Abstract
Introduction
Individual agents used to treat human osteoporosis reduce fracture risk by ~50-60%. Since agents that act with complementary mechanisms are available, sequential therapies that mix anti-resorptive and anabolic agents could improve fracture risk reduction, when compared to monotherapies.
Methods
We evaluated bone mass, bone microarchitecture, and bone strength in adult ovariectomized (OVX), osteopenic rats, during different sequences of vehicle (Veh), parathyroid hormone (PTH), alendronate (Aln), or raloxifene (Ral) in three 90 day treatment periods, over nine months. Differences among groups were evaluated. The interrelationships of bone mass and microarchitecture endpoints, and their relationship to bone strength were studied.
Results
Estrogen deficiency caused bone loss. OVX rats treated with Aln monotherapy had significantly better bone mass, microarchitecture, and bone strength than untreated OVX rats. Rats treated with an Aln drug holiday had bone mass and microarchitecture similar to the Aln monotherapy group, but with significantly lower bone strength. PTH-treated rats had markedly higher bone endpoints, but all were lost after PTH withdrawal without follow-up treatment. Rats treated with PTH followed by Aln had better bone endpoints than those treated with Aln monotherapy, PTH monotherapy, or an Aln holiday. Rats treated initially with Aln or Ral, then switched to PTH, also had better bone endpoints, than monotherapy treatment. Rats treated with Aln, then PTH, and returned to Aln had the highest values for all endpoints.
Conclusion
Our data indicate that anti-resorptive therapy can be coupled with an anabolic agent, to produce and maintain better bone mass, microarchitecture, and strength than can be achieved with any monotherapy.
Keywords: parathyroid hormone, raloxifene, alendronate, trabecular bone, microarchitecture, ovariectomy
Introduction
Osteoporosis is currently treated with effective therapies that act through different tissue level mechanisms to reduce fracture risk. These include anti-resorptive agents like bisphosphonates, a selective estrogen receptor modulator, and a receptor activator of NF kappa B ligand (RANKL) inhibitor, that reduce bone turnover [1], and an anabolic agent, parathyroid hormone [hPTH (1-34)] (PTH) [2], that increases bone formation. Both anti-resorptive and anabolic treatments, that may include both PTH(1-34) and PTH (1-84), raise bone mass, improve bone strength, and reduce fracture risk in postmenopausal women with osteoporosis [3-6].
Since osteoporosis is a chronic disease, long-term management is required. Though many patients respond well to treatment for a number of years, others may need additional therapy. As a result, osteoporosis medications with complementary tissue level mechanisms of action are not infrequently prescribed in a sequential manner. Since bisphosphonates are recommended as the firstline therapy for osteoporosis, many patients that may eventually receive PTH have already received bisphosphonates. Pre-clinical data that compare various treatment sequences could help guide future osteoporosis treatment strategies to achieve better fracture risk reduction than is possible with one agent alone.
Some clinical studies of sequential and/or combined treatment have been reported. PTH treatment has been followed by anti-resorptive agents [7, 8]. Treatment with either raloxifene (Ral) or alendronate (Aln) has been followed by PTH [9, 10] or combined with PTH [10, 11]. Pre-clinical and clinical studies agree that sequential therapy using anti-resorptive agents after PTH is necessary to maintain the bone mass that is added by PTH [7, 12-14]. However, all clinical studies of sequential osteoporosis treatments, not only are of relatively limited duration and sample size, but also lack fracture data. Most report only bone mineral density (BMD) data, that by itself is insufficient to predict completely fracture risk [15].
Studies have also been conducted using sequential therapy on small animal models of osteoporosis [14, 16, 17]. In rats that were ovariectomized (OVX) and left untreated for 11 weeks, the increased bone volume and trabecular BMD seen after 12 weeks of PTH(1-34), was lost within 12 and 24 weeks of PTH withdrawal, respectively [17]. When PTH withdrawal was followed by risedronate, both increased bone mass [17] and bone strength from PTH were maintained [18]. However, a dose of estrogen that prevented OVX-induced bone loss in adult female rats, failed to maintain BV/TV, BMD, and bone strength, after PTH withdrawal [17].
Despite the fact that patients are now cycled through bone active medications, there are very few pre-clinical data addressing how these sequential osteoporosis therapies affect bone mass, microarchitecture, and strength. We propose to address with pre-clinical data, the possibility that properly sequencing current osteoporosis treatment agents, with their complementary mechanisms of action, can produce better fracture risk reduction than can be achieved by any single monotherapy. To do so, we evaluated bone quantity, microarchitecture, and strength in various sequences of anti-resorptive and anabolic therapy that have already been or could be applied clinically. The goal of our study was to compare a set of pre-clinical bone endpoints from approved therapies in which fracture risk data exist for humans, to the same endpoints after sequential therapies in which human fracture risk has not yet been measured.
Materials and Methods
Animals and Experimental Procedures
Six-month-old female ovariectomized (OVX) or sham-operated Sprague-Dawley rats were purchased from and operated on at Harlan Laboratories (Livermore, CA, USA) The rats were shipped to our laboratory two weeks after surgery. They were then maintained on commercial rodent chow (Rodent Diet, Cat# 2918, Teklad; Madison, WI USA) in a 21°C temperature room, with a 12-hour light/dark cycle. Within a week of arrival in our laboratory, pair-feeding of OVX to Sham rats was started. Sham and OVX groups were necropsied at two months post-surgery (Period 0). All remaining OVX rats were then randomized by body weight into ten groups (Table 1), that represent currently-used and potential sequences of anti-osteoporosis medications. OVX rats were treated for three months (Period 1) with vehicle [Veh, normal saline, 1ml/kg/dose, three times per week by subcutaneous (SC) injection] (Life Technologies, Cat# 10010, Grand Island, NY, USA); parathyroid hormone, [hPTH (1-34) (human) acetate 25 μg/kg/dose, SC,5x/wk] (Bachem Biosciences Inc, Cat# H-4835, King of Prussia, PA, USA); alendronate [Aln, 25 μg/kg/dose, SC,2x/wk] (Sigma, Cat# A-4978, St. Louis, MO, USA); or raloxifene [Ral, 5 mg/kg/dose, 3x/wk by oral gavage] (Sigma, Cat# R-1402) (Table 1). No group was orally-dosed three times weekly with Veh to control strictly for oral gavage of Ral rats.
Table 1.
Experimental groups
| Treatment | Period 0 (days –60 to 0) | Period 1 (days 0–90) | Period 2 (days 91–180) | Period 3 (days 181–270) |
|---|---|---|---|---|
| Sham | No treatment (n=12) | No treatment (n=12) | No treatment (n=12) | No treatment (n=7) |
| OVX | ||||
| Veh-Veh-Veh | No treatment (n=10) | Vehicle (n=10) | Vehicle (n=10) | Vehicle (n=10) |
| Aln-Aln-Aln | Alendronate (n=12) | Alendronate (n=12) | Alendronate (n=12) | |
| Ral-Ral-Ral | Raloxifene (n=11) | Raloxifene (n=11) | Raloxifene (n=12) | |
| Aln-Veh-Aln | Alendronate (n=12) | Vehicle (n=12) | Alendronate (n=15) | |
| PTH-Veh-Veh | hPTH(1–34) (n=12) | Vehicle (n=11) | Vehicle (n=12) | |
| PTH-Aln-Veh | hPTH(1–34) (n=12) | Alendronate (n=12) | Vehicle (n=11) | |
| PTH-Ral-Ral | hPTH(1–34) (n=6) | Raloxifene (n=12) | Raloxifene (n=12) | |
| Aln-PTH-Veh | Alendronate (n=12) | hPTH(1–34) (n=12) | Vehicle (n=12) | |
| Aln-PTH-Aln | Alendronate (n=11) | hPTH(1–34) (n=12) | Alendronate (n=10) | |
| Ral-PTH-Ral | – | hPTH(1–34) (n=11) | Raloxifene (n=11) |
Day –60 was the day of ovariectomy (OVX). Day 0 was the first day of dosing. Period 0 (days –60 to 0) allowed establishment of mild-moderate estrogen-deficiency osteopenia. OVX rats were still losing trabecular bone during periods 1 and 2. The number of rats killed after each period from each group is shown. As Ral treatment during period 1 was common to Ral-Ral-Ral and Ral-PTH-Ral groups, no rats from the Ral-PTH-Ral group were killed at the end of period 1. The treatment regimens were: Vehicle (Veh) subcutaneously (SC) at 1 mL kg–1 dose–1, 3×/week; parathyroid hormone (hPTH(1– 34)) (PTH) SC at 25 μg kg–1 dose–1, 5×/week; alendronate (Aln) given SC at 25 μg kg–1 dose–1, 2×/week; and raloxifene (Ral) by oral gavage at 5 mg kg–1 dose–1, 3×/week
The approved dose of PTH(1-34) for the treatment of human osteoporosis that is safe, well-tolerated, and efficacious, is 20μg daily, equating to 2.3μg/kg/wk in a 60kg women. A PTH(1-34) dose that is often used in rat studies is 80μg/kg daily or 560μg/kg/wk [19] . The PTH(1-34) dose of 25μg/kg/d 5d/wk utilized in our rat study is 125μg/kg/wk. Since we administered PTH(1-34) for 90d, we chose a dose that was only ~50-fold, rather than ~240-fold above that used in osteoporotic humans.
The approved dose for raloxifene for the treatment of human osteoporosis is 60mg daily by mouth, or 7mg/kg/wk in a 60kg women. The raloxifene dose (5mg/kg 3d/wk) in our rat study is 2.1mg/kg/wk orally. The minimum raloxifene dose that prevents most OVX-induced bone loss in rats is 1.5mg/kg/d or 10.5mg/kg/wk orally[20] .
The approved dose of alendronate for the treatment of human osteoporosis is 70mg weekly by mouth, or 1.17mg/kg/wk in a 60kg women. Assuming 0.7% bioavailability, this equates to 8.2μg/kg/wk that can be absorbed. Our dose of alendronate (50μg/kg/wk SC) is also based on the minimum dose that has been reported to completely prevent OVX-induced bone loss in rats [21].
After 90 days (Period 1), 6-15 animals were randomly-selected from each group and necropsied, while the remaining animals were switched to their second treatment regimen. At 180 days, another 6-15 animals from each group were necropsied (Period 2), while the remaining animals were switched to their third treatment for ninety days (Period 3), at the end of which time they were necropsied. Experimental groups, with final numbers, are listed (Table 1). During the study, nine rats, randomly-disbursed over the ten groups, died, leaving 383 that reached necropsy. The study protocol was approved by the University of California Davis Institutional Animal Care and Use Committee.
At necropsy, the rats were euthanized by CO2 inhalation. Whole blood was drawn by venipuncture of the left ventricle, then was transferred to a 10cc centrifuge tube and spun to yield serum that was then frozen at −20°C. The uterus was inspected visually to confirm efficacy of OVX. The right femur and the lumbar vertebral (LV) spine 2-6 were excised and cleaned. LV5 and LV6 were dissected free from the vertebral segment. The right femur and LV5 were placed in 10% formalin for 24hrs, then transferred to 70% ethanol. LV6 was wrapped in saline-soaked gauze and frozen at −20°C. The right femur and LV5 were stored in 70% ethanol, while LV6 remained at −20°C until analysis.
MicroCT measurements of bone volume, microarchitecture, and degree of mineralization of bone tissue
The right distal femoral metaphysis (DFM) and fifth lumbar vertebra (LV5) were scanned with μCT (VivaCT 40, Scanco Medical AG, Bassersdorf, Switzerland) at 70 kev and 85 μA with an isotropic resolution of 10.5μm in all three dimensions. Scanning of the DFM was initiated at the level of the growth cartilage-metaphyseal junction and extended proximally for 250 slices. Evaluations were performed on 150 slices beginning 0.2mm proximal to the most proximal point along the boundary of the growth cartilage with the metaphysis. The entire LV5 was scanned. All trabecular bone in the marrow cavity was evaluated. For each slice, the volume of interest was defined as ~0.25mm internal to the boundary of the marrow cavity with the cortex. The methods for calculating bone volume (BV), total volume (TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), structure model index (SMI), degree of anisotropy (DA), and degree of bone mineralization (DBM) have been described [22].
Biomechanical testing
Mechanical properties were determined by a compression test of the sixth lumbar vertebral body (LV6). The posterior elements and transverse processes were removed with a bone cutter (Liston Gross Anatomy Bone Cutter, Fine Science Tools, Inc, Cat# 16104, Foster City, CA, USA). The endplates were removed using a wafer saw and polished to flat, parallel surfaces. Before testing, seven measurements were taken using a digital caliper (Fowler® 0-12″/300mm IP54 Digital Caliper, Global Equipment Company, Inc, Cat# T9FB799807, Buford GA, USA), that is, one length measurement (cranial-caudal direction) and six diameter measurements (major and minor axis at ~0.5 mm from the cranial end, the middle and at ~0.5 mm from the caudal end) of the vertebral body. The average diameter measurements were used with a standard area of a circle equation (π*((d/2)^2)), to calculate cross-sectional area of the test sample.
Test samples were stored in Hanks' Balanced Salt Solution (HBSS) 12 hours prior to testing. They were loaded using an electro servo-hydraulic materials testing system (MTS Model 831, Eden Prairie, MN, USA) in HBSS at 37°C at a displacement rate of 0.01 mm/s. Maximum load, maximum stress, yield stress, stiffness, and energy absorption were calculated from the load-displacement curve. Energy absorption, or work to fracture, was specifically determined from the area under the load-displacement curve divided by twice the cross sectional area of the test specimen.
Statistics
The group means and standard deviations (SDs) were calculated for all variables. When groups were treated identically during the same period, their data were combined and means and SDs for the combined data were calculated for all variables (Table 8).The differences for each variable between the two Period 0 groups were analyzed by two-sided t-test. Differences among the eleven groups for each variable within Periods 1, 2, and 3, respectively, were analyzed by analysis of variance (ANOVA). Differences were considered statistically significant at p-value ≤ 0.05 and inter-group differences were determined by an F-test with the Bonferroni correction, as a post-hoc test for multiple (pair-wise) comparisons.
Table 8.
Determinants of maximum load (multiple regression) in the lumbar vertebral body
| Endpoint | Total R |
|---|---|
| Tb.Th | 0.812 |
| Cross-sectional area | 0.860 |
| DBM | 0.873 |
| SMI | 0.882 |
Tb. Th trabecular thickness, DBM degree of bone mineralization, SMI ure Model Index
The relationship of BV/TV to SMI and DA in both the vertebral body and distal femoral metaphysis was evaluated using linear regression. The bone mass, cross-sectional area, and microarchitectural endpoints that determine vertebral body maximum load were evaluated with multiple regression. All statistical analyses were performed with SAS v9.2 (Cary, NC, USA).
Results
The uteri in OVX rats were routinely observed to be smaller than those of Sham rats at necropsy. Compared to Sham, OVX resulted in a significant increase in body weight within four weeks of surgery (p<0.05). However, once pair-feeding was begun, body weight in Veh-Veh-Veh rats dropped gradually and was not significantly different from Sham rats at the end of any treatment period. Body weight was not affected by any treatment (data not shown).
Bone Volume and Microarchitecture
Fifth Lumbar Vertebral Body
At baseline and all subsequent time periods, lumbar vertebral body bone volume (LV-BV/TV) in Veh-Veh-Veh was less than in Sham rats (Tables 2-5, Figure 1a). At Day 90, all treatment groups except for Ral-Ral-Ral, had higher LV-BV/TV than Veh-Veh-Veh (Table 3). Of the four groups that received Aln monotherapy in Period 1, three (Aln-Aln-Aln, Aln-Veh-Aln, and Aln-PTH-Veh) had LV-BV/TV ranging from 32-36%, while the fourth (Aln-PTH-Aln) had LV-BV/TV of 51%, with a range in individual rats of 42-58%, that was significantly different from the other three Aln-first groups (Figure 1c). At Day 180, LV-BV/TV in PTH-Veh-Veh was the same as Veh-Veh-Veh, whereas all other treatment groups were above Veh-Veh-Veh (Table 4, Figure 1b). LV-BV/TV in PTH-Aln-Veh was higher than in all other treatment groups. By Day 270, all regimens that included PTH, except for PTH-Veh-Veh and PTH-Ral-Ral, had greater LV-BV/TV than the other treatment groups (Table 5, Figures 1b and 1c).
Table 2.
Trabecular bone mass, microarchitecture, and bone strength at end of period 0
| Sham | OVX | |
|---|---|---|
| MicroCT | ||
| Number of rats | 11 | 10 |
| LV-BV/TV (%) | 40.0±4.0* | 27.8±4.7 |
| LV-Tb.N (mm−1) | 4.00±0.36* | 3.36±0.31 |
| LV-Tb.Th (μm) | 90.4±4.8* | 81.0±6.4 |
| LV_SMI | –1.42±0.5* | –0.12±0.50 |
| LV_DA | 1.73±0.06 | 1.77±0.05 |
| LV-DBM (mgHA/cm3) | 907±23* | 871±15 |
| Number of rats | 11 | 8 |
| DF-BV/TV (%) | 31.3±4.0* | 15.0±3.7 |
| DF-Tb.N (mm−1) | 4.84±0.49* | 2.99±0.13 |
| DF-Tb.Th (μm) | 82.3±6.2* | 72.4±6.7 |
| DF_SMI | 0.65±0.38* | 1.88±0.20 |
| DF_DA | 1.42±0.05 | 1.46±0.08 |
| DF-DBM (mgHA/cm3) | 970±14* | 944±12 |
| LV compression | ||
| Number of rats | 12 | 10 |
| Max load (N) | 210±46 | 180±51 |
| Max stress (MPa) | 21.0±4.2 | 17.8±4.0 |
| Yield stress (MPa) | 11.8±3.6 | 12.0±4.6 |
| Stiffness (GPa) | 0.55±0.23 | 0.40±0.16 |
| Energy absorption (kJ/m2) | 5.1±2.5 | 3.9±1.9 |
Mean±SD
LV lumbar vertebral body, DF distal femur, BV/TV bone volume/tissue volume, Tb.Th trabecular thickness, Tb.N trabecular number, SMI Struc- ture Model Index, DA degree of anisotropy, HA hydroxyapatite, DBM degree of bone mineralization, Max load maximum load, Max Stress maximum stress
p<.05, difference from OVX
Table 5.
Trabecular bone mass, microarchitecture, and bone strength at the end of period 3 (day 270)
| Sham | OVX | Aln-Aln-Aln | Ral-Ral-Ral | Aln-Veh-Aln | PTH-Veh-Veh | PTH-Aln-Veh | PTH-Ral-Ral | Aln-PTH-Veh | Aln-PTH-Aln | Ral-PTH-Ral | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group ID | s | * | a | b | c | d | e | f | g | h | i |
| MicroCT | |||||||||||
| Number of rats | 7 | 9 | 12 | 12 | 15 | 8 | 6 | 12 | 12 | 9 | 11 |
| LV-BV/TV (%) | 42.5±9.6* | 20.6±3.5 | 33.0±2.5*bdeghi | 27.2±2.9*cdefghi | 31.8±4.1*deghi | 20.3±3.3efghi | 46.2±2.7*fghi | 32.8±4.5*ghi | 40.0±3.9*h | 54.4±4.7*i | 39.3±6.5* |
| LV-Tb.N (1/mm) | 3.93±0.41* | 2.78±0.27 | 3.34±0.15*bdh | 3.00±0.27cdeghi | 3.38±0.25*dfh | 2.65±0.22efghi | 3.53±0.13*fh | 3.16±0.27*gh | 3.48±0.28*h | 3.85±0.30*i | 3.26±0.29* |
| LV-Tb.Th (μm) | 98.9±15.8* | 71.0±4.3 | 89.7±5.5*deghi | 84.2±2.9*defghi | 89.1±4.4*deghi | 73.2±4.2efghi | 116.3±4.4*fgh | 93.3±7.3*ghi | 103.8±5.8*h | 132.4±7.4*i | 109.3±13.4* |
| LV-SMI | –1.97±1.02* | 0.14±0.31 | –0.65±0.26*degh | –0.30±0.31eghi | –0.32±0.39deghi | 0.23±0.28efghi | –1.96±0.35*fghi | –0.71±0.36*fg | –1.33±0.38*h | –3.02±0.83*i | –1.17±0.62* |
| Number of rats | 7 | 10 | 11 | 12 | 15 | 11 | 11 | 11 | 12 | 10 | 11 |
| DF-BV/TV (%) | 25.4±10.5* | 6.8±1.9 | 14.4±3.2*bcdeghi | 10.0±2.0cefghi | 18.9±3.2*deghi | 7.6±2.3efghi | 26.5±3.7*fh | 16.7±2.7*ghi | 23.5±6.5*h | 36.0±5.0*i | 27.8±7.8* |
| DF-Tb.N (1/mm) | 4.17±0.76* | 2.28±0.24 | 2.91±0.30*bdfh | 2.42±0.33cegh | 3.23±0.34*dfi | 2.11±0.32efghi | 2.93±0.32*fh | 2.51±0.25gh | 2.99±0.56*h | 3.42±0.23*i | 2.75±0.43* |
| DF-Tb.Th (μm) | 75.5±15.7* | 64.0±4.3 | 73.1±3.6*eghi | 72.7±4.0*cefghi | 80.3±5.5*deghi | 66.4±7.5efghi | 97.7±7.8*fhi | 80.7±4.1*ghi | 91.8±7.4*hi | 115.3±10.5* | 110.6±23.5* |
| DF-SMI | 0.74±0.80* | 2.64±0.31 | 2.00±0.29*efghi | 2.38±0.25cefghi | 1.85±0.26*defghi | 2.38±0.44efghi | 0.68±0.34*fh | 1.38±0.24*hi | 1.04±0.48*hi | 0.07±0.49*i | 0.50±0.65* |
| DF-DA | 1.41±0.06 | 1.37±0.08 | 1.47±0.06*bdeghi | 1.41±0.07ch | 1.50±0.04*deghi | 1.41±0.12gh | 1.35±0.05f | 1.46±0.06*ghi | 1.35±0.07 | 1.31±0.07 | 1.35±0.07 |
| DF-DBM (mgHA/cm3) | 968±19 | 956±12 | 982±11*bdfh | 968±10cdehi | 987±13*dfg | 953±16eghi | 984±13*f | 961±8hi | 971±15*h | 997±22* | 989±31* |
| LV compression | |||||||||||
| Number of rats | 7 | 10 | 12 | 12 | 15 | 11 | 11 | 11 | 12 | 10 | 9 |
| Max load (N) | 255±68* | 159±55 | 227±51*bcdegh | 162±21eghi | 171±47eghi | 154±38efghi | 282±45*fhi | 205±43gh | 275±51*hi | 387±68*i | 225±51* |
| Max stress (MPa) | 21.9±6.0* | 13.2±4.1 | 19.1±3.4*bcdegh | 15.5±2.3efghi | 15.2±4.3efghi | 13.0±3.3efghi | 23.6±3.2*fhi | 20.2±4.0*h | 22.7±3.4*h | 31.6±3.8*i | 19.8±4.5* |
| Yield stress (MPa) | 15.5±4.1* | 9.4±3.3 | 15.2±3.1*bcdh | 10.8±2.3cefghi | 7.3±2.0efghi | 10.0±4.1efghi | 15.3±3.0*h | 15.2±3.9*h | 17.5±4.2*h | 23.8±6.1*i | 15.3±4.8* |
| Stiffness (GPa) | 0.73±0.24* | 0.45±0.22 | 0.63±0.22cdh | 0.44±0.14fh | 0.37±0.13fgh | 0.43±0.20fgh | 0.53±0.16fh | 0.73±0.27* | 0.62±0.16h | 0.86±0.25*i | 0.55±0.22 |
| Energy absorption (kJ/m2) | 4.5±1.8 | 2.9±2.7 | 3.3±1.3behi | 5.3±1.7*cdi | 3.2±2.0ehi | 2.3±0.9efghi | 7.1±2.3*fg | 4.5±2.2hi | 4.8±2.2i | 6.5±2.0* | 8.0±2.9* |
Mean±SD. Lowercase letters—difference from group (p<.05)
LV lumbar vertebral body, DF distal femur, BV/TV bone volume/total tissue volume, Tb.N trabecular number, Tb.Th trabecular thickness, SMI Structure Model Index, DA degree of anisotropy, HA hydroxyapatite, DBM degree of bone mineralization, Max load maximum load, Max stress maximum stress
p<.05, difference from OVX
Figure 1.
These figures provide a graphical explanation of the study design and general outcome. The complete dataset with statistical testing is reported in Tables 2-5. The data represent individual groups of rats at each time period rather than longitudinal measurement of one set of rats.
Figure 1a LV-BV/TV data for all groups that received only anti-resorptive monotherapy (A-A-A, R-R-R, and A-V-A) are shown. Sham and Veh-Veh-Veh group data are included for comparison. Veh-Veh-Veh rats had LV-BV/TV ~30% below that in Sham rats at Day 0, signaling established estrogen-deficiency osteopenia as treatments began. By Day 270, osteopenia in Veh-Veh-Veh groups had progressed. Ral-Ral-Ral treated groups did not develop additional osteopenia after Day 90. Both Aln-Aln-Aln and Aln-Veh-Aln rats had significantly higher LV-BV/TV throughout the experiment than either Ral-Ral-Ral or Veh-Veh-Veh rats, but never reached levels seen in age-matched Sham rats.
Figure 1b LV-BV/TV data for all groups that received PTH(1-34) during Period 1 (P-V-V, P-A-V, and P-R-R) are shown, along with from Sham and Veh-Veh-Veh data. All groups experienced a rapid increase in LV-BV/TV by Day 90 that exceede Sham levels, as PTH was given. Failure to follow PTH cessation with anti-resorptive therapy was associated with a rapid decline in LV-BV/TV that reached Veh-Veh-Veh levels by Day 180. Following PTH cessation with raloxifene for 180 days was associated with significantly lower LV-BV/TV that did not, however, reach the Veh-Veh-Veh level by Day 270. Following PTH cessation with alendronate was associated with stable LV-BV/TV for 90d. However, when alendronate was stopped, a slow decline began that did not reach Veh-Veh-Veh levels by Day 270, and was superior to levels seen with raloxifene maintenance.
Figure 1c LV-BV/TV data for groups that began with anti-resorptive monotherapy, then switched to PTH (A-P-V, A-P-A, and R-P-R), are shown with Sham and Veh-Veh-Veh data. The mean of all four groups that received ALN during Period 1 is also plotted in gray. All treated groups experienced a rapid increase in LV-BV/TV between Day 90 and 180, that exceeded Sham levels, as PTH was administered. After PTH cessation, switching rats to alendronate was significantly better than either switching to raloxifene or stopping all treatment. Unlike when PTH was given to treatment-naïve rats in Period 1, when PTH was given to rats pre-treated with anti-resorptive monotherapy, bone mass did not return to Veh-Veh-Veh-levels within 90d.
Table 3.
Trabecular bone mass, microarchitecture, and bone strength at end of period 1 (day 90)
| Sham | O X | Aln-Aln-Aln | Ral-Ral-Ral | Aln-Veh-Aln | PTH-Veh-Veh | PTH-Aln-Veh | P H-Ral-Ral | Aln-PTH-Veh | Aln-PTH-Aln | Ral-PTH-Ral |
|---|---|---|---|---|---|---|---|---|---|---|
| s | * | a | b | c | d | e | f | g | h | i |
| MicroCT | ||||||||||
| Number of rats | 12 | 10 | 12 | 11 | 12 | 12 | 12 | 6 | 12 | 12 |
| LV-BV/TV (%) | 40.9±6.1* | 28.7±5.2 | 32.2±3.0*bdefh | 27.7±2.4cdefgh | 33.4±3.5*defh | 53.4±6.5*g | 51.5±7.5*g | 48.1±6.1*g | 36.1±5.5*h | 51.0±4.4* |
| LV-Tb.N (mm−1) | 4.09±0.54* | 3.21±0.27 | 3.43±0.23*b | 3.19±0.16cdefgh | 3.54±0.25* | 3.55±0.29* | 3.50±0.36* | 3.55±0.31* | 3.42±0.19* | 3.54±0.30* |
| LV-Tb.Th (μm) | 92.0±6.5 | 84.1±6.6 | 88.0±5.5*bdefh | 81.7±4.6cdefgh | 89.4±3.6*defh | 139.5±15.5*g | 134.0±11.4*g | 121.7±10.2*g | 97.5±12.5*h | 132.4±6.4* |
| LV-SMI | –1.71±0.78* | –0.35±0.55 | –0.52±0.36*bdefh | –0.29±0.28cdefgh | –0.48±0.46*defh | –2.29±0.69*g | –2.84±1.14*g | –2.12±0.78*g | –0.90±0.58*h | –2.43±0.60* |
| LV-DA | 0.90±0.81* | 1.76±0.07 | 1.75±0.07 | 1.71±0.04 | 1.76±0.04 | 1.67±0.11 | 1.69±0.08 | 1.67±0.04 | 1.73±0.09 | 1.67±0.08 |
| LV-DBM (mgHA/cm3) | 917±16* | 887±13 | 887±15defh | 904±17defh | 901±18defh | 936±20*egh | 925±26*fgh | 924±23*gh | 908±31h | 957±19* |
| Number of rats | 12 | 10 | 12 | 11 | 12 | 12 | 12 | 5 | 11 | 10 |
| DF-BV/TV (%) | 29.4±7.0* | 12.8±4.4 | 16.9±4.0bcdefh | 8.5±2.8cdefgh | 23.9±6.0*defh | 39.4±6.2*gh | 40.5±9.2*gh | 34.5±4.4*gh | 20.4±5.6*h | 29.8±4.2* |
| DF-Tb.N (mm−1) | 4.62±0.53* | 2.76±0.34 | 3.22±0.42*b | 2.38±0.35*cdefgh | 3.53±0.42*fgh | 3.08±0.46* | 3.37±0.58*h | 2.97±0.22 | 2.90±0.45* | 2.96±0.37* |
| DF-Tb.Th (μm) | 77.7±6.4 | 70.3±7.0 | 73.5±6.5bcdefgh | 65.7±5.8cdefgh | 83.8±7.9*defh | 125.1±7.8*gh | 122.3±8.6*gh | 122.4±8.2*gh | 89.1±16.1*h | 112.9±5.6* |
| DF-SMI | 0.58±0.60* | 1.96±0.45 | 1.76±0.21*bdefh | 2.51±0.36*cdefgh | 1.31±0.50*defh | –0.47±0.64*gh | –0.46±0.95*gh | 0.07±0.41*gh | 1.36±0.59*h | 0.58±0.37* |
| DF-DA | 1.41±0.05 | 1.45±0.06 | 1.43±0.07defh | 1.43±0.09defh | 1.54±0.04defh | 1.31±0.07*g | 1.32±0.07*g | 1.40±0.06*g | 1.37±0.07h | 1.29±0.06* |
| DF-DBM (mgHA/cm3) | 967±20* | 953±11 | 967±22*b | 947±19cdefgh | 972±13* | 990±13* | 983±17* | 978±10* | 983±23* | 1,002±18* |
| LV compression | ||||||||||
| Number of rats | 12 | 10 | 12 | 11 | 12 | 12 | 12 | 5 | 12 | 10 |
| Max load (N) | 203±36 | 220±62 | 242±54bdefh | 178±37cdefgh | 213±23defh | 357±51*fg | 399±112*fgh | 299±79*g | 243±51h | 318±72* |
| Max stress (MPa) | 20.4±2.8 | 19.9±4.0 | 20.8±3.5bdefh | 17.5±3.0cdefgh | 21.5±2.5defh | 31.6±4.8*g | 34.0±7.7*g | 29.7±1.9*g | 20.9±3.9h | 27.2±4.7* |
| Yield stress (MPa) | 11.8±3.3 | 13.4±4.8 | 15.6±4.8defh | 13.0±3.4defh | 15.7±2.5defh | 27.6±5.9*g | 26.7±8.3*g | 20.1±4.1*g | 13.8±4.0h | 22.7±5.2* |
| Stiffness (GPa) | 0.54±0.26 | 0.47±0.15 | 0.61±0.29*bde | 0.48±0.17cdefgh | 0.66±0.21*de | 0.81±0.23*fgh | 1.00±0.40*fgh | 0.61±0.17* | 0.61±0.19* | 0.66±0.19* |
| Energy absorption (kJ/m2) | 4.4±1.8 | 4.7±2.6 | 3.9±1.5bdef | 7.1±3.4*cgh | 5.4±2.8defg | 8.0±3.8*gh | 7.3±2.4*gh | 7.5±1.1*gh | 3.7±1.0 | 6.1±2.5 |
Group ID
Mean±SD. Lowercase letters—difference from group (p<0.05)
LV lumbar vertebral body, DF distal femur, BV/TV bone volume/total tissue volume, Tb.N trabecular number, Tb.Th trabecular thickness, SMI Structure Model Index, DA degree of anisotropy, HA hydroxyapatite, DBM degree of bone mineralization, Max Load maximum load, Max stress maximum stress
p<.05, difference from OVX
Table 4.
Trabecular bone mass, microarchitecture, and bone strength at end of period 2 (day 180)
| Sham | O X | Aln-Aln-Aln | Ral-Ral-Ral | Aln-Veh-Aln | PTH-Veh-Veh | PTH-Aln-Veh | PTH-Ral-Ral | Aln-PTH-Veh | Aln-PTH-Aln | Ral-PTH-Ral | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group ID | s | * | a | b | c | d | e | f | g | h | i |
| MicroCT | |||||||||||
| Number of rats | 12 | 10 | 12 | 11 | 12 | 10 | 12 | 12 | 12 | 12 | 11 |
| LV-Tb.N. (mm−1) | 3.83±0.37* | 2.80±0.25 | 3.39±0.19*bde | 3.11±0.20*ceghi | 3.43±0.18*df | 2.96±0.17eghi | 3.65±0.26*fgh | 3.18±0.23*ghi | 3.43±0.21* | 3.43±0.21* | 3.48±0.36* |
| LV-Tb.Th (μm) | 97.9±15.9* | 68.3±3.7 | 90.0±5.2*deghi | 86.6±5.0*deghi | 92.4±6.5*deghi | 72.3±4.2efghi | 133.7±8.5*f | 91.5±4.5*ghi | 136.2±5.8* | 137.0±7.0* | 138.2±7.4* |
| LV-SMI | –1.67±0.98* | 0.61±0.30 | –0.53±0.47*deghi | –0.33±0.41*deghi | –0.45±0.39*deghi | 0.23±0.17efghi | –2.52±0.70*fgh | –0.70±0.35*ghi | –1.95±0.42*i | –2.15±1.67*i | –2.71±0.85* |
| LV-DA | 1.67±0.06 | 1.74±0.07 | 1.75±0.08h | 1.70±0.05 | 1.75±0.05 | 1.67±0.06 | 1.63±0.06 | 1.68±0.04 | 1.64±0.07 | 1.67±0.05 | 1.64±0.04 |
| LV-DBM (mgHA/cm3) | 934±30* | 857±12 | 892±12*bcdefghi | 917±14*di | 917±28*dei | 860±5efghi | 934±17*fi | 911±14*i | 923±13*i | 926±17*i | 959±16* |
| Number of rats | 12 | 9 | 12 | 11 | 11 | 10 | 12 | 12 | 12 | 12 | 11 |
| DF-BV/TV (%) | 26.1±8.7* | 6.2±3.3 | 13.8±2.3*ceghi | 10.2±3.0cefghi | 23.0±3.3*defghi | 9.7±3.2efghi | 35.7±5.5*f | 17.5±4.7*ghi | 32.3±3.9*i | 33.5±4.2*i | 34.8±4.3* |
| DF-Tb.N (mm−1) | 4.23±0.54* | 2.09±0.55 | 2.91±0.33*cdf | 2.64±0.24*cegh | 3.44±0.35*defi | 2.39±0.23*eghi | 3.10±0.37*f | 2.46±0.44*ghi | 3.17±0.30*i | 3.17±0.30*i | 2.82±0.28* |
| DF-Tb.Th (μm) | 76.5±11.6* | 60.9±4.9 | 71.6±4.2*cdefghi | 66.3±6.6cefghi | 84.3±5.9*deghi | 62.8±7.0efghi | 118.3±8.3*fi | 115.8±7.1*i | 114.3±5.5*i | 128.3±5.2* | |
| DF-SMI | 0.69±0.76* | 2.67±0.55 | 1.98±0.22*cefghi | 2.32±0.35cefghi | 1.49±0.21*deghi | 2.03±0.42*efghi | –0.18±0.48*fgh | 1.34±0.40*ghi | 0.47±0.32*i | 0.36±0.36*i | 0.06±0.36* |
| DF-DA | 1.41±0.07 | 1.38±0.06 | 1.44±0.06cdeghi | 1.38±0.09ceghi | 1.57±0.06*defghi | 1.37±0.08eghi | 1.28±0.05*f | 1.41±0.08ghi | 1.29±0.03* | 1.31±0.05* | 1.30±0.09* |
| DF-DBM (mgHA/cm3) | 970±19* | 954±16 | 975±10*bdefi | 956±15cdeghi | 978±10*defi | 937±19*efghi | 1,004±17*fgh | 958±11ghi | 985±15*i | 981±17*i | 997±10* |
| LV compression | |||||||||||
| Number of rats | 12 | 10 | 12 | 9 | 12 | 11 | 12 | 12 | 12 | 11 | 8 |
| Max load (N) | 217±47 | 174±27 | 241±44*bcdefghi | 188±42eghi | 144±39deghi | 190±36efghi | 366±55*f | 171±28ghi | 368±87*i | 387±50*i | 332±28* |
| Max stress (MPa) | 20.6±4.9* | 14.5±1.1 | 20.9±4.0*cdefgh | 17.6±3.5ceghi | 11.8±4.3defghi | 16.3±2.0eghi | 30.5±3.4*f | 16.8±2.2ghi | 30.5±6.2* | 31.7±4.4* | 30.3±2.1* |
| Yield stress (MPa) | 12.1±4.7 | 12.9±1.8 | 16.3±6.1cdefgh | 12.6±2.6ceghi | 6.6±3.4*defghi | 11.6±3.9eghi | 24.3±4.8*fhi | 24.2±3.5*i | 26.3±5.1*i | 19.1±1.6* | |
| Stiffness (GPa) | 0.66±0.27 | 0.52±0.23 | 0.68±0.28cde | 0.62±0.15ce | 0.29±0.13*efghi | 0.47±0.15egh | 0.89±0.34*fi | 0.50±0.17gh | 0.76±0.34* | 0.86±0.35* | 0.68±0.19 |
| Energy absorption (kJ/m2) | 4.4±2.2* | 2.1±0.9 | 3.7±2.3efghi | 4.0±2.1efghi | 3.9±1.4efghi | 3.2±1.5efghi | 7.2±2.6*hi | 7.9±2.2*hi | 5.8±2.4*i | 6.9±3.6*i | 10.1±4.7* |
Mean±SD. Lowercase letters—difference from group (p<.05)
LV lumbar vertebral body, DF distal femur, BV/TV bone volume/total tissue volume, Tb.N trabecular number, Tb.Th trabecular thickness, SMI Structure Model Index, DA degree of anisotropy, HA hydroxyapatite, DBM degree of bone mineralization, Max load maximum load, Max stress maximum stress
p<.05, difference from OVX
At all times, lumbar vertebral body trabecular number (LV-Tb.N) in Veh-Veh-Veh was less than in Sham rats (Tables 2-5). At all times except Day 90, lumbar vertebral body trabecular thickness (LV-Tb.Th) in Veh-Veh-Veh was less than in Sham rats (Tables 2-5). At Day 90, all treatment groups except for Ral-Ral-Ral, had higher Tb.N and Tb.Th than Veh-Veh-Veh (Table 3). Of the four groups that received Aln monotherapy in Period 1, three (Aln-Aln-Aln, Aln-Veh-Aln, and Aln-PTH-Veh) had LV-Tb.Th that ranged from 88-97μm, while the fourth (Aln-PTH-Aln) had LV-Tb.Th of 132μm, with a range in individual rats of 125-141μm, that was significantly different from the other three Aln-first groups. At Day 180, all groups except for PTH-Veh-Veh had higher LV-Tb.N and LV-Tb.Th than Veh-Veh-Veh (Table 4). By Day 270, all regimens that included PTH, except for PTH-Veh-Veh and PTH-Ral-Ral, had greater LV-Tb.N, and LV-Tb.Th than other groups (Table 5).
At all times, lumbar vertebral body degree of bone mineralization (LV-DBM) in Veh-Veh-Veh was less than in Sham rats (Tables 2-5). At Day 90, all groups except for Aln-Aln-Aln, Aln-Veh-Aln, Aln-PTH-Veh, and Ral-Ral-Ral had higher LV-DBM than Veh-Veh-Veh (Table 3). At Day 180, LV-DBM in PTH-Veh-Veh was the same as Veh-Veh-Veh, whereas all other treatment groups were above Veh-Veh-Veh (Table 4). LV-DBM in Ral-PTH-Ral was higher than all other treatment groups (Table 4). By Day 270, all regimens that included both PTH and Aln had greater LV-DBM than other treated groups, except for Ral-PTH-Ral and Aln-Aln-Aln (Table 5).
At all times, lumbar vertebral body structure model index (LV-SMI) in Veh-Veh-Veh was higher than in Sham rats (Tables 2-5). At Day 90, though all PTH treatment groups had lower SMI than Veh-Veh-Veh, anti-resorptive treatment alone did not affect SMI (Table 3). Of the four groups that received Aln monotherapy in Period 1, three (Aln-Aln-Aln, Aln-Veh-Aln, and Aln-PTH-Veh) had LV-SMI that ranged from -0.48 to -0.9, while the fourth (Aln-PTH-Aln) had LV-SMI of -2.43, with a range in individual rats of -1.45 to -3.51, that was significantly different from the other three Aln-first groups. By Day 180, LV-SMI in PTH-Veh-Veh was the same as Veh-Veh-Veh, whereas all other groups were significantly below Veh-Veh-Veh. In addition, LV-SMI in the anti-resorptive only groups is significantly greater than LV-SMI in all groups that received PTH, except PTH-Ral-Ral (Table 4). At Day 270, PTH-Veh-Veh was the only group whose LVSMI did not differ from Veh-Veh-Veh. Anti-resorptive only groups were significantly below Veh-Veh-Veh, while groups that combined PTH with an anti-resorptive had the lowest values for LV-SMI and were significantly lower than LV-SMI in the anti-resorptive only groups. The lowest value was obtained in Aln-PTH-Aln at Day 270 (Table 5).
Lumbar vertebral body degree of anisotropy (LV-DA) in Veh-Veh-Veh differed from Sham only at Day 90 (Table 2). There were no differences in LV-DA among all treatment groups at any time (Tables 3-5).
Distal Femoral Metaphysis
At all times, distal femur bone volume (DF-BV/TV) in Veh-Veh-Veh was less than in Sham rats (Tables 2-5). At Day 90, all treatment groups except for Aln-Aln-Aln and Ral-Ral-Ral had higher DF-BV/TV than Veh-Veh-Veh (Table 3). At Day 180, DF-BV/TV in PTH-Veh-Veh and Ral-Ral-Ral were not different from Veh-Veh-Veh, whereas all the other treatment groups were above Veh-Veh-Veh (Table 4). PTH-Aln-Veh, Aln-PTH-Veh, Aln-PTH-Aln, and Ral-PTH-Ral had greater DF-BV/TV than anti-resorptive only groups (Table 4). By Day 270, all regimens that included PTH, except for PTH-Veh-Veh, had greater DF-BV/TV than other treatment groups. Aln-PTH-Aln had greater DF-BVTV than any other group (Table 5).
At all times, distal femur trabecular number (DF-Tb.N) in Veh-Veh-Veh was less than in Sham rats (Tables 2-5). At Day 90, all groups except for Ral-Ral-Ral had higher DF-Tb.N than Veh-Veh-Veh (Table 3). Furthermore, though all treatment regimens except Aln-Aln-Aln and Ral-Ral-Ral had higher DF-Tb.Th than Veh-Veh-Veh (Table 3), the greatest positive differences were seen in the PTH-treated groups that were greater than anti-resorptive alone groups. By Day 180, all treatment regimens had higher DF-Tb.N than Veh-Veh-Veh. All treatment regimens that included PTH except for PTH-Veh-Veh and PTH-Ral-Ral had higher DF-Tb.Th than Veh-Veh-Veh (Table 4). Anti-resorptive groups that include Aln, but not Ral had significantly higher DF-Tb.Th than Veh-Veh-Veh. By Day 270, Aln-Veh-Aln plus all regimens that included PTH, except for PTH-Veh-Veh and PTH-Ral-Ral had greater DF-Tb.N than other groups. By Day 270, all regimens that included PTH, except for PTH-Veh-Veh and Ral-Ral-Ral, also had greater DF-Tb.Th than other treatment groups. Treatment groups that included both PTH and Aln, plus Ral-PTH-Ral, had the highest DF-Tb.N values (Table 5).
Before Day 270, distal femur degree of bone mineralization (DF-DBM) in Veh-Veh-Veh was less than in Sham rats (Tables 2-5). At Day 90, all treatment groups except Ral-Ral-Ral had higher DF-DBM than Veh-Veh-Veh (Table 3). At Day 180, DF-DBM in PTH-Veh-Veh, PTH-Ral-Ral and Ral-Ral-Ral was the same as Veh-Veh-Veh, whereas all other groups were greater than Veh-Veh-Veh (Table 4). DF-DBM in PTH-Aln-Veh was higher than all other treatment groups (Table 4). By Day 270, PTH-Aln-Veh, Aln-Aln-Aln, Aln-Veh-Aln, Aln-PTH-Aln and Ral-PTH-Ral had greater DF-DBM than other groups (Table 5).
At all times, distal femur structure model index (DF-SMI) in Veh-Veh-Veh was higher than in Sham rats (Tables 2-5). At Day 90, all treatment groups except for Aln-Aln-Aln and Ral-Ral-Ral had a lower DF-SMI than Veh-Veh-Veh. Groups that involved PTH treatment had the lowest DF-SMI values (Table 3). At Day 180, all groups except for Ral-Ral-Ral had lower DF-SMI than Veh-Veh-Veh. The lowest DF-SMI values were in groups with both PTH and anti-resorptive treatment (Table 4). By Day 270, PTH-Veh-Veh and Ral-Ral-Ral no longer differed from Veh-Veh-Veh. The lowest values were found in groups that had received both PTH and anti-resorptive treatment (Table 5).
Distal femur degree of anisotropy (DF-DA) in Veh-Veh-Veh did not differ from Sham at any time (Table 2-5). At Day 90, DF-DA in all regimens that included PTH was lower than the Veh-Veh-Veh group (Table 3). At Day 180, DF-DA in PTH-Aln-Veh, Aln-PTH-Veh and Aln-PTH-Aln was less than Veh-Veh-Veh (Table 4). DF-DA in Aln-Veh-Aln was higher than all other groups. At day 270, Aln-Veh-Aln had greater DF-DA than all other groups (Table 5).
Mechanical testing (Sixth Lumbar Vertebral Body)
At baseline and Day 90, maximum load, maximum stress and yield stress in Veh-Veh-Veh did not differ from Sham (Tables 2-3). At Day 180, maximum stress in Veh-Veh-Veh was lower than in Sham rats (Table 4). By Day 270, maximum load, maximum stress, and yield stress were all lower in Veh-Veh-Veh than Sham (Table 5). At Day 90, all PTH regimens and Aln-PTH-Aln had higher maximum load, maximum stress, and yield stress than Veh-Veh-Veh. Of the four groups that received Aln monotherapy in Period 1, three (Aln-Aln-Aln, Aln-Veh-Aln, and Aln-PTH-Veh) had maximum load that ranged from 213-243 N, while the fourth (Aln-PTH-Aln) had maximum load 318 N, with a range in individual rats of 217-473, that was significantly different from the other three Aln-first groups. The mean maximum load for the combined groups was 251± 62 N. By Day 180, all PTH regimens, except PTH-Veh-Veh and PTH-Ral-Ral, had greater maximum load, maximum stress, and yield stress than the other groups. Though Aln-Veh-Aln strength did not different from Veh-Veh-Veh, all other groups that received Aln had maximum load, maximum stress and yield stress greater than Veh-Veh-Veh (Table 4). By Day 270, Aln-Aln-Aln and all PTH-containing regimens, except for PTH-Veh-Veh had greater maximum load, maximum stress, and yield stress than other groups. Aln-PTH-Aln had the highest maximum load, maximum stress and yield stress of all the groups (Table 5).
Intrinsic values for 3D microarchitectural variables
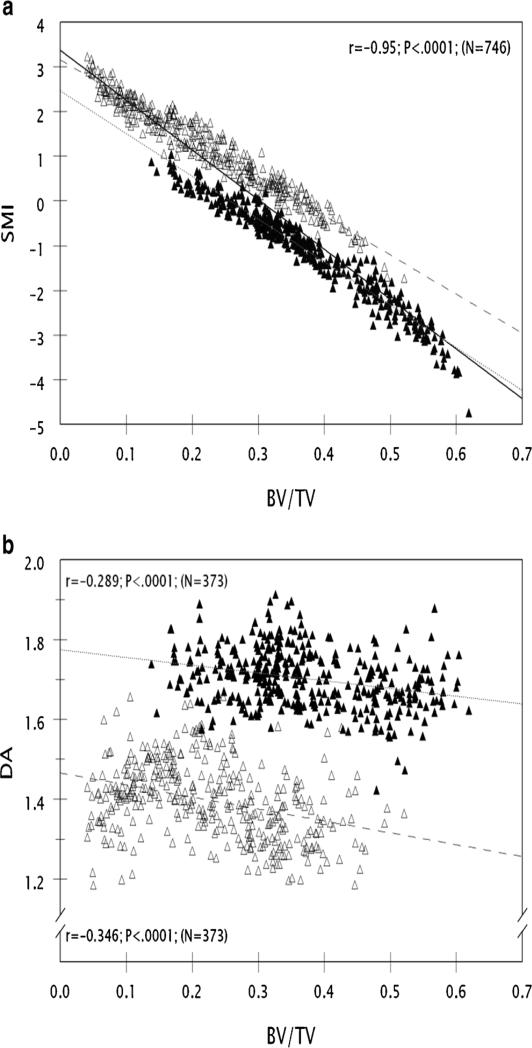
SMI and DA were measured in trabecular bone regions from over 350 samples each from the fifth lumbar vertebral body and distal femoral metaphysis. BV/TV ranged from 2.4-61.9%. SMI ranged from -4.74 to +3.31 and DA ranged from 1.185 to 1.913. SMI was inversely and very strongly correlated to BV/TV (r=-0.954) (Figure 2a). DA was about 20% greater in the vertebral body than the distal femoral metaphysis, and was weakly correlated to BV/TV in both sites (r=-0.289 and -0.346) (Figure 2b).
Figure 2.
a Data describing the relationship of SMI to BV/TV in 752 samples are plotted. Data from the lumbar vertebral body and distal femoral metaphysis are plotted in closed or open triangles respectively. Many specimens exhibit a negative SMI, particularly those with BV/TV greater than 30%. There is a very strong negative linear relationship of SMI to BV/TV, whether considering all samples (r=0.954, solid line), vertebral body only (r=0.972, dotted line) or distal femoral metaphysis only (r=0.971, dashed line). BV/TV explains over 91% of the variation in SMI in the whole dataset. The y-intercepts for the vertebral body and distal femur differ, indicating that the vertebral body has a significantly more negative SMI for any BV/TV value than does the distal femoral metaphysis.
Figure 2b Data describing the relationship of DA to BV/TV in the vertebral body and distal femoral metaphysis, respectively, are plotted as closed or open triangles. The vertebral body and distal femoral metaphysis data are distinct populations, with the vertebral body being ~20% higher than the distal femur. There is a weak negative linear relationship of DA to BV/TV in both the vertebral body only (r=0.289, dotted line) and distal femoral metaphysis only (r=0.35, dashed line). The y-intercepts for the vertebral body and distal femur differ, indicating that the vertebral body has a significantly higher DA for any BV/TV value than the distal femoral metaphysis.
Relationship of Vertebral Body Maximum Load to Measurable Vertebral Body Surrogate
Bone Endpoints
Lumbar vertebral BV/TV, Tb.Th, and SMI were well-correlated to vertebral body maximum load, each explaining at least 55% of the variation in maximum load (Table 6a, Figure 3). However, they were correlated to each other to such an extent that, in multiple regression (Table 6b), Tb.Th, followed by cross-sectional area, with small contributions from DBM and SMI had the strongest association with maximum load. Together, these four endpoints explained 78% of the variation in maximum load (Table 6b). The correlation coefficient of maximum load to DA (r=-0.26) had a smaller absolute value than the correlation coefficients of maximum load to Tb.N, SMI, BV/TV, and Tb.Th, respectively (r=0.37-0.81) (Table 6a).
Table 6.
Data from Combinable Groups in periods 1 and 2
| Variable | Aln-Aln-Aln, Aln-Veh-Aln, Aln-PTH-Veh, and Aln-PTH-Aln (period 1) | PTH-Veh-Veh, PTH-Aln-Veh, and PTH-Ral-Ral (period 1) | Aln-PTH-Veh and Aln-PTH-Aln (period 2) | Ral-Ral-Ral and Ral-PTH-Ral (period 1) |
|---|---|---|---|---|
| MicroCT | ||||
| Number of rats | 47 | 30 | 24 | 11 |
| LV-BV/TV (%) | 37.9±8.5 | 51.6±6.9 | 47.0±4.9 | 27.8±2.4 |
| LV-Tb.N (mm−1) | 3.48±0.25 | 3.52±0.21 | 3.32±0.26 | 3.19±0.17 |
| LV-Tb.Th (μm) | 102.2±19.4 | 133.8±14.2 | 135.6±6.2 | 81.7±4.6 |
| LV-SMI | –1.05±0.92 | –2.48±0.93 | –1.60±0.73 | –0.29±0.28 |
| LV-DA | 1.73±0.08 | 1.68±0.09 | 1.66±0.07 | 1.71±0.04 |
| LV-DBM (mgHA/cm3) | 912±34 | 929±23 | 924±15 | 904±17 |
| Number of rats | 45 | 29 | 24 | 11 |
| DF-BV/TV (%) | 22.5±6.8 | 39.0±7.5 | 32.9±4.0 | 8.5±2.8 |
| DF-Tb.N (mm−1) | 3.17±0.48 | 3.18±0.50 | 3.17±0.30 | 2.38±0.35 |
| DF-Tb.Th (μm) | 88.8±17.1 | 123.4±8.0 | 115.0±6.2 | 65.7±5.8 |
| DF-SMI | 1.29±0.58 | –0.37±0.76 | 0.41±0.34 | 2.51±0.36 |
| DF-DA | 1.41±0.11 | 1.33±0.07 | 1.30±0.04 | 1.43±0.09 |
| DF-DBM (mgHA/cm3) | 980±23 | 985±15 | 983±17 | 947±19 |
| LV compression | ||||
| Number of rats | 46 | 29 | 23 | 11 |
| Max load (N) | 251±62 | 364±91 | 377±71 | 178±37 |
| Max stress (MPa) | 22.4±4.4 | 32.3±5.9 | 31.1±5.3 | 17.5±3.0 |
| Yield stress (MPa) | 16.7±5.2 | 25.9±7.1 | 25.2±4.4 | 13.0±3.4 |
| Stiffness (GPa) | 0.63±0.22 | 0.86±0.33 | 0.81±0.34 | 0.48±0.17 |
| Energy absorption (kJ/m2) | 4.73±2.06 | 7.60±2.85 | 6.32±3.02 | 7.08±3.38 |
Mean±SD
LV lumbar vertebral body, DF distal femur, BV/TV bone volume/total tissue volume, Tb.N trabecular number, Tb.Th trabecular thickness, SMI Structure Model Index, DA degree of anisotropy, HA hydroxyapatite, DBM degree of bone mineralization, Max load maximum load, Max stress maximum stress
Figure 3.
Data describing the relationship of Maximum Load to Tb.Th in the vertebral body are plotted. Non-PTH-treated vs. PTH-treated rats killed immediately at the end of therapy or after anti-resorptive maintenance, are shown as open or closed circles, respectively. These data indicate a very strong relationship of trabecular thickness to vertebral body maximum load, that is driven by treatment with PTH that produces trabecular bone regions with thicker trabeculae and lower SMI than in non-PTH-treated rats.
Discussion
Osteoporosis often requires medical treatment that continues for many years. No single currently-approved medication begins to approach complete elimination of fracture risk. Fortunately, approved agents with complementary mechanisms of action are available. One could theorize that the systematic, sequential application of existing approved medicines that act strongly with complementary tissue level actions, can provide better fracture risk reduction than any single monotherapy.
We investigated the effects of sequential treatment of osteopenic, OVX rats with complementary anti-osteoporosis agents on bone mass, bone microarchitecture, and bone strength endpoints. Each agent is efficacious by itself in reducing vertebral fracture risk in humans [2, 23, 24]. The first two categories of endpoints are generally known to be related to bone strength and provide a non-destructive, measurable surrogate for risk of fracture in humans. We examined all three categories of endpoints in OVX rats treated with each of the three agents as monotherapy. At the same time, we tested the monotherapies head-to-head against a series of sequences that have been or may be used in humans, again assessing the three categories of endpoints.
Our study started with six-month old OVX rats and allowed eight weeks for development of estrogen-deficiency osteopenia. Our data (Tables 2-5) show that estrogen-deficiency bone loss continued throughout the first two three month treatment periods. Because there was ongoing bone loss to prevent, this was a favorable environment for observing efficacy of anti-resorptive monotherapy [21].
Physicians prescribe anti-resorptive treatment as initial monotherapy for osteoporotic patients, because this treatment is proven to reduce fracture risk, is safe, convenient, and cost-effective [23]. Accordingly, several treatment groups addressed such therapy and one even included a bisphosphonate “treatment holiday.” Rats given continuous treatment with a bisphosphonate had better bone strength with modestly better bone mass and trabecular microarchitecture than untreated OVX rats. Though both the bisphosphonate drug holiday and continuous raloxifene groups had better bone mass, trabecular number, and trabecular thickness than untreated OVX rats, these findings were not associated with better bone strength. As in humans, bisphosphonate treatment was associated with lower SMI [25]. Neither DBM nor SMI were as good in drug holiday and continuous raloxifene groups, as in the continuous alendronate group. This may imply that the demonstrated relationship of DBM and SMI to bone strength (Table 6a) is reflected in a practical way in the drug holiday and continuous raloxifene groups. Because bisphosphonate treatment increases DBM above that found in osteopenic subjects [26], some hypothesize that higher DBM accounts for a portion of the increased BMD and reduced fracture risk seen with anti-resorptives [27]. Continuous anti-resorptive therapy also improves the degree and uniformity of bone mineralization and maintains trabecular microstructure [28]. Our results for continuous alendronate agree, showing better bone strength than in untreated OVX rats.
Clinical studies of fracture risk that include a bisphosphonate drug holiday have reported that fracture risk may rise, leading to recommendations that drug holidays be limited to patients with low or moderate fracture risk [29] and then only with intermittent monitoring for bone loss or fracture [30]. Our results show that despite better bone mass and microarchitecture in the drug holiday group than in untreated OVX rats, bone strength is not better. These pre-clinical data agree with reports that drug “holidays” may be associated with less anti-fracture efficacy than continuous bisphosphonate use. Our data also suggest that continuous bisphosphonate use provides better anti-fracture efficacy than continuous raloxifene. Though we increased our raloxifene dose by 150% over one that was efficacious in a previous experiment [31], we cannot rule out that alternate day dosing of raloxifene may have contributed to this finding. It is known that 1.5mg/kg/dose daily raloxifene by oral gavage is the minimum dose that is sufficient to prevent most OVX-induced bone loss in adult rats [20].
Osteopenic, OVX rats treated with PTH had higher bone mass, better microarchitecture, and greater bone strength than untreated OVX rats at the end of PTH treatment. In particular, trabecular thickness was 45-78% higher and trabecular number was 8-22% higher after PTH treatment, based on data from LV5 and the distal femur. However, these anabolic effects were completely lost within three months of PTH discontinuation, leaving the bone the same as untreated OVX rats, consistent with multiple preclinical and clinical studies [7, 32]. For example, PTH cessation in OVX rats caused trabecular bone loss that is detectable by four weeks with almost complete disappearance by 12 weeks [32]. Significant BMD loss is seen in postmenopausal women who receive placebo for one year after cessation of PTH [7].
PTH therapy is only approved by the FDA for a duration of 24 months [6]. For an individual patient, it is a matter of when, not if, PTH is discontinued. In agreement with other reports [7, 12, 13], our data show that post-PTH medical treatment must be provided to avoid loss of PTH's therapeutic benefit. Our study demonstrates that rats given PTH monotherapy followed by alendronate monotherapy had better bone mass, microarchitecture, and strength than rats in which PTH was stopped without follow-up treatment. These results are similar to those reported in clinical studies of PTH followed by a bisphosphonate or raloxifene [7, 12, 13]. We found that alendronate was more efficacious than raloxifene in preserving PTH-related improvements in bone mass, microarchitecture, and strength. As mentioned above, this may be related to our raloxifene dosing regimen. Other pre-clinical studies have investigated sequential therapies in OVX rats in which PTH was followed by estrogen [16], zoledronic acid [14], or risedronate [19]. These follow-up treatments resulted in lack of maintenance by estrogen replacement or maintenance with further gain in bone mass and strength with the bisphosphonates. Though the different times allowed from OVX to treatment initiation may play a role in the different findings, the strength of the anti-resorptives employed may also be responsible. The data suggest that bisphosphonates are generally more efficacious at preserving PTH-related gains in bone strength than estrogen receptor binding anti-resorptives. Our data indicate that initiating osteoporosis treatment with an anabolic agent then following up with bisphosphonate maintenance, could lead to better fracture risk reduction than either anti-resorptive monotherapy or PTH treatment without followup maintenance.
In clinical practice, patients who fracture or lose bone while being treated with one medication require other therapeutic options. Pre-clinical data may guide those choices. We evaluated three groups that started with firstline therapy, then switched to PTH. Two of the three subsequently continued with the types of post-PTH maintenance tested previously here and discussed above.
All groups that switched to PTH from anti-resorptive monotherapy had higher bone mass, higher trabecular thickness, lower SMI, and higher bone strength immediately after the conclusion of treatment, than both untreated OVX rats and rats receiving continuous anti-resorptives. The values were similar to those in rats that began PTH without prior use of anti-resorptives, especially when considering the means of the combined groups with like treatment. This indicates that prior anti-resorptive treatment did not interfere with the skeleton's ability to respond positively to PTH, in agreement with previous pre-clinical findings [33].
Rats treated with PTH that was followed by no maintenance treatment, had lower bone mass, microarchitecture, and bone strength than rats that were switched to alendronate maintenance therapy at the end of PTH treatment. Rats that switched to raloxifene maintenance had bone mass, microarchitecture, and bone strength similar to rats that had no post PTH maintenance treatment. Another pre-clinical study that evaluated PTH combined with a raloxifene analogue to treat osteopenia in OVX rats reported significant reduction in bone mass at several skeletal sites during raloxifene maintenance [34]. Though our results agree with other reports [34], that suggest a bisphosphonate is a better post-PTH maintenance therapy than raloxifene, we cannot rule out that our raloxifene dosing regimen influenced this finding.
At the end of study, rats treated sequentially with alendronate, then PTH, then alendronate had the highest bone mass, microarchitecture, and bone strength of all the groups. These endpoints were at least as high as, and often higher than, Sham rats. The Aln-PTH-Aln group at Day 90 was significantly different from the other three Day 90 groups treated with alendronate for vertebral body endpoints. However, these are not the same rats that comprise the Day 180 and 270 Aln-PTH-Aln groups. Therefore these results need to be evaluated at the end of each treatment period (e.g., 90, 180, or 270 days) and not across the treatment periods. Our pre-clinical data suggest that a treatment plan that starts with a bisphosphonate, then switches to a period of PTH that significantly improves bone mass and microarchitecture, then switches to long-term bisphosphonate maintenance, is likely to have greater anti-fracture efficacy than any monotherapy. These results support the current choice of firstline therapy as anti-resorptives, because one would predict that combining such therapy sequentially with an anabolic agent as needed, can provide more fracture risk reduction than can be achieved with either continuous frontline therapy or starting with PTH and then maintaining.
Our results provide additional practical data about SMI, structural model index, an endpoint that describes the rod or plate-like nature of trabecular lattices [35]. SMI has been thought to range mainly from zero to three, more negative values representing a more plate-like, stronger lattice [36, 37]. We found that, as in humans [38], PTH treatment reduced SMI. Though SMI here was very strongly and inversely correlated to BV/TV (Figure 2a) [39, 40], almost half the specimens had negative SMI [36]. However, most existing SMI studies characterize samples from normal and osteopenic subjects with BV/TV in the range of 2-25% [41], and report positive SMI [39, 42-44]. In contrast, one-third of our specimens were from rats that had received efficacious treatment with anti-osteoporosis agents, that had BV/TV greater than 35%. The BV/TV was greater and SMI was more negative in the lumbar vertebral body than the distal femoral metaphysis (Tables 2-5). Negative SMI indicates pores within high BV/TV trabecular lattices that have a structure with a concave surface [37]. Negative SMI has been seen in the proximal tibial epiphysis where BV/TV is 35-40%, vs. the neighboring metaphysis where SMI is positive and BV/TV is 10-30% [40]. The current data indicate that when specimens with high BV/TV are evaluated, SMI can be negative. SMI is an important determinant of bone strength (Table 6a) [44, 45], and fracture risk [46], negative values being associated with the best strength.
Our data may provide additional insight into DA, degree of anisotropy, an endpoint that describes the orientation of a trabecular lattice, with more positive values representing a more highly-oriented trabecular lattice [35]. Unlike SMI, DA was weakly correlated to BV/TV ( SMI r values mean values of 0.97 compared to mean r values of 0.32) . as reported previously [39, 43], but also did not consistently differ from controls in the treatment groups that had higher bone strength (Tables 2-5), as previously noted [47]. However, DA was about 20% higher in vertebral bodies than in the distal femoral metaphysis, indicating that the vertebral body trabecular lattice is more strongly oriented than that of the distal femur. Others have found that regions such as the tibial epiphyseal and metaphyseal trabecular bone, in which the epiphysis has higher BV/TV than the metaphysis, do not always have higher DA [47]. The current data combine with others’ to suggest that DA describes fundamental properties of trabecular lattices from different anatomic regions [37], better than their bone volume or response to treatment.
We evaluated compressive strength of lumbar vertebral body specimens, in which maximum load ranged from 76-554 N. We also measured non-destructive descriptors of bone strength that included bone volume, trabecular number, trabecular thickness, SMI, DA, cross-sectional area, and DBM. The endpoints themselves were highly correlated and therefore individually correlated to maximum load (Table 6a). Trabecular thickness correlated best to maximum load (r=0.81, Figure 3). Cross-sectional area, an endpoint independent of trabecular microarchitecture, added additional information. Our results suggest that, in rats treated with anti-osteoporosis agents, measuring microarchitectural and bone size endpoints of the lumbar vertebral body appears to be a nondestructive surrogate endpoint for vertebral body compression strength. The principal microarchitectural change affected by PTH treatment in this and other studies of both rats and humans, is trabecular thickening [38, 48] (Figure 3). This probably explains the primary association of bone strength with trabecular thickness here, rather than with SMI [45], and shows that trabecular thickening by an anti-osteoporosis treatment agent is a reasonable microarchitectural property of trabecular bone that can be used to predict bone strength after therapy, particularly with PTH. The results also agree with human data that suggest that bone mass and bone size in the spine predict spine fracture risk [49].
Our pre-clinical study had several strengths. We studied a number of clinical treatment sequences of bone active agents, measuring both non-destructive surrogate measures of bone strength and bone strength itself. We used ninety-day treatment periods, approximately two remodeling periods in mature adult rats, that each may represent up to 12-24 months in humans. We evaluated treatments, such as monotherapy with a bisphosphonate, raloxifene, and PTH, for which clinical fracture risk reduction data exist. We measured surrogate bone strength endpoints in both the approved monotherapies, and other sequences of treatment for which clinical fracture risk data have not yet been collected, to enable predictions about which ones could offer improved fracture risk reduction compared to monotherapy.
However, there were also weaknesses. The dosing regimen of raloxifene at 5mg/kg 3x a week while reported prevent estrogen deficiency bone in previous studies [26, 31] was both a lower dose and less frequently doses than what has been reported to produce the maximum possible effect of raloxifene on prevention of OVX-induced bone loss [20]. Also, we cannot extrapolate our pre-clinical study results to osteoporotic fracture risk in humans. Since we began treatment at eight weeks post-OVX, a time when OVX-related bone loss was still ongoing, the findings may be best applied to women that are within the first few years of menopause.
In summary, we used an osteopenic adult OVX rat model to evaluate various sequential treatments for osteoporosis, using FDA-approved agents with complementary tissue-level mechanisms of action. Sequential treatment for three months each with a bisphosphonate, followed by an anabolic agent, followed by resumption of the bisphosphonate, created the highest trabecular bone mass, highest trabecular thickness, highest trabecular number, lowest SMI, and highest bone strength. Any type of drug holiday, particularly a PTH holiday, resulted in loss of bone strength. These data, if confirmed in clinical studies may assist clinicians in the long-term treatment of postmenopausal osteoporosis.
Table 7.
Correlation coefficients of maximum load to measurable bone endpoints in the lumbar vertebral body
| Endpoint | R |
|---|---|
| BV/TV | 0.768 |
| Tb.Th | 0.812 |
| Tb.N | 0.370 |
| SMI | –0.742 |
| DA | –0.263 |
| DBM | 0.477 |
| Cross-sectional area | 0.443 |
BV/TV bone volume/tissue volume, Tb.Th trabecular thickness, Tb.N trabecular number, SMI Structure Model Index, DA degree of anisotropy, DBM dgree of bone mineralization
Table 9.
Correlation of microarchitectural endpoints of vertebral body to distal femoral metaphysis
| Endpoint | R |
|---|---|
| Tb.Th | 0.92 |
| Tb.N | 0.76 |
| BV/TV | 0.89 |
| SMI | 0.84 |
Tb. Th trabecular thickness, Tb.N trabecular number, BV/TV bone volume/ me, SMI Structure Model Index
Acknowledgements
This work was funded by National Institutes of Health (NIH) grants R01 AR043052, 1K12HD05195801 and 5K24AR048841-09. Statistical support was made possible by grant No. UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research. The involvement of ROR was supported by National Institute of Health (NIH/NIDCR) under grant No. 5R01 DE015633 to the Lawrence Berkeley National Laboratory (LBNL).
Footnotes
Conflicts of interest None.
References
- 1.Mackey DC, Black DM, Bauer DC, McCloskey EV, Eastell R, Mesenbrink P, Thompson JR, Cummings SR. Effects of antiresorptive treatment on nonvertebral fracture outcomes. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:2411–2418. doi: 10.1002/jbmr.446. [DOI] [PubMed] [Google Scholar]
- 2.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. The New England journal of medicine. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 3.Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, Black DM. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. The American journal of medicine. 2002;112:281–289. doi: 10.1016/s0002-9343(01)01124-x. [DOI] [PubMed] [Google Scholar]
- 4.Cosman F, Keaveny TM, Kopperdahl D, Wermers RA, Wan X, Krohn KD, Krege JH. Hip and spine strength effects of adding versus switching to teriparatide in postmenopausal women with osteoporosis treated with prior alendronate or raloxifene. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012 doi: 10.1002/jbmr.1853. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350:550–555. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 6.Black DM, Bilezikian JP, Greenspan SL, Wuster C, Munoz-Torres M, Bone HG, Rosen CJ, Andersen HS, Hanley DA. Improved adherence with PTH(1-84) in an extension trial for 24 months results in enhanced BMD gains in the treatment of postmenopausal women with osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;24:1503–1511. doi: 10.1007/s00198-012-2098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. The New England journal of medicine. 2005;353:555–565. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 8.Keaveny TM, Hoffmann PF, Singh M, Palermo L, Bilezikian JP, Greenspan SL, Black DM. Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23:1974–1982. doi: 10.1359/JBMR.080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004;19:745–751. doi: 10.1359/JBMR.040117. [DOI] [PubMed] [Google Scholar]
- 10.Cosman F, Wermers RA, Recknor C, Mauck KF, Xie L, Glass EV, Krege JH. Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. The Journal of clinical endocrinology and metabolism. 2009;94:3772–3780. doi: 10.1210/jc.2008-2719. [DOI] [PubMed] [Google Scholar]
- 11.Cosman F, Nieves JW, Zion M, Barbuto N, Lindsay R. Retreatment with teriparatide one year after the first teriparatide course in patients on continued long-term alendronate. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:1110–1115. doi: 10.1359/JBMR.081250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurland ES, Heller SL, Diamond B, McMahon DJ, Cosman F, Bilezikian JP. The importance of bisphosphonate therapy in maintaining bone mass in men after therapy with teriparatide [human parathyroid hormone(1-34)]. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2004;15:992–997. doi: 10.1007/s00198-004-1636-z. [DOI] [PubMed] [Google Scholar]
- 13.Rittmaster RS, Bolognese M, Ettinger MP, Hanley DA, Hodsman AB, Kendler DL, Rosen CJ. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. The Journal of clinical endocrinology and metabolism. 2000;85:2129–2134. doi: 10.1210/jcem.85.6.6614. [DOI] [PubMed] [Google Scholar]
- 14.Rhee Y, Won YY, Baek MH, Lim SK. Maintenance of increased bone mass after recombinant human parathyroid hormone (1-84) with sequential zoledronate treatment in ovariectomized rats. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004;19:931–937. doi: 10.1359/JBMR.040123. [DOI] [PubMed] [Google Scholar]
- 15.Felsenberg D, Boonen S. The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clinical therapeutics. 2005;27:1–11. doi: 10.1016/j.clinthera.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Shen V, Birchman R, Xu R, Otter M, Wu D, Lindsay R, Dempster DW. Effects of reciprocal treatment with estrogen and estrogen plus parathyroid hormone on bone structure and strength in ovariectomized rats. The Journal of clinical investigation. 1995;96:2331–2338. doi: 10.1172/JCI118289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwaniec UT, Samnegard E, Cullen DM, Kimmel DB. Maintenance of cancellous bone in ovariectomized, human parathyroid hormone [hPTH(1-84)]-treated rats by estrogen, risedronate, or reduced hPTH. Bone. 2001;29:352–360. doi: 10.1016/s8756-3282(01)00582-8. [DOI] [PubMed] [Google Scholar]
- 18.Samnegard E, Akhter MP, Recker RR. Maintenance of vertebral body bone mass and strength created by human parathyroid hormone treatment in ovariectomized rats. Bone. 2001;28:414–422. doi: 10.1016/s8756-3282(01)00408-2. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Mosekilde L, Sogaard CH, Thomsen JS, Wronski TJ. Parathyroid hormone monotherapy and cotherapy with antiresorptive agents restore vertebral bone mass and strength in aged ovariectomized rats. Bone. 1995;16:629–635. doi: 10.1016/8756-3282(95)00115-t. [DOI] [PubMed] [Google Scholar]
- 20.Black LJ, Sato M, Rowley ER, et al. Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. The Journal of clinical investigation. 1994;93:63–69. doi: 10.1172/JCI116985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seedor JG, Quartuccio HA, Thompson DD. The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1991;6:339–346. doi: 10.1002/jbmr.5650060405. [DOI] [PubMed] [Google Scholar]
- 22.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 23.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA : the journal of the American Medical Association. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 24.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA : the journal of the American Medical Association. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 25.Recker RR, Ste-Marie LG, Langdahl B, Masanauskaite D, Ethgen D, Delmas PD. Oral ibandronate preserves trabecular microarchitecture: micro-computed tomography findings from the oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe study. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2009;12:71–76. doi: 10.1016/j.jocd.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Yao W, Cheng Z, Koester KJ, Ager JW, Balooch M, Pham A, Chefo S, Busse C, Ritchie RO, Lane NE. The degree of bone mineralization is maintained with single intravenous bisphosphonates in aged estrogen-deficient rats and is a strong predictor of bone strength. Bone. 2007;41:804–812. doi: 10.1016/j.bone.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burr DB, Miller L, Grynpas M, Li J, Boyde A, Mashiba T, Hirano T, Johnston CC. Tissue mineralization is increased following 1-year treatment with high doses of bisphosphonates in dogs. Bone. 2003;33:960–969. doi: 10.1016/j.bone.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Rizzoli R, Chapurlat RD, Laroche JM, Krieg MA, Thomas T, Frieling I, Boutroy S, Laib A, Bock O, Felsenberg D. Effects of strontium ranelate and alendronate on bone microstructure in women with osteoporosis. Results of a 2-year study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23:305–315. doi: 10.1007/s00198-011-1758-z. [DOI] [PubMed] [Google Scholar]
- 29.McClung M, Harris ST, Miller PD, Bauer DC, Davison KS, Dian L, Hanley DA, Kendler DL, Yuen CK, Lewiecki EM. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. The American journal of medicine. 2013;126:13–20. doi: 10.1016/j.amjmed.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Ro C, Cooper O. Bisphosphonate drug holiday: choosing appropriate candidates. Current osteoporosis reports. 2013;11:45–51. doi: 10.1007/s11914-012-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng Z, Yao W, Zimmermann EA, Busse C, Ritchie RO, Lane NE. Prolonged treatments with antiresorptive agents and PTH have different effects on bone strength and the degree of mineralization in old estrogen-deficient osteoporotic rats. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:209–220. doi: 10.1359/jbmr.81005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takano Y, Tanizawa T, Mashiba T, Endo N, Nishida S, Takahashi HE. Maintaining bone mass by bisphosphonate incadronate disodium (YM175) sequential treatment after discontinuation of intermittent human parathyroid hormone (1-34) administration in ovariectomized rats. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1996;11:169–177. doi: 10.1002/jbmr.5650110205. [DOI] [PubMed] [Google Scholar]
- 33.Yao W, Su M, Zhang Q, Tian X, Setterberg RB, Blanton C, Lundy MW, Phipps R, Jee WS. Risedronate did not block the maximal anabolic effect of PTH in aged rats. Bone. 2007;41:813–819. doi: 10.1016/j.bone.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Hodsman AB, Watson PH, Drost D, Holdsworth D, Thornton M, Hock J, Bryant H, Fraher LJ. Assessment of maintenance therapy with reduced doses of PTH(1-34) in combination with a raloxifene analogue (LY117018) following anabolic therapy in the ovariectomized rat. Bone. 1999;24:451–455. doi: 10.1016/s8756-3282(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 35.Stauber M, Muller R. Volumetric spatial decomposition of trabecular bone into rods and plates--a new method for local bone morphometry. Bone. 2006;38:475–484. doi: 10.1016/j.bone.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Hildebrand T, Ruegsegger P. Quantification of Bone Microarchitecture with the Structure Model Index. Computer methods in biomechanics and biomedical engineering. 1997;1:15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Y, Zhao J, Liao EY, Dai RC, Wu XP, Genant HK. Application of micro-CT assessment of 3-D bone microstructure in preclinical and clinical studies. Journal of bone and mineral metabolism. 2005;23(Suppl):122–131. doi: 10.1007/BF03026336. [DOI] [PubMed] [Google Scholar]
- 38.Recker RR, Bare SP, Smith SY, Varela A, Miller MA, Morris SA, Fox J. Cancellous and cortical bone architecture and turnover at the iliac crest of postmenopausal osteoporotic women treated with parathyroid hormone 1-84. Bone. 2009;44:113–119. doi: 10.1016/j.bone.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Arlot ME, Burt-Pichat B, Roux JP, Vashishth D, Bouxsein ML, Delmas PD. Microarchitecture influences microdamage accumulation in human vertebral trabecular bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23:1613–1618. doi: 10.1359/jbmr.080517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brouwers JE, van Rietbergen B, Huiskes R, Ito K. Effects of PTH treatment on tibial bone of ovariectomized rats assessed by in vivo micro-CT. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20:1823–1835. doi: 10.1007/s00198-009-0882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brouwers JE, Lambers FM, Gasser JA, van Rietbergen B, Huiskes R. Bone degeneration and recovery after early and late bisphosphonate treatment of ovariectomized wistar rats assessed by in vivo micro-computed tomography. Calcified tissue international. 2008;82:202–211. doi: 10.1007/s00223-007-9084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borah B, Dufresne TE, Chmielewski PA, Johnson TD, Chines A, Manhart MD. Risedronate preserves bone architecture in postmenopausal women with osteoporosis as measured by three-dimensional microcomputed tomography. Bone. 2004;34:736–746. doi: 10.1016/j.bone.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Ito M, Nishida A, Nakamura T, Uetani M, Hayashi K. Differences of three-dimensional trabecular microstructure in osteopenic rat models caused by ovariectomy and neurectomy. Bone. 2002;30:594–598. doi: 10.1016/s8756-3282(02)00684-1. [DOI] [PubMed] [Google Scholar]
- 44.Teo JC, Si-Hoe KM, Keh JE, Teoh SH. Relationship between CT intensity, micro-architecture and mechanical properties of porcine vertebral cancellous bone. Clin Biomech (Bristol, Avon) 2006;21:235–244. doi: 10.1016/j.clinbiomech.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Fields AJ, Eswaran SK, Jekir MG, Keaveny TM. Role of trabecular microarchitecture in whole-vertebral body biomechanical behavior. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:1523–1530. doi: 10.1359/JBMR.090317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melton LJ, 3rd, Christen D, Riggs BL, Achenbach SJ, Muller R, van Lenthe GH, Amin S, Atkinson EJ, Khosla S. Assessing forearm fracture risk in postmenopausal women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21:1161–1169. doi: 10.1007/s00198-009-1047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brouwers JE, Lambers FM, van Rietbergen B, Ito K, Huiskes R. Comparison of bone loss induced by ovariectomy and neurectomy in rats analyzed by in vivo micro-CT. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27:1521–1527. doi: 10.1002/jor.20913. [DOI] [PubMed] [Google Scholar]
- 48.Fox J, Miller MA, Newman MK, Metcalfe AF, Turner CH, Recker RR, Smith SY. Daily treatment of aged ovariectomized rats with human parathyroid hormone (1-84) for 12 months reverses bone loss and enhances trabecular and cortical bone strength. Calcified tissue international. 2006;79:262–272. doi: 10.1007/s00223-006-0108-1. [DOI] [PubMed] [Google Scholar]
- 49.Melton LJ, 3rd, Riggs BL, Keaveny TM, et al. Structural determinants of vertebral fracture risk. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22:1885–1892. doi: 10.1359/jbmr.070728. [DOI] [PubMed] [Google Scholar]