Abstract
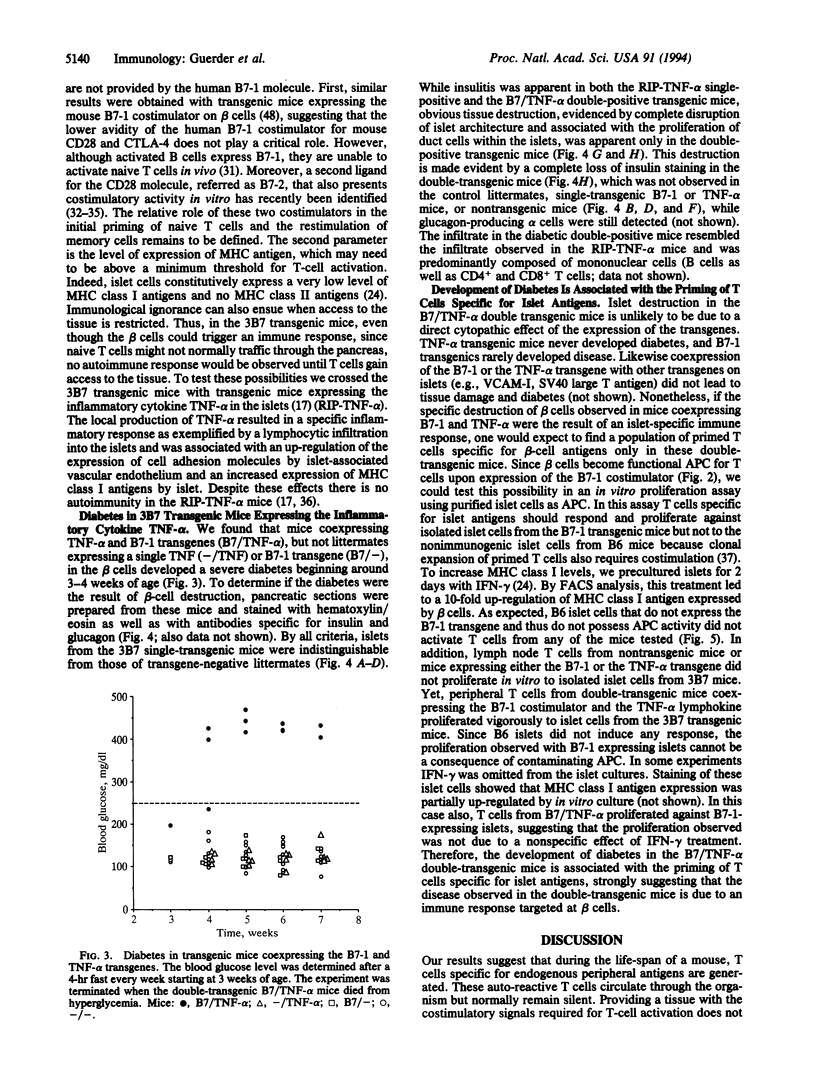
Tolerance to peripheral antigens is thought to result from the inability of parenchymal tissue to stimulate T cells--an inability that is believed to relate to the lack of expression of the costimulatory signal(s) required for T-cell activation. To test this model, we generated transgenic mice expressing costimulatory molecule B7-1 on the B cells of the pancreas. We find that islets from these transgenic mice are immunogenic for naive T cells in vitro and in vivo. Nonetheless, mice expressing the costimulator B7-1 specifically on beta cells do not develop diabetes, suggesting that expression of the B7-1 costimulator is not sufficient to abrogate the tolerance to peripheral antigens. We have reported that tumor necrosis factor alpha subunit (TNF-alpha) expressed by beta cells leads to a local inflammation but no islet destruction. Strikingly, however, the combination of a local inflammation due to the expression of the cytokine TNF-alpha and the expression of B7-1 results in tissue destruction and diabetes.
Full text
PDF

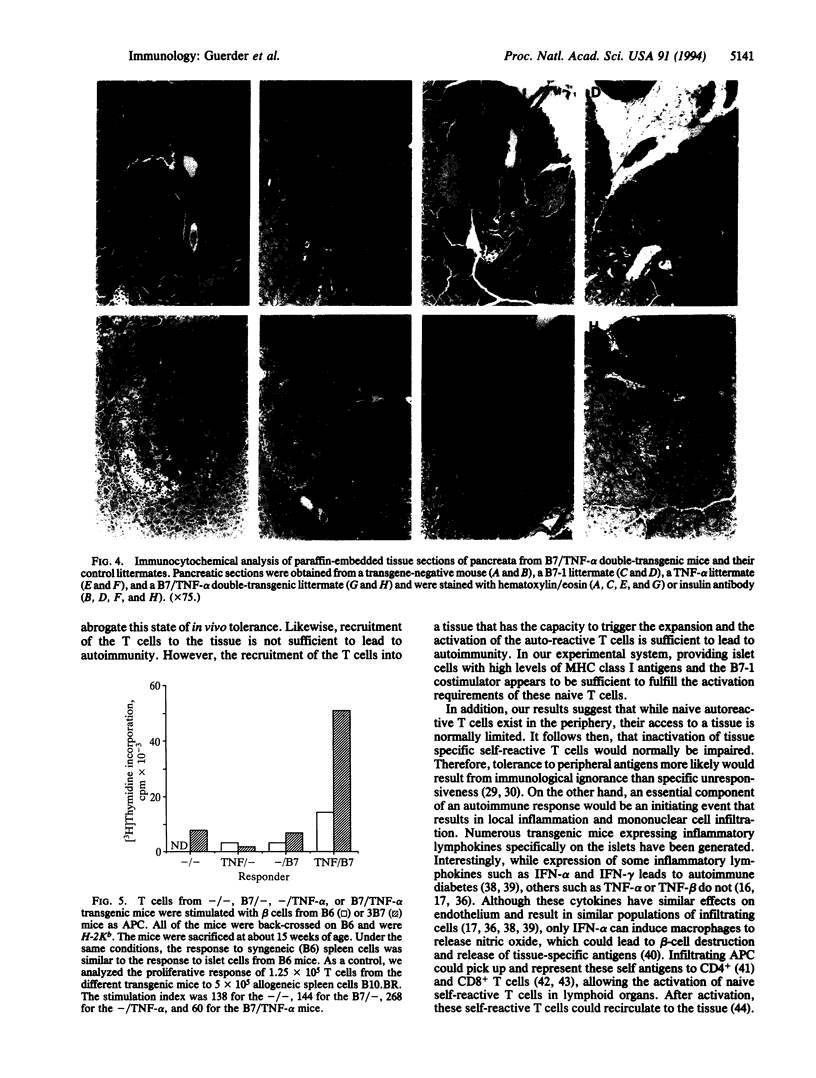

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J., Campbell I. L., Morahan G., Mandel T. E., Harrison L. C., Miller J. F. Diabetes in transgenic mice resulting from over-expression of class I histocompatibility molecules in pancreatic beta cells. Nature. 1988 Jun 9;333(6173):529–533. doi: 10.1038/333529a0. [DOI] [PubMed] [Google Scholar]
- Azuma M., Ito D., Yagita H., Okumura K., Phillips J. H., Lanier L. L., Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993 Nov 4;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- Bevan M. J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976 May 1;143(5):1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M., Guagliardi L. E. The cell biology of antigen processing and presentation. Annu Rev Immunol. 1991;9:707–744. doi: 10.1146/annurev.iy.09.040191.003423. [DOI] [PubMed] [Google Scholar]
- Böhme J., Haskins K., Stecha P., van Ewijk W., LeMeur M., Gerlinger P., Benoist C., Mathis D. Transgenic mice with I-A on islet cells are normoglycemic but immunologically intolerant. Science. 1989 Jun 9;244(4909):1179–1183. doi: 10.1126/science.2499048. [DOI] [PubMed] [Google Scholar]
- Campbell I. L., Wong G. H., Schrader J. W., Harrison L. C. Interferon-gamma enhances the expression of the major histocompatibility class I antigens on mouse pancreatic beta cells. Diabetes. 1985 Nov;34(11):1205–1209. doi: 10.2337/diab.34.11.1205. [DOI] [PubMed] [Google Scholar]
- Chen L., Ashe S., Brady W. A., Hellström I., Hellström K. E., Ledbetter J. A., McGowan P., Linsley P. S. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992 Dec 24;71(7):1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Donohoe J. A., Andrus L., Bowen K. M., Simeonovic C., Prowse S. J., Lafferty K. J. Cultured thyroid allografts induce a state of partial tolerance in adult recipient mice. Transplantation. 1983 Jan;35(1):62–67. doi: 10.1097/00007890-198301000-00012. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Borriello F., Hodes R. J., Reiser H., Hathcock K. S., Laszlo G., McKnight A. J., Kim J., Du L., Lombard D. B. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science. 1993 Nov 5;262(5135):907–909. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Gribben J. G., Boussiotis V. A., Ng J. W., Restivo V. A., Jr, Lombard L. A., Gray G. S., Nadler L. M. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993 Nov 5;262(5135):909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- Fuchs E. J., Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992 Nov 13;258(5085):1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- Galvin F., Freeman G. J., Razi-Wolf Z., Hall W., Jr, Benacerraf B., Nadler L., Reiser H. Murine B7 antigen provides a sufficient costimulatory signal for antigen-specific and MHC-restricted T cell activation. J Immunol. 1992 Dec 15;149(12):3802–3808. [PubMed] [Google Scholar]
- Geiger T., Gooding L. R., Flavell R. A. T-cell responsiveness to an oncogenic peripheral protein and spontaneous autoimmunity in transgenic mice. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2985–2989. doi: 10.1073/pnas.89.7.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Harlan D. M., Hengartner H., Huang M. L., Kang Y. H., Abe R., Moreadith R. W., Pircher H., Gray G. S., Ohashi P. S., Freeman G. J. Mice expressing both B7-1 and viral glycoprotein on pancreatic beta cells along with glycoprotein-specific transgenic T cells develop diabetes due to a breakdown of T-lymphocyte unresponsiveness. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3137–3141. doi: 10.1073/pnas.91.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathcock K. S., Laszlo G., Dickler H. B., Bradshaw J., Linsley P., Hodes R. J. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993 Nov 5;262(5135):905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- Heath W. R., Allison J., Hoffmann M. W., Schönrich G., Hämmerling G., Arnold B., Miller J. F. Autoimmune diabetes as a consequence of locally produced interleukin-2. Nature. 1992 Oct 8;359(6395):547–549. doi: 10.1038/359547a0. [DOI] [PubMed] [Google Scholar]
- Higuchi Y., Herrera P., Muniesa P., Huarte J., Belin D., Ohashi P., Aichele P., Orci L., Vassalli J. D., Vassalli P. Expression of a tumor necrosis factor alpha transgene in murine pancreatic beta cells results in severe and permanent insulitis without evolution towards diabetes. J Exp Med. 1992 Dec 1;176(6):1719–1731. doi: 10.1084/jem.176.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulova L., Clark E. A., Shu G., Dupont B. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991 Mar 1;173(3):759–762. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Andreu J. L., Gonzalo J. A., Gutierrez-Ramos J. C., Martínez C. Interleukin-2, autotolerance, and autoimmunity. Adv Immunol. 1991;50:147–235. doi: 10.1016/s0065-2776(08)60825-1. [DOI] [PubMed] [Google Scholar]
- Lafferty K. J., Prowse S. J., Simeonovic C. J., Warren H. S. Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Annu Rev Immunol. 1983;1:143–173. doi: 10.1146/annurev.iy.01.040183.001043. [DOI] [PubMed] [Google Scholar]
- Landias D., Beck B. N., Buerstedde J. M., Degraw S., Klein D., Koch N., Murphy D., Pierres M., Tada T., Yamamoto K. The assignment of chain specificities for anti-Ia monoclonal antibodies using L cell transfectants. J Immunol. 1986 Nov 1;137(9):3002–3005. [PubMed] [Google Scholar]
- Lenschow D. J., Zeng Y., Thistlethwaite J. R., Montag A., Brady W., Gibson M. G., Linsley P. S., Bluestone J. A. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992 Aug 7;257(5071):789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D., Burkly L. C., Widera G., Cowing C., Flavell R. A., Palmiter R. D., Brinster R. L. Diabetes and tolerance in transgenic mice expressing class II MHC molecules in pancreatic beta cells. Cell. 1988 Apr 8;53(1):159–168. doi: 10.1016/0092-8674(88)90497-7. [DOI] [PubMed] [Google Scholar]
- Markmann J., Lo D., Naji A., Palmiter R. D., Brinster R. L., Heber-Katz E. Antigen presenting function of class II MHC expressing pancreatic beta cells. Nature. 1988 Dec 1;336(6198):476–479. doi: 10.1038/336476a0. [DOI] [PubMed] [Google Scholar]
- Morahan G., Allison J., Miller J. F. Tolerance of class I histocompatibility antigens expressed extrathymically. Nature. 1989 Jun 22;339(6226):622–624. doi: 10.1038/339622a0. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Lee K., Turka L. A., Green J., Thompson C., Shimizu Y. Discordant expression of CD28 ligands, BB-1, and B7 on keratinocytes in vitro and psoriatic cells in vivo. Am J Pathol. 1993 Apr;142(4):1029–1040. [PMC free article] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Nerenberg M., Southern P., Price J., Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991 Apr 19;65(2):319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Picarella D. E., Kratz A., Li C. B., Ruddle N. H., Flavell R. A. Insulitis in transgenic mice expressing tumor necrosis factor beta (lymphotoxin) in the pancreas. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10036–10040. doi: 10.1073/pnas.89.21.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picarella D. E., Kratz A., Li C. B., Ruddle N. H., Flavell R. A. Transgenic tumor necrosis factor (TNF)-alpha production in pancreatic islets leads to insulitis, not diabetes. Distinct patterns of inflammation in TNF-alpha and TNF-beta transgenic mice. J Immunol. 1993 May 1;150(9):4136–4150. [PubMed] [Google Scholar]
- Picker L. J., Butcher E. C. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Rawle F. C., O'Connell K. A., Geib R. W., Roberts B., Gooding L. R. Fine mapping of an H-2Kk restricted cytotoxic T lymphocyte epitope in SV40 T antigen by using in-frame deletion mutants and a synthetic peptide. J Immunol. 1988 Oct 15;141(8):2734–2739. [PubMed] [Google Scholar]
- Rock K. L., Gamble S., Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 1990 Aug 24;249(4971):918–921. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- Sarvetnick N., Shizuru J., Liggitt D., Martin L., McIntyre B., Gregory A., Parslow T., Stewart T. Loss of pancreatic islet tolerance induced by beta-cell expression of interferon-gamma. Nature. 1990 Aug 30;346(6287):844–847. doi: 10.1038/346844a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Schönrich G., Kalinke U., Momburg F., Malissen M., Schmitt-Verhulst A. M., Malissen B., Hämmerling G. J., Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991 Apr 19;65(2):293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Hultgren B., Huang X., Pitts-Meek S., Hully J., MacLachlan N. J. Induction of type I diabetes by interferon-alpha in transgenic mice. Science. 1993 Jun 25;260(5116):1942–1946. doi: 10.1126/science.8100367. [DOI] [PubMed] [Google Scholar]
- Tan R., Teh S. J., Ledbetter J. A., Linsley P. S., Teh H. S. B7 costimulates proliferation of CD4-8+ T lymphocytes but is not required for the deletion of immature CD4+8+ thymocytes. J Immunol. 1992 Nov 15;149(10):3217–3224. [PubMed] [Google Scholar]
- Thompson C. B., Lindsten T., Ledbetter J. A., Kunkel S. L., Young H. A., Emerson S. G., Leiden J. M., June C. H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend S. E., Allison J. P. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993 Jan 15;259(5093):368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Holly R. D., Clark E. A. B lymphoblast antigen (BB-1) expressed on Epstein-Barr virus-activated B cell blasts, B lymphoblastoid cell lines, and Burkitt's lymphomas. J Immunol. 1982 Feb;128(2):823–827. [PubMed] [Google Scholar]