Abstract
Previous neuroimaging research has established that the left ventrolateral prefrontal cortex (VLPFC) is involved in long-term memory (LTM) encoding for individual items. Dorsolateral prefrontal cortex (DLPFC) is implicated less frequently, and one theory that has gained support to explain this discrepancy is that DLPFC is involved in forming item-item relational but not item LTM. Given that neuroimaging results are correlational, complimentary methods such as repetitive Transcranial Magnetic Stimulation (TMS) have been used to test causal hypotheses generated from imaging data. Most TMS studies of LTM encoding have found that disruption of lateral PFC activity impairs subsequent memory. However these studies have lacked methods to precisely localize and directly compare TMS effects from frontal subregions implicated by the neuroimaging literature. Here, we target specific subregions of lateral PFC with TMS to test the prediction from the item/relational framework that temporary disruption of VLPFC during encoding will impair subsequent memory whereas TMS to DLPFC during item encoding will not. Frontal TMS was administered prior to a LTM encoding task in which participants were presented with a list of individual nouns and asked to judge whether each noun was concrete or abstract. After a 40 minute delay period, item recognition memory was tested. Results indicate that VLPFC and DLPFC TMS have differential effects on subsequent item memory. VLPFC TMS reliably disrupted subsequent item memory whereas DLPFC TMS led to numerical enhancement in item memory, relative to TMS to a control region.
Keywords: memory, cognitive control, brain stimulation, TMS, VLPFC, DLPFC
INTRODUCTION
A wealth of neuropsychological and neuroimaging data suggest that prefrontal cortex (PFC) implements control processes that are critical to long-term memory (LTM) function (Shimamura, 1995; and Blumenfeld and Ranganath, 2007 for review). Most neuroimaging studies examining LTM encoding find that ventrolateral regions of PFC (VLPFC) demonstrate enhanced activity during subsequently remembered compared to forgotten trials (for reviews see: Paller and Wagner, 2002; Blumenfeld and Ranganath, 2007). However, activity in more dorsolateral regions of PFC (DLPFC) rarely predicts subsequent LTM, and in some studies DLPFC activity was enhanced during subsequently forgotten compared to remembered trials (Blumenfeld and Ranganath, 2006; Staresina and Davachi, 2006; Summerfield et al., 2006; Murray and Ranganath, 2007; Jenkins and Ranganath, 2010; Blumenfeld et al., 2011 see Figure 1).
Figure 1.
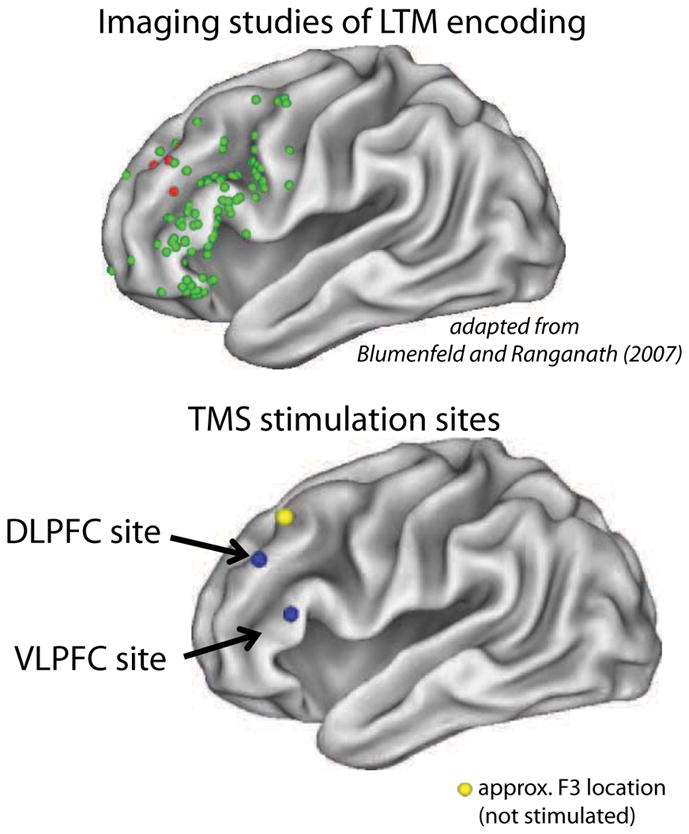
Top: Plot of the local maxima implicated in the fMRI LTM encoding literature. Shown in green are regions implicated in successful subsequent memory. In red are maxima implicated in subsequent forgetting. Bottom: Shown in blue are the DLPFC and VLPFC TMS locations in standardized space. The yellow sphere depicts the approximate stimulation location when F3 electrode is used for TMS localization. Notice that this region is far caudal and dorsal to the DLPFC implicated in the fMRI literature as well as the region stimulated in this study.
Blumenfeld and Ranganath (2006, 2007) hypothesized that this pattern of findings can be explained by the fact that most encoding studies examine subsequent memory for item information, and specific DLPFC regions are critically involved in LTM encoding of inter-item relational information and not item information. In contrast, specific regions of VLPFC are hypothesized to contribute to encoding of item information. This hypothesis has received support from several neuroimaging studies (e.g. Blumenfeld and Ranganath, 2006; Staresina and Davachi, 2006; Summerfield et al., 2006; Murray and Ranganath, 2007; Blumenfeld et al., 2011).
Given that neuroimaging results, such as the subsequent memory effect, are correlational, complimentary methods are needed to establish causal links between functional brain activation and behavior. Repetitive transcranial magnetic stimulation (TMS), a non-invasive technique that temporarily alters neural excitability over a focal cortical region, offers neuroscientists such a method. There is a growing literature using TMS to probe the causal role of PFC in LTM encoding where frontal cortex function is disrupted prior to encoding and the effect on subsequent LTM performance is assessed (Floel and Cohen, 2007 for review). The majority of these studies do indeed find that TMS disrupts subsequent memory. However most previous studies did not use stereotaxic methods that can precisely and reliably target specific frontal subregions, and no study, to the best of our knowledge, has directly compared TMS effects between PFC regions within the same hemisphere. Thus, it is unclear from this literature whether different PFC regions support different LTM encoding functions. Here, we targeted dorsal and ventral subregions of lateral PFC with TMS to test a prediction of the item/relational framework (Blumenfeld and Ranganath, 2007). Specifically, we hypothesize that VLPFC TMS (~BA 45: Petrides and Pandya, 1994) will impair item encoding whereas DLPFC TMS (~BA 9/46 or 46: Petrides and Pandya, 1994) will not. TMS was applied before LTM encoding and a subsequent memory paradigm was used that required deep encoding of single items, but placed minimal demands on encoding relations amongst items (Wagner et al., 1998).
METHODS
Participants
Twenty nine participants were enrolled (14 female, mean age 33.5 ± 3.7). Data from one participant was excluded for not complying with instructions and data from two participants were excluded because their memory performance could not be estimated due to ceiling performance (0 false-alarms). Participants gave informed consent and were paid for their participation.
Study Design
A mixed design was used. Participants were randomly assigned to 1 of 2 groups. Both groups participated in 2 TMS sessions on two separate days. Participants in the VLPFC group received TMS stimulation to VLPFC in one session, and in a separate control session, they received TMS to vertex. Participants in the DLPFC group received TMS to DLPFC in one session and received TMS to vertex in a separate control session. The order of stimulation session (PFC site vs. vertex) was counterbalanced between groups. Age, years of education and gender was matched between groups.
Materials
Stimuli consisted of a list of concrete (200 and mean concreteness 585.4) and abstract nouns (200 and mean concreteness 319) taken from the MRC Psycholinguistic database (Coltheart, 1981). From this master list, two smaller lists of 200 words (100 concrete, 100 abstract words) were constructed. These two lists contained different words and were used on separate sessions per participant. Within these session lists, 100 words (50 concrete, 50 abstract) served as the study list during encoding and 100 (50 concrete, 50 abstract) words served as novel foils during item recognition. The assignments to the study and foil lists were counter-balanced between participant and mean concreteness, frequency, imageability and number of phonemes was matched between all lists. In addition, we categorized each item according to semantic category and distributed the items equally across studied and foil lists. The assignment to session was counterbalanced.
Behavioral procedures
Item encoding task
During study, items were presented individually for 1 sec. Participants were instructed to read the word and decide whether it was concrete or abstract. Participants were told that concrete nouns can be visualized as a whole. Participants were told that abstract nouns do not refer to the whole of an entity and cannot easily be visualized on their own. After initial presentation, a probe was additionally presented for 0.5 sec at the bottom of the screen containing words: “concrete or abstract”. Participants were instructed to make a response at the probe but not before. This was to ensure that participants spent equal time encoding each item. There was a 1 sec inter-trial interval. Participants received instructions and a brief training run before TMS administration.
Item recognition
Nouns were presented individually in the center of the computer screen. Under the noun, the numbers 1 through 6 appeared. Participants were instructed to rate their confidence using a 1 – 6 scale about whether each word was studied or novel. Participants were told that “1” represented high confidence that the item is a novel foil and “6” indicates high confidence that the item was studied. Participants were further instructed to use the entire scale of responses. At the beginning of each session, participants received a brief training on the recognition test after the encoding training. They were told explicitly that their item recognition memory will be tested, but they should focus only on performing the task and not perform any additional mnemonic.
Definition of stimulation sites in standard space
The VLPFC stimulation site was based on standardized coordinates published by Wagner et al. (1998). This study used a nearly identical behavioral task in an fMRI encoding paradigm and found that activity in a VLPFC region (centered on −51, 25, 12 in MNI space (Montreal Neurologic Institute, Montreal, Quebec, Canada)) was enhanced for subsequently recognized item encoding trials compared to item encoding trials that were subsequently forgotten. We used a similar coordinate that was situated more clearly on the cortical surface of pars triangularis (−53, 28, 12). This location was a more reliable landmark for stereotaxy. The coordinate for the DLPFC stimulation site was based on a local maximum in a fMRI study by Blumenfeld et al., 2011. This study found that a region of DLPFC (−44, 35, 26) correlated with subsequent LTM for inter-item relational information but not detailed item information. The present study used a similar coordinate (−43, 35, 30). It should be noted that although the VLPFC and DLPFC stimulation sites are on adjoining gyri, care was taken to ensure that the coordinates chosen were at least 20 mm apart in every direction. Although there is no consensus on the minimum distance needed between sites to prevent stimulation overlap, it has been shown that motor-evoked potentials are not induced in motor areas situated more than 10mm away from the center of coil during stimulation (Brasil-Neto et al., 1992). We therefore considered 10 mm the minimum distance between stimulation sites and ensured that the sites chosen exceeded 10 mm. It should be further noted that the two stimulation sites were at similar rostro-caudal positions. 10 mm spherical masks were constructed at the VLPFC and DLPFC stimulation sites (see Figure 1) in MNI-space.
Procedure for defining stimulation site for each subject
The standard-space DLPFC and VLPFC masks were reversed normalized into individual subject space for each participant using their high-resolution structural image. The DLFC and VLPFC subject-space masks for each subject were visually reviewed to ensure that there was no overlap between them. Depending on group assignment, either the subject-space DLPFC or VLPFC mask was selected as the stimulation site and this mask overlaid on top of the structural image was used for frameless stereotaxy.
Theta-burst TMS Protocol
We used a recently developed TMS protocol, theta-burst stimulation (Huang et al., 2005), to cause transient reductions in cortical excitability. Compared to other repetitive methods, such as 1 Hz TMS, theta-burst stimulation requires a relatively brief stimulation period and yields a longer and less variable (Gangitano et al., 2002) period of reduced cortical excitability. This method is ideal for lateral frontal stimulation as longer stimulations are not tolerated by some participants due to uncomfortable concomitant stimulation of facial muscles. In this method, 50 Hz trains of three TMS pulses are repeated every 200 ms continuously over a period of seconds at 80% of active motor threshold (AMT). In the current study, we used a theta-burst stimulation protocol of 30 sec (450 pulses) which likely produces reductions lasting 30 to 40 minutes (Huang et al., 2005). TMS was applied using a hand-held figure-eight coil with an outer winding diameter of 70 mm (Magstim Co., Whitland, Dyfed, UK). All pulses were delivered using a Magstim rapid stimulator connected to 4 booster modules that produce biphasic pulses.
TMS procedure
After training on the behavioral tasks, the active motor threshold (AMT) was determined for each participant based on the procedure described in (Rossini et al., 1994). A stimulation intensity of 80% AMT was used for TMS.
Next, we used Brainsight (Rogue Research, Montreal, Canada), a computerized frameless sterotaxy system to localize stimulation targets. Head locations are related in real time to the participant’s previously acquired structural MRI data after the data are co-registered to a set of anatomical locations. Reflective markers are attached to the coil and the participant, so that relative positions of the coil to the head (and the MRI) can be tracked, allowing precise positioning of the coil with respect to previously chosen MRI locations.
After the stimulation site was stereotaxically localized, TMS was administered for 30 sec and then the LTM encoding task was administered, lasting 10 minutes. Forty minutes after the end of the encoding session (50 minutes after TMS), item recognition was administered. This delay ensured that TMS effects had subsided before LTM testing.
Statistical Analyses
The main behavioral effects of TMS were evaluated for subsequent item recognition memory by computing d′ across all confidence judgments. The TMS effects were also evaluated for reaction time (RT) and accuracy (% correct) during the concrete/abstract encoding task. D′ values, encoding task % correct and encoding task RT were entered into separate mixed effects ANOVAs. In these ANOVAs, site of PFC stimulation (VLPFC vs. DLPFC) served as the between group factor and TMS session (vertex vs. PFC) was a within subject factor. The number of days between TMS sessions served as a co-variate of no-interest.
Very few (3 out of 29) participants had a sufficient number (>10) of high confidence false alarm responses to allow for reliable estimation of memory measures, such as da, that control for differences in confidence level or bias. These measures, based Receiver Operating Characteristics (ROCs), become unstable with low trial numbers in the extreme bins (ie. “1” and “6”) and are therefore ill-advised in such cases (Yonelinas and Parks, 2007).
RESULTS
Encoding task
Participants were highly accurate in making concrete/abstract decisions on all sessions. VLPFC TMS led to a small decrease in accuracy and increase in reaction times (RT) compared to vertex TMS(mean VLPFC session accuracy = 91.8% +/− 1.2, mean vertex accuracy = 93.5% +/− 1.6; mean VLPFC session RT =444 ms +/− 30, mean vertex RT =495 ms +/− 62 ). DLPFC TMS led to a small increase in accuracy and slowing of RT compared to vertex TMS (mean DLPFC session accuracy = 92% +/− 2.0, mean vertex accuracy = 88.6% +/− 1.5; mean DLPFC session RT =503 ms +/− 33, mean vertex RT =452 ms +/− 32. Accuracy and RT were submitted into separate 2x2 mixed factorial ANOVAs. For accuracy, no main effects were found (Group: F(1,22) = 1.513, p > 0.05, Session: F(1,22) = 0.500, p > 0.05). There was a trend toward a significant Group (VLPFC or DLPFC) by TMS session (PFC vs. vertex stimulation) interaction (F(1,22)=3.99, p=0.058). Planned t-tests revealed a trend toward a significant accuracy reduction following VLPFC vs. vertex TMS (t(12) = 2.004, p= 0.068 ). No significant differences were found between DLPFC and vertex TMS conditions (t(11) = 1.57 p = 0.14). For RTs, no significant main effects nor interaction was found (Group: F(1,22) = 1.513, Session: F(1,22) = 0.003, Group x Session interaction = F(1,22) = 2.054, all p’s > 0.05).
Subsequent Item Recognition Memory
Mean d′ estimates on the subsequent item recognition task is shown in Figure 2. VLPFC TMS disrupted subsequent item memory performance compared to vertex TMS (mean VLPFC session d′ = 1.91 +/− 0.17, mean vertex session d′ = 2.15 +/− 0.15). 10/13 participants in this group exhibited this reduction (VLPFC – Vertex individual difference scores are plotted in the top panel of Figure 3). In contrast, DLPFC TMS caused a numerical enhancement of subsequent item memory performance compared to vertex TMS (mean DLPFC session d′ = 2.05 +/− 0.17, mean vertex session d′ = 1.86 +/− 0.18). 8/12 participants in this group exhibited this enhancement (DLPFC –Vertex individual difference scores are plotted in bottom panel of Figure 3). D′ estimates (i.e. raw scores) were entered into a 2x2 mixed factorial ANOVA with PFC group as the between group factor and TMS session as the within group factor. No main effects were found (group: F(1,22)= 0.075, p > 0.05; session: F(1,22)=0.57, p> 0.05). A significant group x session interaction (F(1,22)=10.58, p < 0.005) was found indicating that the PFC TMS effect compared to vertex was different between the two different frontal sites. Planned t-tests comparing PFC site d′ – Vertex d′ revealed a significant reduction in d′ following VLPFC TMS compared to vertex TMS (t(12)=3.20, p < 0.01). DLPFC TMS d′ did not reliably differ from vertex TMS d′ (t(11) = 1.67, p > 0.05).
Figure 2.
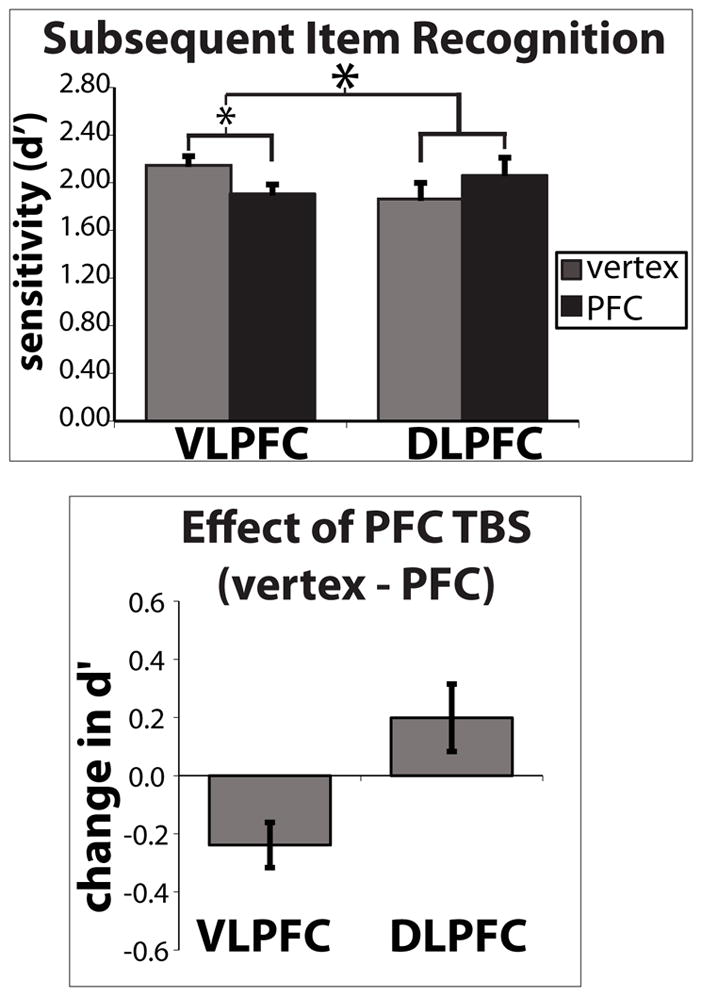
Mean subsequent LTM performance. Top: mean raw d′ values computed for DLPFC, VLPFC and their respective vertex sessions. The mixed effects ANOVA revealed a significant PFC group x session interaction indicating that the difference between PFC TMS and vertex differed as a function of which PFC site was stimulated (VLPFC or DLPFC). Planned t-tests comparing PFC site d′ – Vertex d′ revealed a significant reduction in d′ following VLPFC TMS compared to vertex TMS, however DLPFC TMS d′ did not reliably differ from vertex TMS d′. Bottom: difference in DLPFC – vertex and VLPFC – vertex performance. These difference scores illustrate that VLPFC TMS led to a disruption in the majority of participants in this group, yet DLPFC TMS in fact led to an enhancement in the majority of participants.
Figure 3.
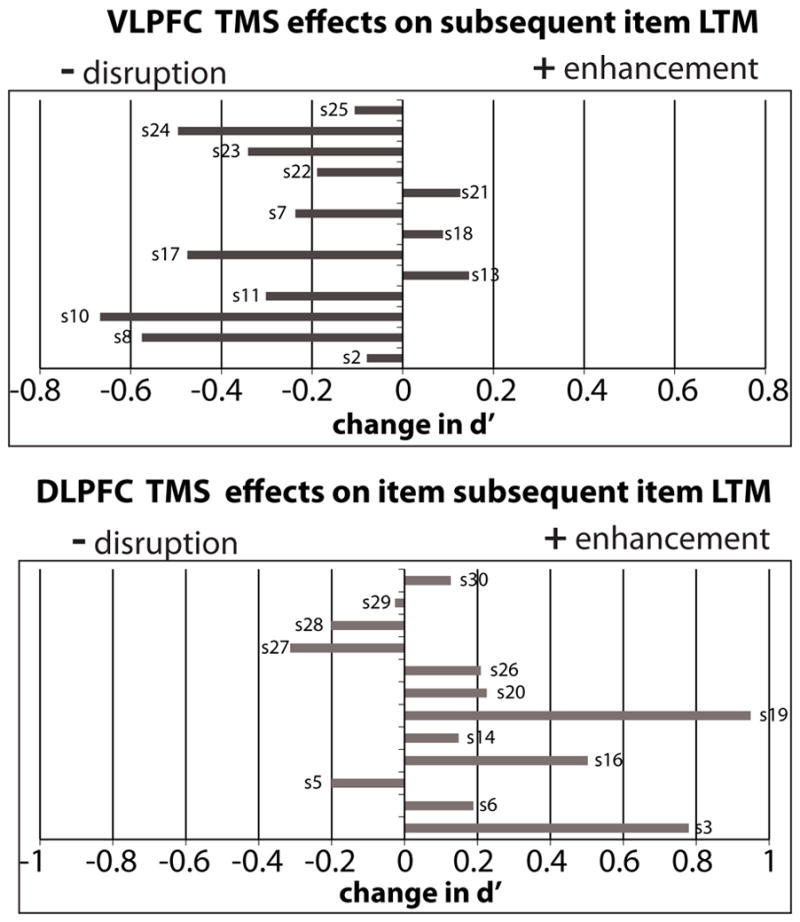
TMS effects for individual participants. Each plot depicts difference in d′ comparing PFC TMS versus vertex TMS. Negative values (disruption) are plotted towards the left and positive values (enhancement) are plotted toward the right. Top: majority of participants in VLPFC group show item LTM disruption with VLPFC TMS. Bottom: majority of participants in DLPFC group show item LTM enhancement with DLPFC TMS.
To further examine the effect of VLPFC TMS, we analyzed the TMS-vertex difference score separately for subsequent hits and false alarms. We found that TMS to VLPFC significantly increased false alarm rate (mean % false alarm difference = 4.92%; t(12)=2.39, p <0.05) but not hit rate ( mean % hit rate difference = 0.39%; t(12)=0.18, p> 0.05). Similar analyses for DLPFC TMS did not produce significant differences for hit rate (mean % hit rate difference = 0.030, t(10)=1.45, p=.18) nor false-alarm rate (mean % false alarm difference = −0.029, t(10)=1.47, p= 0.27).
DISCUSSION
In this study, we compared the effects of TMS to specific regions of lateral PFC on LTM encoding implicated by functional neuroimaging studies. We show that TMS to a region of mid-VLPFC (~BA 45; pars triangularis) before LTM encoding disrupts subsequent memory for item information. Interestingly, VLPFC TMS disproportionately led to an increase in subsequent false-alarms, but did not affect item hit-rate. TMS to mid-DLPFC (~BA 9/46, 46; middle frontal gyrus) did not reliably disrupt subsequent item memory. As a whole, the dissociation that we observed demonstrates that the encoding functions of PFC subregions can be differentiated using TMS. Moreover, the specific pattern of results that we obtained is consistent with the proposal that VLPFC, through its role in selecting and/or retrieving goal-relevant item information (Badre and Wagner, 2007), is critical for the goal-directed encoding of detailed item information (Blumenfeld and Ranganath, 2007).
TMS has been used in numerous studies to explore the causal role of PFC in LTM encoding. These studies have evaluated a variety of different relevant topics and predictions, mostly generated from neuroimaging data. Results are varied, but across the literature it is relatively clear that TMS to the left lateral frontal cortex before LTM encoding impairs subsequent memory (e.g. Rossi et al., 2001, 2004, 2011; Sandrini et al., 2003; Kahn et al., 2005; Turriziani et al., 2008; Gagnon et al., 2010; Innocenti et al., 2010, 2013; Machizawa et al., 2010; Manenti et al., 2011). However, the majority of these studies utilize scalp-based landmarks, based on the EEG 10–20 system, to target TMS to the frontal lobe. Although adequate for the hypotheses of these studies, scalp landmarks cannot be used to reliably and precisely target specific PFC regions. Moreover, even within the studies that used MRI-based stereotaxic methods to precisely localize PFC regions, no previous study has directly compared PFC TMS effects within different PFC subregions within a hemisphere on subsequent LTM. Thus it is less clear from the TMS literature what the causal roles of different PFC subregions are in LTM encoding, and in particular whether different regions along the dorso-ventral PFC axis make different contributions as shown in imaging (Blumenfeld and Ranganath, 2006; Murray and Ranganath, 2007; Jenkins and Ranganath, 2010; Blumenfeld et al., 2011).
The present experiment was designed to address this limitation. We used MRI-based stereotaxic localization to target specific regions of VLPFC and DLPFC implicated in the cognitive control and LTM encoding literature. We stimulated these regions in separate participant groups prior to the performance of an LTM encoding task that emphasized the selection of specific item but not relational information. Our results show the encoding functions of left VLPFC and DLPFC can be differentiated.
In particular, we found that TMS to a mid-VLPFC region (pars triangularis or BA 45), prior to encoding disrupted subsequent LTM item memory. This disruption was evident in the performance in a large majority of participants and was specific to the false-alarm rate. That is VLPFC TMS increased subsequent item false-alarms, but did not reliably affect hit-rate. These results are best understood from the framework that VLPFC supports item LTM encoding through its role in cognitive control.
During cognitive control tasks, pars triangularis has been linked to retrieval and/or selection processes that direct attention toward goal-relevant item information and inhibit the influence of irrelevant information on behavior (Thompson-Schill et al., 1997; Jonides and Nee, 2006; Badre and Wagner, 2007). For instance, activation in pars triangularis is enhanced when participants must select amongst two highly competing responses to perform a task goal (Badre et al., 2005; Badre and Wagner, 2007) and also when successfully resolving interference from noisy mnemonic item representations (Thompson-Schill et al., 1997; D’Esposito et al., 1999; Nelson et al., 2003; Badre and Wagner, 2005; Jonides and Nee, 2006; Nee et al., 2007) or distractions (Wais and Gazzaley, 2011). This cognitive control selection process is essential for encoding distinctive, item-specific information into LTM (e.g. encode “lion” and not just “big cat” see: Hunt and Einstein, 1981) and given that most fMRI encoding studies focus solely on item memory, it is therefore not surprising that pars triangularis is the most frequently implicated region in the fMRI encoding literature (Blumenfeld and Ranganath, 2006; Spaniol et al., 2009; Kim, 2011).
In the present study, participants were required to encode semantic information about each item. Importantly, as mentioned in the Methods, our design ensured that items from the same semantic category or items that had similar semantic features were distributed equally across studied and foil lists. This manipulation increased similarity and interference amongst the items (Hunt and Mcdaniel, 1993; Elfman et al., 2008; Parks et al., 2011) and in so doing taxed cognitive control selection processes; participants needed to encode item-specific information to avoid false alarming to related foils (e.g. reject “tiger”). TMS to VLPFC specifically disrupted the ability to correctly reject foil items. That is, participants were less able to select and encode specific item details. These participants relied more on non-specific or gist-based information during retrieval, and as a result, they were more likely to false-alarm. Thus our study provides causal evidence that VLPFC implements goal-directed selection processes that subserve LTM for item-specific information.
Despite finding clear item LTM impairments with VLPFC TMS, TMS to DLPFC did not impair subsequent LTM for item information. Similarly, fMRI studies rarely implicate DLPFC in LTM encoding, so one possible explanation is that DLPFC does not contribute to LTM encoding. However, Blumenfeld and Ranganath (2007), based on several fMRI studies that emphasize relational but not item encoding (e.g. Blumenfeld and Ranganath, 2006; Staresina and Davachi, 2006; Summerfield et al., 2006; Blumenfeld et al., 2011), suggest the alternate hypothesis that DLPFC activity guides the building of goal-relevant item-item relationships during cognitive control and LTM encoding. For example, DLPFC activity has been shown to be more active during cognitive control tasks that require forming relationships amongst items held in mind and that this activity predicts later subsequent LTM for item-item relationships but not for item information encoded in a shallow (Blumenfeld and Ranganath, 2006) or deep manner (Blumenfeld et al., 2011). Given that we did not have a relational memory condition, this study was not designed to test this DLPFC hypothesis. Nonetheless, the fact that we did not see robust LTM encoding effects in this area is supportive of the notion that DLPFC is not necessary for LTM encoding of goal-relevant item information (Blumenfeld and Ranganath, 2007). Future TMS studies using relational encoding designs are needed to directly test this possibility.
As mentioned above, few TMS studies have targeted specific PFC regions implicated by the neuroimaging literature. Only 4 studies have targeted left VLPFC (out of 19 studies total). One of these studies (Kahn et al., 2005) found no change in subsequent LTM hit-rate, one found LTM hit-rate facilitation (Köhler et al., 2004) and two found small but reliable reductions in LTM hit-rate (Floel et al., 2004; Machizawa et al., 2010). Although the results from these four studies are mixed, it is important to note that Köhler et al., 2004, Kahn et al., 2005 and Machizawa et al., 2010 measured TMS effects based solely on differences in hit-rate. Therefore they could not detect TMS effects on subsequent false-alarms nor could they estimate memory discriminability or sensitivity (d′). Measurements of d′ directly compare hit and false-alarm rates as a means of separating mnemonic signal from sources of noise (Macmillan and Creelman, 1991). Given that, on a conceptual level, VLPFC is thought to be critical for selecting relevant item information (i.e. signal) from amongst interference, competition or distraction, the activity in this region should in general relate most strongly to item d′. Moreover, the fact that we found reliable effects in both d′ and false alarms but not hit-rate, further highlight the need for future to studies to use designs that allow for estimating d′.
One limitation in the present study is a lack of a no-stimulation control or sham control condition. Although rarely used in the extant TMS literature, this additional control session would allow us to isolate the generalized effects of stimulation compared to vertex stimulation and would provide a more neutral baseline condition for the within PFC group comparisons.
Finally, we note that in the extant TMS literature many studies use the location of the “F3” EEG electrode on the scalp to target TMS to the frontal lobe and found impairments in subsequent LTM (including for item information Rossi et al., 2001, 2004, 2011; Gagnon et al., 2010, 2011; Innocenti et al., 2010). In these studies, this site is often referred to as “DLPFC”, yet it has been shown that although variable between subjects, F3 overlays a frontal region roughly on the border of supplementary motor area (BA 8) and BA 9 (Herwig et al., 2003 and see Figure 1) which is quite posterior and dorsomedial to not only the region of middle frontal gyrus stimulated here and implicated in the fMRI LTM encoding literature. It is also posterior and dorsomedial to what is defined as DLPFC by neuroanatomists (e.g. Petrides and Pandya, 1994). Thus it is quite likely that the region stimulated here and the region stimulated in prior “F3” landmarked studies are different. It was beyond our aims to investigate exactly why TMS to the “F3” region (~supplementary motor cortex) can in certain contexts disrupt subsequent LTM but this is an important question for future study. One speculative possibility is that the supplementary motor area carries out more low-level operations that are equally necessary for both subsequently remembered and forgotten encoding/cognitive control trials. Thus this area would not show enhanced activity during remembered vs. forgotten trials, but would be nonetheless critical for performance on the task in general.
Highlights.
Applied TMS to left ventrolateral and dorsolateral PFC during item memory encoding
TMS sites were localized using MRI
Left ventrolateral TMS but not dorsolateral PFC TMS impaired subsequent item memory
The encoding functions of PFC subregions can be differentiated using TMS
Acknowledgments
We would like to thank James Lim and Antonio Fidalgo for help in testing participants and designing stimuli, Colleen M. Parks for giving feedback on an earlier draft of this paper, and our two anonymous reviewers for their feedback. Supported by National Institute of Health (Grants F32MH087047 to R.S.B., MH63901 and NS40813 to M.D.)
Footnotes
Statement of Contribution: RSB and MD designed the experiment and wrote the paper, RSB and TL performed the research
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Frontal Lobe Mechanisms that Resolve Proactive Interference. Cereb Cortex. 2005;15:2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Parks CM, Yonelinas AP, Ranganath C. Putting the pieces together: the role of dorsolateral prefrontal cortex in relational memory encoding. J Cogn Neurosci. 2011;23:257–265. doi: 10.1162/jocn.2010.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26:916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. The Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, McShane LM, Fuhr P, Hallett M, Cohen LG. Topographic mapping of the human motor cortex with magnetic stimulation: factors affecting accuracy and reproducibility. Electroencephalogr Clin Neurophysiol. 1992;85:9–16. doi: 10.1016/0168-5597(92)90095-s. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. The Quarterly Journal of Experimental Psychology Section A. 1981;33:497–505. [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proc Natl Acad Sci U S A. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfman KW, Parks CM, Yonelinas AP. Testing a neurocomputational model of recollection, familiarity, and source recognition. J Exp Psychol Learn Mem Cogn. 2008;34:752–768. doi: 10.1037/0278-7393.34.4.752. [DOI] [PubMed] [Google Scholar]
- Floel A, Cohen LG. Contribution of noninvasive cortical stimulation to the study of memory functions. Brain Res Rev. 2007;53:250–259. doi: 10.1016/j.brainresrev.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Floel A, Poeppel D, Buffalo EA, Braun A, Wu CW-H, Seo H-J, Stefan K, Knecht S, Cohen LG. Prefrontal cortex asymmetry for memory encoding of words and abstract shapes. Cereb Cortex. 2004;14:404–409. doi: 10.1093/cercor/bhh002. [DOI] [PubMed] [Google Scholar]
- Gagnon G, Blanchet S, Grondin S, Schneider C. Paired-pulse transcranial magnetic stimulation over the dorsolateral prefrontal cortex interferes with episodic encoding and retrieval for both verbal and non-verbal materials. Brain Res. 2010;1344:148–158. doi: 10.1016/j.brainres.2010.04.041. [DOI] [PubMed] [Google Scholar]
- Gagnon G, Schneider C, Grondin S, Blanchet S. Enhancement of episodic memory in young and healthy adults: a paired-pulse TMS study on encoding and retrieval performance. Neurosci Lett. 2011;488:138–142. doi: 10.1016/j.neulet.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Valero-Cabré A, Tormos JM, Mottaghy FM, Romero JR, Pascual-Leone A. Modulation of input-output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol. 2002;113:1249–1257. doi: 10.1016/s1388-2457(02)00109-8. [DOI] [PubMed] [Google Scholar]
- Herwig U, Satrapi P, Schönfeldt-Lecuona C. Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16:95–99. doi: 10.1023/b:brat.0000006333.93597.9d. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Hunt RR, Einstein GO. Relational and Item-Specific Information in Memory. Journal of Verbal Learning and Verbal Behavior. 1981;20:497–514. [Google Scholar]
- Hunt RR, Mcdaniel MA. The Enigma of Organization and Distinctiveness. Journal of Memory and Language. 1993;32:421–445. [Google Scholar]
- Innocenti I, Cappa SF, Feurra M, Giovannelli F, Santarnecchi E, Bianco G, Cincotta M, Rossi S. TMS interference with primacy and recency mechanisms reveals bimodal episodic encoding in the human brain. J Cogn Neurosci. 2013;25:109–116. doi: 10.1162/jocn_a_00304. [DOI] [PubMed] [Google Scholar]
- Innocenti I, Giovannelli F, Cincotta M, Feurra M, Polizzotto NR, Bianco G, Cappa SF, Rossi S. Event-related rTMS at encoding affects differently deep and shallow memory traces. Neuroimage. 2010;53:325–330. doi: 10.1016/j.neuroimage.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Jenkins LJ, Ranganath C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J Neurosci. 2010;30:15558–15565. doi: 10.1523/JNEUROSCI.1337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Kahn I, Pascual-Leone A, Theoret H, Fregni F, Clark D, Wagner AD. Transient disruption of ventrolateral prefrontal cortex during verbal encoding affects subsequent memory performance. J Neurophysiol. 2005;94:688–698. doi: 10.1152/jn.01335.2004. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. Neuro Image. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Köhler S, Paus T, Buckner RL, Milner B. Effects of left inferior prefrontal stimulation on episodic memory formation: a two-stage fMRI-rTMS study. J Cogn Neurosci. 2004;16:178–188. doi: 10.1162/089892904322984490. [DOI] [PubMed] [Google Scholar]
- Machizawa MG, Kalla R, Walsh V, Otten LJ. The time course of ventrolateral prefrontal cortex involvement in memory formation. J Neurophysiol. 2010;103:1569–1579. doi: 10.1152/jn.90937.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user’s guide. New York: 1991. [Google Scholar]
- Manenti R, Cotelli M, Miniussi C. Successful physiological aging and episodic memory: a brain stimulation study. Behav Brain Res. 2011;216:153–158. doi: 10.1016/j.bbr.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci. 2007;27:5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Jonides J, Berman MG. Neural mechanisms of proactive interference-resolution. Neuroimage. 2007;38:740–751. doi: 10.1016/j.neuroimage.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JK, Reuter-Lorenz PA, Sylvester CY, Jonides J, Smith EE. Dissociable neural mechanisms underlying response-based and familiarity-based conflict in working memory. Proc Natl Acad Sci U S A. 2003;100:11171–11175. doi: 10.1073/pnas.1334125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Parks CM, Murray LJ, Elfman K, Yonelinas AP. Variations in recollection: the effects of complexity on source recognition. J Exp Psychol Learn Mem Cogn. 2011;37:861–873. doi: 10.1037/a0022798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Handbook of Neuropsychology. Amsterdam: Elsevier; 1994. Comparitive architectonic analysis of the human and macaque frontal cortex; pp. 59–82. [Google Scholar]
- Rossi S, Cappa SF, Babiloni C, Pasqualetti P, Miniussi C, Carducci F, Babiloni F, Rossini PM. Prefrontal [correction of Prefontal] cortex in long-term memory: an “interference” approach using magnetic stimulation. Nat Neurosci. 2001;4:948–952. doi: 10.1038/nn0901-948. [DOI] [PubMed] [Google Scholar]
- Rossi S, Innocenti I, Polizzotto NR, Feurra M, De Capua A, Ulivelli M, Bartalini S, Cappa SF. Temporal dynamics of memory trace formation in the human prefrontal cortex. Cereb Cortex. 2011;21:368–373. doi: 10.1093/cercor/bhq103. [DOI] [PubMed] [Google Scholar]
- Rossi S, Miniussi C, Pasqualetti P, Babiloni C, Rossini PM, Cappa SF. Age-related functional changes of prefrontal cortex in long-term memory: a repetitive transcranial magnetic stimulation study. J Neurosci. 2004;24:7939–7944. doi: 10.1523/JNEUROSCI.0703-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Sandrini M, Cappa SF, Rossi S, Rossini PM, Miniussi C. The role of prefrontal cortex in verbal episodic memory: rTMS evidence. J Cogn Neurosci. 2003;15:855–861. doi: 10.1162/089892903322370771. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. Memory and the prefrontal cortex. Ann N Y Acad Sci. 1995;769:151–159. doi: 10.1111/j.1749-6632.1995.tb38136.x. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. The Journal of neuroscience. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Greene M, Wager T, Egner T, Hirsch J, Mangels J. Neocortical connectivity during episodic memory formation. PLoS biology. 2006;4:e128. doi: 10.1371/journal.pbio.0040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turriziani P, Oliveri M, Salerno S, Costanzo F, Koch G, Caltagirone C, Carlesimo GA. Recognition memory and prefrontal cortex: dissociating recollection and familiarity processes using rTMS. Behav Neurol. 2008;19:23–27. doi: 10.1155/2008/568057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wais PE, Gazzaley A. The impact of auditory distraction on retrieval of visual memories. Psychon Bull Rev. 2011;18:1090–1097. doi: 10.3758/s13423-011-0169-7. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: a review. Psychol Bull. 2007;133:800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]


