Abstract
In the United States, there are significant geographic disparities in the time to transplantation for patients with hepatocellular carcinoma (HCC); it is possible that rapid transplantation contributes to higher rates of posttransplant HCC recurrence because there is insufficient time for the tumor biology to manifest. In this study, we compared HCC recurrence in rapid transplant patients and their slower transplant counterparts. We identified adult liver transplantation (LT) candidates in the Organ Procurement and Transplantation Network/United Network for Organ Sharing (UNOS) data set who were granted an initial exception for an HCC diagnosis between January 1, 2006 and September 30, 2010 and underwent transplantation in the same time window. Patients were followed until HCC recurrence, non–HCC-related death, or last follow-up. The cumulative incidence of HCC recurrence was compared for patients waiting ≤120 days and patients waiting >120 days from an HCC exception to LT. The association between the risk of posttransplant recurrence and the wait time was further evaluated via competing risks regression with the Fine and Gray model. For 5002 LT recipients with HCC, the median wait time from an exception to LT was 77 days, and it varied from 30 to 169 days by UNOS region. The cumulative incidence of posttransplant HCC recurrence was 3.3% [95% confidence interval (CI) = 2.8%–3.8%] and 5.6% (95% CI = 5.0%–6.3%) within 1 and 2 years, respectively. The rate of observed recurrence within 1 year of transplantation was significantly lower for patients waiting >120 days versus patients waiting ≤120 days (2.2% versus 3.9%, P = 0.002); however, the difference did not persist at 2 years (5.0% versus 5.9%, P = 0.09). After we accounted for clinical factors, the HCC recurrence risk was reduced by 40% for patients waiting >120 days (subhazard ratio = 0.6, P = 0.005). In conclusion, the risk of HCC recurrence within the first year after transplantation may be lessened by the institution of a mandatory waiting time after an exception is granted.
The Model for End-Stage Liver Disease (MELD) scoring system has been used for prioritizing patients for liver transplantation (LT) since its implementation in February 2002.1 This model is validated for predicting 3-month mortality for patients with chronic liver disease,2 but it is not a good predictor of survival for patients with hepatocellular carcinoma (HCC)3 because they are at risk of death from progression of their cancer while their liver function is potentially maintained. To compensate for the expected waiting-list dropout due to cancer progression, in 2002, HCC patients meeting the Milan criteria for transplantation4 were given a MELD score of 29 in an attempt to match the dropout risk for non-HCC patients.1 Subsequent studies, though, demonstrated that this provided an unfair advantage to patients with HCC,5 and this led to a progressive reduction of exception scores. The current MELD priority score for T2 HCC (1 lesion of 2–5 cm or 2–3 lesions, each 1–3 cm) is 22 points, and there are quarterly increases corresponding to 10% increases in pretransplant mortality.
On the whole, the MELD exception system has proven advantageous for patients with HCC: in comparison with the pre-MELD era, the wait-list time has decreased from 1.3 to 0.6 years, and the dropout rate has decreased from 25.9% to 6.7%. Twenty-six percent of LT procedures are now performed for HCC.5–7 This trend has been sustainable because of several studies demonstrating 5-year posttransplant survival rates higher than 70%.4,8–10 However, concern is growing that HCC patients are still unfairly privileged in comparison with patients with other indications for transplantation because the survival rates may be lower than previously thought.11–13 More recent analyses have also demonstrated that non-HCC patients have higher wait-list mortality.14,15
Some have speculated that shorter waiting times before transplantation have led to an increased recurrence HCC rate because shorter waiting times may allow for transplantation in patients with existing but undetected extrahepatic disease that may be revealed if the patients wait longer,16 although the evidence is not conclusive. The living donor liver transplantation (LDLT) literature, for instance, suggests that shorter waiting times may be playing a role in the higher recurrence rates seen in this population.16–18 To date, only 1 study has specifically evaluated the waiting time as a risk factor for HCC recurrence after deceased donor liver transplantation (DDLT): that 2007 single-center study did not find a shorter waiting time to be a risk factor for recurrence after either DDLT or LDLT.19 Another study combining LDLT and DDLT found that a waiting time >3 months reduced the odds of recurrence in a univariate analysis.20
Regional disparities in organ availability allow a natural experiment: half of US transplant centers perform transplantation within 3 months, and the other half perform transplantation beyond 3 months. In this study, we compared HCC recurrence in rapid transplant patients and their slower transplant counterparts to determine whether the time to transplantation could be used as a predictor of HCC recurrence. We have recently published results suggesting that the recurrence estimates from this data set appear to be reliable.21
PATIENTS AND METHODS
Data on adults (18 years old or older) who were assigned an exception for an HCC diagnosis meeting policy 3.6.4.4 criteria (stage T2: 1 lesion 2–5 cm in size or 2 lesions 1–3 cm in size with late arterial enhancement on computed tomography or magnetic resonance imaging) and underwent LT for the first time between January 1, 2006 and September 30, 2010 were obtained from United Network for Organ Sharing (UNOS) Standard Transplant Analysis Research files (created March 02, 2012) so that we could investigate predictors of posttransplant HCC recurrence. Patients with cholangiocarcinoma as the cause of death (HCC likely misdiagnosed during the initial evaluation; n = 11) or with a laboratory MELD score ≥22 at transplant (likely other underlying liver complications in addition to HCC; n = 25) were excluded from the analysis. Recurrence was defined as either a diagnosis of HCC recurrence [determined by a physician’s review (J.P.R.) of indications of recurrence in malignancy follow-up data] or a posttransplant HCC-related death (when HCC was a primary or contributory cause of posttransplant death).
HCC was designated as the primary diagnosis for 34% of the patients. To identify the underlying causes of liver disease in these patients, the secondary diagnosis at listing and the diagnosis at transplant (when the secondary diagnosis was unavailable or was also HCC) were evaluated. Patients with only a diagnosis of HCC and evidence of viral hepatitis (hepatitis C virus–seropositive or hepatitis B virus surface antigen– positive) were categorized according to the appropriate viral hepatitis diagnosis.
Frequencies and proportions and medians and interquartile ranges (IQRs) for recipient, donor, and tumor characteristics were described for the total population and by the wait time (≤120 or >120 days from the assignment of an HCC exception to transplant), with significant differences assessed with the chisquare or Wilcoxon rank-sum tests. We derived the 120-day wait-time cutoff by plotting HCC recurrence in 30-day wait-time intervals. The cumulative incidence curves separated around 120 days, and this suggested a reasonable cutoff. We calculated the tumor volume in cubic centimeters as the volume of a sphere:
where tumor radius was half of the reported tumor size; tumor volumes were added together for patients with multiple tumors. We calculated the donor risk index in accordance with Feng et al.22 An alpha-fetoprotein (AFP) cutoff of 500 ng/mL was used in accordance with studies showing that an AFP level of approximately 500 ng/mL is predictive of poor posttransplant survival23 and increased waiting-list dropout.24
The risk of posttransplant HCC recurrence was evaluated via competing risks regression with the Fine and Gray model.25 Posttransplant follow-up terminated in HCC recurrence (an event) or death due to other causes (a competing risk). The time to an event was measured as years from LT to (1) the date of the diagnosis of HCC recurrence (if reported) or HCC-related death, (2) the date of death due to non-HCC causes for patients with a competing event, or (3) the date of the last follow-up for patients alive or lost to follow-up (censored). For patients subsequently undergoing LT for the second or third time, the follow-up was evaluated from the date of the first transplant to the date of death, recurrence, or the last follow-up after retransplantation. The posttransplant follow-up status and the date of death were updated when valid Social Security death certificate master file data were available.
Observed cumulative incidences of posttransplant HCC recurrence and 95% confidence intervals (CIs) were calculated while we accounted for competing risks, and they were evaluated by the wait time. Single-predictor estimates for the risk of posttransplant HCC recurrence [subhazard ratios (SHRs)] were first estimated via the modeling of the cumulative incidence function with competing risks regression for tumor, recipient, and donor characteristics. Characteristics with P < 0.1 were further evaluated in the multivariate model. The final model included the wait time and factors for which multivariate P values were <0.05, and accounted for center-level clustering of outcomes. We evaluated the assumption of proportional subdistribution hazards and modeled covariates violating the assumption as time-varying covariates. We also evaluated potential interactions between the wait time and AFP levels and ablative therapy (P > 0.05; data not shown).
Data manipulation and analyses were completed with SAS 9.3 (SAS Institute, Inc., Cary, NC). Competing risks regression was completed with Stata/IC 11.1 (StataCorp, College Station, TX). This study received approval from the University of California San Francisco committee on human research.
RESULTS
There were 5002 LT recipients with HCC eligible for study inclusion; the recipients were primarily male and white with a median age of 57 years (IQR = 53–62 years) at transplant. Hepatitis C virus was the most common underlying cause of liver disease (62.1%). The majority of the patients underwent transplantation with a MELD score of 22 (Table 1). At the assignment of the HCC exception, the median tumor volume was 9.2 cm3 (IQR = 5.6–17.2 cm3), with 43.3% of the patients having received ablative therapy and with 5.9% having an AFP level >500 ng/mL (Table 2). Similar proportions of patients waiting ≤120 days and patients waiting >120 days received ablative therapy at the time of their exception (P = 0.50). Ablative therapy continued while patients waited for transplantation, with more frequent use among the patients waiting >120 days (72.4% for >120 days versus 46.7% for ≤120 days, P < 0.001) and with a greater tumor load [46.2% for >1 tumor, all <2 cm, versus 56.8% for 1 tumor ≥2 cm (P < 0.001) and 58.6% for 2–3 tumors ≥2 cm (P < 0.001)].
TABLE 1.
Recipient and Donor Characteristics for LT Recipients With HCC by the Time to Transplantation
| Characteristics | Total Population (n = 5002) |
Waiting Time ≤ 120 Days (n = 3278) |
Waiting Time > 120 Days (n = 1724) |
P Value |
|---|---|---|---|---|
| Recipient sex: male [n (%)]* | 3872 (77.4) | 2569 (78.4) | 1303 (75.6) | 0.02 |
| Recipient ethnicity [n (%)]* | ||||
| White | 3373 (67.4) | 2337 (71.3) | 1036 (60.1) | <0.001 |
| Black | 447 (8.9) | 308 (9.4) | 139 (8.1) | 0.12 |
| Hispanic/Latino | 684 (13.7) | 378 (11.5) | 306 (17.7) | <0.001 |
| Asian | 435 (8.7) | 222 (6.8) | 213 (12.4) | <0.001 |
| Other/multiracial | 63 (1.3) | 33 (1.0) | 30 (1.7) | <0.001 |
| MELD score at transplant [n (%)]* | ||||
| 22 | 2666 (53.3) | 2614 (79.7) | 52 (3.0) | <0.001 |
| 23–24 | 44 (0.9) | 38 (1.2) | 6 (0.3) | 0.04 |
| 25 | 1199 (24.0) | 491 (15.0) | 708 (41.1) | <0.001 |
| 26–27 | 110 (2.2) | 23 (0.7) | 87 (5.0) | <0.001 |
| 28 | 471 (9.4) | 13 (0.4) | 458 (26.6) | <0.001 |
| 29–30 | 245 (4.9) | 22 (0.7) | 223 (12.9) | <0.001 |
| 31 | 117 (2.3) | 17 (0.5) | 100 (5.8) | <0.001 |
| 32–40 | 150 (3.0) | 60 (1.8) | 90 (5.2) | <0.001 |
| Intensive care unit at transplant [n (%)] | 72 (1.4) | 39 (1.2) | 33 (1.9) | 0.04 |
| Diagnosis [n (%)] | ||||
| Hepatitis C virus | 3108 (62.1) | 2065 (63.0) | 1043 (60.5) | 0.08 |
| Alcoholic cirrhosis | 422 (8.4) | 275 (8.4) | 147 (8.5) | 0.87 |
| Noncholestatic cirrhosis | 287 (5.7) | 190 (5.8) | 97 (5.6) | 0.81 |
| Hepatitis B virus† | 290 (5.8) | 166 (5.1) | 124 (7.2) | 0.002 |
| Nonalcoholic steatohepatitis | 215 (4.3) | 150 (4.6) | 65 (3.8) | 0.18 |
| Other | 680 (13.6) | 432 (13.2) | 248 (14.4) | 0.24 |
| Recipient age at transplant (years)*‡ | 57 (53–62) | 57 (53–62) | 57 (53–62) | 0.02 |
| Donor risk index‡ | 1.37 (1.13–1.68) | 1.37 (1.12–1.68) | 1.38 (1.14–1.69) | 0.49 |
The MELD score at transplant, age at transplant, sex, and ethnicity were not associated with HCC recurrence (P>0.05) and were not included in the multivariate model.
The hepatitis B virus status was included in the multivariate model.
The data are presented as medians and IQRs.
TABLE 2.
HCC Tumor Characteristics for LT Recipients With HCC by the Time to Transplantation
| Characteristics | Total Population (n = 5002) |
Waiting Time ≤ 120 Days (n = 3278) |
Waiting Time > 120 Days (n = 1724) |
P Value |
|---|---|---|---|---|
| Tumor number and size [n (%)]* | ||||
| >1 tumor, all < 2 cm | 630 (12.6) | 392 (12.0) | 238 (13.8) | 0.06 |
| 1 tumor ≥2 cm or multiple tumors | 4077 (81.5) | 2668 (81.4) | 1409 (81.7) | 0.77 |
| with only 1 ≥2 cm | ||||
| 2–3 tumors ≥2 cm | 295 (5.9) | 218 (6.7) | 77 (4.5) | 0.002 |
| Milan criteria at exception [n (%)]† | 4946 (98.9) | 3233 (98.6) | 1713 (99.4) | 0.02 |
| Ablative therapy at exception [n (%)] | 2167 (43.3) | 1409 (43.0) | 758 (44.0) | 0.50 |
| AFP level > 500 ng/mL at exception [n (%)]* | 293 (5.9) | 219 (6.7) | 74 (4.3) | <0.001 |
| HCC recurrence [n (%)] | 324 (6.5) | 230 (7.0) | 94 (5.4) | 0.03 |
| Non-HCC death [n (%)] | 866 (17.3) | 600 (18.3) | 266 (15.4) | 0.01 |
| Total tumor volume at exception (cm3)‡§ | 9.2 (5.6–17.2) | 9.5 (5.6–17.2) | 8.6 (4.8–17.2) | 0.001 |
| Tumor size of main nodule (cm)‡ | 2.4 (2.0–3.1) | 2.4 (2.0–3.1) | 2.4 (2.0–3.1) | 0.08 |
| Time from exception to transplant (days)‡ | 77 (27–158) | 39 (16–74) | 198 (154–274) | <0.001 |
| Posttransplant follow-up (days)‡ | 753 (383–1323) | 837 (390–1407) | 730 (373–1110) | <0.001 |
The tumor number and size and an AFP level > 500 ng/mL at exception were included in the multivariate model.
The Milan criteria were not associated with HCC recurrence (P = 0.11) and were not included in the multivariate model.
The data are presented as medians and IQRs.
The total tumor volume was related to the tumor number and size and thus was not included in the model.
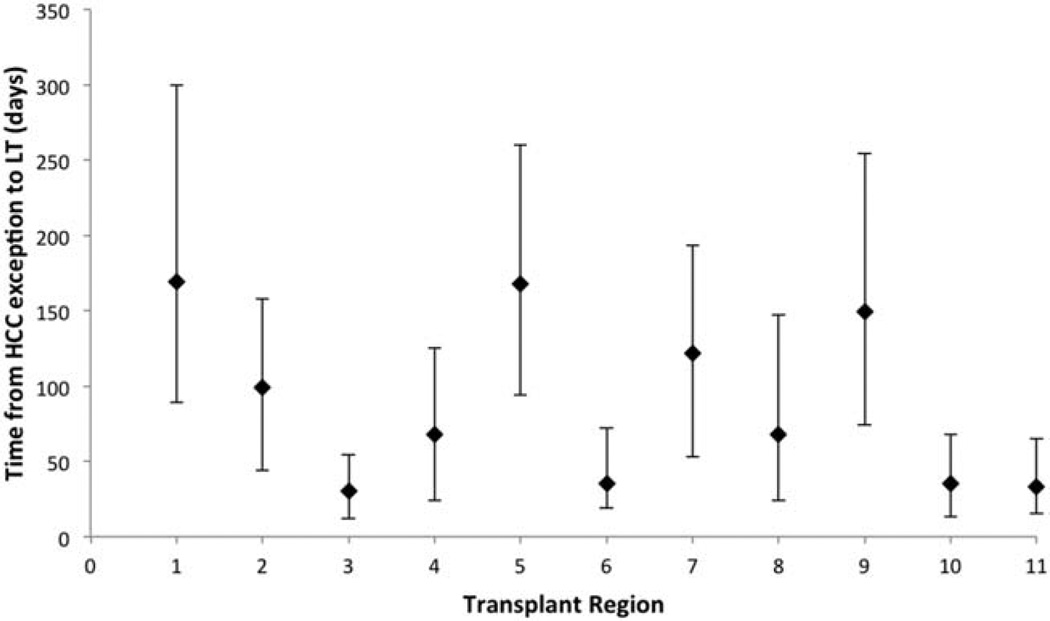
Patients waited for a median of 77 days (IQR = 27–158 days) from the HCC exception to LT; 66% of the patients underwent transplantation ≤120 days after the exception. The median wait time varied by UNOS region, with the shortest median wait times in regions 3, 6, 10, and 11 (30, 35, 35, and 33 days, respectively) and with the longest median wait times in regions 1, 5 and 9 (169, 168, and 149 days, respectively; Fig. 1).
Figure 1.
Wait time from an HCC exception to LT by UNOS transplant region. The median wait time for each region is plotted, with bars indicating IQRs.
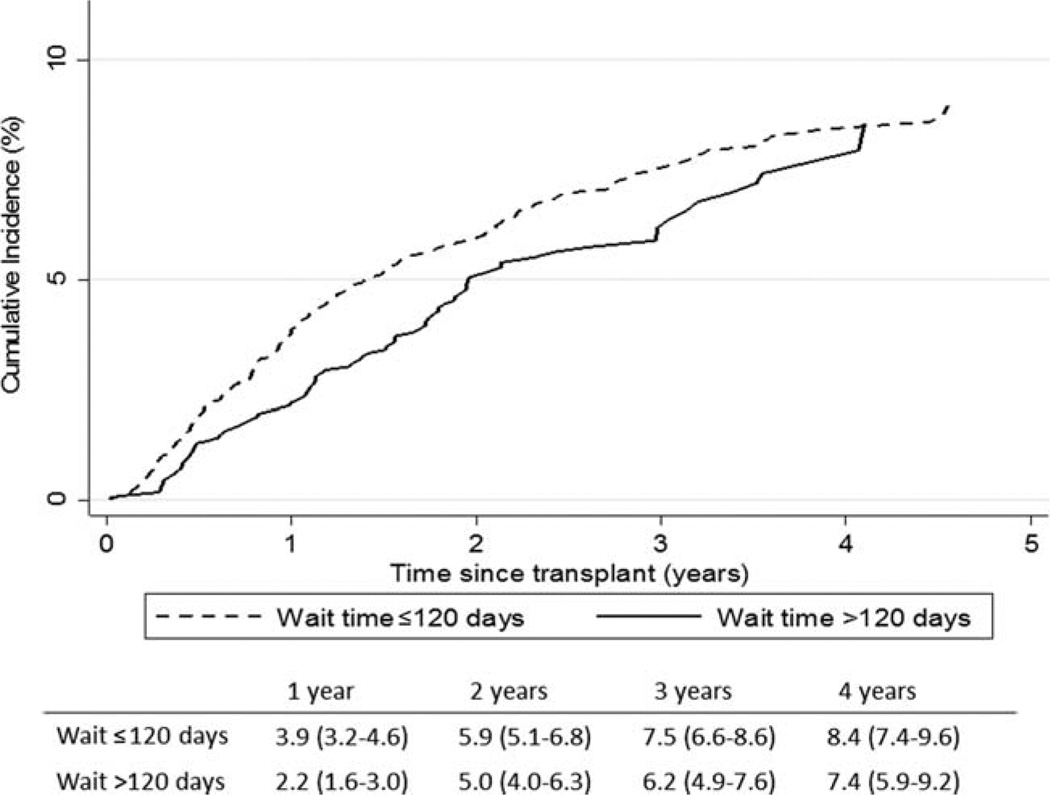
The cumulative incidence of HCC recurrence was 3.3% (95% CI = 2.8%–3.8%) within 1 year of transplantation and 5.6% (95% CI = 5.0%–6.3%) within 2 years of transplantation. The rate of HCC recurrence was lower for patients waiting >120 days versus patients waiting ≤120 days for LT: the cumulative incidences within 1 year were 2.2% (95% CI = 1.6%–3.0%) and 3.9% (95% CI = 3.2%–4.6%), respectively (P = 0.002). This difference was no longer significant 2 years after transplantation (P = 0.09; Fig. 2).
Figure 2.
Observed cumulative incidence of HCC recurrence by the wait time from an HCC exception to LT for patients waiting ≤120 days and patients waiting >120 days. The table in the figure provides estimates and 95% CIs for HCC recurrence within 1, 2, 3, and 4 years of LT.
After adjustments for the tumor size and number, the use of ablative therapy, an AFP level >500 ng/mL, the diagnosis, and the donor risk index, the risk of HCC recurrence was significantly decreased for patients with waiting times >120 days (SHR = 0.6, 95% CI = 0.42–0.85, P = 0.005). However, the association between HCC recurrence and the wait time varied with the time after transplantation. Within 1 year of transplantation, the HCC recurrence risk was reduced by 22% for patients waiting >120 days (P = 0.049). At 2 years after transplantation, the risks were similar for the 2 groups (SHR = 1.03, P = 0.85; Table 3). Non– HCC-related deaths were not significantly reduced by a prolonged waiting time (P > 0.05; data not shown).
TABLE 3.
Risk of Posttransplant HCC Recurrence Estimated With the Fine and Gray Competing Risks Regression Model
| Waiting Time From Exception to LT* | SHR (95% CI) | P Value | ||
|---|---|---|---|---|
| ≤120 days | 1.00 | |||
| >120 days | 0.60 (0.42–0.85) | 0.005 | ||
| Waiting Time From Exception to LT | SHR (95% CI) at a Given Time After LT | |||
| 0.5 Years | 1 Year | 2 Years | 3 Years | |
| ≤120 days | 1.00 | 1.00 | 1.00 | 1.00 |
| >120 days | 0.68 (0.51–0.91) | 0.78 (0.61–0.998) | 1.03 (0.77–1.37) | 1.35 (0.86–2.12) |
| P value | 0.009 | 0.049 | 0.85 | 0.19 |
| SHR (95% CI) With a Given Tumor Size and Number | |||
|---|---|---|---|
| Waiting Time From Exception to LT | >1 Tumor, All < 2 cm (n = 630) |
1 Tumor > 2 cm (n = 4077) |
2–3 Tumors > 2 cm (n = 295) |
| ≤120 days | 1.00 | 1.00 | 1.00 |
| >120 days | 0.66 (0.19–2.28) | 0.64 (0.44–0.95) | 0.19 (0.02–2.11) |
| P value | 0.50 | 0.03 | 0.18 |
| Characteristic | SHR (95% CI) | P Value |
|---|---|---|
| Tumor number and size | ||
| >1 tumor, all < 2 cm | 1.00 | |
| 1 tumor ≥2 cm or multiple tumors with only 1 ≥2 cm | 1.62 (1.13–2.35) | 0.009 |
| 2–3 tumors ≥2 cm | 1.84 (1.08–3.12) | 0.02 |
| Ablative therapy at exception | 1.39 (1.10–1.75) | 0.005 |
| AFP level > 500 ng/mL at exception (versus ≤500 ng/mL)* | 4.68 (2.75–7.95) | <0.001 |
| Diagnosis* | ||
| Hepatitis C virus | 1.00 | |
| Alcoholic cirrhosis | 0.24 (0.10–0.60) | 0.002 |
| Noncholestatic cirrhosis | 0.90 (0.34–2.41) | 0.84 |
| Hepatitis B virus | 1.12 (0.61–2.07) | 0.71 |
| Nonalcoholic steatohepatitis | 0.41 (0.14–1.21) | 0.10 |
| Other | 0.84 (0.51–1.40) | 0.51 |
| Donor risk index | 1.91 (1.43–2.55) | <0.001 |
NOTE: The model accounted for center clustering.
There was a significant interaction with the time after transplantation.
We detected a significant interaction between the wait time and the tumor number and size. The benefit of waiting >120 days for transplantation was increased for patients with multiple large tumors (2–3 tumors ≥2 cm) versus patients with multiple small tumors (>1 tumor, all <2 cm, P = 0.048) and was statistically similar to the benefit for patients with 1 large tumor (1 tumor ≥2 cm, P = 0.30). The observed cumulative incidences of HCC recurrence within 1 year of transplantation for patients waiting >120 days and patients waiting ≤120 days were 1.3% and 7.1%, respectively, for multiple large tumors, 2.4% and 3.8%, respectively, for 1 large tumor, and 1.3% and 2.3%, respectively, for multiple small tumors. Within 2 years of transplantation, the cumulative incidences of HCC recurrence for patients waiting >120 days and patients waiting ≤120 days increased to 3.2% and 8.2%, respectively, for multiple large tumors, 5.1% and 6.2%, respectively, for 1 large tumor, and 5.2% and 2.9%, respectively, for multiple small tumors. Although the observed cumulative incidence of HCC recurrence was numerically higher for patients with multiple small tumors waiting >120 days, a further evaluation of this subgroup showed no benefit from waiting (SHR = 1.64, 95% CI = 0.78–3.44, P = 0.19). Estimates, however, were based on a small number of recurrences (n = 25).
We further investigated wait times and ablative therapy, although no significant interaction was detected between the 2 variables (P = 0.41). HCC recurrence was greater for those waiting ≤120 days with ablative therapy versus those waiting >120 days with ablative therapy (SHR = 2.22, 95% CI = 1.51–3.26, P < 0.001), and it was similar for those waiting ≤120 days without ablative therapy (SHR = 1.20, 95% CI = 0.79–1.83, P = 0.40). Among patients waiting ≤120 days for transplantation, the risk of recurrence was elevated for those with ablative therapy (SHR = 1.85, 95% CI = 1.18–2.88, P = 0.007) versus those without ablative therapy. The benefit of reduced HCC recurrence among patients waiting >120 days was limited to the first year after transplantation. Patients receiving ablative therapy at exception and waiting ≤120 days had a 37% higher risk of HCC recurrence within 1 year after transplantation than their longer waiting counterparts with ablative therapy (P = 0.02), although the risk was statistically similar within 2 years (SHR = 0.85, 95% CI = 0.58–1.24, P = 0.40).
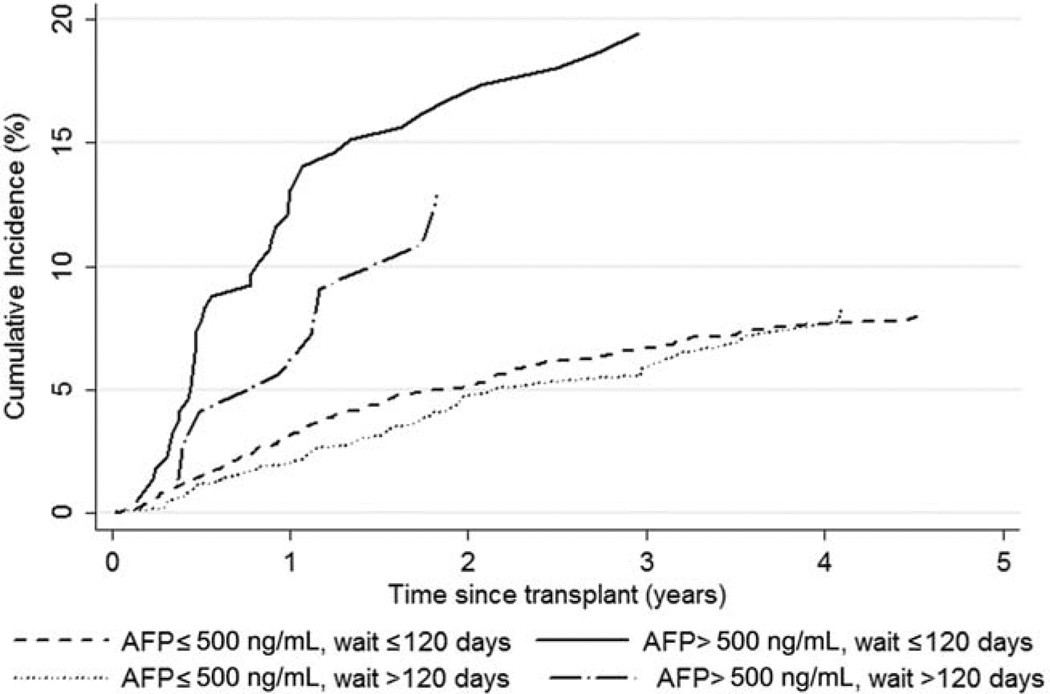
Finally, Fig. 3 shows the cumulative incidence of HCC recurrence by AFP level, although no significant interaction was identified (P = 0.66). Interestingly, the association between HCC recurrence and an AFP level > 500 ng/mL dissipated as the time after transplantation increased. HCC recurrence was no longer significantly different by AFP level within 2.5 years of transplantation (P = 0.24). The SHR for an AFP level > 500 ng/mL versus an AFP level ≤500 ng/mL was 3.71 (95% CI = 2.51–5.51, P < 0.001) within 6 months of transplantation and 1.46 (95% CI = 0.78–2.75, P = 0.24) within 2.5 years of transplantation.
Figure 3.
Observed cumulative incidence of HCC recurrence by the time after transplantation for patients waiting ≤120 days and patients waiting >120 days with AFP levels > or ≤500 ng/mL.
DISCUSSION
The expected increase in the number of HCC cases in the United States over the next decade,26 coupled with the MELD advantage, means that patients with HCC may be getting more livers than is fair, particularly if there is a subgroup with unfavorable outcomes. Roberts et al.27 previously suggested that delaying LT may allow the exclusion of patients with existing metastases after their primary disease is controlled. Up to this point, however, the question of the waiting time as a risk factor for HCC recurrence has mainly been addressed by comparisons of recurrence in the LDLT population, for which there is no wait list, to recipients of cadaveric organ transplants. Early studies finding HCC recurrence to be increased in the LDLT population suggested that this effect was due in part to the fast-tracking of living donor cases.16–18 These studies were hampered by small sample sizes and by difficulty in controlling for tumor characteristics. More recent analyses have not reached a consensus: several studies demonstrated no significant increase in HCC recurrence in the LDLT population after they had controlled for other risk factors,28–30 although a 2013 meta-analysis31 did find LDLT recipients to have lower disease-free survival. Gondolesi et al32 also failed to find that waiting time was a significant predictor of recurrence in LDLT recipients. Comparing LDLT and DDLT, however, is difficult because of unavoidable differences in surgical techniques and graft characteristics that may influence the course of disease.
Our analysis demonstrated that after we had controlled for other predictors, the risk of HCC recurrence after DDLT was significantly lower for patients waiting >120 days from the exception to transplantation, although this advantage dissipated over time. Patients who had received ablative therapy by the time of their HCC exception were at higher risk of recurrence, possibly because of the more aggressive nature of the cancers selected for ablation. Among the patients receiving ablation, those waiting ≤120 days had a significantly higher risk of recurrence than their longer waiting counterparts in the first year after transplantation. The reduction in early recurrence in particular suggests that a significant proportion of early recurrences are due to extrahepatic disease present at the time of transplantation that cannot be eliminated with ablative therapy. Because this difference did not persist after 2 years, it is possible that the tumors in the later transplant patients were also metastasizing but, being more indolent in nature, took longer to grow large enough to be clinically apparent. However, the number of HCC recurrences reported 3, 4, and 5 years after transplantation was relatively low within the wait-time subgroups (≤120 days, 33, 12, and 3, respectively; >120 days, 9, 6, and 2, respectively), and this limited our power to detect differences at these time points.
Our study was limited by the characteristics of the data on which it was built. Although the UNOS/Organ Procurement and Transplantation Network HCC recurrence data have been validated to exclude systematic underreporting and overreporting by centers,33 no mandate requires centers to report HCC recurrence: some cases of recurrence may have been misclassified, and this could have led to an underestimation of the cumulative incidence of HCC recurrence and attenuated risk ratio estimates. This misclassification limited our power to detect differences within subgroups, including wait-time subgroups. This would, however, alter our wait-time findings only if there were systematic underreporting by wait time, and this seems implausible. Other factors, including the blood type and sex, may affect the wait time, although these were not associated with HCC recurrence (P > 0.05; data not shown) and were, therefore, not included in the model. Additionally, UNOS data inadequately describe candidate selection for ablative therapy, and this makes it difficult to critically evaluate ablation on the basis of our findings. An elevated HCC recurrence risk may be associated with ablation, or ablation may be a surrogate marker for an unmeasured component of tumor biology or disease severity.
We found that a waiting time >120 days reduced recurrence in patients receiving ablative therapy; we also found a longer time to be more beneficial for patients with larger (and potentially more aggressive) tumors. In addition, in a recent multicenter study, Lai et al.33 observed that disease progression after local regional therapy based on the modified Response Evaluation Criteria in Solid Tumors predicted poor posttransplant outcomes across all groups within and beyond the Milan criteria. In another study from our institution with prolonged wait-list times, those with progressive disease after ablative therapy based on the modified Response Evaluation Criteria in Solid Tumors had an 85% cumulative risk of dropout from the waiting list at 1 year,34 and this suggests that ablative therapy along with longer wait-list times help to select out patients with tumors with worse biology.35 We could not definitively evaluate the benefit of waiting for transplantation in the nonablated subgroup because infrequent HCC recurrences among patients waiting >120 days without ablative therapy limited our power. However, the lack of a significant interaction between the wait-time and the ablation variables in our model suggests that both groups may benefit from waiting.
Our results support the ablate-and-wait approach and suggest the implementation of an effective waiting time of 120 days. This is not a novel concept: a waiting time of 3 months has been suggested after the down-staging of tumors outside the Milan criteria to allow time for the manifestation of the tumor biology,36 although this has not yet been put into policy. However, as was evident with the development of the point-adjusted MELD system, prolonging the time to transplantation for HCC patients comes with a risk of dropout due to tumor progression. Within our suggested time frame and for carefully selected low-grade tumors, this risk does not appear excessive: a 2003 study by Yao et al.37 showed the rate of wait-list dropout for HCC patients to be negligible in the first 3 months, and more recent studies have shown this rate to be approximately 4%.15,38 Additionally, it is likely that early dropout related to metastasis is due to extrahepatic disease already present at the time of listing; this is supported by the finding that the early wait-list dropout rate is not reduced by ablation therapy.39,40
In conclusion, our study demonstrates that longer waiting times significantly decrease the risk of HCC recurrence within the first year after DDLT. We suggest the implementation of a 120-day waiting period after listing before LT for HCC is performed. More work must be done to further optimize the selection of patients for LT on the basis of factors beyond the tumor size and number.
Acknowledgments
This work was supported by the Biostatistics Core of the University of California San Francisco Liver Center (P30 DK026743) and by the Dean’s Office Medical Student Research Program at the University of California San Francisco.
Abbreviations
- AFP
alpha-fetoprotein
- CI
confidence interval
- DDLT
deceased donor liver transplantation
- HCC
hepatocellular carcinoma
- IQR
interquartile range
- LDLT
living donor liver transplantation
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- SHR
subhazard ratio
- UNOS
United Network for Organ Sharing
Footnotes
Additional Supporting Information may be found in the online version of this article.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567–580. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 2.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. for United Network for Organ Sharing Liver Disease Severity Score Committee. Model for End-Stage Liver Disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 3.Freeman RB., Jr MELD/PELD: one year later. Transplant Proc. 2003;35:2425–2427. doi: 10.1016/j.transproceed.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P, Balan V, Hernandez JL, Harper AM, Edwards EB, Rodriguez-Luna H, et al. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004;10:36–41. doi: 10.1002/lt.20012. [DOI] [PubMed] [Google Scholar]
- 6.Wiesner RH. Patient selection in an era of donor liver shortage: current US policy. Nat Clin Pract Gastroenterol Hepatol. 2005;2:24–30. doi: 10.1038/ncpgasthep0070. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi M. Liver transplantation in the MELD era—analysis of the OPTN/UNOS registry. Clin Transpl. 2012:41–65. [PubMed] [Google Scholar]
- 8.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation: impact on long-term survival. Ann Surg. 2007;245:51–58. doi: 10.1097/01.sla.0000225255.01668.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 10.Figueras J, Jaurrieta E, Valls C, Benasco C, Rafecas A, Xiol X, et al. Survival after liver transplantation in cirrhotic patients with and without hepatocellular carcinoma: a comparative study. Hepatology. 1997;25:1485–1489. doi: 10.1002/hep.510250629. [DOI] [PubMed] [Google Scholar]
- 11.Facciuto ME, Rochon C, Pandey M, Rodriguez-Davalos M, Samaniego S, Wolf DC, et al. Surgical dilemma: liver resection or liver transplantation for hepatocellular carcinoma and cirrhosis. Intention-to-treat analysis in patients within and out with Milan criteria. HPB (Oxford) 2009;11:398–404. doi: 10.1111/j.1477-2574.2009.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charpentier KP, Cheah YL, Machan JT, Miner T, Morrissey P, Monaco A. Intention to treat survival following liver transplantation for hepatocellular carcinoma within a donor service area. HPB (Oxford) 2008;10:412–415. doi: 10.1080/13651820802392320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mailey B, Buchberg B, Prendergast C, Artinyan A, Khalili J, Sanchez-Luege N, et al. A disease-based comparison of liver transplantation outcomes. Am Surg. 2009;75:901–908. doi: 10.1177/000313480907501008. [DOI] [PubMed] [Google Scholar]
- 14.Washburn K. Model for End Stage Liver Disease and hepatocellular carcinoma: a moving target. Transplant Rev (Orlando) 2010;24:11–17. doi: 10.1016/j.trre.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg D, French B, Abt P, Feng S, Cameron AM. Increasing disparity in waitlist mortality rates with increased Model for End-Stage Liver Disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver Transpl. 2012;18:434–443. doi: 10.1002/lt.23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127(suppl 1):S277–S282. doi: 10.1053/j.gastro.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 17.Fisher RA, Kulik LM, Freise CE, Lok AS, Shearon TH, Brown RS, Jr, et al. for A2ALL Study Group. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007;7:1601–1608. doi: 10.1111/j.1600-6143.2007.01802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vakili K, Pomposelli JJ, Cheah YL, Akoad M, Lewis WD, Khettry U, et al. Living donor liver transplantation for hepatocellular carcinoma: increased recurrence but improved survival. Liver Transpl. 2009;15:1861–1866. doi: 10.1002/lt.21940. [DOI] [PubMed] [Google Scholar]
- 19.Chao SD, Roberts JP, Farr M, Yao FY. Short waitlist time does not adversely impact outcome following liver transplantation for hepatocellular carcinoma. Am J Transplant. 2007;7:1594–1600. doi: 10.1111/j.1600-6143.2007.01800.x. [DOI] [PubMed] [Google Scholar]
- 20.Lai Q, Avolio AW, Lerut J, Singh G, Chan SC, Berloco PB, et al. Recurrence of hepatocellular cancer after liver transplantation: the role of primary resection and salvage transplantation in East and West. J Hepatol. 2012;57:974–979. doi: 10.1016/j.jhep.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 21.Samoylova ML, Dodge JL, Vittinghoff E, Yao FY, Roberts JP. Validating posttransplant hepatocellular carcinoma recurrence data in the United Network for Organ Sharing database. Liver Transpl. 2013;19:1318–1323. doi: 10.1002/lt.23735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 23.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant. 2010;10:1643–1648. doi: 10.1111/j.1600-6143.2010.03127.x. [DOI] [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 26.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 27.Roberts JP, Venook A, Kerlan R, Yao F. Hepatocellular carcinoma: ablate and wait versus rapid transplantation. Liver Transpl. 2010;16:925–929. doi: 10.1002/lt.22103. [DOI] [PubMed] [Google Scholar]
- 28.Fan ST. Live-donor liver transplantation for hepatocellular carcinoma. Hepatol Res. 2007;37(suppl 2):S275–S276. doi: 10.1111/j.1872-034X.2007.00196.x. [DOI] [PubMed] [Google Scholar]
- 29.Sandhu L, Sandroussi C, Guba M, Selzner M, Ghanekar A, Cattral MS, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transpl. 2012;18:315–322. doi: 10.1002/lt.22477. [DOI] [PubMed] [Google Scholar]
- 30.Todo S, Furukawa H for Japanese Study Group on Organ Transplantation. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240:451–459. doi: 10.1097/01.sla.0000137129.98894.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant RC, Sandhu L, Dixon PR, Greig PD, Grant DR, McGilvray ID. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant. 2013;27:140–147. doi: 10.1111/ctr.12031. [DOI] [PubMed] [Google Scholar]
- 32.Gondolesi GE, Roayaie S, Mu~noz L, Kim-Schluger L, Schiano T, Fishbein TM, et al. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg. 2004;239:142–149. doi: 10.1097/01.sla.0000109022.32391.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai Q, Avolio AW, Graziadei I, Otto G, Rossi M, Tisone G, et al. for European Hepatocellular Cancer Liver Transplant Study Group. Alpha-fetoprotein and modified Response Evaluation Criteria in Solid Tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19:1108–1118. doi: 10.1002/lt.23706. [DOI] [PubMed] [Google Scholar]
- 34.Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl. 2013;19:1343–1353. doi: 10.1002/lt.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta N, Yao FY. Moving past “one size (and number) fits all” in the selection of candidates with hepatocellular carcinoma for liver transplantation. Liver Transpl. 2013;19:1055–1058. doi: 10.1002/lt.23730. [DOI] [PubMed] [Google Scholar]
- 36.Yao FY, Breitenstein S, Broelsch CE, Dufour JF, Sherman M. Does a patient qualify for liver transplantation after the down-staging of hepatocellular carcinoma? Liver Transpl. 2011;17(suppl 2):S109–S116. doi: 10.1002/lt.22335. [DOI] [PubMed] [Google Scholar]
- 37.Yao FY, Bass NM, Nikolai B, Merriman R, Davern TJ, Kerlan R, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl. 2003;9:684–692. doi: 10.1053/jlts.2003.50147. [DOI] [PubMed] [Google Scholar]
- 38.Park SJ, Freise CE, Hirose R, Kerlan RK, Yao FY, Roberts JP, Vagefi PA. Risk factors for liver transplant waitlist dropout in patients with hepatocellular carcinoma. Clin Transplant. 2012;26:E359–E364. doi: 10.1111/j.1399-0012.2012.01668.x. [DOI] [PubMed] [Google Scholar]
- 39.Maddala YK, Stadheim L, Andrews JC, Burgart LJ, Rosen CB, Kremers WK, Gores G. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: outcome with chemoembolization. Liver Transpl. 2004;10:449–455. doi: 10.1002/lt.20099. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi PH, Ludkowski M, Forman LM, Osgood M, Johnson S, Kugelmas M, et al. Hepatic artery chemoembolization for hepatocellular carcinoma in patients listed for liver transplantation. Am J Transplant. 2004;4:782–787. doi: 10.1111/j.1600-6143.2004.00413.x. [DOI] [PubMed] [Google Scholar]