Abstract
The elevation of serum creatinine levels is a concern with telavancin therapy. We examined the onset of kidney injury associated with telavancin in an animal model. Urine samples were collected at baseline and daily to determine the concentrations of kidney injury molecule 1 (KIM-1), a marker for early kidney injury. When a clinically relevant exposure of telavancin was given daily to rats, some differences in kidney injury were attributed to the dosing regimen. Further investigations of alternative telavancin dosing regimens are warranted.
TEXT
Telavancin is a lipoglycopeptide antibiotic which exerts its antibacterial activity by inhibiting peptidoglycan synthesis and causing membrane depolarization (1). In view of its activity against organisms nonsusceptible to vancomycin, daptomycin, or linezolid, it has great potential for use against drug-resistant bacterial infections. Pharmacokinetic-pharmacodynamic studies in an animal infection model demonstrated that the 24-h area under the concentration-time curve (AUC)/MIC ratio was the best predictor of efficacy (2), which suggested that dosing frequency had little influence on antibacterial activity. In the registration trials, all patients randomized to telavancin therapy were given a standard dose of 10 mg/kg of body weight once daily. An elevation in serum creatinine levels was found in an unexpectedly high proportion of the patients (6% to 16%) (3, 4). The impact on these elevations by the dosing frequency is unknown, and the responsible renal histological changes are not well characterized. Our clinical experience suggested that vancomycin nephrotoxicity can be delayed with a nonconventional drug administration schedule (5). The objectives of this study were to (i) investigate the relationship between the pharmacokinetics of telavancin and the onset of kidney injury and (ii) characterize the renal histological changes associated with telavancin in an animal model.
Sprague-Dawley CD IGS rats (female, 200 to 250 g; male, 325 to 375 g) were used (Charles River, Raleigh, NC). The jugular veins of the animals were cannulated to facilitate blood sampling. The study was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Houston. Each animal was given a single telavancin dose of 15 mg/kg, 30 mg/kg, or 60 mg/kg intravenously (n = at least 3 in each group). Six blood samples were obtained from each animal serially (0.5, 1, 2, 4, 6, and 8 h) after the end of drug infusion. The plasma samples were spiked with an internal standard, AMI-13616 (Covance, Princeton, NJ), and assayed for telavancin concentrations by the qualified liquid chromatography tandem mass spectrometry method (6). The assay was linear over the range of 0.0125 μg/ml to 250,000 μg/ml. The intra-assay and interassay precision levels for the calibration standards were within 10%. The concentrations observed at each time point for each dosing group were averaged, and a pharmacokinetic model was fit to each average concentration-time profile using ADAPT 5 (University of Southern California, Los Angeles, CA). The drug exposures observed were expressed as area under the concentration-time curves (AUC) over 24 h and correlated to those achieved with clinically relevant doses in humans.
In pilot studies, telavancin (up to 150 mg/kg) was given intravenously once daily. Serum and urine samples (postdose and cumulative over 24 h) were collected at baseline (prior to the first dose) and serially for up to 14 days to determine the concentrations of various kidney injury biomarkers (i.e., creatinine, kidney injury molecule 1 [KIM-1], neutrophil gelatinase-associated lipocalin [NGAL], and N-acetyl-β-d-glucosaminidase [NAG]). For comparison, saline and formulation-vehicle-only (no active drug content) controls were used for up to 7 days. Serum creatinine levels were measured using a clinical chemistry analyzer (Piccolo Xpress, Union City, CA). Urine KIM-1, NGAL, and NAG concentrations were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits. Based on the preliminary findings, a dose fractionation design was subsequently adopted. A clinically relevant exposure of telavancin (60 mg/kg) was given intravenously to each animal as a single dose or as 4 daily divided doses over 1 h. Eight animals (4 of each gender) were used in each dosing group. Urine samples were collected at baseline and daily over 5 days; for each animal, the KIM-1 concentration was determined and expressed as a ratio to its baseline value. Kidney injury was defined as a significant (more than 3 times the baseline) elevation in KIM-1 concentrations. The onset of kidney injury was compared using Kaplan-Meier analysis and the log-rank test; right censoring was used for events not observed. P values of ≤0.05 were considered significant.
Animals with significant elevations in urinary KIM-1 concentrations were further examined to ascertain the histological features of the kidney injury. At the end of the dose fractionation experiments, representative animals (1 gender from each dosing group) were anesthetized, and their kidneys were whole-body perfusion fixed by Dulbecco's phosphate-buffered saline (Mediatech, Inc., Manassas, VA) and preserved in 10% formaldehyde (Avantor, Center Valley, PA) for 24 h. For examinations by light microscopy, 4-μm sections along the coronal plane were cut and submitted to hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) staining. To characterize the injuries further, examinations by electron microscopy were also performed. Pieces of kidney tissue measuring less than 1 mm were obtained from either the cortex or the medulla; they were fixed in 2.5% glutaraldehyde and 2% formaldehyde in 0.1 M cacodylate buffer (pH 7.4) overnight and subsequently postfixed with freshly prepared 2% osmium tetroxide. Dehydrated tissue was embedded in EMbed 812 epoxy resin (Electron Microscopy Sciences, Hatfield, PA) and polymerized overnight. Thick (1 μm) sections were cut and stained with toluidine blue (Electron Microscopy Sciences, Hatfield, PA) to select areas for electron microscopic study. These areas were included in the thin sections (60 nm), which were submitted to staining with lead citrate for 1 min followed by 4% aqueous uranyl acetate for 5 min. Thin sections were examined with an EM 12 JEOL 1400 transmission electron microscope (JEOL, Japan).
The serum concentration-time profiles observed after different doses are shown in Fig. 1. The profiles were satisfactorily characterized by a one-compartment intermittent infusion model (r2 > 0.98 for each profile), and the best-fit parameter estimates are shown in Table 1. In comparison, in healthy volunteers given a single intravenous dose (10 mg/kg) of telavancin, the mean AUC0–∞ was 747 mg · h/liter (95% confidence interval [CI], 494 to 1,000 mg · h/liter) (data on file). Consequently, 60 mg/kg of telavancin daily (approximately 1.5 times the mean clinical exposure, close to the upper end of the 95% confidence interval) was used in the subsequent dose fractionation study.
FIG 1.
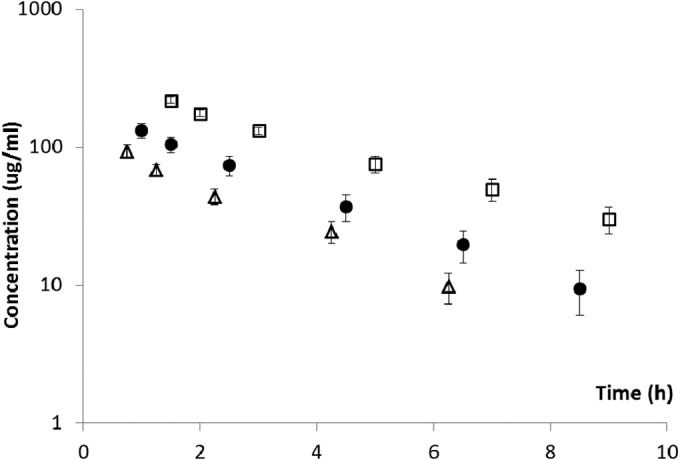
Pharmacokinetic profiles after intravenous administration. Data shown are means ± standard deviation. Open squares, 60 mg/kg over 60 min; filled circles, 30 mg/kg over 30 min; open triangles, 15 mg/kg over 15 min.
TABLE 1.
Best-fit pharmacokinetic parameters
| Parametera | Value at an intravenous dose of: |
||
|---|---|---|---|
| 15 mg/kg | 30 mg/kg | 60 mg/kg | |
| r2 | 0.986 | 0.998 | 0.993 |
| kel (h−1) | 0.429 | 0.362 | 0.285 |
| t1/2 (h) | 1.62 | 1.91 | 2.43 |
| V (liter/kg) | 0.128 | 0.177 | 0.215 |
| AUC0–∞ (mg · h/liter) | 273 | 468 | 979 |
r2, coefficient of correlation; kel, elimination rate constant; t1/2, terminal elimination half-life; V, volume of distribution; AUC0–∞, area under the concentration-time curve from 0 h to infinity.
Despite using an AUC drug exposure that was much higher than the human equivalent (telavancin doses up to 150 mg/kg once daily) for 14 days, no significant fluctuations in serum creatinine or urinary NGAL levels were observed (data not shown). Urine production was not dramatically different among the animals, and the pHs of the urine were found to be consistently between 7.0 and 8.0. Urinary NAG concentrations were consistently below the limit of detection of the ELISA kit and thus were not further pursued. Conversely, considerable elevations in urinary KIM-1 concentrations were observed when telavancin doses of ≥60 mg/kg were given once daily. Typically, the elevations in KIM-1 concentrations were the most prominent in the first 5 days of therapy (peak around day 3) and gradually regressed toward baseline levels despite continuous administration (Fig. 2). In contrast to active telavancin administration, no significant elevations in urinary KIM-1 levels were observed with saline or the formulation vehicle, as shown in Fig. 2. In view of these findings, dose fractionation studies were performed for 5 days in order to capture the peak rise in urinary KIM-1 concentrations. When the same daily doses were given, significant elevations in urinary KIM-1 concentrations were seen in a greater proportion of the animals given telavancin at a dose of 60 mg/kg every 24 h than in animals given a dose of 15 mg/kg every 6 h (87.5% versus 25%; P = 0.04). In addition, the onset of kidney injury was also significantly earlier if the same daily dose of telavancin was given once every 24 h than when given once every 6 h (P = 0.007), as shown in Fig. 3.
FIG 2.
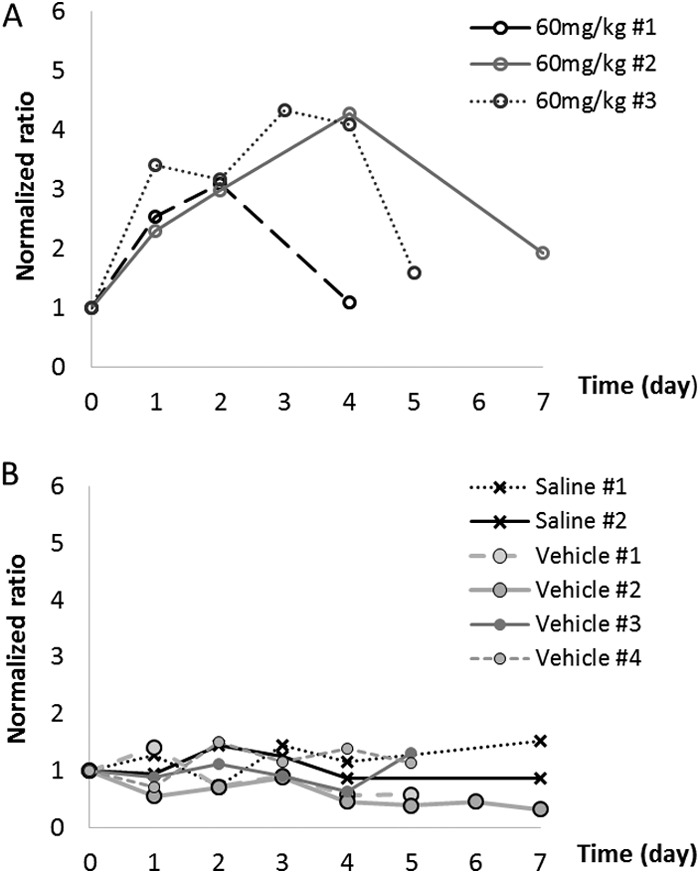
Typical urinary KIM-1 profiles. (A) Telavancin; (B) negative controls. Each agent was given intravenously once every 24 h.
FIG 3.
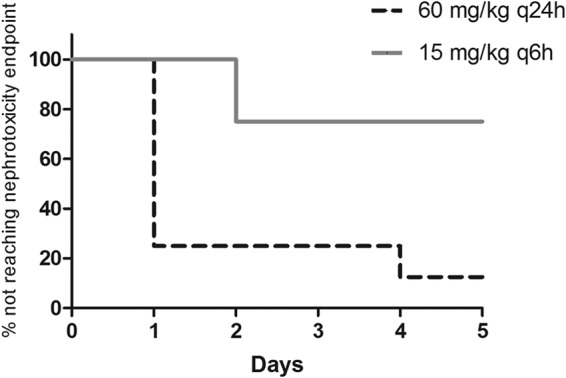
Onset of kidney injury (P = 0.007). q6h, every 6 hours; q24h, every 24 hours.
The renal changes are illustrated in Fig. 4 and 5. The histopathological findings were generally consistent with the elevation of urinary KIM-1 concentrations. Administering the formulation vehicle to the animals did not result in any significant renal changes (Fig. 4A). In animals given telavancin, the changes were primarily seen in proximal tubular cells; there were no significant changes in other portions of the nephron, glomeruli, or blood vessels. The findings of the electron microscopic study corroborate those of the light microscopic study. The injury was limited to proximal tubular cells and included cytoplasmic clearing, loss of organelles, formation of lysosomes, alteration of tubular cell surface microvilli (brush border), and individual cell necrosis (Fig. 5). Collectively, the morphological findings suggest that telavancin was associated with acute injury to the proximal tubular cells. Our data also suggest that with daily exposure to telavancin for 14 days, histological evidence of renal injury remained despite the regression of urinary KIM-1 concentrations (data not shown). Based on the limited number of animals examined, injuries appeared more pronounced in male animals than in female animals. The injuries also appeared more pronounced with 24-hour dosing than with 6-hour dosing.
FIG 4.
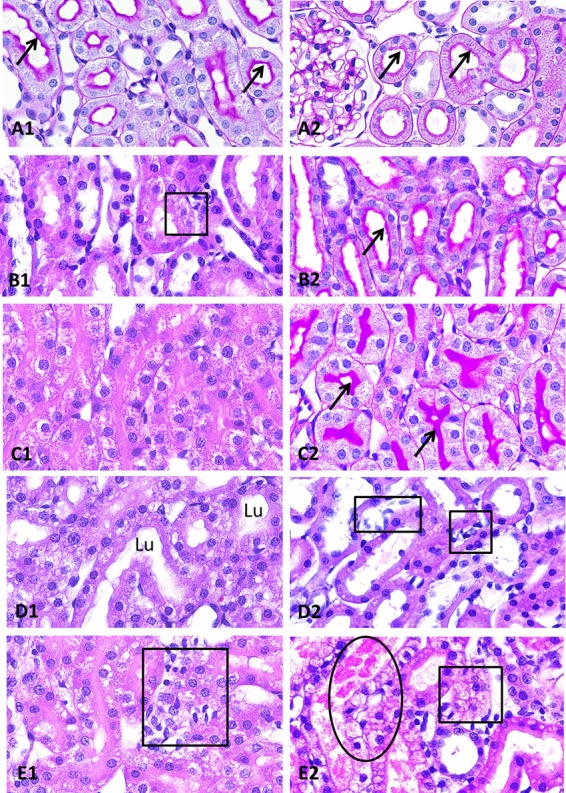
Key histopathological findings (light microscopy). (A) Female rat, formulation vehicle. The tubules, including the S3 portion of proximal tubules, are normal, with intact brush border (arrows) and open lumens (A1). The S1 and S2 portions of the proximal tubules are also normal, with intact brush border (arrows) and open lumens (A2). There are no glomerular changes (A2) (PAS stain, ×40 [A1 and A2]). (B) Female rat, 15 mg/kg q6h. The S3 portions of the proximal tubules show mild focal cytoplasmic vacuolization (square) (B1); however, their tubular lumens remain open, and brush borders (arrows) are intact (B2) (H&E stain, ×40 [B1]; PAS stain, ×40 [B2]). (C) Male rat, 15 mg/kg q6h. The S3 portions of the proximal tubules show marked diffuse cytoplasmic vacuolization with obliterated tubular lumens (C1); however, the brush borders (arrows) remain intact (C2) (H&E stain, ×40 [C1]; PAS stain, ×40 [C2]). (D) Female rat, 60 mg/kg q24h. The S3 portions of the proximal tubules show marked diffuse cytoplasmic vacuolization, but their lumens (Lu) remain open (D1). S1 and S2 portions of the proximal tubules are intact, but there are focal interstitial edema and inflammatory cell infiltrates (squares) among them (D2) (H&E stain, ×40 [D1 and D2]). (E) Male rat, 60 mg/kg q24h. The S3 portions of the proximal tubules show marked diffuse cytoplasmic vacuolization with obliterated tubular lumens, as seen in panel C1, together with interstitial inflammation (square) (E1). There is frank necrosis (oval) or cytoplasmic vacuolization (square) involving S1 and S2 portions of the proximal tubules (E2); the changes are not seen in animals under other experimental conditions (H&E stain, ×40 [E1 and E2]).
FIG 5.
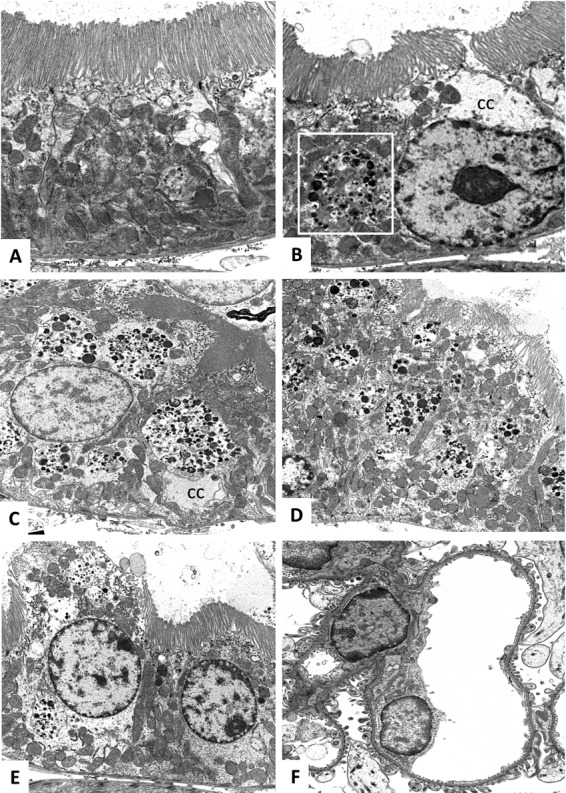
Detailed histopathological findings (transmission electron microscopy). (A) Female rat, formulation vehicle. A normal proximal tubular cell with intact brush border (top) and normal distribution of organelles, including mitochondria (×8,000). (B) Female rat, 15 mg/kg q6h. A proximal tubular cell in the S3 portion shows a rare lysosome (square) and mild focal cytoplasmic clearing (cc); however, the tubular lumen remains open, and the brush border (top) is intact (×8,000). (C) Male rat, 15 mg/kg q6h. A proximal tubular cell in the S3 portion shows numerous lysosomes. There is focal cytoplasmic clearing (cc). The brush border is intact (×5,000). (D) Female rat, 60 mg/kg q24h. A proximal tubular cell in the S3 portion shows numerous lysosomes. The brush border shows focal disorganization (top right) (×5,000). (E) Male rat, 60 mg/kg q24h. A proximal tubular cell in the S3 portion (left) shows necrotic changes, including loss of cell membrane and brush border and organelles, marked cytoplasmic clearing, and numerous lysosomes. An adjacent tubular cell (right) displays much less severe changes (×5,000). (F) Male rat, 60 mg/kg q24h. The glomerular capillaries are normal (×3,000).
We previously used an animal model to study the pharmacokinetics and nephrotoxicity associated with polymyxin B (7, 8). However, using telavancin in the same model, we were unable to demonstrate an elevation in serum creatinine levels, as was shown in previous clinical studies. We reasoned that this may be attributed to the insensitivity of creatinine to reflect early and subtle renal injury within the experimental time frame. Consequently, we evaluated several investigational early renal injury biomarkers in search of a useful signal for this proof-of-concept study. While KIM-1 was found to be the most reliable biomarker, the concentration elevations in the urine were transient and more prominent early in the course of therapy. The regression trend of urinary KIM-1 concentrations over time was unlikely to be fully explained by the variability in pH and urine volume. The utility of KIM-1 as a biomarker for early kidney injury in drug development was investigated previously (9, 10). Whereas the injury site is specific to the proximal tubules, it remains unclear if the magnitude of KIM-1 elevation is well correlated to the extent of kidney injury. Consequently, we selected an empirical threshold (more than 3 times the baseline value) as a surrogate endpoint of nephrotoxicity. Using a dose fractionation design, the onset of kidney injury was found to be earlier if the same daily dose was given once daily. In addition to examining the dynamic changes of various renal injury biomarkers over time, we also reported the corresponding histological findings observed in the animals. To the best of our knowledge, this is the first detailed report to characterize the histological changes in renal tissue associated with telavancin administration. These findings may be instrumental in future studies of the underlying mechanism of kidney injury.
There are several limitations to this study. First, we recognize that the utility of urinary KIM-1 levels in routine clinical practice is not well established. Therefore, we felt compelled to substantiate the clinical relevance of our findings with microscopic evidence of kidney injury. Second, while the elimination of telavancin was considerably more rapid in rats, the human equivalent drug exposure attained in the animals was based primarily on the overall AUC. To achieve a more realistic humanized drug exposure, a more sophisticated experimental setup delivering a continuous infusion of variable rates over time would be necessary (11). Third, dose fractionation studies were performed with only a single daily dose level that was deemed to be clinically relevant. The generalizability of our findings to other daily dose levels remains to be established. Finally, the mechanism of kidney injury was not investigated, as it was beyond the scope of this study.
In summary, when a clinically relevant exposure of telavancin was given daily, some differences in renal injury were attributed to the dosing regimen. Based on these preliminary findings, further investigations on the clinical utility of alternative telavancin dosing regimens are warranted.
ACKNOWLEDGMENT
Theravance Biopharma Antibiotics, Inc., provided funding support and drug/placebo and conducted telavancin assays on deidentified plasma samples.
REFERENCES
- 1.Saravolatz LD, Stein GE, Johnson LB. 2009. Telavancin: a novel lipoglycopeptide. Clin Infect Dis 49:1908–1914. doi: 10.1086/648438. [DOI] [PubMed] [Google Scholar]
- 2.Hegde SS, Reyes N, Wiens T, Vanasse N, Skinner R, McCullough J, Kaniga K, Pace J, Thomas R, Shaw JP, Obedencio G, Judice JK. 2004. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against Gram-positive bacteria. Antimicrob Agents Chemother 48:3043–3050. doi: 10.1128/AAC.48.8.3043-3050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubinstein E, Lalani T, Corey GR, Kanafani ZA, Nannini EC, Rocha MG, Rahav G, Niederman MS, Kollef MH, Shorr AF, Lee PC, Lentnek AL, Luna CM, Fagon JY, Torres A, Kitt MM, Genter FC, Barriere SL, Friedland HD, Stryjewski ME, ATTAIN Study Group . 2011. Telavancin versus vancomycin for hospital-acquired pneumonia due to Gram-positive pathogens. Clin Infect Dis 52:31–40. doi: 10.1093/cid/ciq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stryjewski ME, Graham DR, Wilson SE, O'Riordan W, Young D, Lentnek A, Ross DP, Fowler VG, Hopkins A, Friedland HD, Barriere SL, Kitt MM, Corey GR, Assessment of Telavancin in Complicated Skin and Skin-Structure Infections Study. 2008. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by Gram-positive organisms. Clin Infect Dis 46:1683–1693. doi: 10.1086/587896. [DOI] [PubMed] [Google Scholar]
- 5.Ingram PR, Lye DC, Fisher DA, Goh WP, Tam VH. 2009. Nephrotoxicity of continuous versus intermittent infusion of vancomycin in outpatient parenteral antimicrobial therapy. Int J Antimicrob Agents 34:570–574. doi: 10.1016/j.ijantimicag.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Gotfried MH, Shaw JP, Benton BM, Krause KM, Goldberg MR, Kitt MM, Barriere SL. 2008. Intrapulmonary distribution of intravenous telavancin in healthy subjects and effect of pulmonary surfactant on in vitro activities of telavancin and other antibiotics. Antimicrob Agents Chemother 52:92–97. doi: 10.1128/AAC.00875-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelraouf K, Braggs KH, Yin T, Truong LD, Hu M, Tam VH. 2012. Characterization of polymyxin B-induced nephrotoxicity: implications for dosing regimen design. Antimicrob Agents Chemother 56:4625–4629. doi: 10.1128/AAC.00280-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelraouf K, He J, Ledesma KR, Hu M, Tam VH. 2012. Pharmacokinetics and renal disposition of polymyxin B in an animal model. Antimicrob Agents Chemother 56:5724–5727. doi: 10.1128/AAC.01333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs TC, Frick K, Emde B, Czasch S, von Landenberg F, Hewitt P. 2012. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicol Pathol 40:1031–1048. doi: 10.1177/0192623312444618. [DOI] [PubMed] [Google Scholar]
- 10.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. 2010. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blatter M, Fluckiger U, Entenza J, Glauser MP, Francioli P. 1993. Simulated human serum profiles of one daily dose of ceftriaxone plus netilmicin in treatment of experimental streptococcal endocarditis. Antimicrob Agents Chemother 37:1971–1976. doi: 10.1128/AAC.37.9.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


