electron microscopy and Thioflavin T (ThT) fluorescence. Having established that curcumin modulates IAPP misfolding in vitro at micromolar concentrations, we then tested the efficacy of curcumin to protect islet cells from h-IAPP cytotoxicity under those conditions. This was undertaken both in a β-cell line (INS 832/13 cells) as well as in isolated islets from rats over-expressing h-IAPP.
Methods
Chemicals and peptides
Hexafluoroisopropanol (HFIP) and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich. Curcumin was obtained in powdered form from Sigma-Aldrich. Synthetic wild type human IAPP (lot no. E-0509) was purchased from Polypeptide Laboratories (Wolfenbuettel, Germany). The spin label, 1-oxyl-2,2,5,5-tetramethyl-Δ3-pyrroline-3-methyl methanethiosulfonate (MTSL), was obtained from Toronto Research Chemicals (Toronto, Canada). Single cysteine mutants of full-length IAPP were purchased from Biomer Technology (Pleasanton, CA). The native cysteine residues at position 2 and 7 were substituted with an alanine to create a C2A C7A double-mutant as described previously [18].
Spin labelling and EPR
For spin labelling, single cysteine-containing peptides were reacted with 3-fold molar excess of MTSL for 1 h at room temperature. Unreacted MTSL was removed using a Toyopearl cation exchange column. The spin-labelled peptide was then desalted using a C18 reverse phase SpinColumn from Harvard Apparatus (Holliston, Massachusetts) and eluted with 100% HFIP. Peptide concentrations were calculated by UV absorbance at 280 nm in 6 M guanidine HCl using an extinction coefficient of 1400 M−1 cm−1. Labelled peptides were stored at −80°C until use. Stock solutions of spin-labelled IAPP and wild type IAPP were lyophilised. For preparation of stock solutions the lyophilised peptides were reconstituted with deionised water containing 0.5% acetic acid. For fibril formation, aliquots of stock IAPP was added to 10 mM phosphate buffer with 100 mM NaCl (pH 7.4) for a total volume of 100 ml and 100 mM concentration. Curcumin was freshly dissolved in DMSO prior to use. The samples were incubated with or without curcumin (1–100 mM) at room temperature for 4 days to allow fibril formation. To test the effect of curcumin on preformed fibrils, 16R1 labelled IAPP was prepared to form fibrils for 3–7 days at a concentration of 100 mM. Curcumin was added to the preformed fibrils, allowed to incubate for either 1 h or 24 h. The samples were then harvested for EPR spectroscopy.
EPR spectroscopy
For EPR spectroscopy, samples were pipetted into a glass capillary (0.6 mm inner diameter, 0.84 outer diameter, VitroCom, Mountain Lakes, NJ) sealed at one end. The sample was centrifuged and all supernatant was removed to eliminate smaller aggregates and monomeric protein. EPR spectra of fibrils were recorded on a Bruker (Billerica, MA) EMX spectrometer with an HS resonator at 12 mW incident microwave power with a magnetic field scan range of 150 Gauss.
Electron microscopy
To examine the effect of curcumin on fibril growth, 10 ml of samples were adsorbed onto carbon and formvar-coated copper grids and negatively stained with 2% (w/v) uranyl acetate solution for 5 min. The stained grids were examined and photographed using a JEOL JEM-1400 electron microscope at 100 kV.
Thioflavin T assay
To test the effect of curcumin on ThT fluorescence intensity of IAPP fibrils, 20 μl aliquots of wild type IAPP peptide solutions in 0.5% acetic acid were mixed with 500 μl of 10 mM phosphate buffer (pH 7.4) to yield 50 mM IAPP concentration in the presence and absence of 10 mM and 50 mM curcumin concentrations. To each fibrilisation reaction, 25 mM ThT was added and emission intensities were measured at 482 nm with excitation at 450 nm for 16 h. Measurements were performed at room temperature with excitation and emission slit widths of 1 and 10 nm, respectively. To assess the effect of curcumin on preformed IAPP fibrils, h-IAPP fibrils were allowed to undergo complete fibrilisation for 4 days in 10 mM phosphate buffer (pH 7.4). Preformed h-IAPP fibrils were incubated with ThT for 30 min, curcumin was added at 0, 1, 10 or 100 mM and fluorescence was monitored for 8 h. All fluorescence measurements were carried out using a Jasco FP-6500 spectrofluorometer.
Cell culture
Rat insulinoma cell line INS 832/13 was kindly provided by Dr. C. Newgard, Durham, NC [19]. INS cells were grown in RPMI 1640 medium supplemented with 10 mM HEPES, 1 mM sodium pyruvate, 100 IU/ml penicillin and 100 mg/ml streptomycin (Invitrogen, Carlsbad, CA), 10% heat-inactivated FBS (Gemini, West Sacramento, CA), 50 μM β-mercaptoethanol (Sigma, St. Louis, MO), at 37°C in a humidified 5% CO2 atmosphere.
Adenovirus generation and transduction experiments
The complementary cDNA encoding the full-length human and rat preproIAPP were used as templates to generate the human and rat IAPP adenovirus. KpnI and XhoI or EcoRI and EcoRV restriction sites were, respectively, introduced in front of the ATG and after the stop codon of the human and rat cDNA using PCR. A 290 bp human prepro-IAPP PCR fragment was digested with KpnI and XhoI and a 300 bp rat preproIAPP PCR fragment was digested with EcoRI and EcoRV. The fragments were ligated into the pENTR2B vector (Invitrogen, CA) and the entry clones were subsequently subcloned into the pAd/cytomegalovirus/DEST adenovirus vector (Invitrogen). Recombinant adenovirus expressing human and rat preproIAPP (Ad h-IAPP and Ad r-IAPP, respectively) were produced and purified according to the manufacturer’s instructions (Invitrogen). For the transduction experiments, INS cells were plated on 96-well or 6-well tissue culture plates at a density of 2.5 × 104 or 1.0 × 106 cells/well, respectively, and cultured overnight. Cells were transduced with r-IAPP or h-IAPP adenoviruses at 400 moi for 48 h in complete RPMI medium. Two hours after cell transduction, curcumin or DMSO (vehicle) was added to cells at desired concentrations. At the end of the transduction, cells plated on 96-well plates were used to assess cell viability by MTT assay (see below) and cells plated on 6-well plates were washed in PBS and lysed in NP40 buffer (50 mM Tris-Hcl, pH 7.4, 250 mM NaCl, 1 mM EDTA, 0.5% NP40, 1 mM DTT and protease inhibitors (Sigma)).
h-IAPP preparation and toxicity assays
Lyophilised h-IAPP was dissolved in 0.5% acetic acid to prepare a 2 mM stock solution and vortexed for 30 s. To assess the effect of curcumin on cell death induced by exogenous h-IAPP, INS cells were seeded on a 96-well plate at 5 × 104 cells/well and cultured for 20 h. On the following day, the medium was replaced by DME medium (Irvine Scientific, Santa Ana, CA) containing 15 mM of freshly dissolved h-IAPP, vehicle or curcumin. After 20 h of incubation, cell viability was assessed.
MTT assay
The reduction of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) was used to assess cell viability. At the end of the incubation or transduction time, cell medium was changed to 1 mg/ml MTT (Sigma) containing medium according to the manufacturer’s instructions. Colorimetric measurements were performed 2 h after addition of the MTT reagent at 570 nm with an ELISA plate reader (Spectramax 250; Molecular Devices, Sunnyvale, CA). The background wavelength at 690 nm was subtracted from the 570 nm measurement.
Rat islet isolation
The generation of h-IAPP transgenic rats (HIP rats) has been described in detail previously [20]. Rats were bred and housed at the animal housing facility of the University of California Los Angeles. Animal studies were approved by the UCLA Animal Research Committee. Five-month old WT (n = 5) and HIP (n = 6) rats were euthanised using isofluorane. The bile duct was cannulated, and the pancreas was perfused with a collagenase solution (HBSS supplemented with 25 mM HEPES (Invitrogen), 0.23 mg/ml liberase (Roche, Penzberg, Germany), 0.1 mg/ml DNAse (Roche)). The pancreas was then removed, incubated for 20 min in collagenase solution at 37°C, and dispersed by shaking for 30 s. The suspension was transferred into 30 μl of ice cold HBSS. After 5 min of incubation on ice, ¾ of supernatant was removed by aspiration, and fresh ice-cold HBSS was added to the pellet. Islets were washed three times, then manually picked and incubated in RPMI 1640 medium supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin and 10% heat-inactivated FBS at 37°C in a humidified 5% CO2 atmosphere. Rat islets were cultured for 48 h in the absence or presence of curcumin and either lysed in NP40 buffer or used for propidium iodide (PI) staining.
Propidium iodide staining
Aliquots of 30 islets were incubated 48 h with or without curcumin (0.5, 1, 2 and 5 mM). Subsequently, islets were stained with 50μg/μl PI (Molecular Probes, Eugene, OR) for 30 min at 37° C. Islets were then washed in PBS and fixed in 4% paraformaldehyde for 30 min at room temperature. Islets were imaged using a Leica DM600 microscope (Leica Microsystems, Wetzlar, Germany), and images of 25 islets per condition were acquired using OpenLab software (Improvision). The fractional area of the islets positive for PI was digitally quantified using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD).
Western blot analysis
Proteins (25–40μg/lane) were separated on a 4–12% Bis-Tris NuPAGE gel and blotted onto a nitrocellulose membrane (Whatman, Germany). Membranes were blocked in TBS-Tween 20 (0.1%) – Blotting-Grade Blocker (5%) (Bio-Rad, Hercules, CA), probed overnight at 48C with pro and cleaved caspase 3, PARP, β actin and GAPDH antibodies (Cell Signalling Technology, Beverly, MA), followed by three washes and 1-h incubation with horseradish peroxidase-conjugated secondary antibodies (Zymed Laboratories, South San Francisco, CA). For IAPP western blot, membranes were blocked in PBS-TritonX100 (0.25%) – gelatin (1%), probed over-night with IAPP antibody, (25–37 aa; specific for both r-IAPP and h-IAPP, Peninsula Laboratories, San Carlos, CA; 1/1000 in PBS-Triton X100 (0.25%) – gelatin (1%)), followed by three washes and 1-h incubation with anti-rabbit horseradish peroxidase-conjugated secondary antibody (Zymed Laboratories, South San Francisco, CA) diluted in TBS-Tween 20 (0.1%) – Blotting-Grade Blocker (5%). Proteins were visualised by enhanced chemiluminescence (Millipore) and protein expression levels were quantified using Labworks software (UVP, Upland, CA).
Statistical analysis
Data are presented as the means ± SEM. Statistical analyses were carried out by ANOVA followed by Duncan’s post hoc test using Statistica (Statsoft, Tulsa, OK). A p value of 50.05 was taken as evidence of statistical significance.
Results
Curcumin alters h-IAPP misfolding
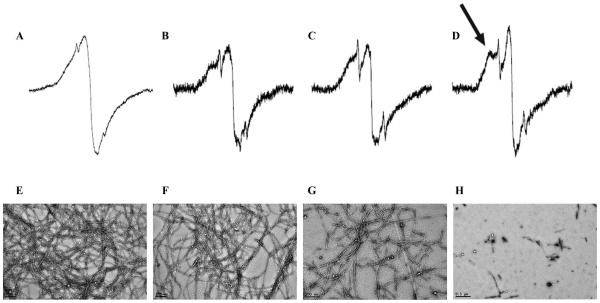
We sought to test whether curcumin can modulate h-IAPP misfolding in vitro using a combination of biophysical methods including EPR, electron micro-scopy (EM) and ThT fluorescence. The EPR experiments were based on the introduction of a single spin label into the peptide. Previous spin labelling studies on h-IAPP, Abeta, α-synuclein, tau and the human prion protein have shown that fibrils from these proteins give rise to a parallel, in-register structure in which same residues stack on top of each other [18,21–24]. As illustrated with the example of h-IAPP spin labelled at position 16, the stacking of the same residues from different molecules gave rise to strong spin-exchange (Figure 1A), as reflected by a predominantly single line EPR spectrum. In order to test whether curcumin can affect the misfolding and fibril formation of h-IAPP, spin-labelled h-IAPP was incubated with curcumin (1–100 mM) for 4 days and the EPR spectra of the resulting h-IAPP aggregates were recorded. The addition of 1 mM and 10 mM curcumin resulted in EPR spectra (Figure 1B,C, respectively) that showed subtle, but clearly detectable, changes as indicated by the formation of additional peaks in the EPR spectra. This spectral change, which was even more pronounced in the presence of 100 mM curcumin, (Figure 1D, black arrow), reflects a gradual loss of spin–spin interaction and indicates that curcumin alters the misfolding of h-IAPP in vitro.
Figure 1.
EPR spectra and EM images of spin-labelled h-IAPP in the presence and absence of curcumin. EPR spectra of 16R1 h-IAPP aggregates formed after incubation with 0 μM (A), 1 μM (B), 10 μM (C) and 100 μM (D) curcumin. All spectra were obtained at a scan width of 150 Gauss and are rescaled to the same amplitude. The arrow in panel (D) denotes a hyperfine component that arises from strong immobilisation and loss of spin exchange that is caused by curcumin-dependent alteration of h-IAPP misfolding. Representative spectra from four independent experiments are shown. The corresponding EM images of 16R1 h-IAPP incubated with 0 μM (E), 1 μM (F), 10 μM (G) and 100 μM (H) curcumin are also shown.
These findings were supported by EM analysis of the same samples (Figure 1E–H), illustrating that the addition of curcumin at high concentrations decreased fibril formation and altered the morphology of the aggregates. The most significant changes were observed at 100 mM curcumin concentration (Figure 1H), resulting in decreased fibril formation as well as a change to shorter and more dysmorphic structures. Thus, the EPR and EM data demonstrated that micromolar concentrations of curcumin have a strong effect on h-IAPP misfolding.
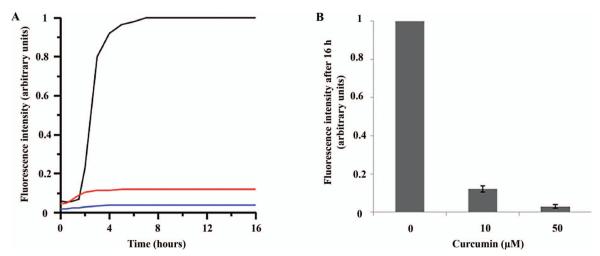
In addition, we also used ThT fluorescence to monitor the effect of curcumin on h-IAPP misfolding and fibril formation. This commonly used assay is based upon the fluorescence enhancement that occurs as ThT binds to amyloid fibrils, such as those formed by h-IAPP [25,26]. As expected from the aforementioned curcumin-dependent reduction of h-IAPP fibril formation, the ThT fluorescence of h-IAPP was strongly reduced in the presence of 10 μM and 50 μM curcumin (Figure 2). While these data are consistent with the EPR and EM data, it is important to note that ThT fluorescence, in contrast to EM and EPR, is an indirect readout that cannot readily distinguish between a reduction in fibril formation or competitive inhibition of ThT binding to the fibrils (see below).
Figure 2.
Thioflavin T assay for h-IAPP in the presence and absence of curcumin. h-IAPP was incubated with curcumin at 0 μM (black line), 10 μM (red line) and 50 μM (blue line) during fibril formation. A: Representative spectra for the given curcumin concentrations. B: Fluorescence intensity quantification (n = 6). Six separate measurements yielded a highly reproducible curcumin dependent loss in signal intensity. This is evidenced by the standard deviation for the final plateau values after 16 h, which were 0.02 and 0.01 (arbitrary units; equivalent to 2 and 1% of the control amplitude without curcumin) for 10 and 50 μM curcumin, respectively.
The effect of curcumin on preformed fibrils
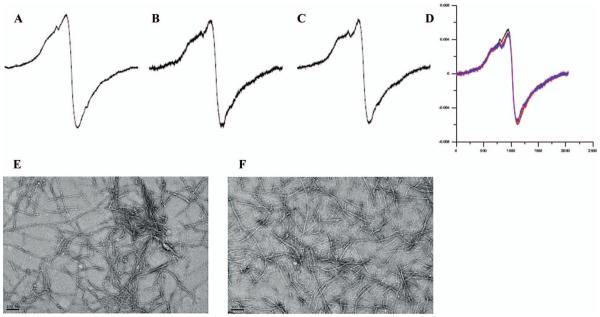
Having shown that curcumin had pronounced effects on h-IAPP misfolding when added during the misfolding process, we next tested whether curcumin might also be similarly effective at reversing fibril formation when added to preformed fibrils. In order to address this question, we again turned to the EPR based assay using IAPP spin labelled at position 16. As shown in Figure 3, the addition of curcumin to pre-formed, spin-labelled h-IAPP fibrils did not significantly change their EPR spectra after 1 h (Figure 3B) or 24 h (Figure 3C). This finding was further supported by negative staining EM, which revealed largely unchanged fibril morphology and density (Figure 3E,F). Thus, curcumin did not rapidly dissolve fibrils or significantly alter their structure within 24 h. The notion that disruption of fibril structure is slow at best is further supported by later time points, which show only spectral changes during a 2–4 week incubation period (data not shown).
Figure 3.
Curcumin does not change the fibrillar structure of preformed h-IAPP fibrils. A: h-IAPP control (B) h-IAPP incubated with 100 μM curcumin for 1 h (C) h-IAPP incubated with 100 μM curcumin for 24 h (D) overlay of h-IAPP fibrils (16R1) incubated with 100 μM curcumin for 24 h (red line) and compared to control fibrils (black line). Each spectrum was normalised to represent the same number of spins. E: EM of h-IAPP control (F) EM of h-IAPP incubated with 100 μM curcumin for 24 h. Representative spectra from four independent experiments are shown.
In contrast to the negligible changes seen by EPR and EM, the ThT assay resulted in more pronounced changes. In these experiments, curcumin was added to a mixture of h-IAPP fibrils that had been preincubated with ThT for 30 min. While control fibrils (Figure 4, black line) exhibited a steady level of fluorescence intensity, the addition of 1 μM curcumin (Figure 4, blue line) showed a detectable decrease in fluorescence intensity over time. Even more significant changes in fluorescence were observed upon addition of 10 μM (Figure 4, green line) and 100 μM concentrations of curcumin (Figure 4, red line). Interestingly, the strong reduction in ThT fluorescence occurred within only a few hours, at a time when no significant changes in fibril structure and overall amount could be observed by EM or EPR. The rather rapid change in ThT fluorescence in the absence of detectable structural changes in the fibrils suggests that curcumin directly interferes with ThT fluorescence. Such interference could be brought about by a direct competition between curcumin and ThT for the same binding sites on the fibril. In fact, this mechanism has previously been reported in the case of ThT binding to Abeta fibrils [14,15] or in the case of rifampicin, which acts as a competitive inhibitor of ThT binding to IAPP fibrils [27]. This notion is supported by our findings that fibrils which are incubated with curcumin and harvested by centrifugation have a yellow appearance due to the binding of curcumin (data not shown).
Figure 4.
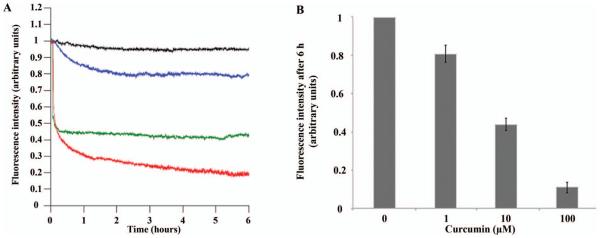
Thioflavin assay for h-IAPP preformed fibrils in the presence and absence of curcumin. h-IAPP fibrils were incubated with curcumin at 1 μM (blue line), 10 μM (green line) and 100 μM (red line) and compared to control (black line). Time = 0 indicates the point at which curcumin was added to the sample. A: Representative spectra for the given curcumin concentrations. B: Fluorescence intensity quantification (n = 6). All experiments reproducibly showed a dose-dependent loss of ThT fluorescence. The standard deviation for the final plateau values after × h were 0.05, 0.03 and 0.03 (arbitrary units equivalent to 5 and 3% of the starting signal) for 1, 10 and 100 μM curcumin, respectively.
Curcumin partially protects INS cells against exogenous h-IAPP toxicity
Having demonstrated that curcumin affects h-IAPP misfolding and aggregates morphology, we next sought to establish whether curcumin protects β-cells against h-IAPP toxicity, which, as described above, is thought to be caused by β sheet containing misfolded oligomeric forms of h-IAPP. Since most studies of amyloidogenic protein toxicity have been performed by exogenous application of the respective proteins to cell lines, we reproduced this by applying h-IAPP to the INS 832/13 β-cell line. We first determined that curcumin concentrations up to 10 μM did not affect cell viability after 20 h treatment, but that 25 μM or more did (Figure 5A), thus we assessed the potential protective effect of 0.1–25 μM curcumin on exogenous h-IAPP-induced apoptosis. Synthetic h-IAPP (15 mM) was applied on INS 832/13 cells for 20 h in the presence or absence of increasing concentrations of curcumin. Low concentrations of curcumin (0.1 to 5 mM) did not influence the deleterious effect of h-IAPP, whereas higher concentrations, 10 and 25 μM reduced h-IAPP toxicity (p < 0.05 and p < 0.01) (Figure 5B). Curcumin itself is toxic at concentrations that are required to show a protective effect against extrinsic h-IAPP toxicity (Figure 5A,B). The protective effect of curcumin against h-IAPP cytoxicity, therefore, has a narrow therapeutic window.
Figure 5.
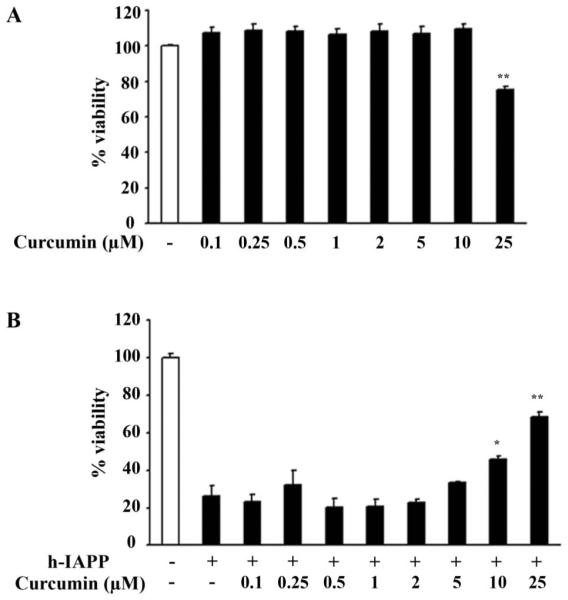
Curcumin partially protects INS cells against exogenous h-IAPP toxicity. A: INS 832/13 cells were treated for 24 h with increasing concentrations of curcumin and cell viability was assessed by MTT assay. B: Viability of INS 832/13 cells was assessed by MTT assay after 20 h treatment with a solution of h-IAPP at a final concentration of 15 mM, alone or in combination with curcumin. Data are expressed as mean ± SEM (n = 3). *p < 0.05, **p < 0.01, significant differences, curcumin vs. vehicle in h-IAPP treated cells.
Curcumin does not prevent apoptosis induced by h-IAPP over-expression
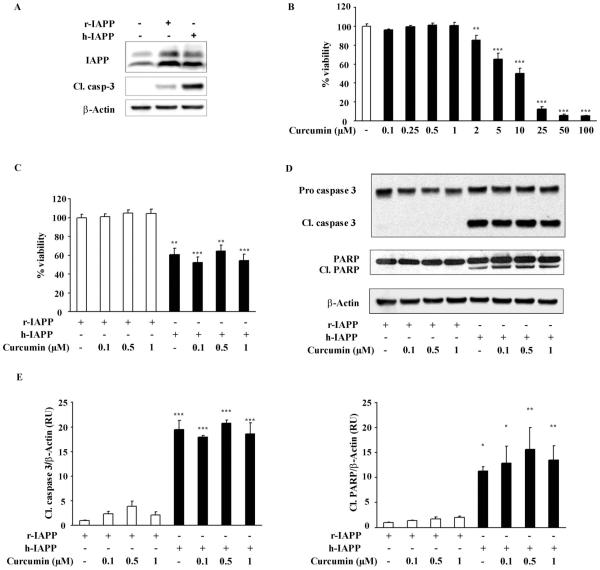
We next investigated a potential protective effect of curcumin against apoptosis induced by endogenous over-expression of h-IAPP, since accumulating evidence suggests that h-IAPP toxicity initially occurs intracellularly [28]. To discriminate the deleterious effect of h-IAPP aggregation from a potential toxicity due to protein over-expression and ER overload, r-IAPP and h-IAPP adenovirus titers were adjusted to obtain the same expression rate of r-IAPP and h-IAPP (400 moi, 48 h) (Figure 6A). In these conditions, apoptosis was assessed by immunoblotting for the cleaved form of caspase 3, which reflects the activated state of caspase 3. As shown in Figure 6A, h-IAPP over-expression induced INS 832/13 cell apoptosis compared to control or r-IAPP transduced cells. To a lesser extent, r-IAPP adenovirus displayed certain toxicity, which may be due to ER overload or adenoviral transduction.
Figure 6.
Curcumin does not prevent apoptosis induced by h-IAPP over-expression in INS cells. A: IAPP and cleaved caspase 3 (Cl. Casp-3) western blots were used to assess IAPP expression and apoptosis in INS 832/13 cells non-transduced or transduced for 48 h with 400 moi of r-IAPP as control or h-IAPP adenoviruses. B: INS 832/13 cells were treated for 46 h with increasing concentrations of curcumin and cell viability was assessed by MTT assay. C,D: INS 832/13 cells were transduced for with h-IAPP or r-IAPP adenoviruses (400 moi, 48 h). Apoptosis induced by h-IAPP over-expression in the presence or absence of curcumin was assessed by MTT assay (C), cleaved caspase 3 and cleaved PARP immunoblotting (D). E: Western blot quantification. Data are expressed as mean ± SEM (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001, significant differences vs. control.
Before assessing its ability to prevent h-IAPP-induced apoptosis, we used curcumin’s auto fluorescence property and ensured by confocal microspy that curcumin effectively enters INS 832/13 cells (data not shown). We also performed a dose response experiment on INS 832/13 cells treated or not with curcumin for 46 h, which corresponds to the time frame required to detect h-IAPP-induced apoptosis. This experiment revealed that after 46 h treatment, curcumin toxicity was present at concentrations of 2 μM or more (Figure 6B).
INS 832/13 cells were then transduced with h-IAPP or r-IAPP adenoviruses (400 moi, 48 h) and treated with curcumin from 0.1 to 1 μM for the last 46 h of transduction. As expected, h-IAPP over-expression led to a 40% decrease in cell viability when compared to cells transduced with r-IAPP (Figure 6C). The addition of curcumin at 0.1, 0.5 and 1 μM did not exert any protective effect on h-IAPP-induced cell death (Figure 6C). These observations were confirmed with the analysis of the apoptotic markers, caspase 3 and PARP by western blotting. h-IAPP over-expression in INS cells induced apoptosis as illustrated by an increase in the cleavage of both caspase 3 and PARP that was not prevented by curcumin (Figure 6D,E). Thus, curcumin failed to protect INS 832/13 cells against apoptosis induced by h-IAPP over-expression.
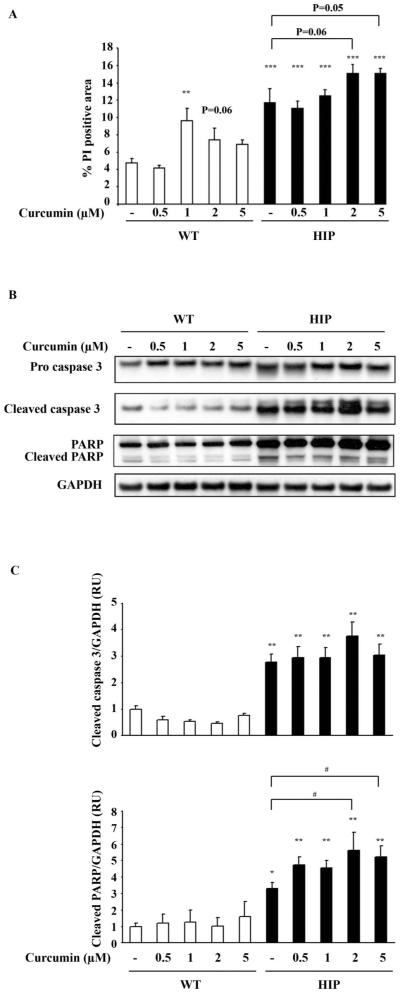
Curcumin does not protect HIP rat islets against apoptosis
Having established that physiological concentrations of curcumin, which do not by itself induce toxicity were ineffective in preventing β-cell apoptosis mediated by h-IAPP adenovirus over-expression in INS 832/13 cells, we sought to examine any potential benefit in primary β-cells. Replicating cells have increased vulnerability to h-IAPP toxicity, which might have obscured benefit. To accomplish this, we examined the effect of curcumin on cell death in pancreatic islets isolated from h-IAPP transgenic (HIP) rats, a rat model for T2DM. The islet in the HIP rat recapitulates the phenotype present in humans with T2DM, with loss of β-cells through endoplasmic reticulum stress induced apoptosis, formation of islet amyloid, and impaired insulin secretion leading to diabetes [9]. Islets were isolated from 5-month-old control rats (WT) or HIP rats. As expected, islet cell death was increased in HIP versus WT islets (10.3% ± 1.9 vs 4.8% ± 0.5, p < 0.05, Figure 7A). No protective effect of curcumin against apoptosis was observed in HIP islets as assessed by PI staining (Figure 7A) and by caspase 3 and PARP cleavage (Figure 7B,C).
Figure 7.
Effect of curcumin on WT and h-IAPP transgenic (HIP) rat islets. WT and h-IAPP transgenic (HIP) rat islet viability was assessed after 48 h treatment with or without curcumin (0.5, 1, 2 and 5 mM) by PI staining (A), cleaved caspase 3 and cleaved PARP immunoblotting (B). C: Western blot quantification. Data are expressed as mean ± SEM (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001, significant differences vs. WT + vehicle. #p < 0.05 significant differences vs. vehicle treatment.
ThT staining did not reveal any difference in the extent of amyloid deposition in HIP rat islets treated for 48 h with or without curcumin (data not shown), suggesting that at low concentration, curcumin did not affect de novo fibril formation and/or rapidly dissolve preformed fibrils. These results are, however, limited by the presence of extensive amyloid deposits in HIP rat islets and the fact that islets were only cultured for a short period of time with curcumin.
In these experiments, not only did curcumin fail to protect islet cells in the HIP rat from h-IAPP toxicity, but at 2 μM (p = 0.06) and 5 μM (p = 0.05) tended to further increase β-cell death (Figure 7A). In WT islets, 1 μM curcumin was in addition sufficient to induce a significant increase in the percentage of PI positive area (Figure 7A).
Discussion
There is interest in the concept that the proteotoxicity caused by amyloidogenic proteins involved in protein misfolding diseases might be prevented by the use of molecules that constrain the formation of toxic, misfolded forms. Curcumin has been proposed as a potential such molecule on the basis that it has been shown to inhibit amyloid formation in vitro [14–17]. Since protein misfolding and aggregation of h-IAPP has been implicated in β-cell apoptosis in T2DM, we examined the potential for curcumin to favourably influence h-IAPP aggregation and cytoxicity.
In order to establish the therapeutic potential of such an approach, it is important to first determine at what concentration and by what means a candidate compound influences protein aggregation. With the use of EPR and EM, we found that 10–100 μM concentrations of curcumin are required to significantly alter misfolding. At these concentrations, curcumin altered the misfolding process by reducing h-IAPP fibril formation, resulting in aggregates with alternative morphology and structure. We also found that the commonly used ThT fluorescence did not give reliable readouts for misfolding, as curcumin appears to compete for the same sites that ThT binds to.
Having established the concentration range of curcumin required to influence h-IAPP aggregation, we examined the actions of curcumin on h-IAPP toxicity. Since most screening studies of the toxicity of amyloidogenic proteins are carried out in vitro in cell lines after application of the protein, we also employed this approach making use of INS 832/13 cells. Perhaps, not surprisingly, the conditions under which both curcumin and h-IAPP were added extracellularly and could freely interact with each other produced the strongest protective effect. Even under these conditions, however, there is, at best, a narrow therapeutic window whereby curcumin can act to decrease h-IAPP toxicity and not by itself induce toxicity. Unfortunately, in the case of INS 832/13 cells transduced with adenoviruses to express h-IAPP, curcumin caused toxicity at concentrations lower than those required to exert protective effects. Since tumour cell lines may differ from primary cells in vulnerability to pro apoptotic signals, we also examined primary islet cells isolated from wild type rats or rats transgenic for h-IAPP (HIP rats). The HIP rat islet recapitulates the pathology of the islet in humans with T2DM, and by so doing offers an attractive model for investigating the potentially beneficial actions of candidate compounds to protect against h-IAPP toxicity. As expected HIP rat islets had increased apoptosis compared to those of WT rats, but curcumin failed to protect against this. In isolated islets, a curcumin concentration of 1 μM was sufficient to induce toxicity. This lower concentration, compared to INS cells may reflect the high expression rate of anti apoptotic factors, such as Bcl2, characteristic of tumour cells, or increased vulnerability of isolated islets that are prone to anoxia.
As with all studies, the present one has limitations that should be considered. The present in vitro approach only monitors the overall misfolding process without specifically evaluating the formation of transient oligomeric species, which are of yet unresolved structure [9,29]. To directly address whether curcumin can modulate the formation of a toxic (presumably oligomeric) species, we examined whether curcumin suppresses the cytoxicity of h-IAPP both when applied to cells and when overexpressed by them. Another consideration was that the cytotoxicity studies were carried out in culture. The conditions in vivo might be considerably different, including the question of bioavailability. Curcumin is poorly absorbed from the gut [30], but, once absorbed, would likely be delivered to pancreatic islets initially bound to plasma proteins and then via proteins in the extracellular fluid. Thus, while cell culture media to some extent recapitulate these conditions, there are obviously differences that might be important both for biodelivery and actions. It should also be noted that the prevalence of type 2 diabetes in India, where curcumin is most abundantly consumed, is one of the highest in the world, perhaps supporting the overall conclusions of the present studies that curcumin ingestion is unlikely to be protective against development of type 2 diabetes. Taking both the biophysical and biological aspects of this study into consideration, we have found that curcumin has the ability to alter h-IAPP misfolding and fibril formation, while also showing protective effects against h-IAPP cytoxicity at ranges that in itself seem harmful to the cells. Curcumin clearly has the ability to interact with and inhibit the formation of amyloid proteins, such as Abeta [14] and h-IAPP, but it does not seem to have useful therapeutic characteristics. While the present findings suggest that curcumin is unlikely to be an effective treatment to prevent or delay onset of type 2 diabetes, the property of curcumin to interact with IAPP and inhibit amyloid formation might be explored through preparation of curcumin analogs, if these can be developed to enhance the property to inhibit misfolding, enhance bioavailability and inhibit cytotoxicity.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (AG027936) to RL and PCB (DK59579), The Larry Hillblom Foundation (RL, PCB) and from Fondation pour la Recherche Médicale (FRM) to MD. We are grateful to Matthew Schibler (Brain Research Institute, Advanced Microscopy/Spectroscopy Core Facility, UCLA) for his help with confocal microscopy experiments.
Abbreviations
- EM
electron microscopy
- EPR
electron paramagnetic resonance
- HBSS
Hank’s buffered salt solution
- HFIP
hexafluoroisopropanol
- h-IAPP
human islet amyloid polypeptide
- IAPP
islet amyloid polypeptide
- INS
insulinoma cell line
- MTSL
1-oxyl-2,2,5,5-tetramethyl-Δ3-pyrroline-3-methyl methanethiosulfonate
- T2DM
type 2 diabetes mellitus
- ThT
Thioflavin T
References
- 1.Stefani M. Protein misfolding and aggregation: new examples in medicine and biology of the dark side of the protein world. Biochim Biophys Acta. 2004;1739:5–25. doi: 10.1016/j.bbadis.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Westermark P, Wernstedt C, Wilander E, Hayden DW, O’Brien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci USA. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark A, de Koning EJ, Hattersley AT, Hansen BC, Yajnik CS, Poulton J. Pancreatic pathology in non-insulin dependent diabetes (NIDDM) Diabetes Res Clin Pract. 1995;28(Suppl):S39–S47. doi: 10.1016/0168-8227(95)01075-o. [DOI] [PubMed] [Google Scholar]
- 4.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 5.Gepts W, Lecompte PM. The pancreatic islets in diabetes. Am J Med. 1981;70:105–115. doi: 10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- 6.Huang CJ, Haataja L, Gurlo T, et al. Induction of endoplasmic reticulum stress-induced β-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am J Physiol Endocrinol Metab. 2007;293:E1656–E1662. doi: 10.1152/ajpendo.00318.2007. [DOI] [PubMed] [Google Scholar]
- 7.Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, et al. Selective β-cell loss and α-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 8.Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 11.Ammon HPWM. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 13.Mazumder ARK, Weinstein J, Kohn KW, Pommier Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem Pharmacol. 1995;49:1165–1170. doi: 10.1016/0006-2952(95)98514-a. [DOI] [PubMed] [Google Scholar]
- 14.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 16.Pandey N, Strider J, Nolan WC, Yan SX, Galvin JE. Curcumin inhibits aggregation of α-synuclein. Acta Neuropathol. 2008;115:479–489. doi: 10.1007/s00401-007-0332-4. [DOI] [PubMed] [Google Scholar]
- 17.Hafner-Bratkovic I, Gaspersic J, Smid LM, Bresjanac M, Jerala R. Curcumin binds to the α-helical intermediate and to the amyloid form of prion protein – a new mechanism for the inhibition of PrP(Sc) accumulation. J Neurochem. 2008;104:1553–1564. doi: 10.1111/j.1471-4159.2007.05105.x. [DOI] [PubMed] [Google Scholar]
- 18.Jayasinghe SA, Langen R. Identifying structural features of fibrillar islet amyloid polypeptide using site-directed spin labeling. J Biol Chem. 2004;279:48420–48425. doi: 10.1074/jbc.M406853200. [DOI] [PubMed] [Google Scholar]
- 19.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K + channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 20.Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC. Diabetes due to a progressive defect in β-cell mass in rats transgenic for human islet amyloid polypeptide (HIP Rat): a new model for type 2 diabetes. Diabetes. 2004;53:1509–1516. doi: 10.2337/diabetes.53.6.1509. [DOI] [PubMed] [Google Scholar]
- 21.Chen M, Margittai M, Chen J, Langen R. Investigation of α-synuclein fibril structure by site-directed spin labeling. J Biol Chem. 2007;282:24970–24979. doi: 10.1074/jbc.M700368200. [DOI] [PubMed] [Google Scholar]
- 22.Der-Sarkissian A, Jao CC, Chen J, Langen R. Structural organization of α-synuclein fibrils studied by site-directed spin labeling. J Biol Chem. 2003;278:37530–37535. doi: 10.1074/jbc.M305266200. [DOI] [PubMed] [Google Scholar]
- 23.Margittai M, Langen R. Template-assisted filament growth by parallel stacking of tau. Proc Natl Acad Sci USA. 2004;101:10278–10283. doi: 10.1073/pnas.0401911101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torok M, Milton S, Kayed R, Wu P, McIntire T, Glabe CG, Langen R. Structural and dynamic features of Alzheimer’s Abeta peptide in amyloid fibrils studied by site-directed spin labeling. J Biol Chem. 2002;277:40810–40815. doi: 10.1074/jbc.M205659200. [DOI] [PubMed] [Google Scholar]
- 25.Kudva YC, Mueske C, Butler PC, Eberhardt NL. A novel assay in vitro of human islet amyloid polypeptide amyloidogenesis and effects of insulin secretory vesicle peptides on amyloid formation. Biochem J. 1998;331(Part 3):809–813. doi: 10.1042/bj3310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naiki H, Higuchi K, Hosokawa M, Takeda T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal Biochem. 1989;177:244–249. doi: 10.1016/0003-2697(89)90046-8. [DOI] [PubMed] [Google Scholar]
- 27.Meng F, Marek P, Potter KJ, Verchere CB, Raleigh DP. Rifampicin does not prevent amyloid fibril formation by human islet amyloid polypeptide but does inhibit fibril Thioflavin-T interactions: implications for mechanistic studies of β-cell Death. Biochemistry. 2008;47:6016–6024. doi: 10.1021/bi702518m. [DOI] [PubMed] [Google Scholar]
- 28.Lin CY, Gurlo T, Kayed R, Butler AE, Haataja L, Glabe CG, Butler PC. Toxic human islet amyloid polypeptide (h-IAPP) oligomers are intracellular, and vaccination to induce anti-toxic oligomer antibodies does not prevent h-IAPP-induced β-cell apoptosis in h-IAPP transgenic mice. Diabetes. 2007;56:1324–1332. doi: 10.2337/db06-1579. [DOI] [PubMed] [Google Scholar]
- 29.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 30.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]