Abstract
Mosquito-borne virus infections, such as dengue and chikungunya, are continuously expanding their geographical range. The dengue virus, which is known to be a common cause of febrile illness in tropical areas of the Old World, is now widespread in the Americas. In most affected areas, all the four dengue virus serotypes have circulated. Recently, small clusters of dengue have been identified also in Southern Europe during the hot season. The chikungunya virus, initially restricted to Central Africa, where is a common cause of sporadic cases or small outbreaks, and Asia, where it is used to cause large epidemics, has recently invaded new territories. After ravaging Indian Ocean Islands and the Indian subcontinent, CHIKV caused an outbreak in north-eastern Italy. Recently, chikungunya has reached the Caribbean, causing for the first time a large epidemic on the American continent. Although Aedes aegypti is the main vector of both viruses, Aedes albopictus, the Asian ‘Tiger’ mosquito, is now playing an increasingly important role, contributing to their spread in temperate climate areas. Hereby, we focus the attention on outbreaks of dengue and chikungunya occurring in previously disease-free areas and discuss factors associated with the long-distance spread of the vector-borne infections, such as mutations increasing viral fitness, climate change, urbanization, and globalization of humans and vectors.
Keywords: Arbovirus, Dengue, Chikungunya, Aedes albopictus, Global health
INTRODUCTION
Arthropod-borne viruses (Arboviruses), in particular mosquito-borne viruses, such as the dengue virus (DENV), chikungunya virus (CHIKV), and West Nile virus (WNV), are becoming an increasingly important global health threats, spreading from their original niche in sub-Saharan Africa to most areas of the world. Differently from the WNV, which has a bird reservoir, DENV and CHIKV are maintained through a primate–mosquito–primate cycle; thus, their spread does not depend on bird migration routes. In this review article, we analyze recent changes in the geographical distribution and describe the main determinants of the global spread of these two viruses, focusing the attention on new territorial ‘conquests’ through long-distance spread, and on outbreaks occurring in temperate climate zones. In fact, although the increased spread of infections in, and around, endemic areas is likely to be the most important modality of disease-burden increase (at least for dengue), long-distance spread, with consequent occurrence of unexpected outbreaks, is not a negligible aspect of current epidemic dynamics of dengue and chikungunya.
DENGUE
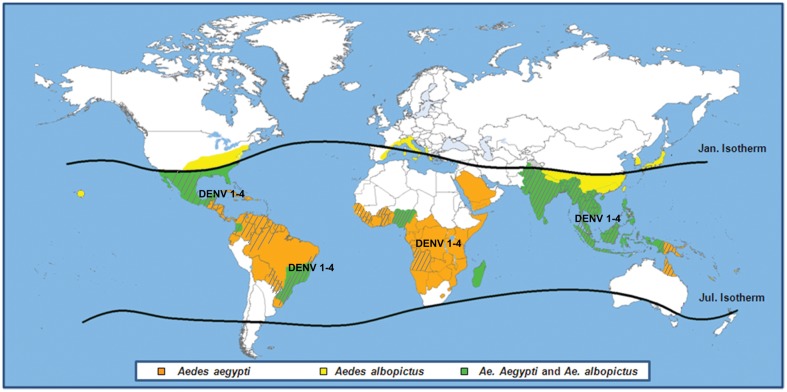
Dengue is caused by an arbovirus belonging to the Flavivirus genus of the Flaviviridae family. There are four DENV strains, referred to as DENV1–4 serotypes. Clinical manifestations range from mild cases of dengue fever to severe cases of dengue hemorrhagic fever and/or dengue shock syndrome. The main vector of DENV is Aedes aegypti, but the infection may be transmitted also by Aedes albopictus. In the past 50 years, the incidence of dengue increased 30-fold, and nowadays it is the most rapidly spreading mosquito-borne viral disease worldwide, accounting for an estimated 50–100 million infections occurring every year.3 The global distribution of Aedes spp and DENV serotypes is shown in Fig. 1; all the four DENV serotypes have been circulating at some point in time in virtually all the affected continents.1–3
Figure 1.
Distribution of Aedes aegypti (orange area), Aedes albopictus (yellow area), or both (green area), and areas where dengue activity has been detected (dotted area). Circulation of all the four dengue virus serotypes has been identified in all affected continents. No details are given on small outbreaks occurred on the Hawaii and sporadic cases of dengue reported in France and Croatia are not reported in the map.
In the following paragraphs, some case descriptions of the re-introduction of dengue in temperate or subtropical climate zones which are not contiguous to known endemic areas are presented.
The threat of dengue to Europe
The last European outbreak of dengue: Athens, 1927–1928
Dengue outbreaks were not uncommon in the Mediterranean area between the end of the IXX and the beginning of the XX century. The disease was reported in Athens in 1889, 1895–1897, and in 1910. Then, in 1927–1928, Athens and neighboring areas of Greece were the site of the last major epidemic on the European continent, causing an unusual large number of severe cases.
The outbreak began in Athens in the summer or early fall of 1927.4 The first wave was mild, affecting a minority of the population, and terminated with the arrival of cold weather. However, sporadic cases continued to be observed through the winter and springtime.5 Then, in August 1928, the number of cases increased dramatically, possibly as a consequence mosquito vectors inside heated houses. The 1928 epidemic was severe and characterized by a high number of cases (approximately 650 000); hemorrhagic manifestations and deaths were common: about 1% of those >60 years old died.6 The findings of several serosurveys suggested that clinical severity was attributable to the sequential circulation of different DENV serotypes (DENV-1 and DENV-2), with the occurrence of secondary infections, which are known to be more severe than primary infections.6–8 The hypothesis was criticized by a study showing that almost one-third of those born between 1914 and 1928 had antibodies against DENV-1, while DENV-2 had occurred in Greece only after 1928.9
Whatever the cause of the severe 1928 Athens outbreak, dengue apparently abandoned the European continent for a long time. Aedes aegypti, which was the vector of dengue in Athens and Piraeus,10 almost disappeared from Eastern Mediterranean after 1935.11
The return of dengue: small clusters in Southern France and Croatia
In the late summer of 2010, two autochthonous cases of dengue fever were identified in Nice. The first case had friends from the West French Indies staying with him, whereas the second one lived 70 meters from the first autochthonous case.12 Three years later, in October 2013, another autochthonous case of dengue was diagnosed in Bouches-du-Rhône, southern France. The probable source of infection was a man from Guadaloupe who had visited the immediate vicinity of the autochthonous case’s workplace. Aedes albopictus eggs were found in the ovitraps.13 The identification of autochthonous cases clustered in time and space, and the presence of a competent vector, were strongly suggestive of ongoing local transmission of DENV.
On mid-August of the year 2010, after returning from a holiday on the Peljesac peninsula and on the isle of Korĉula, approximately 100 km north-west of the city of Dubrovnik, Croatia, a man from Germany developed a febrile illness which was diagnosed as dengue.14 Following this report, active case-finding and seroepidemiological surveys were conducted, showing DENV-specific antibodies in 5.4% of the serum samples collected in October 2010. The modalities of introduction of DENV remain undefined. Aedes albopictus, for the first time identified in Croatia in 2004, was found to be the dominant species in the area. Mosquito control activities were successfully implemented to reduce the risk of DENV reemergence.15
The Madeira outbreak
In 2012, Madeira reported its first major outbreak of dengue. The outbreak, caused by the DENV-1 serotype, started on 3 October 2012, and resulted in over 2100 cases by March 2013. The autonomous region of Madeira belongs to Portugal; thus, it is considered European territory, but it is an archipelago located in the Atlantic Ocean, on the same latitude as the north coast of Africa. Thanks to its subtropical climate, Aedes aegypti, which was introduced in 2005, has established on the island.16 The origin of the outbreak was investigated applying the importation index (which depends on the incidence rate of dengue in the country of origin and on travelers volume from the country of origin to the outbreak area) and phylogenetic analysis; the findings suggested that DENV was likely to be imported from South America, with a higher probability for Venezuela compared to Brazil.17
The continued menace of dengue in the USA
Since 2001, several autochthonous outbreaks of dengue fever occurred in several areas of the USA: Hawaii (2001), Brownsville, Texas (2005), and southern Florida (2009–2011).18
In the Hawaii, where autochthonous transmission had last been reported in 1944, 122 laboratory-confirmed cases were identified during 2001–2002.19 The outbreak was caused by a DENV-1 strain imported by travelers from French Polinesia, and it was transmitted by Aedes albopictus mosquitoes. In Brownsville, Texas, limited outbreaks of locally acquired dengue had occurred sporadically since 1980 in areas that border Mexico. In 2005, 25 cases were detected, but only 3 were considered autochthonous cases, while 22 persons had traveled to Mexico, where a large epidemic was ongoing. DENV-2 was the most commonly detected serotype, which was likely transmitted by Aedes aegypti. A high proportion of residents in Brownsville (38%) were positive for IgG against DENV, suggesting past circulation of the infection.20 Finally, in Key West, Florida, where dengue was not reported since 1934, 90 cases were identified since 2009 and a recent DENV infection was detected in 5.4% of residents. DENV-1 was detected both in human cases and in pools of Aedes aegypti mosquitoes.18,21 The repeated episodes of locally transmitted dengue and the fact that both the major dengue vectors, and the wide distribution of Aedes aegypti and Ae. albopictus in the USA urge public health awareness about vector-borne infection control.22
Dengue reemergence in Queensland, Australia
Queensland is a state located in the tropical and subtropical northeastern Australia, where dengue was present in the late nineteenth century to disappear for most of the twentieth century; then, in the last 20 years, virus importation and transmission has been increasingly observed.23 The main vector of DENV in this area is Aedes aegypti mosquitoes, which was previously established in other Australian states, but it is now almost exclusively found in Queensland. Despite repeated transmission events, dengue is not endemic, and outbreaks occur when the virus is imported by a viremic traveler.24 An analysis of imported cases shows that most index cases reported traveling to Asia, Papua New Guinea, and Pacific Islands, and the outbreaks were due to different DENV serotypes.25
CHIKUNGUNYA
Chikungunya fever is caused by an arbovirus belonging to the Alphavirus genus of the Togaviridae family, first isolated in the Newala district of Tanzania in 1952–1953.26 CHIKV is transmitted to humans by the bite of Aedes spp. (i.e. Aedes aegypti and Aedes albopictus) mosquitoes. In western and central Africa, CHIKV is maintained in a sylvatic cycle involving non-human primates and forest-dwelling Aedes spp. mosquitoes. In Asia, the predominant vector is the urban, peri-domestic, anthropophilic, Aedes aegypti mosquito, which is responsible for large-scale outbreaks characterized by long inter-epidemic periods which may last several decades.27
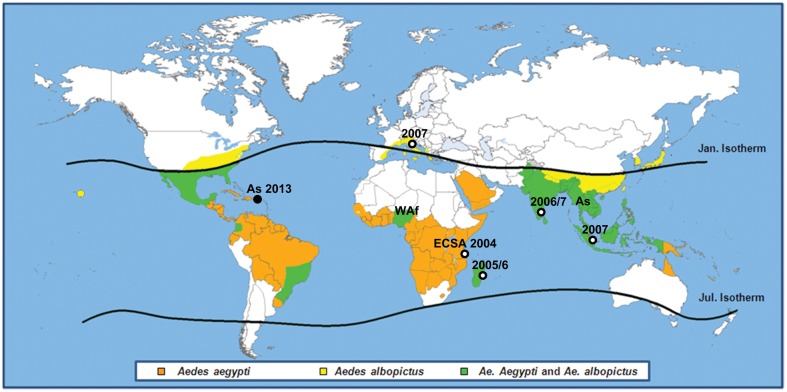
Three different genotypes of CHIKV have been identified: the western African, the east-central-south African (ECSA), and the Asian genotype;28,29 evolutionary studies have suggested an African origin for CHIKV.30 Since its identification, sporadic cases and outbreaks have been reported in several African countries, on the Indian subcontinent, and in southeast Asia.26 Whether there is sustainability of the human–mosquito cycle at a local scale in the absence of continued importation in Asia remains undefined.27,28,30 In the last decade, CHIKV has re-emerged, causing a series of large outbreaks, which started in Kenya in 2004, and ravaged the Comoros Islands, the island of La Réunion, and other islands in the southwest Indian Ocean in early 2005, followed by an epidemic in the Indian subcontinent in 2005/2006.31,32 Last year, autochthonous transmission of CHIKV was reported for the first time in the Americas. The geographical spread of CHIKV and the distribution of the different genotypes are presented in Fig. 2.3,33,34
Figure 2.
Distribution of Aedes aegypti (orange area), Aedes albopictus (yellow area), or both (green area), and areas where chikungunya activity has been detected. Chikungunya virus genotype (As = Asian, ECSA = East/Centre/South African, WAf = West-African) distribution and dates of recent outbreaks due to the ECSA (Indian Ocean) and Asian genotype (America) are also shown in the map. Sporadic cases of chikungunya reported in France are not reported in the map.
The following case presentations are paradigmatic of the combined effect of human mobility, vector globalization, and virus adaptation on the long-distance spread of CHIKV.
CHIKUNGUNYA OUTBREAKS IN THE INDIAN OCEAN: THE CHANGING RELATIONSHIP BETWEEN VIRUS AND VECTOR
CHIKV strains which caused the large outbreaks in the Indian Ocean area belong to the ECSA genotype.28,35,36 During the epidemic, two different lineages were identified, suggesting independent introductions of CHIKV strains from Kenya into Indian Ocean islands and India.28 A viral variant, presenting a substitution of the amino-acid alanine with valine in the position 226 of the E1 protein (one of the two major envelope surface glycoproteins) of CHIKV (A226V), selected during the course of the epidemic, originated in the Indian Ocean and became predominant in affected areas where Ae. albopictus was largely predominant compared to Ae. Aegypti, such as La Reunion and the Kerala district in India, allowing an efficient replication and dissemination of the A226V variant,37
While the 226A strain was the only genotype observed during the first period of the outbreak (March to June 2005), the E1 A226V genotype started to be observed from September 2005. On la Reunion, the identification of A226V variant circulation preceded by at least 3 months the explosive epidemic peak of mid-December, suggesting an increase in viral transmission.38 In the Indian region of Kerala, CHIKV strains isolated in 2007 presented the A226V mutation, which had not been found in strains isolated in Kerala and in other Indian regions in 2006.39,40 Thus, a single amino-acid substitution may have influenced vector specificity, increasing the fitness of CHIKV for specific vector species and consequently, CHIKV transmission.41 The same variant caused the outbreak propagated by Aedes albopictus in north-eastern Italy.42
Chikungunya in Europe: the Italian outbreak and other small clusters in southern France
At the end of August 2007, a fever outbreak was reported in two small villages in north-eastern Italy.42 The epidemiological investigation revealed that the outbreak started about ten days after a few hours visit of a viremic person from Kerala, India, to his relatives living in one of the affected villages, which resulted heavily infested by Aedes albopictus mosquitoes. Overall, more than 200 cases were identified. During the course of the epidemic, small clusters of cases occurred in neighbouring towns, and even in Bologna, which is about 75 km away from the epicentre of the epidemic. As mentioned above, CHIKV strains presented the A226V mutation detected in other areas where the Tiger mosquito was the predominant vector. The implementation of vector control measures and the decline of mosquito activity at the beginning of the cold season ended the outbreak.33,42
In September 2010, autochthonous transmission of CHIKV was identified in south-eastern France, where the Aedes albopictus mosquito vector is present. Two girls living nearby the house of another girl who developed a febrile illness after returning from Rajasthan, India, developed dengue fever due to viral strains closely related to strains from India within the ECSA lineage.43
Another event occurred in southern France in 2014, at the end of the summer season, when four autochthonous cases of chikungunya, living in Montpellier, in the vicinity of a case imported from central Africa, were identified, confirming the persistent threat posed by this tropical virus to temperate climate countries (ECDC, unpublished data).
Chikungunya reaches America
On 5 December 2013, autochthonous chikungunya cases were confirmed on Saint Martin island, French West Indies; then, about 50 cases were reported on Martinique island, and in January 2014, other cases due to local transmission were reported from Guadeloupe, Saint Barthelemy, Dominica, and the British Virgin Islands.34,44 In February 2014, chikungunya cases were reported also in French Guiana.45 The virus causing the outbreak in the Caribbeans does not belong to the ECSA genotype but to the Asian genotype, which was identified in late 1950s in southeast Asia., and it is phylogenetically related to strains recently identified in several Asian countries (i.e. in Indonesia in 2007, in China in 2012, and in the Philippines in 2013). The only competent vector in this area is Ae. aegypti. The spread of chikungunya in the Caribbean area represents a public health menace for all Latin America. Moreover, since the Caribbean islands are a popular travel destination for European and North American tourists, the emergence of chikungunya in this area opens new channels for intercontinental transmission of the infection.46
HUMAN MOBILITY AND OTHER DRIVERS OF THE GLOBAL SPREAD OF DENGUE AND CHIKUNGUNYA
The case presentations reported above are paradigmatic of the capacity of long-distance spread of vector-borne infections, such as DENV and CHIKV infection. The unprecedented frequency and the large size of outbreaks which occurred in previously disease-free areas of the world, with no contiguity with known affected areas, are impressive and needs to be investigated in depth.
Increased human mobility is a key factor for the long-distance spread of infectious diseases. In most outbreaks, DENV and/or CHIKV were introduced by infected persons coming from endemic or epidemic areas, playing the role of a Trojan horse for these germs. Then, local mosquitoes (either native or relatively newly invaders) acted as competent vectors for viral infections. Once the virus has been imported in a new area, other factors, such as climate change, virus evolution, deteriorating vector control, and socio-demographic and environmental changes, in particular population growth with rapid uncontrolled urbanization, play an important role for the geographic spread of mosquito-borne infections, with special regard to dengue.1 All these drivers have been examined in detail in the international literature. However, there is still uncertainty about the role played by some of these factors and to what extent they may influence future epidemic dynamics. In particular, three main questions need to be answered: (1) what is the impact of climatic changes on the occurrence of mosquito-borne diseases?; (2) to what extent Aedes albopictus-vectored infections may represent a permanent threat for temperate zones?; and (3) are we prepare to deal with new challenges posed by the globalization of humans, vectors, and disease agents? Hereby, we discuss shortly these issues taking into account our case presentations.
Is climate change a major driver of the spread of vector-borne infections?
Climate change, which may consist in changes not only in temperature but also in the pattern of humidity and precipitation, may influence the activity of some vector-borne infections. In general, increased temperatures and humidity favor the growth of mosquito populations, but paradoxical effects, such as the association between drought and the emergence of chikungunya along coastal East Africa, have been also reported.47 Sometimes, the effect of global climatic changes on vector-borne diseases spread has been overemphasized: for example, the scientific debate following the chikungunya outbreak in Italy tended to attribute a role to climate change,48 without considering that the Italian climate has always been suitable for Ae. Albopictus to flourish during the warm season.49 Moreover, it should be mentioned that the urban ‘heat island effect’ due to land-use changes from rapid urbanization may have played a major role compared to global climate change in favoring dengue outbreaks after the introduction of an exotic strain.
Thus, the real question is ‘can climate change, at the global or the local level, influence epidemic dynamics and favor the establishment of vector-borne infections in temperate zones?’. This question is difficult to respond. Increased temperatures and humidity might favor the establishment of Ae. aegypti, which does not survive in areas where the mean temperature during the coldest month is below 10°C, or the geographical spread of Ae. albopictus in Europe beyond the Mediterranean area, but long periods of high temperatures are required for virus replication.50 Moreover, even in areas where Ae. Albopictus is established, milder winters are needed to maintained a low level of human–mosquito–human transmission, otherwise outbreaks tend to dye out and do not recur in the absence of continues virus importation. Unless major changes in the climate will occur, sporadic cases or relatively small outbreaks of dengue or chikungunya may be expected during the hot season, without endemic persistence of the infection, due to low mosquito activity during the cold season.
Global vector spread and viral adaptability: what is the role of the ‘Tiger’ mosquito in the reemergence of dengue and chikungunya?
Ae. albopictus, the Asian ‘Tiger’ mosquito, is considered a less efficient vector, since it is not as well adapted to urban domestic environment and is less anthropophilic than Aedes aegypti.51 However, Ae albopictus represents an alternative vector, whose importance is constantly growing as a consequence of rapid changes in its overall distribution.52 For example, the ‘Tiger’ mosquito was involved in several dengue outbreaks, such as those occurred in Japan from 1942 to 1945,53 Hawaii from 2001 to 2002,19 La Reunion in 2004,54 and Donnguan, China.55
The ‘Tiger’ mosquito originally lived at the edges of the forests of East and Southeast Asia. In recent decades, it spread to all continents, as a result of increased global air travel and seaborne trade. Shipment of used tires infested with mosquito’s eggs is the main modality of spread of Ae. albopictus,56 but other ways of introduction have been reported.57
As mentioned before, the ‘globalization’ of Ae. albopictus is fueling vector-borne infections spread in areas which were previously unaffected by dengue and/or chikungunya. Since Ae. albopictus adapts better than Ae aegypti to temperate climates, it may cause outbreaks in areas where Ae. aegypti is not established. However, dengue and chikungunya outbreaks caused by Ae. albopictus tend to be milder for several reasons: (1) Ae. albopictus feeds both on humans and on animals; thus, the probability that it feeds on humans is decreased and usually restricted to a single individual, while Ae. aegypti is more anthropophilic and tends to feed on more humans; (2) since the ‘Tiger’ mosquito tends to be present also outside the tropics, in temperate climate areas, the outbreaks are usually mitigated by the arrival of cooler winter temperature; (3) Ae. aegypti is more likely to rest/bite inside houses, and its daily flight times better match human activity patterns; (4) vertical transmission of DENV and CHIKV from ‘Tiger’ mosquitoes to their offspring does not seems to be very efficient: in La Reunion Island, CHIKV was isolated only in two out of 500 pools of larvae of Ae. Albopictus, demonstrating a low level of vertical transmission.58 Since mosquito activity heavily declines during the cold season, the low efficiency of transovarial transmission may explain the self-limiting nature of outbreaks occurring in temperate climate areas; and (5) DENV fitness for Ae. albopictus is not negligible, but it is lower than that of CHIKV (especially when compared to the A226V CHIKV variant).59 For the reasons reported above, long-term sustained transmission of DENV in areas where Ae. albopictus is the predominant vector is unlikely.
Prevention and control of vector-borne infections in the globalization era: are we adequately prepared?
Since human mobility cannot be reduced, in order to contrast the long-distance spread of dengue and chikungunya, a series of prevention and control activities should be plan in areas at risk of vector-borne transmission. Such plans should include (1) prevention activities (from vector control to public awareness and education); (2) outbreak control activities (from vector control to personal protection); and (3) information and training for medical professionals in order to favor early detection of exotic diseases. Recent experience of epidemic events has contributed to the adoption of surveillance and preparedness activities; however, it remains unknown whether the knowledge of vector-borne infections due to exotic viruses is adequate. To this regard, it should be kept in mind that early intervention is key in order to end an outbreak in areas with relatively cold and dry winter.
CONCLUSIONS
Dengue and chikungunya are two paradigmatic examples of emerging infections, showing similar epidemic dynamics. Because of higher human mobility and the increasingly wider geographical distribution of the Aedes spp. mosquito vectors, these arboviruses are expanding their area of activity, conquering new continents and starting to affect areas that were previously considered disease-free. In this context, the role played by long-distance spread through human and vector mobility is not negligible in the globalization era. Owing to the global threat posed by these arboviral infections, it is mandatory to explore the feasibility of innovative mosquito control strategies and the adoption of measures addressed to epidemic impact mitigation in critical geographical areas.
DISCLAIMER STATEMENTS
Funding None.
Conflicts of interest No conflict of interest.
Ethics approval Ethical clearance not needed.
Acknowledgments
Thanks to Stefanno Boros for maps editing.
REFERENCES
- 1.Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop Med Health. 2011;39(4 Suppl):3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halstead SB. Dengue. Lancet. 2007;370:1644–52. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: WHO; 2009. [PubMed] [Google Scholar]
- 4.Anon. The dengue epidemic in Greece. League Nations Monthly Epidemiol Rep. 1928;7:334. [Google Scholar]
- 5.Cardamatis JP. La dengue in Greece. Bull Soc Path Exot. 1929;22:272–92. [Google Scholar]
- 6.Papaevangelou G, Halstead SB. Infections with two dengue viruses in Greece in the 20th century. Did dengue hemorrhagic fever occur in the 1928 epidemic? Am J Trop Med Hyg. 1977;80:46–51. [PubMed] [Google Scholar]
- 7.Theiler M, Casals J, Moutousses C. Etiology of the 1927–28 epidemic of dengue in Greece. Proc Soc Exp Biol Med. 1960;103:244–6. doi: 10.3181/00379727-103-25474. [DOI] [PubMed] [Google Scholar]
- 8.Halstead SB, Papaevangelou G. Transmission of dengue 1 and 2 viruses in Greece in 1928. Am J Trop Med Hyg. 1980;29:635–7. doi: 10.4269/ajtmh.1980.29.635. [DOI] [PubMed] [Google Scholar]
- 9.Rosen L. Dengue in Greece in 1927 and 1928 and the pathogenesis of dengue hemorrhagic fever: new data and a different conclusion. Am J Trop Med Hyg. 1986;35:642–53. doi: 10.4269/ajtmh.1986.35.642. [DOI] [PubMed] [Google Scholar]
- 10.Blanc G, Caminopetros J. Recherches expérimentales sue la dengue. Ann Inst Pasteur (Paris). 1930;44:367–436. [Google Scholar]
- 11.Curtin TJ. Status of Aedes aegypti in the Eastern Mediterranean. J Med Entomol. 1967;4:48–50. doi: 10.1093/jmedent/4.1.48. [DOI] [PubMed] [Google Scholar]
- 12.La Ruche G, Souarès Y, Armengaud A, Peloux-Petiot F, Delaunay P, Desprès P, et al. First two autochthonous dengue virus infections in Metropolitan France, September 2010. Euro Surveill. 2010;15(39):19676. [PubMed] [Google Scholar]
- 13.Marchand E, Prat C, Jeannin C, Lafont E, Bergmann T, Flusin O, et al. Autochthonous case of dengue in France, October 2013. Euro Surveill. 2013;18(50):20661. doi: 10.2807/1560-7917.es2013.18.50.20661. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Chanasit J, Haditsch M, Schöneberg I, Günther S, Stark K, Frank C. Dengue vuirus infection in a traveler returning from Croatia to Germany. Euro Surveill. 2010;15(40):19677. doi: 10.2807/ese.15.40.19677-en. [DOI] [PubMed] [Google Scholar]
- 15.Gjenero-Margan I, Aleraj B, Krajcar B, Lesnikar V, Klobučar A, Pem-Novosel I, et al. Autochthomous dengue fever in Croatia, August–September 2010. Euro Surveill. 2011;16(9):19805. [PubMed] [Google Scholar]
- 16.Alves MJ, Fernandes PL, Amaro F, Osório H, Luz T, Parreira P, et al. Clinical presentation and laboratory findings for the first autochthonous cases of dengue fever in Madeira Island, Portugal, October 2012. Euro Surveill. 2013;18(6):20398. [PubMed] [Google Scholar]
- 17.Wilder-Smith A, Quam M, Sessions O, Rocklov J, Liu-Helmersson J, Franco L, et al. The 2012 dengue outbreak in Madeira: exploring the origins. Euro Surveill. 2014;19(8):20718. doi: 10.2807/1560-7917.es2014.19.8.20718. [DOI] [PubMed] [Google Scholar]
- 18.Adalja AA, Sell TK, Bouri N, Franco C. Lessons learned during dengue outbreaks in the United States, 2001–2011. Emerg Infect Dis. 2012;18:608–14. doi: 10.3201/eid1804.110968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Effler PV, Pang L, Kitsutani P, Vorndam V, Nakata M, Ayers T, et al. Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis. 2005;11:742–9. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC. Dengue hemorrhagic fever — US–Mexico border, 2005. MMWR Morb Mortal Wkly Rep. 2007;56:785–9. [PubMed] [Google Scholar]
- 21.CDC. Locally acquired dengue – Key West, Florida, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:577–81. [PubMed] [Google Scholar]
- 22.Halstead SB. More dengue, more questions. Emerg Infect Dis. 2005;11:740–1. doi: 10.3201/eid1105.050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warrilow D, Northill JA, Pyke AT. Sources of dengue viruses imported into Queensland, Australia, 2002–2010. Emerg Infect Dis. 2012;18:1850–7. doi: 10.3201/eid1811.120014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBride WJ. Dengue fever: is it endemic in Australia? Intern Med J. 2010;40:247–9. doi: 10.1111/j.1445-5994.2010.02196.x. [DOI] [PubMed] [Google Scholar]
- 25.Ritchie SA, Pyke AT, Hall-Mendelin S, Day A, Mores CN, Christofferson RC, et al. An explosive epidemic of DENV-3 in Cairn, Australia. PLoS One. 2013;8(7):e68137. doi: 10.1371/journal.pone.0068137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond). 1956;54:177–91. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chevillon C, Briant L, Renaud F, Devaux C. The Chikungunya threat: an ecological and evolutionary perspective. Cell. 2007;16:80–8. doi: 10.1016/j.tim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet. 2007;7:319–27. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 29.Ng LC, Hapuarachchi HC. Tracing the path of chikungunya virus — evolution and adaptation. Infect Genet Evol. 2010;10:876–85. doi: 10.1016/j.meegid.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Volk SM, Chen R, Tsetsarkin KA, Adams AP. Garcia TI. Sall AA, et al. Genome-scale phylogenetic analyses of Chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol. 2010846497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Chikungunya and dengue, south-west Indian Ocean. Wkly Epidemiol Rec. 2006;81:105–16. [PubMed] [Google Scholar]
- 32.Charrel RN, de Lamballerie X, Raoult D. Chikungunya outbreaks — the globalization of vectorborne diseases. N Engl J Med. 2007;356:769–71. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- 33.Rezza G.Chikungunya fever virus In: Ergonul O, Can F, Akova M, Madoff L, editors. Emerging infectious diseases. Amsterdam: Elsevier; 2014163–174. [Google Scholar]
- 34.Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lambellerie X. Chikungunya in the Americas. Lancet. 2014;383:514. doi: 10.1016/S0140-6736(14)60185-9. [DOI] [PubMed] [Google Scholar]
- 35.Yergolkar PN, Tandale BV, Arankalle VA, Sathe PS, Sudeep AB, Gandhe SS, et al. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12:1580–3. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Njenga MK, Nderitu L, Ledermann JP, Ndirangu A, Logue CH, Kelly CH, et al. Tracking epidemic Chikungunya virus into Indian Ocean from East Africa. J Gen Virol. 2008;89:2754–60. doi: 10.1099/vir.0.2008/005413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vazeille M, Moutailler S, Coudrier D, Rousseaux C, Khun H, Huerre M, et al. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS One. 2007;2(11):e1168. doi: 10.1371/journal.pone.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, et al. Genome microevolution of Chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3(7):e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar NP, Joseph R, Kamaraj T, Jambulingam P. A226V mutation in virus during the 2007 Chikungunya outbreak in Kerala, India. J Gen Virol. 2008;89:1945–8. doi: 10.1099/vir.0.83628-0. [DOI] [PubMed] [Google Scholar]
- 40.Bordi L, Carletti F, Castilletti C, Chiappini R, Sambri V, Cavrini F, et al. Presence of the A226V mutation in autochthonous and imported Italian Chikungunya virus strains. Clin Infect Dis. 2008;47:428–9. doi: 10.1086/589925. [DOI] [PubMed] [Google Scholar]
- 41.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with Chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–6. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 43.Grandadan M, Caro V, Plumet S, Thiberge JM, Souarès Y, Failloux AB, et al. Chikungunya virus, Southeastern France. Emerg Infect Dis. 2011;17(5):910–3. doi: 10.3201/eid1705.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassadou S, Boucau S, Petit-Sinturel M, Huc P, Leparc-Goffart I, Ledrans M. Emergence of chikungunya fever on the French side of Saint Martin island, October to December 2013. Euro Surveill. 2014;19(13):20752. doi: 10.2807/1560-7917.es2014.19.13.20752. [DOI] [PubMed] [Google Scholar]
- 45.van Bortel W, Dorleans F, Rosine J, Blateau A, Rousset D, Matheus S, et al. Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. Euro Surveill. 2014;19(13):20759. doi: 10.2807/1560-7917.es2014.19.13.20759. [DOI] [PubMed] [Google Scholar]
- 46.Editorial. Chikungunya — coming to America. Lancet. 2014;383:488. doi: 10.1016/S0140-6736(14)60167-7. [DOI] [PubMed] [Google Scholar]
- 47.Chretien JP, Anyamba A, Bedno SA, Breiman RF, Sang R, Sergon K, et al. Drought-associated Chikungunya emergence along coastal East Africa. Am J Trop Med Hyg. 2007;76:405–7. [PubMed] [Google Scholar]
- 48.Anonymous. Chikungunya reaches northern Italy: effect of global warming? Arch Dis Child. 2008;93:312. [PubMed] [Google Scholar]
- 49.Rezza G. Re-emergence of chikungunya and other scourges: the role of globalization and climate change. Ann Ist Super Sanita. 2008;44:315–8. [PubMed] [Google Scholar]
- 50.ECDC. Climate change and communicable diseases in the EU Member States. ECDC Technical Document. Stockholm: European Centre for Disease Prevention and Control; 2010. [Google Scholar]
- 51.Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;4:e646. doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benedict MQ, Levine RS, Hawley WA, Lounibos P. Spread of the Tiger: global risk of invasion by the mosquto Aedes albopicus. Vector Borne Zoonotic Dis. 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hotta S. Dengue epidemics in Japan, 1942–45. J Trop Med Hyg. 1953;56:83. [PubMed] [Google Scholar]
- 54.Delatte H, Paupy C, Dehecq JS, Thiria J, Failloux AB, Fontenille D. [Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control]. Parasite. 2008;15:3–13. doi: 10.1051/parasite/2008151003. French. [DOI] [PubMed] [Google Scholar]
- 55.Peng HJ, Lai HB, Zhang BY, Xu BY, Zhang H, Liu WH, et al. A local outbreak of dengue caused by an imported case in Dongguan, China. BMC Public Health. 2012;12:83. doi: 10.1186/1471-2458-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rezza G. Aedes albopictus and the reemergence of Dengue. BMC Public Health. 2012;12:72. doi: 10.1186/1471-2458-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scholte EJ, Dijkstra E, Blok H, de Vries A, Takken W, Hofhuis A, et al. Accidental importation of the mosquito Aedes albopictus into the Netherlands: a survey of mosquito distribution and the presence of dengue virus. Med Vet Entomol. 2008;22(4):352–8. doi: 10.1111/j.1365-2915.2008.00763.x. [DOI] [PubMed] [Google Scholar]
- 58.Reiter P. Aedes albopictus and the world trade in used tires, 1988–1995: the shape of things to come? J Am Mosq Cont Assoc. 1998;14:83–94. [PubMed] [Google Scholar]
- 59.Moutailler S, Barré H, Vazeille M, Failloux AB. Recently introduced Aedes albopictus in Corsica is competent to chikungunya virus and in a lesser extent to dengue virus. Trop Med Int Health. 2009;14:1105–9. doi: 10.1111/j.1365-3156.2009.02320.x. [DOI] [PubMed] [Google Scholar]