Abstract
Clostridium difficile infection (CDI) is a gastrointestinal disease caused by C. difficile, a spore-forming bacterium that in its spore form is tolerant to standard antimicrobials. Ramoplanin is a glycolipodepsipeptide antibiotic that is active against C. difficile with MICs ranging from 0.25 to 0.50 μg/ml. The activity of ramoplanin against the spores of C. difficile has not been well characterized; such activity, however, may hold promise, since posttreatment residual intraluminal spores are likely elements of disease relapse, which can impact more than 20% of patients who are successfully treated. C. difficile spores were found to be stable in deionized water for 6 days. In vitro spore counts were consistently below the level of detection for 28 days after even brief (30-min) exposure to ramoplanin at concentrations found in feces (300 μg/ml). In contrast, suppression of spore counts was not observed for metronidazole or vancomycin at human fecal concentrations during treatment (10 μg/ml and 500 μg/ml, respectively). Removal of the C. difficile exosporium resulted in an increase in spore counts after exposure to 300 μg/ml of ramoplanin. Therefore, we propose that rather than being directly sporicidal, ramoplanin adheres to the exosporium for a prolonged period, during which time it is available to attack germinating cells. This action, in conjunction with its already established bactericidal activity against vegetative C. difficile forms, supports further evaluation of ramoplanin for the prevention of relapse after C. difficile infection in patients.
INTRODUCTION
Clostridium difficile infection (CDI) remains a clinically important disease, with increasing incidence and mortality rates in the United States; it now surpasses methicillin-resistant Staphylococcus aureus as the most common health care-associated infection (HAI) and results in an annual cost burden to the health care system of $4.8 billion (1–3). A prolonged cure for treated patients remains elusive, as evidenced by the high rates of relapse (same ribotype within 30 days) and reinfection (different ribotype within 30 days). Recurrence rates have ranged from 15% to 40% (4–6) and are most commonly associated with the ribotype 027 epidemic strain (7). One contributing factor for high relapse rates is the presence of spores that persist within the intestinal lumen and evade elimination by standard anti-CDI treatments (8, 9). C. difficile spores are tolerant to standard antimicrobial therapy; hence, antimicrobial interventions targeting vegetative cells alone are unlikely to eradicate this disease reservoir (10).
Ramoplanin is a glycolipodepsipeptide antibiotic produced from Actinoplanes, which inhibits peptidoglycan biosynthesis by limiting lipid II availability (11); it is bactericidal to many Gram-positive aerobic and anaerobic bacteria, including C. difficile and vancomycin-resistant Enterococcus (11). Ramoplanin has known bactericidal activities against wild-type C. difficile and strains with reduced susceptibilities to vancomycin and has been studied in an open-label phase 2 clinical trial evaluating early clinical efficacy (12, 13). Ramoplanin was also previously reported to have activity against C. difficile spores in an in vitro gut model and an in vivo hamster model (14). Since residual intraluminal spores are considered essential for subsequent disease relapse, we have used in vitro models to define in more detail the activities of ramoplanin against C. difficile spores.
MATERIALS AND METHODS
Bacterial strain and spore preparation.
C. difficile spores (ribotype 027) were prepared as previously described (15). Spore pellets were resuspended in 1/10 the original volume (50 ml) in deionized water. The spores were counted by serial 10-fold dilution in prereduced anaerobically sterilized (PRAS) dilution blanks (Anaerobe Systems, Morgan Hill, CA) and subsequently plated on brain heart infusion (BHI) agar (anaerobic incubation for 48 h at 37°C ± 2°C) supplemented with horse blood and sodium taurocholate, a known germinant (15).
Antimicrobial survey.
In this study, 105 spores/ml were stored in deionized water anaerobically at 37°C ± 2°C for 6 days and continuously exposed to fecal-level concentrations of metronidazole (10 μg/ml), vancomycin (500 μg/ml), or ramoplanin (300 μg/ml) (11, 16, 17). One milliliter of spores was sampled daily from day 0 (D0) to D6 and serially diluted 10-fold, as described above. Neither metronidazole nor vancomycin demonstrated spore-specific activity; hence, such activity was assessed for only ramoplanin (using the same ribotype, 027). The spores (105 spores/ml) were exposed to 0, 2, 300, or 600 μg/ml of ramoplanin for 30 min at 37°C ± 2°C. After 30 min, excess ramoplanin was removed by six sequential centrifugation steps, each requiring 10 min at 15,000 × g with subsequent resuspension in deionized water. After the last wash, spores were reconstituted to the original volume (50 ml) with deionized water and stored at 37°C ± 2°C for 28 days. At each time point (days 0, 1, 7, 14, and 28), samples were centrifuged at 10,000 × g for 2 min to separate spores from the unbound ramoplanin. The spores were reconstituted to their original sampling volume, diluted, and plated as previously described. Supernatants were held for the ramoplanin bioassay, as described below.
Ramoplanin bioassay.
The procedures used for the bioassay followed those described by Carman et al. (18). Briefly, lawns of 106 CFU/ml Streptococcus salivarius were plated on unsupplemented blood agar plates. Thirty microliters of each wash supernatant (days 0, 1, 7, 14, and 28) was pipetted aseptically onto blank sterile paper discs, which were transferred to the lawn of S. salivarius, in triplicate. Plates were incubated aerobically for 24 h at 37°C ± 2°C. Zones of inhibition around each disc were measured using calipers and compared to standard curves of ramoplanin for which known concentrations of ramoplanin and measured zones of inhibition had been established.
Exosporium processing.
Spore exosporia were removed as described by Escobar-Cortes et al. (19); “intact” spores did not undergo such processing. Briefly, spores were washed four times by centrifugation (10 min at 10,000 × g) and resuspended in deionized water followed by final resuspension in 15 ml of phosphate-buffered saline (PBS) and sonication for 90 s. Three milliliters of 10% Sarkosyl (detergent) was added to each preparation and subsequently incubated for 15 min at room temperature with rocking. Preparations were then centrifuged for 10 min at 10,000 × g, and pellets were resuspended in 10 ml PBS with 0.1 ml of 1 M Tris and 10 mg of lysozyme. Spores were rocked overnight (approximately 18 h) at 37 ± 2°C and then sonicated for 90 s, passed through a 50% solution of sucrose using a swinging bucket rotor for 20 min at 4,000 × g, and resuspended in a solution containing 3 ml PBS, 200 mM EDTA, 300 ng/ml proteinase K, and 1% Sarkosyl. The spores were further rocked at room temperature for 20 min and were passed through a 50% solution of sucrose as previously described by Escobar-Cortes et al. (19). Next, the spores were washed in deionized water by two centrifugation steps for 10 min at 10,000 × g and resuspended in deionized water; plating and incubation were carried out as described in the foregoing text.
For comparisons among different samples, Microsoft Excel (2010) was used for the statistical evaluation of one-way analysis of variance (ANOVA) (single factor) as well as post hoc t testing (two samples, assuming unequal variances).
RESULTS
Spore stability in water.
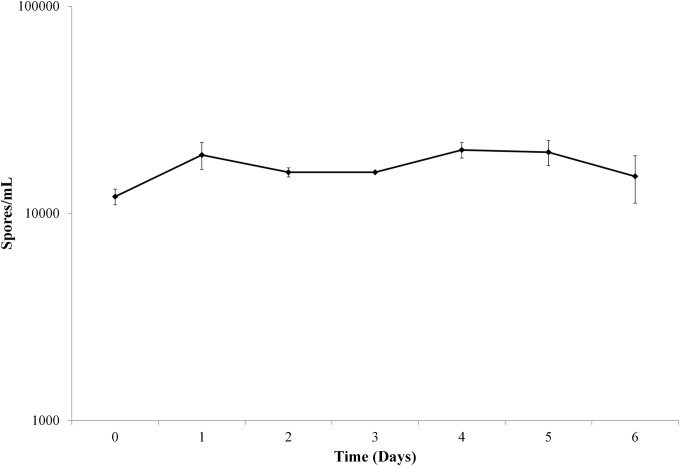
Incubation in water at 37°C for 6 days resulted in spore persistence with limited variation in spore counts from D0 through D6 (Fig. 1). The mean spore counts (spores/ml) on D0 and D6 were 1.2 × 104 (±0.11 × 104) and 1.5 × 104 (±0.39 × 104), respectively, with no significant differences noted (P = 0.240). Stable spore counts permitted the assessment of continuous antimicrobial exposure in water with the expectation that no effect on spores would be identified, a finding consistent with previous observations (10).
FIG 1.
Spores incubated in water over the course of 6 days showed no significant variation in counts (P = 0.24).
Antimicrobial activity on spores.
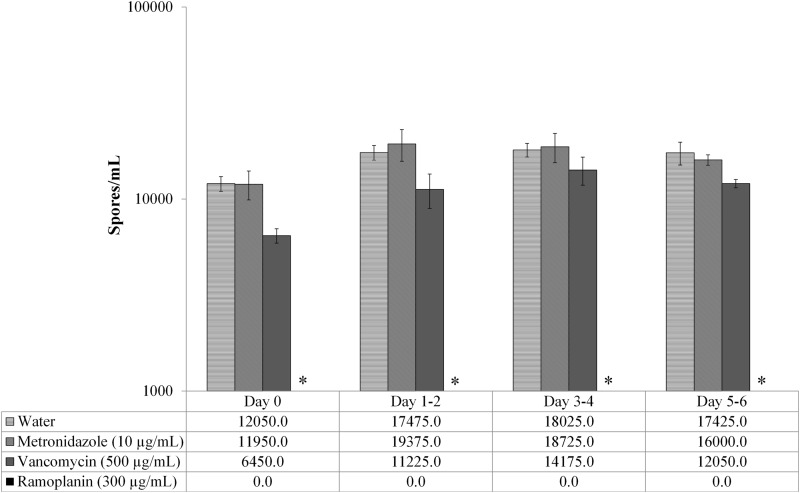
The effects of the assessed antimicrobials on spores were distinct, with ramoplanin clearly having a more pronounced effect than did vancomycin, metronidazole, or the water controls. Continuous exposure to vancomycin (500 μg/ml), metronidazole (10 μg/ml), and water did not significantly reduce spore counts over that at baseline. The spores exposed to ramoplanin (300 μg/ml) showed no growth after plating (Fig. 2), resulting in a significant decline (denoted by the asterisks in Fig. 2) of spore counts over time based on ANOVA at each time point after baseline (D1 to D2, P = 0.0002; D3 to D4, P = 0.0001; D5 to D6, P < 0.0001). Post hoc, two-sample t tests confirmed significant differences in spore counts between ramoplanin and comparators (water, metronidazole, and vancomycin) at the evaluated time points (all P values, <0.02). Not only were the differences in spore counts significant between ramoplanin and each comparator, but the magnitude of the effect was striking in that ramoplanin-exposed spores, when plated on agar, exhibited no observable growth. There was no statistical evidence of spore growth suppression by vancomycin or metronidazole compared to the baseline (as assessed by ANOVA; P = 0.18 and 0.49, respectively).
FIG 2.
Continuous exposure of spores in water to metronidazole, vancomycin, or ramoplanin. Only ramoplanin-exposed spores yielded no counts (P < 0.001 for all time points).
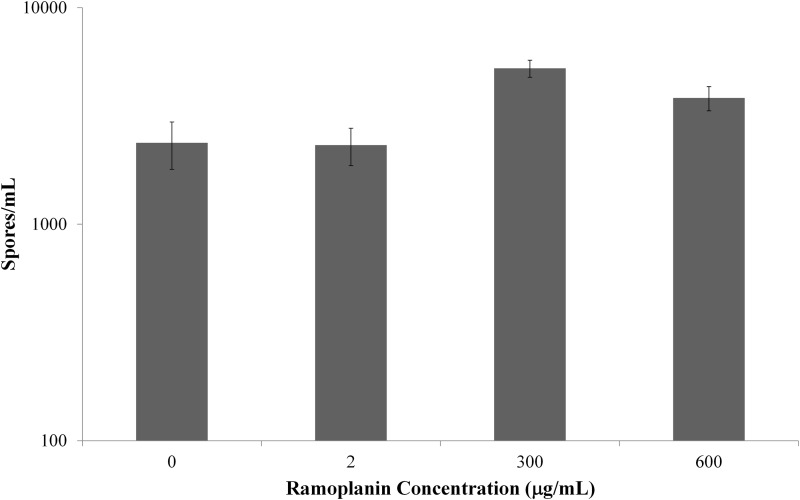
Persistence of ramoplanin activity.
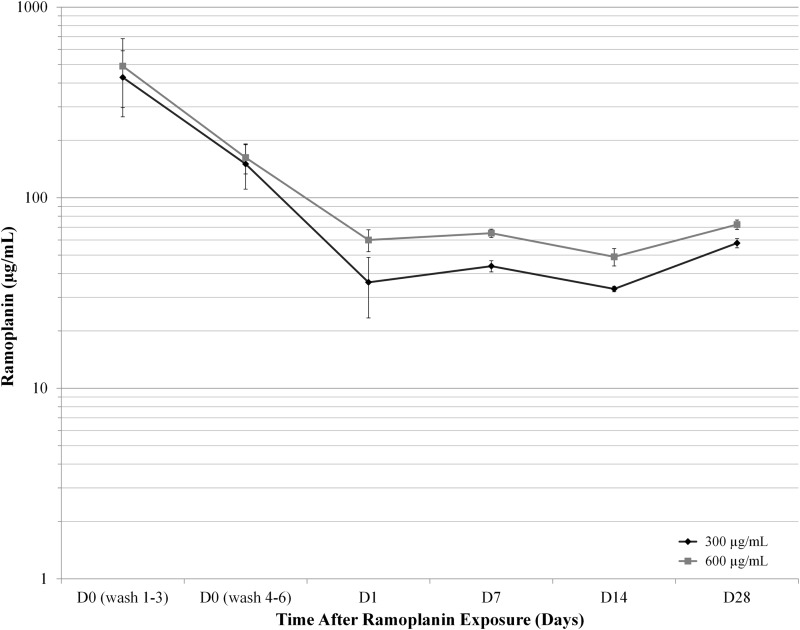
The observed effect of ramoplanin on spores was prolonged. Ramoplanin concentrations were varied (0 μg/ml, 2 μg/ml, 300 μg/ml, and 600 μg/ml) to characterize a concentration-response relationship for the drug's effects. Exposure to ramoplanin was brief and limited to 30 min. Ramoplanin-exposed spores were washed six times on D0 to remove unbound drug and repelleted once at each time point. At set intervals (D0, D1, D7, D14, and D28), spore-bound and free ramoplanin concentrations were assayed (Fig. 3), and spores were counted (Fig. 4, no growth is denoted by the asterisks). From D1 through D28, the supernatant concentrations of ramoplanin remained stable, ranging from (mean ± standard error of the mean [SEM]) 33.2 ± 1.1 to 57.8 ± 3.2 μg/ml for the spores exposed to 300 μg/ml and from 49.0 ± 5.1 to 72.3 ± 4.1 for the those exposed to 600 μg/ml (Fig. 3). There were no significant differences in supernatant ramoplanin concentrations among the evaluated time points for spores exposed to 300 μg/ml (P = 0.116) or to 600 μg/ml (P = 0.065).
FIG 3.
Unbound ramoplanin was recovered from spores exposed to two concentrations of ramoplanin for 30 min (300 and 600 μg/ml). Concentrations were stable from day 1 (D1) to D28.
FIG 4.
Summary spore counts for all time points at day 28 after 30 min of ramoplanin exposure. Higher concentrations (300 and 600 μg/ml) resulted in no counts for the duration of the incubations in water.
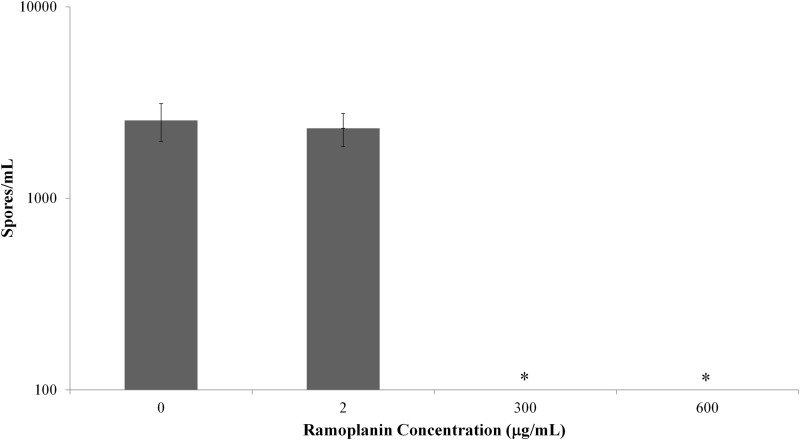
At each time point for which the ramoplanin drug concentrations were assessed, spore pellets free of unbound ramoplanin were plated for spore counts to determine if the spore-related activity would persist past the short-term time points initially evaluated (D0 to D6 of the antimicrobial survey). Specifically, the intent was to determine whether a brief 30-min ramoplanin exposure that was followed by multiple washes can inhibit spore growth over the course of 28 days. Those spores exposed to low concentrations of ramoplanin (2 μg/ml) demonstrated no inhibition relative to the controls (0 μg/ml ramoplanin) over the course of 28 days (Fig. 4), with mean count ± SEM values of 2.3 × 104 ± 4.6 × 102 and 2.5 × 104 ± 5.71 × 102, respectively. Those spores exposed to higher concentrations of ramoplanin (300 μg/ml and 600 μg/ml) had no growth observed when plated undiluted. The difference in spore counts after ramoplanin exposure compared to deionized water controls was significant (P < 0.0001). However, diluting these samples 10-fold resulted in spore counts similar to those for the spores exposed to 0 and 2 μg/ml (Fig. 5). At an additional dilution (10×), spore counts of samples exposed to higher concentrations of ramoplanin (300 μg/ml and 600 μg/ml) did not demonstrate an effect that was similar to those with the lower dilution spore counts relative to those of the water controls (P = 0.19 and 0.53, respectively, paired two-sample t-tests for means).
FIG 5.
Diluting ramoplanin-exposed samples 10-fold resulted in growth not measurable at previous dilution.
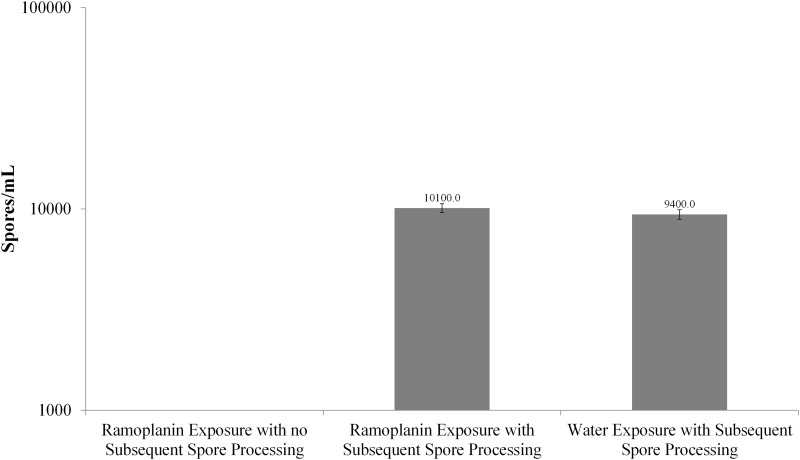
Adherence of ramoplanin to C. difficile exosporium.
In order to determine whether the exosporium was essential to the observed growth-inhibiting effects of ramoplanin, the exosporia were processed as previously described (19), resulting in spores with “stripped” exosporia, and then compared to unprocessed spores (with “intact” exosporia) under three separate conditions (the ramoplanin concentration was 300 μg/ml in each condition): (i) ramoplanin exposure with no subsequent spore processing (i.e., “intact”), (ii) ramoplanin exposure with subsequent spore processing (i.e., “stripped”), and (iii) water exposure with subsequent spore processing (Fig. 6). Ramoplanin-exposed spores that had not been processed yielded no growth when plated; in contrast, when ramoplanin-exposed spores were processed (i.e., their exosporia had been removed), the spore counts were similar to that for control water-exposed spores. After ramoplanin exposure, the difference in spore counts between samples that were not processed (with presumed intact exosporia) and samples that were processed (without presumed exosporia) was significant (P < 0.0001). No difference in spore count was noted when comparing processed spores that had been exposed to ramoplanin to spores exposed only to water (P = 0.389).
FIG 6.
Removal of exosporia after exposure to ramoplanin resulted in counts equivalent to those after water exposure; unprocessed spores, similarly exposed, exhibited no measurable counts.
DISCUSSION
C. difficile infection, as a leading cause of hospital-acquired infection and as a particular source of morbidity and mortality among the elderly, is a cause for concern to clinicians across multiple specialties. Unlike most acute illnesses from infections, for which treatment results in a prolonged cure, C. difficile infections carry with them a high risk of disease recurrence that can affect approximately 25% of successfully treated patients. This disease recurrence is, at least in part, a consequence of C. difficile spores that persist in the gut after the acute infection has been treated. These forms of C. difficile remain tolerant to standard antimicrobials and, if present in sufficient quantity, can reemerge as a threat to the host not long after hospital discharge.
Ramoplanin has demonstrable in vitro activities against many pathogens of clinical concern, such as Staphylococcus aureus, Mycobacterium tuberculosis, Bacillus anthracis, and C. difficile. While its previously described mechanism of action, the inhibition of cell wall synthesis through peptidoglycan binding, remains distinct from those of other agents that affect the cell wall, such as β-lactams and cell wall-active glycopeptides (e.g., vancomycin), the effect of ramoplanin on the spore, the antimicrobial-tolerant form of C. difficile, has not been well characterized. Chilton et al. speculated that certain antimicrobial agents, such as nisin and oritavancin, adhere to the exosporium of C. difficile because of electrostatic charges resulting from cross-linkages on the spore surface (20). It is possible that such exosporial adherence might persist and permit sufficient concentrations of antimicrobial activity to kill any vegetating organisms. This would explain the observations reported here, where ramoplanin was adherent to the exosporial surface and prevented emergence of viable C. difficile organisms.
We have presented evidence supporting the concept that antimicrobial spore binding may play a role in the activity of ramoplanin against C. difficile. Based on the prolonged suppression of spore counts and the presence of assayable ramoplanin in the supernatant samples, ramoplanin appears to bind to the C. difficile exosporium for an extended period of time. We postulate that ramoplanin remains adherent to the exosporium and achieves a concentration equilibrium with the supernatant over the course of the 28-day incubation. Because ramoplanin is a relatively large molecule with a molecular mass of 2,554 Da (21), it is unlikely to penetrate the exosporium. Instead, it remains adherent to the exosporium and only acts as an effective antimicrobial as it ambushes the germinating cell. Supporting this mechanism further is the observation that removal of the exosporium after ramoplanin exposure, prior to plating, resulted in spore counts no different from those of control water-exposed spores with intact exosporia (Fig. 6). More challenging to explain are increasing spore counts when the same samples are diluted 10-fold (Fig. 5). We suggest that, with a 10-fold dilution, the local ramoplanin concentration on the agar plate falls below the MIC, thereby permitting spore growth.
Firm conclusions cannot yet be made from these data since observed counts can be confounded by cell turnover. This seems unlikely since initial assessment with a glutamate dehydrogenase assay did not support a high turnover rate (data not shown). Future experiments should, however, include an alternative evaluation of spore germination, such as phase contrast (22). Furthermore, although “fecal concentrations” were used for this in vitro evaluation, these studies were carried out without the presence of fecal material, a limitation which will need to be addressed in follow-up studies.
Treatment of recurrent C. difficile infection includes the use of pulsed vancomycin (23), which is, in part, an attempt to eliminate the intraluminal spores that are germinating at different times. Emerging data indicate that sporulation rates may vary based on ribotype, with a heightened rate of sporulation for C. difficile ribotype 027 (24), which is associated with the higher disease recurrence rate for this particular ribotype (25). Enhancing the therapeutic options to address such disease recurrence is clearly an unmet need that is currently being evaluated with strategies such as fecal microbiota transplantation (FMT) and C. difficile vaccines (26–30). Should the activity observed in these in vitro experiments mirror activity in the human gut, then the use of ramoplanin as an agent for secondary prophylaxis may be a therapeutic option for clinicians.
Given the prior clinical experience with ramoplanin that includes a phase II study in CDI (13), the need to identify strategies for relapse reduction in patients with CDI, and the evidence reported here that supports an ambush type of activity against CDI spore forms, definitive determination of the clinical efficacy of ramoplanin in the secondary prophylaxis of CDI is appropriate and timely.
ACKNOWLEDGMENTS
We thank R. G. McAllister for manuscript review and editing.
This study was funded by Nanotherapeutics, Inc.
REFERENCES
- 1.Dubberke ER, Olsen MA. 2012. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 55(Suppl):S88–S92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lessa FC, Gould CV, McDonald LC. 2012. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis 55(Suppl):S65–S70. doi: 10.1093/cid/cis319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller BA, Chen LF, Sexton DJ, Anderson DJ. 2011. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol 32:387–390. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 4.Figueroa I, Johnson S, Sambol SP, Goldstein EJ, Citron DM, Gerding DN. 2012. Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clin Infect Dis 55(Suppl):S104–S109. doi: 10.1093/cid/cis357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson S, Adelmann A, Clabots CR, Peterson LR, Gerding DN. 1989. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis 159:340–343. doi: 10.1093/infdis/159.2.340. [DOI] [PubMed] [Google Scholar]
- 6.Kamboj M, Khosa P, Kaltsas A, Babady NE, Son C, Sepkowitz KA. 2011. Relapse versus reinfection: surveillance of Clostridium difficile infection. Clin Infect Dis 53:1003–1006. doi: 10.1093/cid/cir643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsh JW, Arora R, Schlackman JL, Shutt KA, Curry SR, Harrison LH. 2012. Association of relapse of Clostridium difficile disease with BI/NAP1/027. J Clin Microbiol 50:4078–4082. doi: 10.1128/JCM.02291-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarland LV, Elmer GW, Surawicz CM. 2002. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 97:1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 9.Walters BA, Roberts R, Stafford R, Seneviratne E. 1983. Relapse of antibiotic associated colitis: endogenous persistence of Clostridium difficile during vancomycin therapy. Gut 24:206–212. doi: 10.1136/gut.24.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baines SD, O'Connor R, Saxton K, Freeman J, Wilcox MH. 2009. Activity of vancomycin against epidemic Clostridium difficile strains in a human gut model. J Antimicrob Chemother 63:520–525. doi: 10.1093/jac/dkn502. [DOI] [PubMed] [Google Scholar]
- 11.Farver DK, Hedge DD, Lee SC. 2005. Ramoplanin: a lipoglycodepsipeptide antibiotic. Ann Pharmacother 39:863–868. doi: 10.1345/aph.1E397. [DOI] [PubMed] [Google Scholar]
- 12.Pelaez T, Alcala L, Alonso R, Martin-Lopez A, Garcia-Arias V, Marin M, Bouza E. 2005. In vitro activity of ramoplanin against Clostridium difficile, including strains with reduced susceptibility to vancomycin or with resistance to metronidazole. Antimicrob Agents Chemother 49:1157–1159. doi: 10.1128/AAC.49.3.1157-1159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leach T, Pullman J, Prieto J. 2004. Ramoplanin vs vancomycin in the treatment of Clostridium difficile diarrhea: a phase II study, abstr K-985a. Abstr 44th Intersci Conf Antimicrob Agents Chemother. [Google Scholar]
- 14.Freeman J, Baines SD, Jabes D, Wilcox MH. 2005. Comparison of the efficacy of ramoplanin and vancomycin in both in vitro and in vivo models of clindamycin-induced Clostridium difficile infection. J Antimicrob Chemother 56:717–725. doi: 10.1093/jac/dki321. [DOI] [PubMed] [Google Scholar]
- 15.Wilson KH, Kennedy MJ, Fekety FR. 1982. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J Clin Microbiol 15:443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolton RP, Culshaw MA. 1986. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut 27:1169–1172. doi: 10.1136/gut.27.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong SS, Woo PC, Luk WK, Yuen KY. 1999. Susceptibility testing of Clostridium difficile against metronidazole and vancomycin by disk diffusion and Etest. Diagn Microbiol Infect Dis 34:1–6. doi: 10.1016/S0732-8893(98)00139-4. [DOI] [PubMed] [Google Scholar]
- 18.Carman RJ, Simon MA, Petzold HE III, Wimmer RF, Batra MR, Fernandez AH, Miller MA, Bartholomew M. 2005. Antibiotics in the human food chain: establishing no effect levels of tetracycline, neomycin, and erythromycin using a chemostat model of the human colonic microflora. Regul Toxicol Pharmacol 43:168–180. doi: 10.1016/j.yrtph.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Escobar-Cortes K, Barra-Carrasco J, Paredes-Sabja D. 2013. Proteases and sonication specifically remove the exosporium layer of spores of Clostridium difficile strain 630. J Microbiol Methods 93:25–31. doi: 10.1016/j.mimet.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Chilton CH, Freeman J, Baines SD, Crowther GS, Nicholson S, Wilcox MH. 2013. Evaluation of the effect of oritavancin on Clostridium difficile spore germination, outgrowth and recovery. J Antimicrob Chemother 68:2078–2082. doi: 10.1093/jac/dkt160. [DOI] [PubMed] [Google Scholar]
- 21.Walker S, Chen L, Hu Y, Rew Y, Shin D, Boger DL. 2005. Chemistry and biology of ramoplanin: a lipoglycodepsipeptide with potent antibiotic activity. Chem Rev 105:449–476. doi: 10.1021/cr030106n. [DOI] [PubMed] [Google Scholar]
- 22.Burns DA, Heap JT, Minton NP. 2010. SleC is essential for germination of Clostridium difficile spores in nutrient-rich medium supplemented with the bile salt taurocholate. J Bacteriol 192:657–664. doi: 10.1128/JB.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. 2013. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 108:478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 24.Akerlund T, Persson I, Unemo M, Noren T, Svenungsson B, Wullt M, Burman LG. 2008. Increased sporulation rate of epidemic Clostridium difficile type 027/NAP1. J Clin Microbiol 46:1530–1533. doi: 10.1128/JCM.01964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrella LA, Sambol SP, Cheknis A, Nagaro K, Kean Y, Sears PS, Babakhani F, Johnson S, Gerding DN. 2012. Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C. difficile BI strain. Clin Infect Dis 55:351–357. doi: 10.1093/cid/cis430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagdasarian N, Rao K, Malani PN. 2015. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 313:398–408. doi: 10.1001/jama.2014.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baliban SM, Michael A, Shammassian B, Mudakha S, Khan AS, Cocklin S, Zentner I, Latimer BP, Bouillaut L, Hunter M, Marx P, Sardesai NY, Welles SL, Jacobson JM, Weiner DB, Kutzler MA. 2014. An optimized, synthetic DNA vaccine encoding the toxin A and toxin B receptor binding domains of Clostridium difficile induces protective antibody responses in vivo. Infect Immun 82:4080–4091. doi: 10.1128/IAI.01950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karczewski J, Zorman J, Wang S, Miezeiewski M, Xie J, Soring K, Petrescu I, Rogers I, Thiriot DS, Cook JC, Chamberlin M, Xoconostle RF, Nahas DD, Joyce JG, Bodmer JL, Heinrichs JH, Secore S. 2014. Development of a recombinant toxin fragment vaccine for Clostridium difficile infection. Vaccine 32:2812–2818. doi: 10.1016/j.vaccine.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Mathur H, Rea MC, Cotter PD, Ross RP, Hill C. 2014. The potential for emerging therapeutic options for Clostridium difficile infection. Gut Microbes 5:696–710. doi: 10.4161/19490976.2014.983768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. 2014. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 312:1772–1778. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]