Abstract
Background:
Millions of people in the world have diabetes mellitus and its prevalence is growing. Oxidative stress, advanced glycation end-products (AGEs) and receptor for advanced glycation end-products (RAGE) play key role in the pathogenesis of diabetes. New and safe strategies of remedy are needed for this disease.
Objectives:
We hypothesized that resveratrol may exert a renal protective effect on diabetic rats.
Materials and Methods:
Male rats with diabetes were treated with or without resveratrol as 1, 5, 10 mg/kg of body weight for 30 days. The total AGEs and malondialdehyde levels in kidney tissues were determined by spectrofluorimetric method and the insulin level was assayed using ELISA. The total antioxidant capacity contents in kidney and the glucose in plasma were measured by a colorimetric assay. The expression of RAGE was assayed in kidneys of all animals using quantitative PCR.
Results:
In resveratrol-treated rats with diabetes, malondialdehyde levels, plasma glucose and expression of RAGE were significantly reduced compared with the untreated group. Moreover, the total antioxidant and insulin levels significantly increased in treated rats. There was no significant difference in the AGEs contents among all the groups.
Conclusions:
These results revealed that resveratrol has beneficial effects on kidney by extenuating the oxidative stress and down-regulation of RAGE expression.
Keywords: Resveratrol, Diabetes Mellitus, Advanced Glycation End-product, Oxidative Stress
1. Background
Diabetes mellitus (DM) is one of the greatest threats to modern global health and based on epidemiological studies, diabetes is a huge and growing problem. In 2013, more than 382 million people were affected by diabetes; every six seconds, a person dies from diabetes (1). Hyperglycemia in diabetic condition leads to microvascular (retinopathy, nephropathy, and neuropathy) and macrovascular (cardiac, cerebral) complications and early death (2).
Oxidative stress is one of the major factors in the pathogenesis of diabetic complications; particularly, diabetic nephropathy and hyperglycemia are important sources of reactive oxygen species (ROS) (3). Advanced glycation end-products (AGEs), resulting from binding of sugars to proteins, lipids and nucleic acids by nonenzymatic reactions have been implicated as key pathogenic factors in initiation and progression of diabetic complications (4).
One of the important pathways through which AGEs may exert their pathological effects is via interaction with the receptor for advanced glycation end product (RAGE) (5). RAGE as a member of the immunoglobulin superfamily of cell surface molecules is composed of three extracellular domains and a fourth transmembrane domain which is connected to a highly-charged fifth intracellular domain (6). Ligand–RAGE interactions activate cell signaling cascades, which control several genes through NF-kB (nuclear factor kappa-light-chain B) factor. Such interactions have causative effects on many complications of diabetes (7).
To reduce the complications of diabetes, several strategies including diet, exercise, oral antidiabetic drugs and insulin therapy are used. Chemical drugs such as biguanides, sulphonylureas and ɑ-glucosidase inhibitors have many side effects (8). Therefore, new therapeutic strategies are required to diminish the health problems of this increasing disease. In the recent years, many plants have been used to improve hyperglycemia in diabetic rats (9); among them, resveratrol (3, 5, 4-trihydroxylstilbene, RSV) is of the highest interest. RSV possesses antidiabetic effects (10).
Recently, some studies have emphasized on the role of resveratrol in prevention or treatment of diabetic nephropathy (11-15), since RAGE and oxidative stress have key roles in pathogenesis of kidney dysfunction.
2. Objectives
This study was designed to determine the effects of RSV on mRNA expression of RAGE and oxidative stress status in kidney of rats with diabetes.
3. Materials and Methods
3.1. Experimental Design
Male Wistar rats (Razi Institute, Tehran, Iran), weighing 150 - 200 g (ranging from 8 to 10 weeks of age), were housed at room temperature (22 - 25°C) with 12:12-hour light/dark cycles and free access to food and water. All the procedures for the treatment of animals were approved by the Research Committee of Hamedan University of Medical Sciences, Iran. Type 2 diabetes was induced by injection of streptozotocin (STZ, Sigma) (60 mg/kg; intraperitoneal (IP)) dissolved in 0.1 M citrate buffer (pH 4.5), 15 minutes after the prescription of nicotinamide (Sigma) (110 mg/kg; IP) in 12-hour-fasted rats. Citrate buffer was injected alone in control rats. Nicotinamide preserves the pancreas β-cells (up to 40%) from STZ cytotoxicity and produces diabetes type 2 (16). Afterwards, 72-hour blood glucose levels were measured using a strip-operated blood glucose sensor (Accuchek; Roche, Germany). Animals were considered diabetic if fasting blood glucose levels exceeded 250 mg/dL. Rats with diabetes were randomly divided into four groups (n = 8 in each group) and treated without or with RSV. The rats were divided into five groups (n = 8 in each): normal (C), diabetic (D), diabetic treated with RSV 1 mg/kg/day (D + RSV1), diabetic treated with RSV 5 mg/kg/day (D + RSV5), diabetic treated with RSV 10 mg/kg/day (D + RSV10). Resveratrol treatment was performed orally for 30 days. The dosage was regulated every week. At the end of the experimental period, fasted rats were anesthetized with ketamine (50 mg/kg) and blood samples (5 mL) were collected from each rat for biochemical measurements. Thereafter, the rats were scarified and the kidneys were quickly frozen in liquid nitrogen and kept at -80°C. Fasting blood glucose, urea and creatinine were measured using proper kits (Pars Azmun, Iran) and the rat insulin ELISA kit (Alpco, USA; sensitivity = 3.1 μU /mL) was used to determine serum insulin levels. Homeostatic model assessment (HOMA) (insulin resistance index) was performed using the following formula: insulin (μU/mL) × glucose (mmol/L)/22.5] (17). Scientists are interested in addressing the best dosage of RSV essential to take benefits. Different doses of RVS have been tested in rats, but overall, it is divided to low dose (2.5 - 5 mg/kg/day) and high dose (25 - 50 mg/kg/day) (18). In our experiments, we examined low doses (1 and 5 mg/kg) and a moderate dose (10 mg/kg).
3.2. Preparation of Kidney Tissue Homogenate
Kidney tissues from control and experimental groups of rats were excised and rinsed with ice-cold saline and grounded to a fine powder using liquid nitrogen. The homogenate was resuspended in ice cold lysis buffer (10 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid ), 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 0.1% triton X100, 0.5 mM Dithiothreitol, protease inhibitor cocktail, pH 7.9) and incubated on ice for 20 minutes. The tissue homogenates were then vortex and centrifuged (20000 g; 10 minutes; 4°C), and the supernatants were retained for analysis (19). The protein concentration for tissue homogenates was determined by Bradford assay using bovine serum albumin (BSA) as a standard (20).
3.3. Antioxidant Potential Measurement
Total antioxidant capacity was determined using ferric reducing antioxidant potential (FRAP) assay. This procedure involves the reduction of ferric tripyridyltriazine (Fe III-TPTZ) to a blue colored Fe II-TPTZ by biological antioxidants. The change of absorbance at 600 nm of the sample was compared with the change of absorbance of a known standard (FeSO4 7H2O) (21).
3.4. Lipid Peroxidation
The malondialehyde (MDA) content in kidney tissue homogenate was measured spectrofluorometrically as a thiobarbiuric acid reactive substances (TBARS) according to Yagi’s methods (22). The results were reported per milligram of protein content of the samples.
3.5. AGEs Assay
Determination of AGEs (ie, some fluorescent products from the AGEs family) was based on the spectrofluorimetric detection according to Kalousova et al. method (23). Fluorescence intensity was expressed in arbitrary units per milligram protein (AU/mg protein).
3.6. Real-time qPCR Analysis of Receptor for Advanced Glycation End-product
Total RNA was extracted from the renal tissues of control and experimental groups of rats by TRIzol® reagent (Invitrogen), according to the manufacturer’s protocol. Total RNA was reverse transcribed into single-strand cDNA using RevertAid™ first strand cDNA synthesis kit (Thermo Scientific) according to the manufacturer's instructions and analyzed by CFX96 real-time PCR detection system (BioRad, USA) using the SYBR premix Ex Taq 2 kit (Takara). The amplification protocol comprised one cycle at 95°C for one minute, followed by 40 cycles at 95°C for 30 seconds, 58°C for 30 seconds, and then 72°C for 30 seconds. The primer sequences were designed and synthesized by Takapou Zist Company (Iran). The primer sequences were as follows: RAGE: forward: 5′-GAGTCCGAGTCTACCAGATTCC-3′, reverse: 5′-GGTCTCCTCCTTCACAACTGTC-3′; GAPDH: forward: 5′-AAGGTCGGTGTGAACGGATTTGG-3′, reverse: 5′-TCCTGGAAGATGGTGATGGGTT-3′. Relative copy numbers were obtained from standard curve values and were normalized to values obtained for the internal control GAPDH. The fold change in expression was then calculated by 2-ΔΔCT formula.
3.7. Statistical Analysis
All the data were expressed as mean ± SD. The statistical differences in studying parameters among the groups were evaluated by SPSS 11 software by one-way analysis of variance; Tukey’s post hoc test was used to evaluate the difference in the means of data between related groups. In all the analyses P < 0.05 was considered significant.
4. Results
4.1. General Measurements
Body weights and biochemical parameters are summarized in Table 1. Body weights of the diabetic group showed significant decreases in comparison with the control (P < 0.001). Weight losses in RSV-treated diabetic groups were significantly lower compared with the diabetic group (P < 0.01). Fasting blood glucose, urea and creatinine levels were significantly higher in the diabetic group in comparison with the control (P < 0.001); but in treated groups, they were significantly lower compared with the diabetic group (P < 0.01). Insulin concentration in the diabetic group was significantly lower than that of the control group (P < 0.001); however, in RSV (5 mg/kg and 10 mg/kg)-treated groups, the insulin level was markedly higher than the diabetic group. Insulin resistance in the RSV-treated groups was significantly lower compared with the diabetic group (P < 0.05). Our results demonstrated that there were no significant differences between the effects of two doses of 5 and 10 mg/kg in all the measurements.
Table 1. Effects of Resveratrol on Body Weight and Biochemical Parameters of Control Diabetic and RSV-Treated Rats With Diabetes a, b, c, d.
| Parameters/Groups | C | D | D + RSV1 | D + RSV5 | D + RSV10 |
|---|---|---|---|---|---|
| Body Weight, g | 260.6 ± 14.7 | 191.2 ± 14.4 e | 222.6 ± 17.6 f | 221.5 ± 14.9 f | 211.0 ± 18.01 f |
| Fasting Blood Glucose, mg/dL | 92.0 ± 10.8 | 303.3 ± 92.1 e | 271.3 ± 77.5 | 192.5 ± 84.8 f | 190.3 ± 68.6 f |
| Insulin, μU/mL | 27.7 ± 2.3 | 18.1 ± 2.7 e | 20.3 ± 2.4 | 23.8 ± 2.6 f | 24.5 ± 2.2 f |
| Urea, mg/dL | 23.2 ± 6.9 | 63.5 ± 16.9 e | 38.1 ± 7.5 f | 33.4 ± 9.1 f | 48.1 ± 5.1 |
| Creatinine, mg/dL | 0.48 ± 0.07 | 0.69 ± 0.07 e | 0.53 ± 0.03 f | 0.53 ± 0.04 f | 0.47 ± 0.08 f |
| HOMA-IR | 6.29 ± 0.06 | 13.6 ± 0.61 e | 13.5 ± 0.45 | 11.3 ± 0.54 g | 11.4 ± 0.37 g |
aData are presented as mean ± SD.
b n = 8.
c C, control; D, diabetic; D + RSV1, diabetic treated with RSV (1 mg/kg body weight); D + RSV5, diabetic treated with RSV (5 mg/kg body weight); D + RSV10, diabetic treated with RSV (10 mg/kg body weight).
d 1 U of insulin is equal to 0.04167 mg and 1 mg/mL is about 25 μU/mL.
e P < 0.001 versus control.
f P < 0.01 versus diabetic group.
g P < 0.05 versus diabetic group.
4.2. Effects of Resveratrol on Lipid Peroxidation
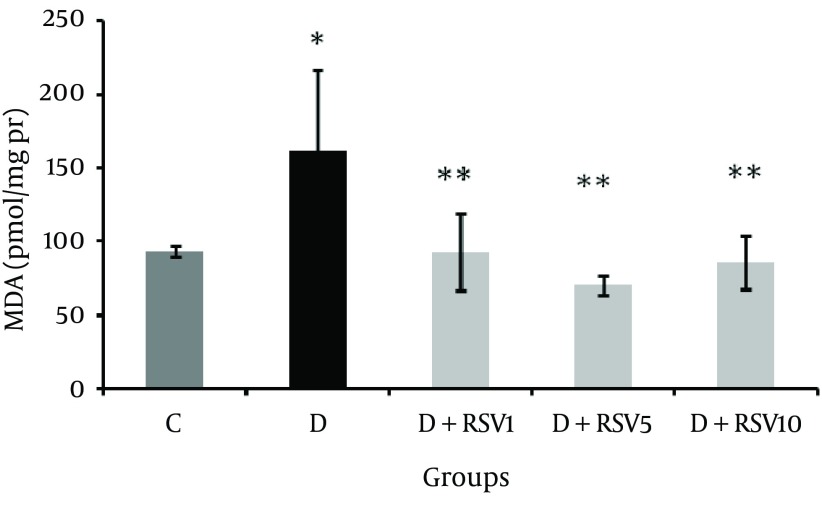
MDA levels as a lipid peroxidation indicator showed significant (P < 0.05) increase in kidney tissue of the diabetic group compared with the control group (Figure 1). MDA concentration in the resveratrol groups was significantly lower than the diabetic group (P < 0.05).
Figure 1. Effect of Resveratrol on MDA Level of Kidney Tissue in Diabetic Rats.
The data represent mean ± SD of 8 rats. *p < 0.05 versus control; **p < 0.05 versus diabetes group. MDA: malondialehyde,C: control normal, D: diabetic, D + RSV1: diabetic treated with RSV (1 mg/kg body weight), D + RSV5: diabetic treated with RSV (5 mg/kg body weight), D + RSV10: diabetic treated with RSV (10 mg/kg body weight).
4.3. Effects of Resveratrol on Total Antioxidant Capacity
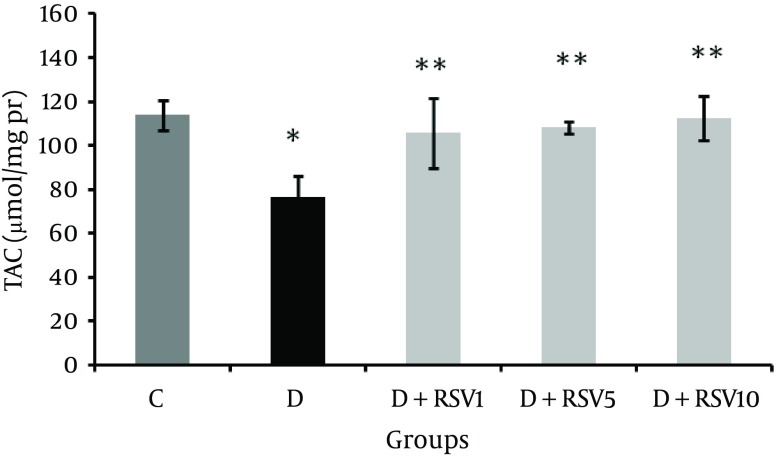
Renal tissue total antioxidant capacity (TAC) in the diabetic group was significantly lower than that of the control group (P < 0.05, Figure 2). TAC in all the RSV-treated groups was significantly higher compared to the untreated diabetic group (P < 0.05).
Figure 2. Effects of Resveratrol on Total Antioxidant Capacity in Kidney of Diabetic Rats.
The data represent mean ± SD of 8 rats. *p < 0.05 versus control; **p < 0.05 versus diabetic group. TAC: Total Antioxidant Capacity , C: control normal, D: diabetic, D + RSV1: diabetic treated with RSV (1 mg/kg body weight), D + RSV5: diabetic treated with RSV (5 mg/kg body weight), D + RSV10: diabetic treated with RSV (10mg/kg body weight).
4.4. Effects of Resveratrol on Advanced Glycation End-products
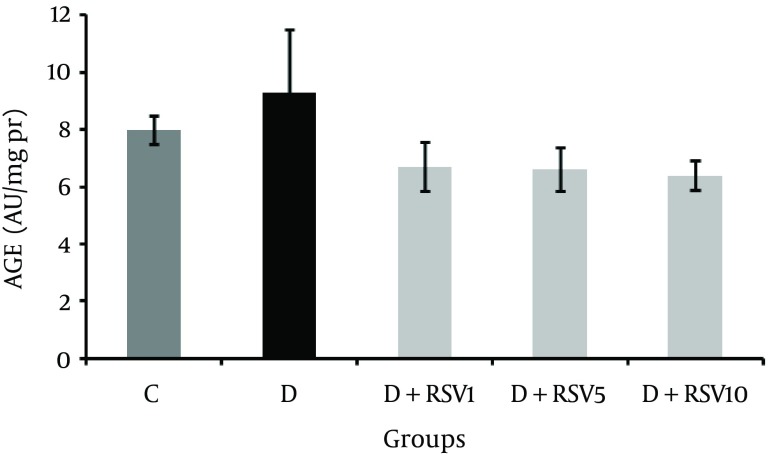
The AGEs level in renal tissue did not show significant difference among all the studied groups (Figure 3).
Figure 3. Effects of Resveratrol on Advanced Glycation End-products in Kidney of Diabetic Rats.
The data represent mean ± SD of 8 rats. AGE: Advanced Glycation End-Products, C: control normal, D: diabetic, D+RSV1: diabetic treated with RSV (1 mg/kg body weight), D + RSV5: diabetic treated with RSV (5 mg/kg body weight), D + RSV10: diabetic treated with RSV (10 mg/kg body weight).
4.5. Effects of Resveratrol on Receptor for Advanced Glycation End-products Expression
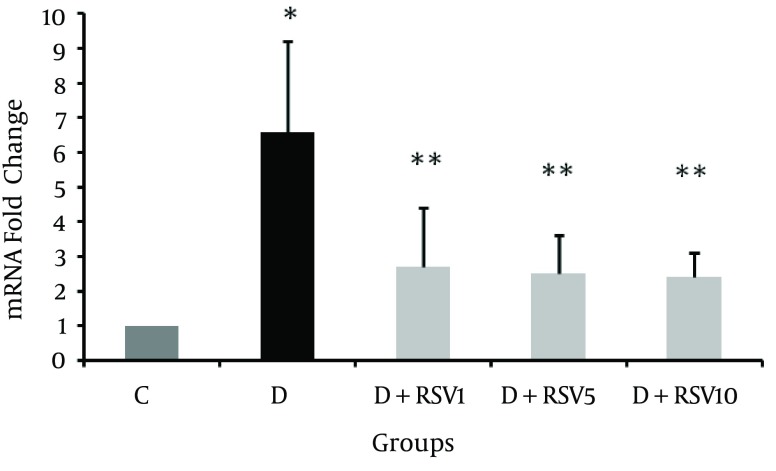
The mRNA levels of RAGE in kidneys of all the groups were determined. As shown in Figure 4, RAGE expression significantly increased in the diabetic group compared with the control (P < 0.001). The RAGE levels in RSV-treated groups significantly decreased in comparison to the diabetic group (P < 0.05).
Figure 4. mRNA Folding Changes of RAGE in Renal Tissue of Diabetic and Resveratrol Treated Diabetic Rats.
The data represent mean ± SD of 8 rats. *p < 0.001 versus control; **p < 0.05 versus diabetic group. C: control normal, D: diabetic, D + RSV1: diabetic treated with RSV (1 mg/kg body weight), D + RSV5: diabetic treated with RSV (5 mg/kg body weight), D + RSV10: diabetic treated with RSV (10 mg/kg body weight).
5. Discussion
In this investigation, we evaluated the useful effects of RSV on kidneys of male rats with type 2 diabetes induced by streptozotocin (STZ) and nicotinamide. The results of the present study revealed that serum urea, creatinine and glucose levels decreased after treatment with RSV. RSV increases the glucose uptake in cells and stimulates glucose transporters to uptake more glucose in an insulin-independent manner (24, 25). In this study, diabetic rats developed insulin resistance and homeostatic model assessment - insulin resistance (HOMA-IR) in RSV-treated groups was lower than the diabetic group, indicating that this group had higher insulin sensitivity, which was in part because of the RSV effects on improvement of the insulin level in rats with diabetes; this can be another way to reduce blood glucose and it exerts its anti-hyperglycemic effects. A recent study demonstrated that RSV treatment significantly reduced HOMA-IR (26)
RSV can increase insulin secretion from beta cells (27-29), which is in supporting of our data, although Szkudelska et al. reported reduction of insulin secretion (30). In our opinion, it is dependent on the animal condition; in decreased insulin level condition, RSV stimulates secretion of insulin and in increased insulin level condition, resveratrol reduces insulin secretion. Based on our condition, RSV stimulated insulin secretion.
In obesity and high fat conditions, because of diminishing of the total body fat, RSV reduce weight gain, which is a beneficial effect of RSV in obesity; but in diabetes, because of improvement of diabetic complication and antidiabetic effects of RSV, weight gain improves with RSV administration (31, 32)
Our finding showed increased lipid peroxidation in renal tissue of diabetic rats. RSV hindered the production of MDA in kidney of diabetic groups. Oxidative stress has a key role in the progress of diabetic complications, particularly diabetic nephropathy, and is central in the mechanisms of the disease pathogenesis. Hyperglycemia in diabetic conditions causes an elevated flux of electron in mitochondria, which inhibits complex III and consequently increases superoxide radical production (33). Other studies support our findings (11, 34).
Free radicals such as reactive oxygen species are formed in body as a result of metabolic pathways and the environment. There are antioxidant defenses that delete these free radicals enzymatically and nonenzymatically (vitamin C, vitamin E, glutathione and carotenoids) (35). Based on the findings of this study, the total antioxidant capacity was lower in the diabetic group and RSV administration elevated the antioxidant content in diabetic rats. RSV chemically includes two isomers of Z and E (cis and trans); that trance isomer (E form) shows a higher antioxidant power (36). Leonard et al. demonstrated that RSV had radical scavenging properties (37). There are other studies supporting our results. Palsamy et al. demonstrated improvement of antioxidant enzyme activity such as superoxide dismutase, glutathione peroxidase, and catalase with short-term administration of resveratrol (38). Khamneh et al. also reported same results with long-term RSV treatment (39). We measured the total antioxidant power instead of each antioxidant separately, because measuring all the antioxidants is difficult and they have different interactions. In this study, the FRAP assay was used, which is convenient and practically simple. We performed experiments with three different concentrations of RSV in a short period; in each treated group, the antioxidant capacity levels were normalized.
Our results revealed no significant difference in AGEs levels between all the groups; although, AGEs level in the diabetic group was higher than other groups. AGEs formation is a nonenzymatic process which includes multiple chemical reactions with condensing carbonyl and amino groups and progress slowly in the body tissue under normal conditions. Half-life of macromolecules (proteins), duration of hyperglycemia conditions, and hyperglycemia level are key factors in the formation of AGEs (40).
Results of the present study demonstrated significant elevation of receptor for AGEs (RAGE) in kidney tissues of the diabetic group. Treatment with RSV significantly reduced mRNA transcripts of RAGE in renal tissue of the three RSV-treated groups. Jing et al. reported a decrease in RAGE expression in aortic tissue of RSV-treated diabetic rats (31). RAGE plays an important role in diabetes complications and AGE/RAGE axis triggers signaling cascades leading to deleterious consequents (41). Expression of RAGE in physiological conditions is at minimum levels and with accretion of its ligands it is up-regulated. Since RAGE recognizes the tertiary structure of ligands, its ligands are numerous (AGEs, S100 proteins, macrophage-1, high-mobility box group-1, and oxidized low-density lipoprotein (LDL)) (42). AGE-RAGE interaction activates various signaling pathways. One of the consequences is the generation of ROS (such as superoxide and hydrogen peroxide) by activation of NADPH oxidase (43, 44). Increased lipid peroxidation and decreased total antioxidant in the diabetic group of the present study supports this idea. RAGE down-regulation mechanisms in RSV-treated groups are not elucidated. RSV may inhibit AGEs formation or may involve in downstream of RAGE signaling, which need further research to reveal details.
In conclusion, our observations disclosed the profitable nature of RSV in kidney of diabetic rats and clarified that the useful effects are because of the reduction of oxidative stress and hyperglycemia and increase of the total antioxidant. Furthermore, RSV administration decreases RAGE expression in renal tissues of diabetic rats and may be used as a useful remedy for diabetes in future after clinical trial investigations and finding a suitable dose of resveratrol. For exploring the exact mechanisms, more studies are required.
Acknowledgments
We would like to thank Ms. Noushin Shabab for qPCR assistance and Ms. Taibeh Ghiasvand. This study was supported by Hamadan University of Medical Sciences. This study was a part of the MSc thesis of Heresh Moridi.
References
- 1.International Diabetes Federation. 2014 Available from: http://www.idf.org/diabetesatlas.
- 2.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–88. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 3.Ha H, Hwang IA, Park JH, Lee HB. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82 Suppl 1:S42–5. doi: 10.1016/j.diabres.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 5.Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1(1):1–15. doi: 10.1016/s0047-6374(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 6.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl). 2005;83(11):876–86. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 7.Bierhaus A, Nawroth PP. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia. 2009;52(11):2251–63. doi: 10.1007/s00125-009-1458-9. [DOI] [PubMed] [Google Scholar]
- 8.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinent M, Blay M, Blade MC, Salvado MJ, Arola L, Ardevol A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology. 2004;145(11):4985–90. doi: 10.1210/en.2004-0764. [DOI] [PubMed] [Google Scholar]
- 10.Vallianou NG, Evangelopoulos A, Kazazis C. Resveratrol and diabetes. Rev Diabet Stud. 2013;10(4):236–42. doi: 10.1900/RDS.2013.10.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbe H, Vardi N, Esrefoglu M, Ates B, Yologlu S, Taskapan C. Amelioration of streptozotocin-induced diabetic nephropathy by melatonin, quercetin, and resveratrol in rats. Hum Exp Toxicol. 2015;34(1):100–13. doi: 10.1177/0960327114531995. [DOI] [PubMed] [Google Scholar]
- 12.Xu F, Wang Y, Cui W, Yuan H, Sun J, Wu M, et al. Resveratrol Prevention of Diabetic Nephropathy Is Associated with the Suppression of Renal Inflammation and Mesangial Cell Proliferation: Possible Roles of Akt/NF-kappaB Pathway. Int J Endocrinol. 2014;2014:289327. doi: 10.1155/2014/289327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji H, Wu L, Ma X, Ma X, Qin G. The effect of resveratrol on the expression of AdipoR1 in kidneys of diabetic nephropathy. Mol Biol Rep. 2014;41(4):2151–9. doi: 10.1007/s11033-014-3064-2. [DOI] [PubMed] [Google Scholar]
- 14.Wen D, Huang X, Zhang M, Zhang L, Chen J, Gu Y, et al. Resveratrol attenuates diabetic nephropathy via modulating angiogenesis. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang B, Guo L, Li BY, Zhen JH, Song J, Peng T, et al. Resveratrol attenuates early diabetic nephropathy by down-regulating glutathione s-transferases Mu in diabetic rats. J Med Food. 2013;16(6):481–6. doi: 10.1089/jmf.2012.2686. [DOI] [PubMed] [Google Scholar]
- 16.Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, et al. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47(2):224–9. doi: 10.2337/diab.47.2.224. [DOI] [PubMed] [Google Scholar]
- 17.Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, Araki-Sasaki R, et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24(2):362–5. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee S, Dudley JI, Das DK. Dose-dependency of resveratrol in providing health benefits. Dose Response. 2010;8(4):478–500. doi: 10.2203/dose-response.09-015.Mukherjee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming TH, Theilen TM, Masania J, Wunderle M, Karimi J, Vittas S, et al. Aging-dependent reduction in glyoxalase 1 delays wound healing. Gerontology. 2013;59(5):427–37. doi: 10.1159/000351628. [DOI] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239(1):70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 22.Yagi K. Assay for blood plasma or serum. Meth Enzymol. 1984;105:328–31. doi: 10.1016/s0076-6879(84)05042-4. [DOI] [PubMed] [Google Scholar]
- 23.Kalousova M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res. 2002;51(6):597–604. [PubMed] [Google Scholar]
- 24.Palsamy P, Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother. 2008;62(9):598–605. doi: 10.1016/j.biopha.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 25.Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290(6):E1339–46. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Sun X, Li X, Dong X, Li P, Zhao L. Resveratrol attenuates intermittent hypoxia-induced insulin resistance in rats: involvement of Sirtuin 1 and the phosphatidylinositol-4,5-bisphosphate 3-kinase/AKT pathway. Mol Med Rep. 2015;11(1):151–8. doi: 10.3892/mmr.2014.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen WP, Chi TC, Chuang LM, Su MJ. Resveratrol enhances insulin secretion by blocking K(ATP) and K(V) channels of beta cells. Eur J Pharmacol. 2007;568(1-3):269–77. doi: 10.1016/j.ejphar.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 28.Palsamy P, Subramanian S. Modulatory effects of resveratrol on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Chem Biol Interact. 2009;179(2-3):356–62. doi: 10.1016/j.cbi.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Rouse M, Younes A, Egan JM. Resveratrol and curcumin enhance pancreatic beta-cell function by inhibiting phosphodiesterase activity. J Endocrinol. 2014;223(2):107–17. doi: 10.1530/JOE-14-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010;635(1-3):1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 31.Jing YH, Chen KH, Yang SH, Kuo PC, Chen JK. Resveratrol ameliorates vasculopathy in STZ-induced diabetic rats: role of AGE-RAGE signalling. Diabetes Metab Res Rev. 2010;26(3):212–22. doi: 10.1002/dmrr.1076. [DOI] [PubMed] [Google Scholar]
- 32.Chen KH, Cheng ML, Jing YH, Chiu DT, Shiao MS, Chen JK. Resveratrol ameliorates metabolic disorders and muscle wasting in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2011;301(5):E853–63. doi: 10.1152/ajpendo.00048.2011. [DOI] [PubMed] [Google Scholar]
- 33.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97(22):12222–6. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu L, Zhang Y, Ma X, Zhang N, Qin G. The effect of resveratrol on FoxO1 expression in kidneys of diabetic nephropathy rats. Mol Biol Rep. 2012;39(9):9085–93. doi: 10.1007/s11033-012-1780-z. [DOI] [PubMed] [Google Scholar]
- 35.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Pangeni R, Sahni JK, Ali J, Sharma S, Baboota S. Resveratrol: review on therapeutic potential and recent advances in drug delivery. Expert Opin Drug Deliv. 2014;11(8):1285–98. doi: 10.1517/17425247.2014.919253. [DOI] [PubMed] [Google Scholar]
- 37.Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, et al. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309(4):1017–26. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 38.Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta. 2011;1812(7):719–31. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Khamneh S, Ghadiri SF, Afshar F. Long-term Resveratrol Administration Reduces Renal Oxidative Stress and Apoptosis Rate In Experimental Model of Type 2 Diabetes. 2012;9(4):2997–3001. [Google Scholar]
- 40.Prasad C, Imrhan V, Marotta F, Juma S, Vijayagopal P. Lifestyle and Advanced Glycation End Products (AGEs) Burden: Its Relevance to Healthy Aging. Aging Dis. 2014;5(3):212–7. doi: 10.14336/AD.2014.0500212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bierhaus A, Humpert PM, Stern DM, Arnold B, Nawroth PP. Advanced glycation end product receptor-mediated cellular dysfunction. Ann N Y Acad Sci. 2005;1043:676–80. doi: 10.1196/annals.1333.077. [DOI] [PubMed] [Google Scholar]
- 42.Farmer DG, Kennedy S. RAGE, vascular tone and vascular disease. Pharmacol Ther. 2009;124(2):185–94. doi: 10.1016/j.pharmthera.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280(5):E685–94. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 44.Karimi J, Goodarzi MT, Tavilani H, Khodadadi I, Amiri I. Increased receptor for advanced glycation end products in spermatozoa of diabetic men and its association with sperm nuclear DNA fragmentation. Andrologia. 2012;44 Suppl 1:280–6. doi: 10.1111/j.1439-0272.2011.01178.x. [DOI] [PubMed] [Google Scholar]