Abstract
Malaria in the China-Myanmar border region is still severe; local transmission of both falciparum and vivax malaria persists, and there is a risk of geographically expanding antimalarial resistance. In this research, the pfmdr1, pfcrt, pvmdr1, and K13-propeller genotypes were determined in 26 Plasmodium falciparum and 64 Plasmodium vivax isolates from Yingjiang county of Yunnan province. The pfmdr1 (11.5%), pfcrt (34.6%), and pvmdr1 (3.1%) mutations were prevalent at the China-Myanmar border. The indigenous samples exhibited prevalences of 14.3%, 28.6%, and 14.3% for pfmdr1 N86Y, pfcrt K76T, and pfcrt M74I, respectively, whereas the samples from Myanmar showed prevalences of 10.5%, 21.1%, and 5.3%, respectively. The most prevalent genotypes of pfmdr1 and pfcrt were Y86Y184 and M74N75T76, respectively. No pvmdr1 mutation occurred in the indigenous samples but was observed in two cases coming from Myanmar. In addition, we are the first to report on 10 patients (38.5%) with five different K13 point mutations. The F446I allele is predominant (19.2%), and its prevalence was 28.6% in the indigenous samples of Yingjiang county and 15.8% in samples from Myanmar. The present data might be helpful for enrichment of the molecular surveillance of antimalarial resistance and useful for developing and updating guidance for the use of antimalarials in this region.
INTRODUCTION
Malaria is a serious public health problem in the Greater Mekong Subregion (GMS), which includes Cambodia, Laos, Thailand, Vietnam, Myanmar, and Yunnan province of China (1). Within this region, malaria transmission is particularly intense in international border areas. The malaria prevalence along the China-Myanmar border is particularly high, and malaria outbreaks have occurred frequently (2). In 2010, China initiated the National Malaria Elimination Action Plan, which aims to eliminate local malaria transmission nationwide by 2015 with the exception of the border region in Yunnan province and to completely eliminate malaria from China by 2020 (3). Despite a great reduction in the number of local malaria cases recently, both Plasmodium falciparum and Plasmodium vivax infections persist in the counties at the China-Myanmar border.
Effective chemotherapy is essential for malaria control, but the emergence and spread of multidrug-resistant (MDR) P. falciparum strains have led to the adoption of artemisinin-based combination therapies (ACTs) as the first-line treatment for uncomplicated falciparum malaria in the GMS. However, the confirmed emergence of artemisinin resistance in western Cambodia is a major threat for malaria control and elimination (4). Artemisinin resistance has since spread or emerged independently or both in other areas of mainland Southeast Asia (5). Because this area has been the origin of both chloroquine (CQ) and sulfadoxine-pyrimethamine (SP) resistance (6, 7), the consequences of a similar spread of artemisinin resistance will be catastrophic. Therefore, the World Health Organization (WHO) is coordinating a large-scale elimination campaign in this region aiming to contain artemisinin resistance (8).
The national drug policy of China was updated in 2006; since then the first-line drugs used to treat uncomplicated falciparum malaria have been ACTs, including dihydroartemisinin-piperaquine (DHA-PPQ), artesunate-amodiaquine (AS-AQ), artimisinin-naphthoquine phosphate (ART-NQ), and artemisinin-piperaquine (ART-PPQ) (9). However, the China-Myanmar border area has the longest history of artemisinin monotherapy; it has been used for more than 3 decades, and in vitro studies have detected the reduced susceptibility of P. falciparum to artemisinins (10). In the city of Laiza, Myanmar, near the border, P. falciparum strains have a certain level of resistance in vitro to artesunate and dihydroartemisinin, and the resistance is gradually increasing (11). Moreover, the increasing border migration is a great challenge for the elimination of malaria in this region, and the mobile population remains a risk factor for the spread of ACT resistance (12). Therefore, close surveillance of artemisinin resistance in this area is necessary to detect and deter P. falciparum resistance development.
CQ was adopted as the first-line drug for treatment of the blood-stage infection of P. vivax in China more than 50 years ago. Although high-level resistance of P. vivax to CQ and SP was reported more than a decade ago in the north of Myanmar, the resistance in the border counties of China remains unknown. Therefore, there is an urgent need to monitor drug resistance of vivax malaria in this area.
Several genetic polymorphisms can provide reliable data about the prevalence of drug resistance related P. falciparum and P. vivax. The P. falciparum chloroquine resistance transporter gene (pfcrt) T76 mutation and multidrug resistance 1 gene (pfmdr1) Y86 mutation have been linked to chloroquine and amodiaquine resistance (13, 14). Similarly, the P. vivax multidrug resistance 1 gene (pvmdr1) F976 mutation, which was shown to be associated with reduced susceptibility to chloroquine, was selected to evaluate the resistance of P. vivax (15). As for artemisinin resistance, the slowly clearing infections were strongly associated with single point mutations in the “propeller” region of the P. falciparum kelch protein gene on chromosome 13 (kelch13) (16). Recently, the WHO stated that artemisinin resistance should be suspected when ≥5% of patients carry K13 resistance-associated mutations (17).
Here we report an assessment of antimalarial resistance marker polymorphisms including pfmdr1, pfcrt, pvmdr1, and K13 propeller in samples collected from the China-Myanmar border region. The results might provide basic evidence for further molecular surveillance of drug-resistant P. falciparum and P. vivax strains in this region.
MATERIALS AND METHODS
Study site.
The study was conducted in Yingjiang county, one of the 18 counties at the China-Myanmar border, in western Yunnan province. It has a long borderline of 214.6 km with the Kachin state of Myanmar, which is a tier II area according to the Global Plan for Artemisinin Resistance Containment (GPARC) by the WHO (17). The population of Yingjiang county is 307,960, and cross-border trade, logging, quarry, and plantation activities are frequent. In 2013, there were 72 malaria cases, including 18 indigenous cases in this county, which accounted for 21.2% of the total indigenous cases in China.
Sample collection.
The blood samples at enrollment from confirmed malaria cases were collected from January 2013 to July 2014 in Yingjiang county. Approximately 200 μl of finger-prick blood was spotted on a piece of Whatman filter paper (3MM) and air dried. The samples were labeled with study numbers, names, and dates and stored at −20°C until DNA extraction.
Preparation of DNA template from blood samples.
Parasite genomic DNA from all blood spot samples collected in microcentrifuge tubes was extracted by use of a QIAamp DNA blood kit (Qiagen, Valencia, CA), following the dried blood spot protocol provided in the kit. The known polymorphisms pfmdr1 and pfcrt were assessed. Also, we investigated the mutation of the PF3D7_1343700 kelch propeller domain (PF13_0238, also called K13 propeller), a molecular marker of artemisinin resistance. Sequences were evaluated using nested PCR followed by restriction fragment length polymorphism (RFLP) analysis, as described previously (14, 18). The pvmdr1 single nucleotide polymorphisms (SNPs) at 976 were detected using a DNA template mismatch primer method (19). Polymorphisms were analyzed by Shanghai DNA BioTechnologies Co., Ltd. (Shanghai, China). Sequences were analyzed by the BLAST program (http://blast.ncbi.nlm.nih.gov/). Multiple nucleotide sequence alignments and analysis were performed using the BioEdit sequence alignment editor (http://www.mbio.ncsu.edu/BioEdit/bioedit.html).
Data analysis.
Data were analyzed using Microsoft Excel 2003 and SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). The map was created by using ArcGIS 10.1 (Environmental Systems Research Institute, Inc.). The Fisher exact test was used to assess the differences in the gene polymorphisms between indigenous cases and cases from Myanmar. The P values were calculated, and results were considered statistically significant when P was <0.05.
Ethical considerations.
The study was reviewed and approved by the ethical committee of the Chinese Centre for Disease Control and Prevention (China CDC).
RESULTS
Study samples.
A total of 90 malaria cases were included in this study: 64 P. vivax and 26 P. falciparum. The P. vivax cases were composed of 18 indigenous cases and 46 cases from Myanmar, while the P. falciparum cases were composed of 7 indigenous cases and 19 cases from Myanmar (Fig. 1).
FIG 1.
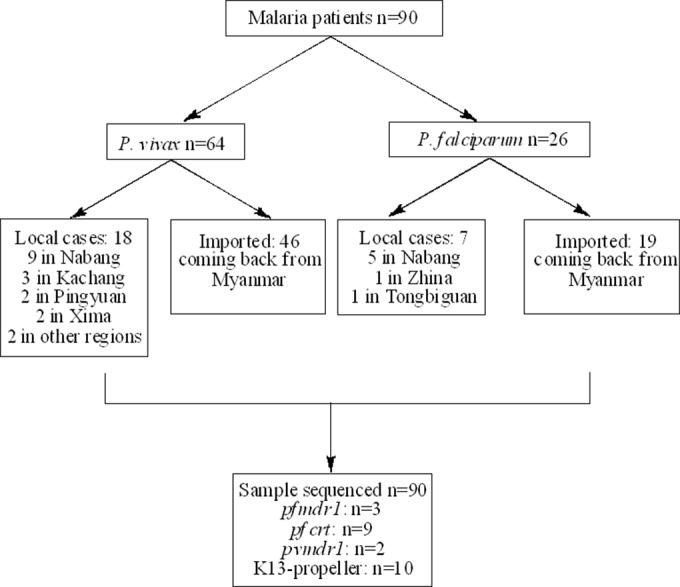
Screening, enrollment, and follow-up of subject patients.
pfmdr1.
The pfmdr1 gene was sequenced successfully in 3 isolates of all P. falciparum samples (11.5%, 3/26) that covered codons 86 and 184. Among all of the mutational types, no N1042D, S1034C, and D1246Y mutations were found. Mutations at codon N86Y (11.5%, 3/26) were common; Y86Y184 was the most prevalent (66.7%, 2/3) of all haplotypes (Tables 1 and 2). Further, one patient harboring the N86Y mutation was found in Nabang, China; the other two patients with this mutation were from Myanmar (P = 1.0000) (Table 1).
TABLE 1.
Selection of P. falciparum and P. vivax polymorphisms
| SNP | In China |
From Myanmar |
P | ||||
|---|---|---|---|---|---|---|---|
| No. | Total no. | % | No. | Total no. | % | ||
| pfmdr1 N86Y (n = 3) | 1 | 7 | 14.3 | 2 | 19 | 10.5 | 1.0000 |
| pfmdr1 Y184F (n = 1) | 0 | 7 | 0 | 1 | 19 | 5.3 | NSa |
| pfcrt M74I (n = 2) | 1 | 7 | 14.3 | 1 | 19 | 5.3 | 0.4738 |
| pfcrt N75E (n = 2) | 0 | 7 | 0 | 2 | 19 | 10.5 | NS |
| pfcrt K76T (n = 6) | 2 | 7 | 28.6 | 4 | 19 | 21.1 | 1.0000 |
| pvmdr1 Y976F (n = 2) | 0 | 18 | 0 | 2 | 46 | 4.3 | NS |
NS, not significant.
TABLE 2.
Prevalence of genotypes of candidate genes pfmdr1, pfcrt, and pvmdr1
| Candidate gene | Prevalence (% [no.]) | In China |
From Myanmar |
||
|---|---|---|---|---|---|
| Genotypeb | Proportion (%) | Genotypeb | Proportion (%) | ||
| pfmdr1 (n = 3) | 11.5 (3/26) | Y86Y184 | 33.3 | Y86F184 | 33.3 |
| Y86Y184 | 33.3 | ||||
| pfcrt (n = 9) | 34.6 (9/26) | I74N75K76 | 11.1 | I74N75K76 | 11.1 |
| M74N75T76 | 22.2 | M74N75T76 | 33.4 | ||
| M74E75K76 | 11.1 | ||||
| M74E75T76 | 11.1 | ||||
| pvmdr1 (n = 2) | 3.1 (2/64) | NDa | ND | Y976F | 100.0 |
ND, no mutated genotype detected in pvmdr1 in the local samples.
Mutations are in bold type.
pfcrt.
Sequencing of the pfcrt gene was successful in 9 isolates (34.6%, 9/26) that covered codons 74, 75, and 76. Of all three mutated codons, K76T was the most prevalent (23.1%, 6/26) (Table 1). Two patients with the mutated genotype K76T were observed in Nabang, China; the other four patients with K76T were from Myanmar (P = 1.0000). Four different pfcrt genotypes were found, among which M74N75T76 was the most common (55.6%, 5/9) (Table 2). One patient harboring I74N75K76 was detected in Tongbiguan, China, and the other one was returning from Myanmar (P = 0.4738). Another two haplotypes, including M74E75K76 and M74E75T76, were all in patients from Myanmar.
pvmdr1.
Regarding pvmdr1, 64 P. vivax samples were assayed. Only 2 patients (3.1%, 2/64) harbored the Y976F allele, and the patients were returning from Myanmar (Table 2).
K13 propeller.
To investigate the K13 propeller polymorphism, all 26 P. falciparum samples were assayed and sequenced. Ten patients (38.5%, 10/26) with five different point mutations, including two reported mutation sites and three unreported mutation sites, were observed (Table 3). Of all five nonsynonymous mutations, F446I was more frequent (19.2%, 5/26) than the others, and its prevalence was 28.6% (2/7) in the indigenous samples in Yingjiang county and 15.8% (3/19) in samples from Myanmar. Two out of five patients harboring the mutated condon F446I were found in Nabang, China, and one of these was found as a double-mutated allele of pfmdr1 N86Y and K13 propeller F446I. The other three patients with mutated F446I were returning from Myanmar (P = 0.5875). In addition, one patient with the mutated condon A676D was observed in Zhina, China. The other four patients with other mutated condons were all returning from Myanmar.
TABLE 3.
Polymorphisms observed in the K13 propeller in P. falciparum isolates
| Codon position | Amino acid reference | Nucleotide reference | Amino acid mutation | Nucleotide mutationa | Prevalence (% [no.]) | Location(s) |
|---|---|---|---|---|---|---|
| 446 (n = 5)b | F | Ttt | I | Att | 19.2 (5/26) | 2 in China, 3 from Myanmar |
| 511 (n = 2)b | I | Ata | M | atG | 7.7 (2/26) | Myanmar |
| 537 (n = 1) | N | Aat | I | aTt | 3.8 (1/26) | Myanmar |
| 574 (n = 1) | P | Cct | L | cTt | 3.8 (1/26) | Myanmar |
| 676 (n = 1)b | A | Gcc | D | gAc | 3.8 (1/26) | China |
Mutations are in bold type.
A mutated site which was not reported before.
DISCUSSION
Yunnan province is located in southern China, and malaria is one of the most important public health problems (20). The incidence of malaria transmission is more severe in the China-Myanmar border counties. The total number of malaria cases in Yunnan province according to the annual reported data was 576 in 2013, including 460 P. vivax cases, 106 P. falciparum cases, and 10 cases of other species. Most of them (n = 463, 80.4%) were observed in the 18 China-Myanmar border counties. The situation in Yingjiang county was the most severe, with an incidence rate of 2.3 per 10,000 people, and 18 local transmission cases were reported, representing 21.2% of the total local cases in China, with 54 other cases imported from Myanmar. Based on these facts, it was selected as the study site.
Resistance to antimalarial drugs has been a long-standing problem in the GMS. MDR P. falciparum strains that have emerged in the Thai-Cambodian border region, as well as the emerging resistance to chloroquine (CQ), to sulfadoxine-pyrimethamine (SP), and then to mefloquine (MQ), are gradually spreading in the tropical world (21). Malaria in the China-Myanmar border region is a topic of regional and national public health concern. The development and spread of MDR P. falciparum have led to the adoption of artemisinin-based combination therapy (ACT) as the first-line treatment for uncomplicated P. falciparum malaria in this region to improve treatment outcomes. However, widespread artemisinin resistance has been observed in the GMS (4, 22); this poses a great threat of resistant parasites being brought into China with the migrant population, immediately affecting therapy efficacy. In the China-Myanmar border area, artemisinins have been used for more than 30 years, mostly as monotherapies prior to 2005. Earlier in vitro assays had already detected a trend of declining sensitivity to artemisinins in the border area of Yunnan province (23, 24). Therefore, an understanding of whether artemisinin-resistant parasites have spread to the neighboring regions or emerged elsewhere in this area is essential for coordinating containment efforts.
In addition, P. vivax malaria persists in areas of Yunnan province along the China-Myanmar border (25), and CQ was adopted more than 50 years ago in China as the first-line drug used for treatment of the blood-stage P. vivax infection. Although high-level resistance of P. vivax to CQ and SP was reported more than a decade ago in the north of Myanmar (26, 27), the resistance in the border counties of China remains unknown.
The malaria parasite encodes many transporters, and some of them such as pfmdr1 and pfcrt have been strongly connected with antimalarial drug resistance (28). The pfmdr1 genotype is correlated with resistance of P. falciparum to CQ, MQ, and artemisinins, whereas knockdown of pfmdr1 expression leads to increased susceptibility (29). The N86Y pfmdr1 mutation that confers CQ resistance is also associated with decreased sensitivity to artemisinins (18). In Thailand and Cambodia, the pfmdr1 gene has become increasingly prevalent in field parasite populations and was responsible for the declining efficacy of ACTs (30, 31). In our assay, two mutated pfmdr1 alleles, N86Y and Y184F, were observed, and one patient with a Y86Y184 amplification was found in the port city of Nabang, China. These findings are contradictory to those of Wang et al., who reported that pfmdr1 N86Y had not been observed in this region (32). The possibility that patients take antimalarials with insufficient compliance cannot be excluded, although MQ was not frequently used in this region (33). Moreover, we have found one patient with the double mutation of pfmdr1 N86Y and K13 propeller F446I, which may further indicate significance of pfmdr1 mutations in artemisinin resistance. This uncommon pfmdr1 polymorphism from this region offers opportunities for investigations of the mechanisms of artemisinins.
The pfcrt K76T mutation has been widely used as a reliable marker for CQ resistance, and it was found to have a high prevalence in China (34). Furthermore, it also influences P. falciparum susceptibility to MQ, halofantrine, and artemisinin (18). This is consistent with our study showing that pfcrt K76T played a predominant role in the pfcrt genotypes. Molecular assays have shown high prevalence of the M74N75T76 (55.6%) genotype, which was similar to the findings of Huang et al. (35). No significant difference was observed between the local samples from Yingjiang county and those from Myanmar, since migration occurs in both directions. Despite the fact that China has not used CQ to treat P. falciparum infections for more than 30 years, the stable and high prevalence of this mutation may be a result of the continued use of CQ as a first-line drug for P. vivax infection over several decades. Another factor that may contribute to the high prevalence of pfcrt K76T is the use of CQ as a first-line drug for P. vivax infection for several decades in Myanmar, especially in the Myanmar-Thailand border area, where a high prevalence of pfcrt K76T was found, thus suggesting that the natural selection against CQ pressure for the maintenance of the pfcrt mutation in P. falciparum is still retained in the region (36, 37). However, we also found three other haplotypes (I74N75K76, M74E75K76, and M74E75T76) in our study, suggesting that selection of other pfcrt haplotypes was still needed.
Resistance to common antimalarial drugs has been reported for P. vivax in the GMS, including Myanmar and Vietnam, and also in Indonesia (38–40). A trend for a gradual decline in the in vitro sensitivity of this parasite to CQ had also been reported during 2005 to 2008 around the China-Myanmar border and in central China (41, 42). Our findings revealed that the presence of the pvmdr1 Y976F mutation in Yingjiang county in China is consistent with previous reports of declining sensitivity to CQ; this was also observed in Myanmar and Xishuangbanna (Yunnan province), which exhibit high frequencies of the pvmdr1 Y976F allele (43, 44). The long history of CQ use and the frequent population movement across the borders may contribute to the CQ-resistant P. vivax strains detected in Yingjiang county. Close surveillance at sentinel sites in this region should continue so that the emergence and spread of P. vivax resistance can be carefully monitored.
Artemisinin and its derivatives have been used for falciparum malaria treatment in China since the late 1970s (45). In vitro assays showed that the susceptibility of P. falciparum to artemisinins was declining in China, but no evidence of the artemisinin resistance has been detected (46). In our study, five nonsynonymous mutations were found, and three of them were not reported previously. Furthermore, our study showed that F446I was the predominant allele (19.2%, 5/26), and two cases with this mutation were found in the port city of Nabang, China. Another mutation, K13 propeller A676D, was also observed in Zhina, China (Fig. 2). However, the C580Y allele, which was widely found in Cambodia (16), was not found in this study. Nevertheless, our results indicate that the mutated K13 propeller gene alleles exist in the China-Myanmar border area, and their presence should raise concerns regarding the risks of emerging artemisinin resistance in the GMS. We recommend further clinical trials associated with K13 propeller mutations, which might be useful for identifying additional genetic loci involved in monitoring the threat of artemisinin resistance.
FIG 2.
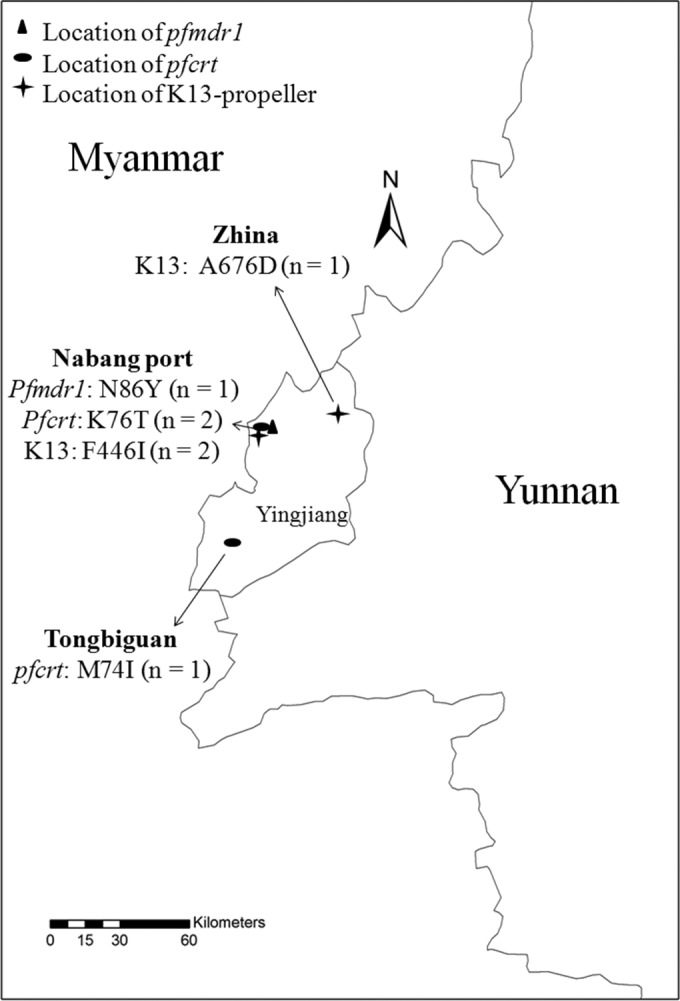
Location of antimalarial resistance marker polymorphisms in Yingjiang county. The numbers of mutated samples are shown in parentheses. The map was created by using ArcGIS 10.1 (Environmental Systems Research Institute, Inc.).
The prevalence of the K13 propeller polymorphism detected in Yingjiang county indicates that ACTs should be used in the China-Myanmar border area, and rational use of antimalarials against P. falciparum strains imported from Southeast Asia should be adopted. In addition, routine monitoring and surveillance, as recommended by the WHO Global Plan for Artemisinin Resistance Containment, should continuously be strengthened. Additional clinical investigations to complement sentinel surveillance, including either analysis of the drug markers or risk factors or new approaches to monitor resistance, are required.
In conclusion, the present data might be helpful for enrichment of molecular surveillance of antimalarial resistance and for developing and updating guidance for the use of antimalarials in the region.
ACKNOWLEDGMENTS
We thank the staffs of the provincial and county centers for disease control and prevention in China for assistance.
We declare no conflicts of interest.
REFERENCES
- 1.Delacollette C, D'Souza C, Christophel E, Thimasarn K, Abdur R, Bell D, Dai TC, Gopinath D, Lu S, Mendoza R, Ortega L, Rastogi R, Tantinimitkul C, Ehrenberg J. 2009. Malaria trends and challenges in the Greater Mekong Subregion. Southeast Asian J Trop Med Public Health 40:674–691. [PubMed] [Google Scholar]
- 2.Li HX, Zhang ZX, Du ZW, Chen GW, Chen ZW. 2005. Investigation into an outbreak of malaria in Lincang prefecture on China-Myanmar border and the neighboring area outside China. Zhongguo Re Dai Yi Xue 5:55–57. (In Chinese.) [Google Scholar]
- 3.Ministry of Health. 2010. Action plan of China malaria elimination (2010-2020). Ministry of Health, Beijing, China. [Google Scholar]
- 4.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wellems TE, Plowe CV. 2001. Chloroquine-resistant malaria. J Infect Dis 184:770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- 7.Roper C, Pearce R, Nair S, Sharp B, Nosten F, Anderson T. 2004. Intercontinental spread of pyrimethamine-resistant malaria. Science 305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. 2009. Development of a strategy towards elimination of Plasmodium falciparum parasites with altered responses to artemisinins, p 1–52. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 9.Ministry of Health. 2006. Antimalarial drug policy in China. Ministry of Health, Beijing, China. [Google Scholar]
- 10.Yang HL, Liu DQ, Yang YM, Fan B, Yang PF, Li XL, Li CF, Dong Y, Yang CJ. 2003. Changes in susceptibility of Plasmodium falciparum to artesunate in vitro in Yunnan Province, China. Trans R Soc Trop Med Hyg 97:226–228. doi: 10.1016/S0035-9203(03)90127-1. [DOI] [PubMed] [Google Scholar]
- 11.Sun XD, Wang J, Li XL, Yang J, Deng Y, Lasi JH. 2014. In vitro sensitivity of Plasmodium falciparum to 6 antimalarials in the City of Laiza, Burma. Zhongguo Bing Yuan Sheng Wu Xue Za Zhi 9:561–563. (In Chinese.) [Google Scholar]
- 12.Carrara VI, Lwin KM, Phyo AP, Ashley E, Wiladphaingern J, Sriprawat K, Rijken M, Boel M, McGready R, Proux S, Chu C, Singhasivanon P, White N, Nosten F. 2013. Malaria burden and artemisinin resistance in the mobile and migrant population on the Thai-Myanmar border, 1999-2011: an observational study. PLoS Med 10:e1001398. doi: 10.1371/journal.pmed.1001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babiker HA, Pringle SJ, Abdel-Muhsin A, Mackinnon M, Hunt P, Walliker D. 2001. High-level chloroquine resistance in Sudanese isolates of Plasmodium falciparum is associated with mutations in the chloroquine resistance transporter gene pfcrt and the multidrug resistance gene pfmdr1. J Infect Dis 183:1535–1538. doi: 10.1086/320195. [DOI] [PubMed] [Google Scholar]
- 14.Djimdé A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourte Y, Coulibaly D, Dicko A, Su XZ, Nomura T, Fidock DA, Wellems TE, Plowe CV. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med 344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 15.Suwanarusk R, Chavchich M, Russell B, Jaidee A, Chalfein F, Barends M, Prasetyorini B, Kenangalem E, Piera KA, Lek-Uthai U, Anstey NM, Tjitra E, Nosten F, Cheng Q, Price RN. 2008. Amplification of pvmdr1 associated with multidrug-resistant Plasmodium vivax. J Infect Dis 198:1558–1564. doi: 10.1086/592451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. 2014. Status report on artemisinin resistance. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 18.Duraisingh MT, Jones P, Sambou I, von Seidlein L, Pinder M, Warhurst DC. 2000. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol Biochem Parasitol 108:13–23. doi: 10.1016/S0166-6851(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 19.Brega S, Meslin B, de Monbrison F, Severini C, Gradoni L, Udomsangpetch R, Sutanto I, Peyron F, Picot S. 2005. Identification of the Plasmodium vivax mdr-like gene (pvmdr1) and analysis of single-nucleotide polymorphisms among isolates from different areas of endemicity. J Infect Dis 191:272–277. doi: 10.1086/426830. [DOI] [PubMed] [Google Scholar]
- 20.Yang HL. 2001. Malaria control and research in Yunnan province. Zhongguo Ji Sheng Chong Bing Fang Zhi Za Zhi 14:54–56. (In Chinese.) [Google Scholar]
- 21.Hastings IM. 2004. The origins of antimalarial drug resistance. Trends Parasitol 20:512–518. doi: 10.1016/j.pt.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, et al. 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang HL, Liu DQ, Huang KG, Dong Y, Yang PF, Yang YM, Liao MZ, Zhang CY, Liu RJ. 1997. In vitro sensitivity of Plasmodium falciparum to derivatives of artemisinin, pyronardine and chloroquine in Yunnan. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 15:292–296. (In Chinese.) [Google Scholar]
- 24.Yang HL, Gao BH, Huang KG. 1999. Comparison of sensitivity of artesunate-sensitive and artesunate-resistant Plasmodium falciparum to chloroquine and amodiaquine. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 17:353–355. (In Chinese.) [PubMed] [Google Scholar]
- 25.Zhou G, Sun L, Xia R, Duan Y, Xu J, Yang H, Wang Y, Lee MC, Xiang Z, Yan G, Cui L, Yang Z. 2014. Clinical malaria along the China-Myanmar border, Yunnan Province, China, January 2011-August 2012. Emerg Infect Dis 20:675–678. doi: 10.3201/eid2004.130647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wongsrichanalai C, Lin K, Pang LW, Faiz MA, Noedl H, Wimonwattrawatee T, Laoboonchai A, Kawamoto F. 2001. In vitro susceptibility of Plasmodium falciparum isolates from Myanmar to antimalarial drugs. Am J Trop Med Hyg 65:450–455. [DOI] [PubMed] [Google Scholar]
- 27.Smithuis F, Shahmanesh M, Kyaw MK, Savran O, Lwin S, White NJ. 2004. Comparison of chloroquine, sulfadoxine/pyrimethamine, mefloquine and mefloquine-artesunate for the treatment of falciparum malaria in Kachin State, North Myanmar. Trop Med Int Health 9:1184–1190. doi: 10.1111/j.1365-3156.2004.01323.x. [DOI] [PubMed] [Google Scholar]
- 28.Mu J, Ferdig MT, Feng X, Joy DA, Duan J, Furuya T, Subramanian G, Aravind L, Cooper RA, Wootton JC, Xiong M, Su XZ. 2003. Multiple transporters associated with malaria parasite responses to chloroquine and quinine. Mol Microbiol 49:977–989. doi: 10.1046/j.1365-2958.2003.03627.x. [DOI] [PubMed] [Google Scholar]
- 29.Sidhu AB, Uhlemann AC, Valderramos SG, Valderramos JC, Krishna S, Fidock DA. 2006. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J Infect Dis 194:528–535. doi: 10.1086/507115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wongsrichanalai C, Meshnick SR. 2008. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia-Thailand border. Emerg Infect Dis 14:716–719. doi: 10.3201/eid1405.071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim P, Alker AP, Khim N, Shah NK, Incardona S, Doung S, Yi P, Bouth DM, Bouchier C, Puijalon OM, Meshnick SR, Wongsrichanalai C, Fandeur T, Le Bras J, Ringwald P, Ariey F. 2009. Pfmdr1 copy number and artemisinin derivatives combination therapy failure in falciparum malaria in Cambodia. Malar J 8:11. doi: 10.1186/1475-2875-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Parker D, Meng H, Wu L, Li J, Zhao Z, Zhang R, Fan Q, Wang H, Cui L, Yang Z. 2012. In vitro sensitivity of Plasmodium falciparum from China-Myanmar border area to major ACT drugs and polymorphisms in potential target genes. PLoS One 7:e30927. doi: 10.1371/journal.pone.0030927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong Y, Li CL, Wang XR, Guo XF, Li WY, Li CF, Li L, Liu H, Li JS, Lv SS. 2005. The prevalence of malaria and control situation in Jiangcheng County of Yunnan Province. Zhongguo Re Dai Yi Xue 5:709–712. (In Chinese.) [Google Scholar]
- 34.Zhang GQ, Guan YY, Zheng B, Wu S, Tang LH. 2009. Molecular assessment of Plasmodium falciparum resistance to antimalarial drugs in China. Trop Med Int Health 14:1266–1271. doi: 10.1111/j.1365-3156.2009.02342.x. [DOI] [PubMed] [Google Scholar]
- 35.Huang F, Tang L, Yang H, Zhou S, Sun X, Liu H. 2012. Therapeutic efficacy of artesunate in the treatment of uncomplicated Plasmodium falciparum malaria and anti-malarial, drug-resistance marker polymorphisms in populations near the China-Myanmar border. Malar J 11:278. doi: 10.1186/1475-2875-11-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muhamad P, Chaijaroenkul W, Phompradit P, Rueangweerayut R, Tippawangkosol P, Na-Bangchang K. 2013. Polymorphic patterns of pfcrt and pfmdr1 in Plasmodium falciparum isolates along the Thai-Myanmar border. Asian Pac J Trop Biomed 3:931–935. doi: 10.1016/S2221-1691(13)60181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown T, Smith LS, Oo EK, Shawng K, Lee TJ, Sullivan D, Beyrer C, Richards AK. 2012. Molecular surveillance for drug-resistant Plasmodium falciparum in clinical and subclinical populations from three border regions of Burma/Myanmar: cross-sectional data and a systematic review of resistance studies. Malar J 11:333. doi: 10.1186/1475-2875-11-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guthmann JP, Pittet A, Lesage A, Imwong M, Lindegardh N, Min Lwin M, Zaw T, Annerberg A, de Radigues X, Nosten F. 2008. Plasmodium vivax resistance to chloroquine in Dawei, southern Myanmar. Trop Med Int Health 13:91–98. doi: 10.1111/j.1365-3156.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- 39.Sumawinata IW, Bernadeta, Leksana B, Sutamihardja A, Purnomo, Subianto B, Sekartuti, Fryauff DJ, Baird JK. 2003. Very high risk of therapeutic failure with chloroquine for uncomplicated Plasmodium falciparum and P. vivax malaria in Indonesian Papua. Am J Trop Med Hyg 68:416–420. [PubMed] [Google Scholar]
- 40.Phan GT, de Vries PJ, Tran BQ, Le HQ, Nguyen NV, Nguyen TV, Heisterkamp SH, Kager PA. 2002. Artemisinin or chloroquine for blood stage Plasmodium vivax malaria in Vietnam. Trop Med Int Health 7:858–864. doi: 10.1046/j.1365-3156.2002.00948.x. [DOI] [PubMed] [Google Scholar]
- 41.Lu F, Wang B, Cao J, Sattabongkot J, Zhou H, Zhu G, Kim K, Gao Q, Han ET. 2012. Prevalence of drug resistance-associated gene mutations in Plasmodium vivax in Central China. Korean J Parasitol 50:379–384. doi: 10.3347/kjp.2012.50.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang GL, Sun XD, Wang J, Zhang ZX. 2009. Sensitivity of Plasmodium vivax to chloroquine in Laza City, Myanmar. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 27:175–176. (In Chinese.) [PubMed] [Google Scholar]
- 43.Huang B, Huang S, Su XZ, Tong X, Yan J, Li H, Lu F. 2014. Molecular surveillance of pvdhfr, pvdhps, and pvmdr-1 mutations in Plasmodium vivax isolates from Yunnan and Anhui provinces of China. Malar J 13:346. doi: 10.1186/1475-2875-13-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imwong M, Pukrittayakamee S, Pongtavornpinyo W, Nakeesathit S, Nair S, Newton P, Nosten F, Anderson TJ, Dondorp A, Day NP, White NJ. 2008. Gene amplification of the multidrug resistance 1 gene of Plasmodium vivax isolates from Thailand, Laos, and Myanmar. Antimicrob Agents Chemother 52:2657–2659. doi: 10.1128/AAC.01459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li GQ, Guo XB, Fu LC, Jian HX, Wang XH. 1994. Clinical trials of artemisinin and its derivatives in the treatment of malaria in China. Trans R Soc Trop Med Hyg 88(Suppl 1):S5–S6. [DOI] [PubMed] [Google Scholar]
- 46.Zhang G, Guan Y, Zheng B, Wu S, Tang L. 2008. No PfATPase6 S769N mutation found in Plasmodium falciparum isolates from China. Malar J 7:122. doi: 10.1186/1475-2875-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]


