Abstract
Nontypeable Haemophilus influenzae (NTHI) is an opportunistic pathogen that is an important cause of acute exacerbations of chronic obstructive pulmonary disease (AECOPD). COPD is an inflammatory disease of the airways, and exacerbations are acute inflammatory events superimposed on this background of chronic inflammation. Azithromycin (AZM) is a macrolide antibiotic with antibacterial and anti-inflammatory properties and a clinically proven potential for AECOPD prevention and management. Relationships between AZM efficacy and resistance by NTHI and between bactericidal and immunomodulatory effects on NTHI respiratory infection have not been addressed. In this study, we employed two pathogenic NTHI strains with different AZM susceptibilities (NTHI 375 [AZM susceptible] and NTHI 353 [AZM resistant]) to evaluate the prophylactic and therapeutic effects of AZM on the NTHI-host interplay. At the cellular level, AZM was bactericidal toward intracellular NTHI inside alveolar and bronchial epithelia and alveolar macrophages, and it enhanced NTHI phagocytosis by the latter cell type. These effects correlated with the strain MIC of AZM and the antibiotic dose. Additionally, the effect of AZM on NTHI infection was assessed in a mouse model of pulmonary infection. AZM showed both preventive and therapeutic efficacies by lowering NTHI 375 bacterial counts in lungs and bronchoalveolar lavage fluid (BALF) and by reducing histopathological inflammatory lesions in the upper and lower airways of mice. Conversely, AZM did not reduce bacterial loads in animals infected with NTHI 353, in which case a milder anti-inflammatory effect was also observed. Together, the results of this work link the bactericidal and anti-inflammatory effects of AZM and frame the efficacy of this antibiotic against NTHI respiratory infection.
INTRODUCTION
Nontypeable (noncapsulated) Haemophilus influenzae (NTHI) is a Gram-negative coccobacillus that is a common commensal in the nasopharynx of healthy humans and is also an important cause of respiratory infections, such as acute otitis media, otitis media with effusion, community-acquired pneumonia, and exacerbations of chronic obstructive pulmonary disease (COPD) (1). COPD is characterized by progressive and not fully reversible airflow limitation, accompanied by infiltration of the airways by neutrophils and mucus hypersecretion (2, 3). Acute exacerbations of COPD (AECOPD) are episodes of increased respiratory and systemic symptoms, mostly caused by bacterial and viral infections (4). In this context, infection by NTHI is a marker of severe obstructive airflow and progression of the disease and has been associated with declines in lung function and with mortality (5). Given that patients with frequent AECOPD have fast lung function declines, prolonged times of recovery, and a poor quality of life (4, 6, 7), a reduction in AECOPD would improve patient well-being and survival (8–10).
Macrolide antibiotics are polyketide compounds with a 14-, 15-, or 16-membered macrocyclic lactone to which one or more amino and/or neutral sugars are attached. Macrolides include azithromycin (AZM), a prototypical 15-membered compound with favorable pharmacological properties (11). Features contributing to AZM's success as an antibiotic are its acid resistance, a short time to achieve peak concentration, with a high level of accumulation in phagocytes, and a long half-life (12–14). Macrolides inhibit bacterial growth by binding the 23S rRNA in the 50S subunit of the bacterial ribosome, preventing the transfer of tRNA from the A to the P site of the ribosome. Binding to the A site prevents addition of an incoming amino acid-charged tRNA to the nascent polypeptide chain, aborting polypeptide growth (15). In addition to their direct antibacterial effect, macrolides are immunomodulators, presenting anti-inflammatory activities on cells of the innate and adaptive immunity and on structural cells (16–18). On airway epithelia, macrolides positively regulate tight junctions (19) and suppress the activation of NF-κB, Sp1, and AP-1, leading to a reduction of proinflammatory cytokines (20–25). On phagocytes, besides decreasing the secretion of proinflammatory cytokines, macrolides stimulate monocyte differentiation into alveolar macrophages, improve phagocytic function, and favor expression of the phagocytic mannose receptor in COPD patients (26–31).
Macrolides provide adequate coverage for the most frequently identified pathogens in AECOPD (11). Several clinical studies have examined their potential for AECOPD prevention (32–37). In particular, long-term prophylaxis with AZM in patients with moderate to severe COPD has been associated with a significantly decreased number of exacerbations and an improved quality of life. Importantly, patients receiving AZM were less likely to become colonized with respiratory pathogens but also were more likely to become colonized with macrolide-resistant microorganisms (32). In agreement with this notion, longitudinal studies of nasopharyngeal carriage of respiratory bacteria in indigenous Australian and Alaskan native children with bronchiectasis revealed that macrolide resistance was higher in Australian children, who received more AZM, than in Alaskan children (34).
Although clinical data indicate the potential of AZM in the management of chronic inflammatory airway disorders (9, 38, 39), the impact of AZM on infections caused by bacterial pathogens causing AECOPD is poorly understood. Moreover, the observed increased macrolide resistance by colonizing respiratory pathogens generates doubts about its long-term low-dose prophylactic use. Independent studies with unrelated NTHI isolates displaying different degrees of AZM susceptibility have shown a bactericidal effect of AZM against intracellular NTHI (40–42), and the therapeutic efficacy of macrolides has been suggested by use of mouse and rat NTHI pulmonary infection models (32, 43–46). In this study, we questioned the effects of both prophylactic and therapeutic uses of AZM, the relationship between NTHI AZM resistance and administration effects, and the antimicrobial versus immunomodulatory effects of AZM on NTHI respiratory infection. We employed two NTHI clinical isolates with different AZM susceptibilities (NTHI strain 375 [AZM sensitive] and NTHI strain 353 [AZM resistant]) to systematically evaluate the effects of prophylactic and therapeutic administrations of AZM on (i) NTHI-host cell interplay, by assessing adhesion, invasion/phagocytosis, intracellular localization, and inflammatory responses on cultured bronchial and alveolar epithelial cells and alveolar macrophages; and (ii) NTHI respiratory infection in vivo, by assessing bacterial loads in lungs and bronchoalveolar lavage fluid (BALF), as well as airway inflammatory lesions, in a murine intranasal infection model. This work provides a context for a better understanding of the effects of AZM administration on NTHI respiratory infection.
MATERIALS AND METHODS
Bacteria, media, and growth conditions.
NTHI 375 is an otitis media isolate (47, 48). NTHI 353 is a clinical isolate from a sputum sample recovered from a COPD patient at the Hospital Universitari Bellvitge (HUB; Barcelona, Spain). Both NTHI strains were grown overnight at 37°C and with 5% CO2 on chocolate-agar plates (bioMérieux) or on brain heart infusion (BHI)-agar plates supplemented with 10 μg/ml hemin and 10 μg/ml β-NAD, referred to as sBHI-agar plates.
Antimicrobial agents and antibiotic susceptibility testing.
AZM (azithromycin dihydrate; Zitromax) was purchased from Pfizer and dissolved in sterile water. The susceptibilities of NTHI 375 and NTHI 353 to AZM were determined by a standard MIC microdilution test, using the criteria of the Clinical and Laboratory Standards Institute (CLSI) (49, 50).
Cell culture and bacterial infection.
Human alveolar basal epithelial carcinoma cells (A549; CCL-185) were maintained as described previously (51). Cells were seeded to 6 × 104 cells per well in 24-well tissue culture plates for 32 h and then serum starved for 16 h before infection. Mucoepidermoid pulmonary human epithelial carcinoma cells (NCI H-292; ATCC CRL-1848) and MH-S murine alveolar macrophages (ATCC CRL-2019) were maintained in RPMI 1640 tissue culture medium supplemented with 10 mM HEPES, 10% heat-inactivated fetal calf serum (FCS), and antibiotics (penicillin and streptomycin) in 75-cm2 tissue culture flasks at 37°C in a humidified 5% CO2 atmosphere. NCI H-292 cells were seeded to 4 × 105 cells per well in 24-well plates 24 h before each experiment. MH-S cells were seeded to 7 × 105 cells per well in 24-well plates 16 h before each experiment. In all cases, ∼90% confluence was reached at the time of infection.
NTHI infection was performed as previously described (51, 52). Bacteria were recovered with 1 ml phosphate-buffered saline (PBS) from a chocolate-agar plate after growth for 16 h at 37°C and 5% CO2. Each bacterial suspension was adjusted to an optical density at 600 nm (OD600) of 1 in PBS (∼109 CFU/ml). A549 and NCI H-292 cells were infected in 1 ml Earle's balanced salt solution (EBSS; Gibco) to obtain a multiplicity of infection (MOI) of ∼100:1. MH-S cells were infected in 1 ml RPMI 1640 supplemented with 10 mM HEPES and 10% FCS to obtain an MOI of ∼100:1, or ∼12:1 when necessary. For adhesion experiments, A549 and NCI H-292 cells were incubated with bacteria for 30 min, and MH-S cells were incubated with bacteria for 1 h. In all cases, wells were then washed 5 times with PBS, lysed with 300 μl of PBS-0.025% saponin for 10 min at room temperature, serially diluted, and plated on sBHI-agar plates. For invasion assays, A549 and NCI H-292 cells were incubated with bacteria for 2 h, washed 3 times with PBS, and incubated for 1 h with RPMI 1640 containing 10% FCS, 10 mM HEPES, and 200 μg/ml gentamicin to kill extracellular bacteria. For phagocytosis assays, MH-S cells were infected for 1 h, washed 3 times with PBS, and incubated for 1 h with RPMI 1640 containing 10% FCS, 10 mM HEPES, and 300 μg/ml gentamicin. In all cases, cells were washed 3 times with PBS and lysed as described above. When indicated, cells were pretreated with AZM (with various dose ranges, depending on the NTHI strain and type of assay) or vehicle solution (sterile water) for 30 min; the drug/vehicle was then removed before NTHI infection. AZM doses and regimens were selected according to previous studies (40–42, 53) and according to the susceptibility to AZM of the NTHI strains under study. Alternatively, cells were infected as described above, and AZM (with various dose ranges, depending on the NTHI strain and assay) or vehicle solution (sterile water) was added during the gentamicin incubation period. These treatments did not induce cytotoxicity on the three cell types tested, as verified by measuring the release of lactate dehydrogenase and by microscopy (data not shown). In all cases, parallel controls (CON) were performed: in adhesion assays, cells did not receive AZM; and in invasion assays, cells were treated with gentamicin but did not receive AZM. Adhesion, invasion, and phagocytosis results are expressed as numbers of bacterial CFU per well. Each infection assay was carried out in triplicate and on at least three independent occasions (n ≥ 3).
Immunofluorescence microscopy.
A549 cells were seeded on 13-mm circular coverslips in 24-well tissue culture plates. Infections were performed as described above, and infected cells were incubated in RPMI 1640 containing 10% FCS, 10 mM HEPES, and 200 μg/ml gentamicin for 15 or 60 min in the absence (control) or presence of AZM. When indicated, cells were washed three times with PBS and fixed with 3.7% paraformaldehyde (PFA) in PBS, pH 7.4, for 15 min at room temperature. Staining was carried out in 10% horse serum and 0.1% saponin in PBS. NTHI 375 was stained with rabbit anti-NTHI serum diluted 1:600 (51). Early endosomes were stained with goat anti-EEA1 (N-19) antibody (Santa Cruz Biotechnology) diluted 1:50. Late endosomes were stained with mouse monoclonal anti-human LAMP1 antibody (H4A3; Developmental Studies Hybridoma Bank) diluted 1:100. DNA was stained with Hoechst 33342 (Invitrogen) diluted 1:2,500. Donkey anti-rabbit conjugated to Cy2 and donkey anti-goat or donkey anti-mouse conjugated to rhodamine were purchased from Jackson and were used as secondary antibodies, diluted 1:100.
For immunofluorescence staining, fixed coverslips were washed twice in PBS containing 0.1% saponin and once in PBS and then incubated for 30 min with primary antibodies in 10% horse serum, 0.1% saponin in PBS. Coverslips were then washed twice in 0.1% saponin in PBS and once in PBS and incubated for 30 min with secondary antibodies in 10% horse serum, 0.1% saponin in PBS. Finally, coverslips were washed twice in 0.1% saponin in PBS, once in PBS, and once in water and then mounted on glass coverslips by use of Aqua-Poly/Mount (Polysciences). Samples were analyzed with a Carl Zeiss Axioskop 2 Plus fluorescence microscope and a Carl Zeiss Axio Cam MRm monochrome camera. An NTHI-containing vacuole (NTHI-CV) was considered positive for EEA1 or LAMP1 when the marker was detected throughout the area occupied by the bacterium or around/enclosing the bacterium. To determine the percentage of bacteria that colocalized with each marker, all bacteria located inside a minimum of 150 infected cells were scored in each experiment. Results were calculated from at least two independent experiments and are presented as means and standard deviations (SD) of the percentages of bacterium-subcellular marker association.
Secretion of IL-8.
A549 cells were maintained, seeded, and infected for 2 h, as described above. Cells were washed 3 times with PBS and incubated for 6 h with fresh medium containing 100 μg/ml gentamicin. Supernatants were collected from the wells, cell debris was removed by centrifugation, and samples were frozen at −80°C until use. Interleukin-8 (IL-8) levels in the supernatants were measured by an enzyme-linked immunosorbent assay (ELISA) (Abnova KA0115) with a sensitivity of <2 pg/ml, performed according to the manufacturer's instructions. When necessary, cells were pretreated with 50 μg/ml AZM or vehicle solution (sterile water) for 30 min, and the antibiotic was removed before NTHI infection. Alternatively, cells were pretreated with 1 µM dexamethasone (DEX; Sigma-Aldrich) for 2 h, which was kept during infection, including the gentamicin incubation period (see above). In all cases, infections were carried out in duplicate and on at least two independent occasions (n ≥ 2). Results are expressed as the means and SD of IL-8 levels (in picograms per milliliter).
Mouse assays.
A mouse model of NTHI pulmonary infection was used as described previously (54, 55). CD1 female mice (18 to 20 g) aged 4 to 5 weeks were purchased from Charles River Laboratories (France), housed under pathogen-free conditions at the Institute of Agrobiotechnology facilities (registration number ES/31-2016-000002-CR-SU-US), and used at 22 to 25 g. Animal handling and procedures were performed in accordance with the current European (directive 86/609/EEC) and national (Real Decreto 53/2013) legislations, following the FELASA and ARRIVE guidelines, and with the approval of the Universidad Pública de Navarra (UPNa) Animal Experimentation Committee (Comité de Ética, Experimentación Animal y Bioseguridad) and the local government. Following the 3Rs principles, we first performed a pilot assay to determine the most adequate AZM administration pattern in the mouse model. NTHI 375 was used for lung infection, and mice were randomly divided into the following six groups (n = 5): (i) control (administered vehicle solution); (ii) treated with AZM 24 h before infection; (iii) treated with AZM 12 h before infection; (iv) treated with AZM 1 h before infection; (v) treated with AZM 24 and 1 h before infection; and (vi) treated with AZM 24, 12, and 1 h prior to infection and at 6 h postinfection (hpi). AZM treatments were performed at doses of 100 mg/kg of body weight in 0.1 ml water and administered by oroesophageal gavage (Popper & Sons Inc.). NTHI intranasal infections were performed as described previously (55). Briefly, 20 μl of an NTHI suspension containing ∼5 × 109 CFU/ml (1 × 108 CFU/mouse) was placed at the entrance of the nostrils until complete inhalation by the mouse, which was previously anesthetized with ketamine-xylazine (3:1). At 12 hpi, all mice were euthanized by cervical dislocation, and lungs were aseptically removed, individually weighed in sterile bags (Stomacher80; Seward Medical), and homogenized 1:10 (wt/vol) in PBS. Each homogenate was serially 10-fold diluted in PBS, and 100 μl of each dilution was spread in triplicate on sBHI-agar plates to determine the number of viable bacteria. Results are expressed as means ± SD of individual measurements of log10 CFU/lung. A second assay was designed to study the relationship between AZM effects and susceptibility of the NTHI infecting strain. Strains NTHI 375 and NTHI 353 were used separately. Mice were randomly divided into the following three groups (n = 5) for each NTHI strain infection: (i) untreated control; (ii) animals treated with three AZM doses, 24, 12, and 1 h prior to infection; and (iii) animals treated with a single AZM dose, at 6 hpi. AZM oral administration and NTHI intranasal infections were performed as detailed above. At 12 hpi, mice were euthanized, and BALF samples were obtained by perfusion and collection of 0.7 ml of PBS, with the help of a sterile 20G (1.1-mm diameter) Vialon intravenous catheter (Becton-Dickinson) inserted into the trachea. Each recovered BALF fraction was serially 10-fold diluted in PBS, and 100 μl of each dilution was spread on sBHI-agar plates to determine the number of viable bacteria. Results are expressed as means ± SD of individual measurements of log10 CFU/BALF sample. In parallel, the lungs were removed; the left one was processed for viable bacterial counts (as detailed above), and the right lung was fixed in 10% neutral buffered formalin for histological purposes. Heads and necks, containing upper airways, larynxes, and tracheas, were also fixed in the same buffered formalin for histology. Uninfected mice receiving PBS, and AZM when necessary, were used as controls.
Histopathology and lesion scores.
Heads and necks were rinsed in running tap water for 1 h and immersed in 5% nitric acid for 24 to 36 h until complete decalcification, and 7 or 8 transaxial slices were made every 3 to 4 mm, beginning at the nostrils and finishing in the caudal tracheas. Transaxial slices and lungs were embedded in paraffin, and 4- to 6-μm sections were stained with hematoxylin and eosin (H&E) by standard procedures and examined by microscopy to determine the presence and extent of inflammatory lesions. Sections were examined blind as sets by a trained veterinary pathologist (M. Barberán). Parameters characterizing an acute inflammatory reaction in the upper airway, larynx, trachea, and lung, including hemorrhages, hyperemia, polymorphonuclear cell (PMN) infiltrates, and alveolar macrophages, were subjectively scored on a scale of 0 to 3 (0, absent; 1, mild; 2, moderate; and 3, severe). For tissue control, similar organs obtained from noninfected control and AZM-treated mice were processed in a manner identical to that for the infected tissues. The images were observed and digitalized using an Olympus Vanox AHBS3 microscope coupled to an Olympus DP12 digital camera.
Statistical analysis.
For cell infection, IL-8 secretion, and bacterial loads in lungs and BALF samples, means ± SD were calculated, and statistical comparisons of means were performed using the two-tailed t test. For histopathology scoring, means ± SD were also calculated, and statistical comparisons were performed using one-way analysis of variance (ANOVA) followed by Fisher's protected least significant difference (PLSD) multiple-comparison test. In all cases, P values of <0.05 were considered statistically significant. Analyses were performed using the Prism software, version 4 for PC (GraphPad Software), statistical package.
RESULTS
Selection of two NTHI clinical isolates with different susceptibilities to AZM.
We first selected two pathogenic NTHI isolates with different levels of susceptibility to AZM. NTHI 375 is a child otitis media isolate recovered from an ear sample (47, 48), whose interplay with the host was previously characterized (51, 55, 56). The MIC of AZM for NTHI 375 is 0.25 μg/ml, and accordingly, it was classified as susceptible. NTHI 353 is a COPD strain isolated from a sputum sample. The MIC of AZM for NTHI 353 is 8 μg/ml, and it was classified as resistant. Both strains displayed comparable growth rates in sBHI liquid culture (data not shown) and infected cultured respiratory epithelial cells and alveolar macrophages (see control data in Fig. 1 to 3; see Fig. S1 in the supplemental material). Intranasal inoculation of CD1 mice with similar doses of both NTHI strains rendered comparable bacterial loads in lungs and BALF samples at 12 hpi (see control panels in Fig. 4). Histopathological analysis of upper airways, larynxes, tracheas, and lungs from mice infected intranasally with NTHI 375 or with NTHI 353 rendered inflammatory lesions (Table 1). Lungs and airways from control mice instilled with PBS did not show either significant inflammation or pathological changes. In both NTHI 375- and NTHI 353-infected mice, the upper airways showed some subepithelial and intravascular neutrophils, and the lumens of the turbinates were filled with a seropurulent exudate and sloughed lining epithelial necrotic cells. Mild PMN infiltration of the lamina propria and lumens containing red blood cells and PMNs were the main findings in larynxes and tracheas. A tendency toward increased inflammation in NTHI 375-infected compared to NTHI 353-infected animals was apparent, with more accumulation of PMNs in the upper airway, larynx, and trachea, although the differences did not reach statistical significance (Table 1). Lungs in infected mice showed areas of acute bronchopneumonia. In these areas, alveolar septa were thickened with edema and hyperemic septal capillaries. Neutrophils, alveolar macrophages, and scattered small hemorrhages were observed in alveolar spaces. Suppurative bronchitis and bronchiolitis with neutrophilic subepithelial infiltrates and seropurulent exudates in the lumens were also observed. Comparative analysis of scored lesions showed significantly (P < 0.0001) more recruitment of PMNs at the bronchi and bronchioles of mice infected with NTHI 375 than at those infected with NTHI 353. Conversely, both PMNs and alveolar macrophages were found in larger proportions (P < 0.05 and P < 0.001, respectively) at the alveoli of mice infected with NTHI 353 than at those of mice infected with NTHI 375 (Table 1).
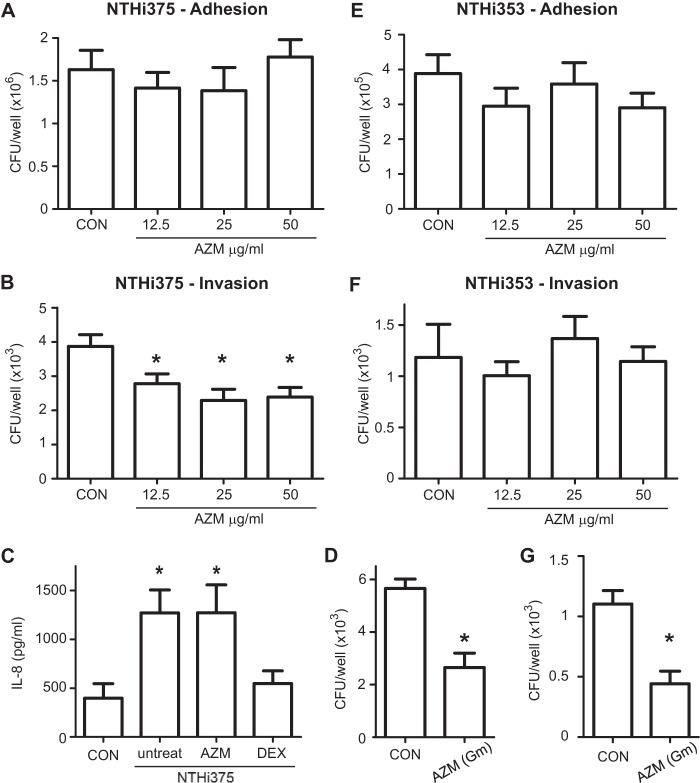
FIG 1.
Effect of AZM administration on NTHI infection of A549 human respiratory epithelial cells. Controls were as follows: in adhesion assays, cells did not receive AZM; and in invasion assays, cells were treated with gentamicin but did not receive AZM. (A and E) Effects of AZM pretreatment on NTHI 375 (A) and NTHI 353 (E) adhesion to A549 type II pneumocytes. Cells were pretreated with AZM at 12.5, 25, or 50 μg/ml. The antibiotic was removed before NTHI infection. Both strains presented similar adhesion rates for AZM-pretreated and control (CON) cells. (B and F) Effects of AZM pretreatment on NTHI 375 (B) and NTHI 353 (F) invasion of A549 cells. Cells were pretreated with AZM at 12.5, 25, or 50 μg/ml. Mean numbers of NTHI 375 entry into AZM-pretreated cells were significantly lower (*) than those obtained for control (CON) cells (for 12.5 μg/ml, P < 0.05; for 25 μg/ml, P < 0.01; and for 50 μg/ml, P < 0.005). Mean numbers of NTHI 353 invasion did not vary upon AZM pretreatment. (C) Effect of AZM or dexamethasone (DEX) on the NTHI 375-mediated inflammatory response, measured by the level of IL-8 secreted into the supernatant by A549 cells. CON, uninfected cells that did not receive AZM; untreat, cells infected with NTHI 375 that did not receive AZM. Quantification of IL-8 was performed by ELISA at 8 h postinfection. AZM did not show an anti-inflammatory effect under the conditions tested. (D and G) Effects of AZM on intracellular NTHI 375 (D) and NTHI 353 (G). A549 cells were infected, and 50 μg/ml AZM was added during cell incubation with gentamicin (Gm). In both cases, the number of intracellular bacteria was significantly lower (P < 0.001 and P < 0.0005, respectively) for AZM-treated cells than for control cells.
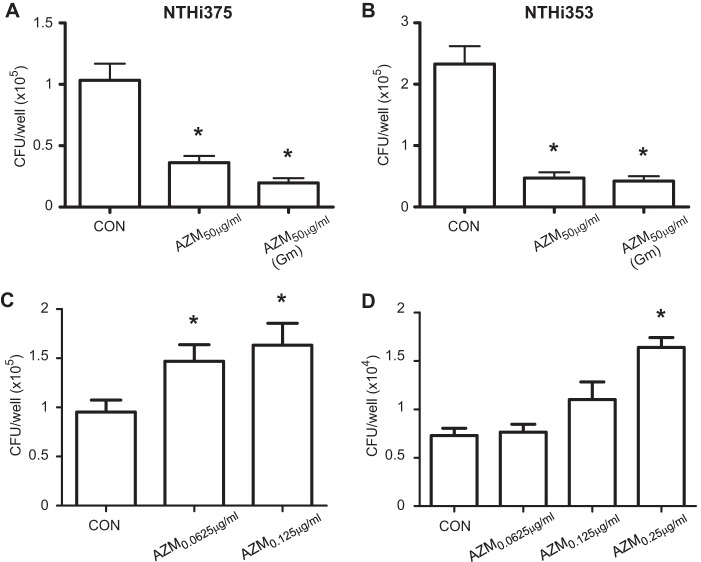
FIG 3.
Effect of AZM on NTHI infection of MH-S alveolar macrophages. Cells were left untreated (CON; cells treated with gentamicin that did not receive AZM), pretreated with AZM at 50 μg/ml, or infected and treated with AZM at 50 μg/ml during cell incubation with gentamicin. Mean numbers for NTHI 375 (A) and NTHI 353 (B) phagocytosis are shown. (A) NTHI 375 phagocytosis levels by AZM-pretreated cells and cells treated with AZM during gentamicin incubation were significantly lower than those obtained for control cells (P < 0.005 and P < 0.0005, respectively). (B) Mean numbers for NTHI 353 phagocytosis by AZM-pretreated cells and cells treated with AZM during gentamicin incubation were significantly lower than those obtained for control cells (P < 0.0005). (C) Cells were left untreated or pretreated with AZM at 0.0625 or 0.125 μg/ml, i.e., concentrations unable to kill intracellular NTHI 375; drug/vehicle was removed prior to infection. Mean numbers for NTHI 375 phagocytosis by cells pretreated with AZM were significantly higher than those obtained for control cells (P < 0.05 and P < 0.01, respectively). (D) Cells were left untreated or pretreated with AZM at 0.0625, 0.125, or 0.25 μg/ml, i.e., concentrations unable to kill intracellular NTHI 375; drug/vehicle was removed prior to infection. Infection was performed at an MOI of ∼12:1. Mean numbers for NTHI 353 phagocytosis by cells pretreated with AZM at 0.25 μg/ml were significantly higher than those obtained for control cells (P < 0.0001).
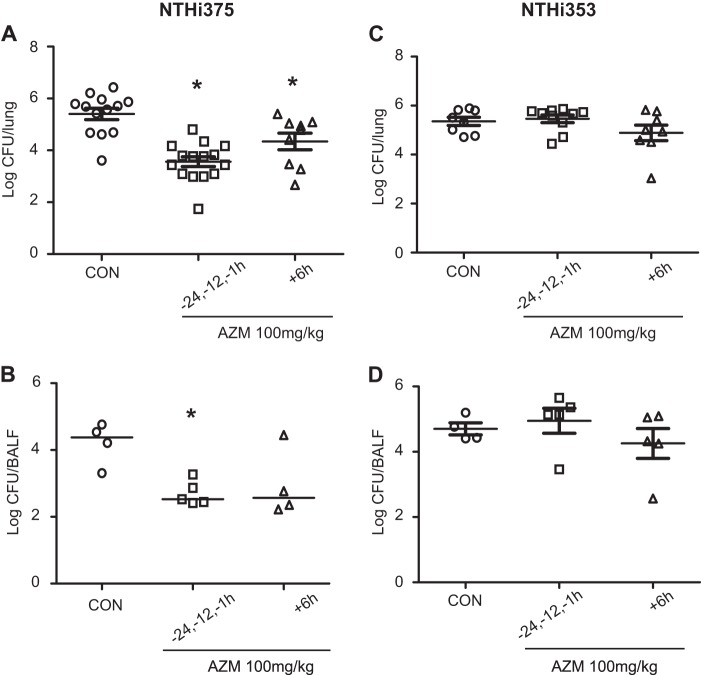
FIG 4.
Effect of AZM administration on bacterial loads in CD1 mice infected by NTHI. Mice were infected intranasally with ∼108 bacteria/mouse. AZM (100 mg/kg/dose) was administered orally. Controls were as follows: animals were administered vehicle solution but did not receive AZM. Bacterial counts were determined at 12 hpi for lungs (log10 CFU/lung) (A and C) and BALF (log10 CFU/BALF sample) (B and D). (A) Impact of AZM on NTHI 375 counts in lungs. NTHI 375 counts were significantly lower in mice treated with AZM prior to (1, 12, and 24 h before infection) and/or after (6 hpi) infection than in control (CON) mice (P < 0.0001 and P < 0.05, respectively). (B) Effect of AZM on NTHI 375 bacterial counts in BALF samples. NTHI 375 counts were significantly (P < 0.005) lower in mice treated with AZM prior to infection than in control mice. (C and D) Impacts of AZM use on NTHI 353 bacterial counts in lungs (C) and BALF (D). NTHI 353 counts were similar for all mouse groups.
TABLE 1.
Scores of histopathological lesions found in the airways of untreated or AZM-treated mice intranasally infected with NTHI 375 or NTHI 353
| Strain | AZM treatmenta | Score (mean ± SD)b |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Upper airways |
Larynx-trachea |
Lung |
||||||||
| PMNs in lumen | PMNs in lamina propria | Hyperemia | PMNs in lumen | PMNs in lamina propria | Bronchial PMNs | Hemorrhage | Alveolar PMNs | Alveolar macrophages | ||
| NTHI 375 | Control | 2.4 ± 0.3 | 2.3 ± 0.3 | 1.4 ± 0.2 | 1 ± 0.5 | 1.4 ± 0.2 | 2c,e | 1.1 ± 0.5e | 0d,f | 0.13 ± 0.13d,f |
| Prior to infection | 1.5 ± 0.4 | 1.2 ± 0.3 | 0.8 ± 0.1 | 0.2 ± 0.1 | 1 | 0.7 ± 0.2e | 0.8 ± 0.2 | 1.1 ± 0.1f | 0.6 ± 0.1 | |
| Postinfection | 2.1 ± 0.4 | 1.6 ± 0.1 | 1.3 ± 0.3 | 0.3 ± 0.1 | 1.4 ± 0.2 | 0.6 ± 0.2e | 0e | 1.5 ± 0.3f | 0.9 ± 0.2f | |
| NTHI 353 | Control | 2 ± 0.1 | 1.6 ± 0.2 | 1.4 ± 0.2g | 0.4 ± 0.1 | 1.2 ± 0.3 | 0.5 ± 0.1c | 0.6 ± 0.2 | 1.3 ± 0.3d | 1.2 ± 0.1d,h |
| Prior to infection | 1.7 ± 0.4 | 1.5 ± 0.2 | 1.3 ± 0.2 | 0.4 ± 0.1 | 1.3 ± 0.3 | 1 ± 0.4 | 0.8 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.1 | |
| Postinfection | 1.8 ± 0.3 | 1.2 ± 0.2 | 0.6 ± 0.2g | 0.5 ± 0.2 | 1.1 ± 0.1 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.4 ± 0.1 | 0.5h | |
Control, animals administered vehicle solution; prior to infection, three AZM doses were administered, 24, 12, and 1 h before infection; postinfection, one AZM dose was administered, at 6 h postinfection.
Statistical comparisons of mean values were performed using one-way ANOVA followed by Fisher's PLSD multiple-comparison test.
More recruitment of PMNs at the bronchi of mice infected with NTHI 375 than at those of mice infected with NTHI 353 (P < 0.0001).
Larger proportions of PMNs and alveolar macrophages at the alveoli of mice infected with NTHI 353 than at those of mice infected with NTHI 375 (P < 0.05 and P < 0.001, respectively).
Reductions of inflammatory lesions for bronchial PMNs in mice treated with AZM prior to and after NTHI 375 infection (P < 0.0005) and of lung hemorrhage in mice treated with AZM after NTHI 375 infection (P < 0.05), compared to controls.
Larger numbers of PMNs (for mice treated prior to and after infection [P < 0.01]) and alveolar macrophages (for mice treated postinfection [P < 0.05]) at the alveoli of AZM-treated animals than at those of controls infected by NTHI 375.
Less hyperemia in the upper airways of mice treated with AZM after NTHI 353 infection than in those of control mice (P < 0.05).
Decreased recruitment of alveolar macrophages during NTHI 353 infection of animals treated with AZM postinfection compared to that of controls (P < 0.0005).
In sum, NTHI 375 and NTHI 353 are two pathogenic isolates with different susceptibilities to AZM that are able to infect cultured respiratory cells and the airways of mice, and these strains were used to assess AZM efficacy in this work.
AZM efficacy against NTHI infection of airway epithelial cells.
We first evaluated the impact of AZM on the interface between NTHI and airway epithelial cells. A549 human type II pneumocytes were pretreated with 50 μg/ml AZM, an AZM dose previously shown to be immunomodulatory by inhibiting NTHI-induced MUC5A expression and secretion (53). AZM was removed before infection, and NTHI 375 adhesion and invasion levels were quantified. NTHI 375 adhesion levels to A549 cells were comparable for control and AZM-pretreated cells (Fig. 1A). In contrast, cell pretreatment with 50 μg/ml AZM reduced the number of intracellular NTHI 375 organisms recovered from A549 cells (P < 0.005) (Fig. 1B). Similar effects were observed when cells were pretreated with 25 μg/ml (P < 0.01) or 12.5 μg/ml (P < 0.05) AZM (Fig. 1B). Controls (CON) consisted of cells that did not receive AZM (adhesion assays) and cells treated with gentamicin that did not receive AZM (invasion assays).
Given that the AZM doses used above have been shown to be immunomodulatory (53), we asked if the observed reduced NTHI 375 invasion could be related to the impact of AZM on host cell signaling. We showed previously that NTHI infection triggers IL-8 secretion by A549 cells (54, 55). We sought to determine the effect of pretreatment with 50 μg/ml AZM on IL-8 secretion by infected A549 cells. NTHI 375 induced secretion of comparable IL-8 levels by untreated and AZM-pretreated cells. As a nonbactericidal anti-inflammatory control, we employed the glucocorticoid dexamethasone (DEX), which decreased the amount of IL-8 secreted by NTHI 375-infected cells to the level displayed by control noninfected cells (Fig. 1C).
Although they were removed prior to infection in this study, macrolides are likely to penetrate epithelial cells (41, 42, 45), and the AZM doses used above are higher than the NTHI 375 AZM MIC (MICAZM). Thus, we asked if the observed reduced NTHI 375 invasion could relate to a bactericidal effect of AZM against intracellular bacteria. A549 cell infection with NTHI 375 was performed in culture medium without supplementation of any antibiotic, followed by subsequent incubation in fresh medium supplemented with gentamicin to kill extracellular bacteria and without (CON) or with AZM to assess intracellular killing by AZM. AZM was effective at reducing (P < 0.001) the number of intracellular bacteria (Fig. 1D).
To assess the relationship between AZM's effect and the susceptibility of the infecting NTHI strain, we next quantified A549 cell adhesion and invasion for NTHI 353. Cell pretreatment with AZM at 12.5, 25, or 50 μg/ml did not modify NTHI 353 cell adhesion or invasion levels compared to those for control cells not receiving AZM (Fig. 1E and F). However, when A549 cell infection with NTHI 353 was performed and followed by subsequent incubation in fresh medium supplemented with gentamicin and AZM, AZM was effective (P < 0.0005) at reducing the number of intracellular bacteria (Fig. 1G).
This AZM effect against NTHI could also be extended to NCI H-292 bronchoepithelial cells. Pretreatment with AZM at 50 μg/ml significantly (P < 0.0001) reduced NTHI 375 invasion but did not modify NTHI 353 invasion compared to that in control cells. In contrast, addition of AZM at 50 μg/ml during gentamicin incubation reduced both NTHI 375 and NTHI 353 intracellular counts (P < 0.0001 and P < 0.005, respectively) (see Fig. S1 in the supplemental material).
We and others previously described that NTHI invades epithelial cells and localizes to an NTHI-containing vacuole (NTHI-CV), a nonproliferative compartment with late endosome features (51, 57, 58). Given that macrolides present good penetration of lung tissue, are acid resistant, and accumulate in lysosome-like compartments (11), we asked if AZM could modulate NTHI intracellular compartmentalization. We determined the percentage of intracellular bacteria that colocalized with the EEA1 or LAMP1 endocytic marker. Table S1 in the supplemental material shows the means ± SD of the percentages of bacterium-marker association. We did not detect significant differences between EEA1- or LAMP1-bacterium colocalization levels between control and AZM-treated infected cells. At 15 min post-gentamicin treatment, a tendency toward an increased percentage of EEA1-NTHI 375 colocalization in AZM-treated cells was apparent but did not reach statistical significance.
In summary, these results suggest a bactericidal effect of AZM on NTHI 375 and NTHI 353 organisms inside epithelial cells. This effect may relate to the AZM susceptibility of the infecting strain. Similar observations were obtained for A549 and NCI H-292 cells.
Intracellular bacteriological effects of various AZM concentrations on NTHI epithelial infection.
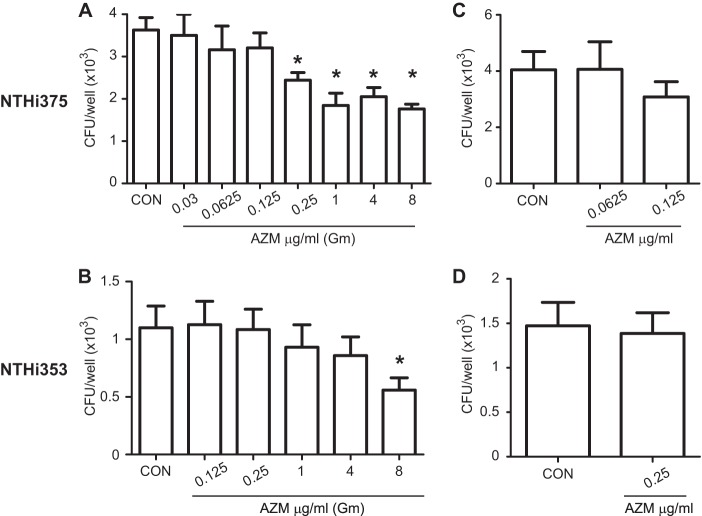
An AZM concentration higher than the MICs of strains NTHI 375 and NTHI 353 was used in the assays described above and showed an intracellular bacteriological effect on epithelial cells. Note that an assessment of the intracellular bactericidal effect of clarithromycin (CLR) against NTHI inside NCI H-292 bronchoepithelial cells previously revealed that CLR reduced the number of viable bacteria at less than half the MIC (45). Considering this evidence, we hypothesized that the bactericidal effect of AZM against intracellular NTHI may relate to the antibiotic dose used and to the MIC of the infecting strain. We compared the intracellular bacteriological effects at various AZM concentrations for both NTHI 375 and NTHI 353. As shown in Fig. 2A, AZM significantly reduced the number of intracellular viable NTHI 375 organisms at AZM concentrations corresponding to the NTHI 375 MIC (P < 0.05) and higher (for 1 μg/ml AZM, P < 0.001; for 4 μg/ml AZM, P < 0.005; and for 8 μg/ml AZM, P < 0.0005). AZM also significantly reduced the number of intracellular NTHI 353 organisms when an AZM dose corresponding to the NTHI 353 MIC was used (P < 0.05) (Fig. 2B). AZM concentrations lower than the NTHI 375 or NTHI 353 MIC did not significantly reduce intracellular bacterial counts.
FIG 2.
Intracellular bactericidal effects of various AZM concentrations against NTHI infection of A549 cells. Controls were as follows: cells were treated with gentamicin but did not receive AZM. (A) A549 cells were infected with NTHI 375. Various AZM concentrations were added during infected cell incubation with gentamicin. The amount of intracellular NTHI 375 decreased significantly upon treatment with AZM at 1×, 4×, 16×, and 32× MIC (P < 0.05, P < 0.001, P < 0.005, and P < 0.0005, respectively) compared to that in control (CON) cells. (B) A549 cells were infected with NTHI 353. Various AZM concentrations were added during infected cell incubation with gentamicin. Intracellular viable bacterial numbers decreased significantly when NTHI 353 was treated with AZM at 1× MIC (P < 0.05). (C) Effect of A549 cell pretreatment with two AZM concentrations unable to kill intracellular NTHI 375 (0.0625 and 0.125 μg/ml). AZM was removed, and infection was performed in AZM-free cell culture medium. Mean numbers for NTHI 375 entry into AZM-pretreated cells were similar to those obtained for control cells. (D) Effect of A549 cell pretreatment with AZM at 0.25 μg/ml, a concentration unable to kill intracellular NTHI 353. AZM was removed, and infection was performed in AZM-free cell culture medium. Mean numbers for NTHI 353 entry into AZM-pretreated cells were similar to those obtained for control cells.
To further support the notion of a bactericidal effect of AZM on intracellular NTHI, we pretreated A549 cells with AZM at concentrations unable to reduce intracellular bacterial loads and evaluated bacterial invasion. For NTHI 375, cells pretreated with AZM at 0.0625 and 0.125 μg/ml did not reduce bacterial invasion (Fig. 2C). For NTHI 353, cells pretreated with AZM at 0.25 μg/ml did not decrease the intracellular bacterial counts (Fig. 2D). In agreement, NCI H-292 cell pretreatment with the highest AZM concentrations unable to reduce intracellular bacterial loads in A549 cells rendered NTHI 375 and NTHI 353 invasion rates similar to those obtained for control cells (see Fig. S1 in the supplemental material).
Together, these data indicate that the effect of AZM on intracellular bacteria depends on the AZM dose and the bacterial MIC, with AZM being able to reduce intracellular NTHI inside respiratory epithelial cells when used at levels corresponding to the MIC of the infecting strain.
Effect of AZM on NTHI alveolar macrophage infection.
AZM has been shown to have a short time to achieve peak concentration, with a high level of accumulation in phagocytes (14, 46), and to improve macrophage phagocytic uptake of bronchial epithelial cells and heat-killed nonpathogenic bacteria (26–28). We assessed the effect of AZM on NTHI infection of MH-S alveolar macrophages. As described above, MH-S cells were first pretreated with 50 μg/ml AZM, which was removed prior to infection by NTHI 375 or NTHI 353. Phagocytosis quantification showed a reduction of counts for both strains in AZM-pretreated compared to control cells (P < 0.005 for NTHI 375 and P < 0.0005 for NTHI 353). A bacterial decrease was also observed for cells infected by NTHI 375 or NTHI 353 when AZM was added during the gentamicin incubation time (P < 0.0005) (Fig. 3A and B). As indicated above, controls (CON) consisted of cells treated with gentamicin that did not receive AZM.
To further explore this observation, together with the previously described improvement of macrophage phagocytic function due to AZM use (26–28), we pretreated MH-S cells with AZM at concentrations unable to reduce intracellular bacterial loads and then evaluated bacterial phagocytosis. The amounts of NTHI 375 phagocytosis by cells pretreated with AZM at 0.125 and 0.0625 μg/ml, i.e., concentrations that do not modify bacterial counts when added at the onset of gentamicin treatment, were higher than those for control cells (P < 0.01 and P < 0.05, respectively) (Fig. 3C). For NTHI 353, when cells were pretreated with AZM at 0.0625, 0.125, and 0.25 μg/ml, i.e., doses unable to modify bacterial counts when added during gentamicin incubation, phagocytosis was similar to that obtained for untreated cells (data not shown). The MH-S phagocytic rate for NTHI 353 was approximately twice that observed for NTHI 375 (see control data in Fig. 3A and B). This observation prompted us to hypothesize that our standard phagocytosis assay is not sensitive enough to reveal a potential effect of AZM on macrophage phagocytic function toward NTHI 353. Following this notion, we lowered the NTHI 353 infecting dose to an MOI of ∼12:1 and observed that NTHI 353 phagocytosis by cells pretreated with 0.25 μg/ml AZM was higher (P < 0.0001) than that by control cells (Fig. 3D).
In conclusion, these results show that AZM has a bactericidal effect on bacteria inside alveolar macrophages. Moreover, AZM concentrations unable to kill intracellular NTHI may enhance alveolar macrophage phagocytic function and therefore increase the NTHI uptake rate.
Bactericidal and anti-inflammatory effects of AZM administration on mouse pulmonary infection with NTHI.
Finally, we sought to determine the impact of oral administration of AZM in vivo by using a mouse model system of NTHI respiratory infection. First, we used different single-dose regimens of oral AZM (100 mg/kg) before infection with NTHI 375, or we used a combined regimen (consisting of three AZM administrations prior to infection plus one at 6 hpi). The results indicated that only the combined AZM regimen was effective against NTHI 375 lung infection in mice (P < 0.01) (see Fig. S2 in the supplemental material). Accordingly, a second experiment was conducted separately with strains NTHI 375 and NTHI 353, using (i) a combined AZM regimen before (−24, −12, and −1 h) infection to mimic the prophylactic use of AZM and (ii) a single AZM treatment at 6 hpi to mimic its therapeutic use. AZM was effective against infection by the AZM-susceptible strain NTHI 375 (Fig. 4A and B) but not against the AZM-resistant strain NTHI 353 (Fig. 4C and D). Thus, both AZM regimens provided significant reductions of NTHI 375 pulmonary infection compared to the case for control mice (for the regimen prior to infection, P < 0.0001; and for the postinfection regimen, P < 0.05) (Fig. 4A), suggesting not only a therapeutic but also a prophylactic effect of AZM against NTHI 375 infection. Fewer NTHI 375 bacteria were also recovered from the BALF of mice treated with AZM; when mice received AZM before NTHI 375 infection, this decrease was significant (P < 0.005) compared to controls (Fig. 4B).
Microscopy scores of average histopathological lesions in samples from mice infected with NTHI 375 or NTHI 353 were also determined along the respiratory tract and compared for untreated and AZM-treated mice. Lungs and airways from control mice instilled with PBS did not show significant inflammation or pathological changes, independently of the absence or presence of AZM. Comparative analysis of scored lesions in upper and lower airways of mice infected with NTHI 375 showed lower inflammatory reaction scores for AZM-treated mice, especially those treated before the infection (Fig. 5). This reduction of inflammatory lesions was significant for bronchial PMNs in both mice treated with AZM preinfection and those treated postinfection (P < 0.0005) and for lung hemorrhage in mice treated with AZM postinfection (P < 0.05) (Table 1). Conversely, we observed significantly larger numbers of PMNs (for mice treated pre- or postinfection [P < 0.01]) and alveolar macrophages (for mice treated postinfection [P < 0.05]) at the alveoli of AZM-treated animals than at those of controls infected by NTHI 375 (Table 1). We also observed a dampening effect of AZM on respiratory inflammatory lesions in mice infected by NTHI 353, which, in general terms, were milder than those observed in NTHI 375-infected animals. We observed significantly (P < 0.05) less hyperemia in the upper airways of mice treated with AZM postinfection than in those of control mice, and the recruitment of alveolar macrophages during NTHI 353 infection was decreased in these AZM-treated animals (P < 0.0005) compared to controls (Table 1).
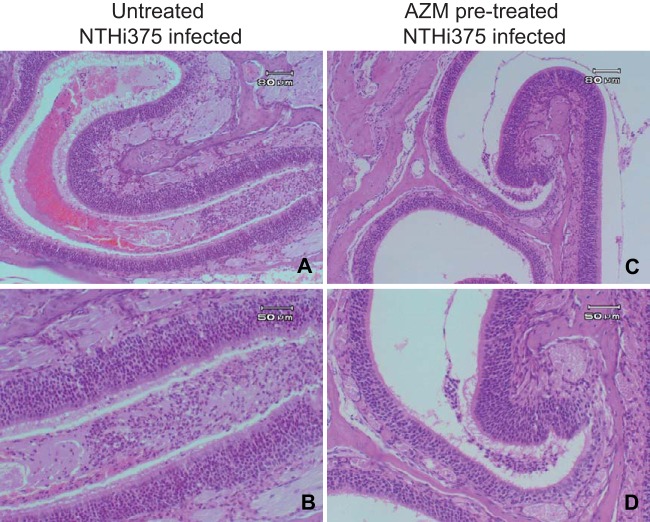
FIG 5.
Effect of AZM on histopathological findings in upper airways of mice infected intranasally with NTHI 375. Tissue sections were stained with H&E. (A) Severe lesions in upper airway from an untreated mouse infected with NTHI 375. The lumen of the turbinate is fully filled with exudate. (B) Detail of the lumen, showing serous exudate, erythrocytes, neutrophils, and scanty detached epithelial cells. (C) Mild lesions in upper airway from an AZM-pretreated mouse infected with NTHI 375, showing small amounts of seropurulent luminal exudate. (D) Detail of the exudate from panel C.
In sum, these results indicate that administration of three prophylactic doses of AZM at 100 mg/kg facilitates pulmonary clearance of the AZM-susceptible strain NTHI 375 but not of the AZM-resistant isolate NTHI 353. The same effect was observed for the use of one therapeutic dose of AZM (100 mg/kg). AZM administration reduced NTHI-triggered inflammation to different extents, depending on the infecting strain, with this anti-inflammatory effect being more prominent in NTHI 375-infected than NTHI 353-infected animals. These observations suggest a link between the antimicrobial and anti-inflammatory effects of AZM on NTHI respiratory infection, which may be modulated by the MICAZM of the infecting strain.
DISCUSSION
This study delineates the effect of AZM administration on respiratory infection by NTHI. By using conditions mimicking preventive or therapeutic AZM administration in cultured respiratory cell and in vivo model systems, we have established the impact of this macrolide on a number of properties related to NTHI pathogenesis in the respiratory tract. NTHI is largely associated with chronic infection in COPD patients and with AECOPD frequency (4), and clinical evidence suggests a potential for AZM in the prevention and management of AECOPD (32). Despite its potential benefit, AZM seems to increase carriage of macrolide-resistant bacteria, thereby generating doubts about long-term administration of AZM (32, 34). Based on the clinical evidence, understanding the impact of AZM on infection by NTHI will inform potential changes in clinical guidelines. Moreover, given that long-term low-dose preventive administration of AZM is under consideration, it is important to determine the impacts of both prophylactic and therapeutic uses of AZM on NTHI infection. Our systematic use of in vitro and in vivo model systems allowed us to comprehensively compare AZM's effects on infection by an AZM-susceptible and an AZM-resistant NTHI clinical isolate and to establish associations between AZM efficacy, dose, bacterial MIC, and bactericidal/immunomodulatory properties.
At the cellular level, our results show a bactericidal effect of AZM on intracellular NTHI, possibly related to AZM's ability to penetrate tissues and cells. This is an interesting notion regarding NTHI infection treatment, given that NTHI invades epithelial cells and can persist intracellularly (51, 58). An antimicrobial effect on intracellular bacteria was observed for NTHI 375 and NTHI 353 and was related to the AZM dose and the MIC of the infecting strain. The efficacy of AZM to clear intracellular NTHI was previously compared to those of a whole range of different antibiotic treatments (41, 42). To our knowledge, this is the first report of AZM's efficacy on intracellular NTHI where both AZM doses and the MIC of the infecting strain have been considered systematically. CLR's intracellular bacteriological effects at various antibiotic concentrations were previously evaluated for a CLR-resistant NTHI strain, establishing that CLR could reduce the number of bacteria inside NCI H-292 cells at less than half the MIC (45). In the present study, we observed a correlation between a significant reduction of bacteria inside A549 cells and the MIC of AZM for both NTHI 375 and NTHI 353. A comparison between previous CLR (45) and current AZM data suggests an advantageous use of CLR, which is able to reduce intracellular bacterial counts at less than half the MIC, compared to AZM, which is able to do so at a dose close to the strain MIC. Overall, the data indicate that macrolides present the potential to eradicate intracellular susceptible and resistant NTHI, and this ability may contribute to preventing persistent and recurrent infections by this pathogen.
Besides its antimicrobial effect, AZM has been shown to have additional properties on host cells. First, AZM is a lysosomotropic antibiotic, whose accumulation is associated with a vacuolation of endosomes in macrophages and fibroblasts (59, 60). Our immunofluorescence-based analysis did not reveal significant differences in the percentage of NTHI-EEA1/LAMP1 colocalization in A549 cells, limiting a possible AZM-triggered modulation of NTHI's intracellular location. Second, AZM has been shown to suppress inflammation in a range of cell model systems (20, 21, 23–25, 29, 30, 61). Moreover, AZM suppresses inflammation induced by Pseudomonas aeruginosa (62), Fusobacterium nucleatum (63), and Klebsiella pneumoniae (64). Although AZM has been suggested to dampen inflammation during NTHI infection (53, 65), it should be noted that these two studies were carried out by stimulating host cells with bacterial extracts, which may differ substantially from using viable bacteria. We did not observe any modification in the level of NTHI-triggered IL-8 secreted by AZM-treated A549 cells compared to untreated cells. However, our results may not exclude AZM-driven modulation of other inflammatory parameters upon NTHI cell infection. Third, AZM has been shown to improve macrophage phagocytic function of bronchial epithelial cells and heat-killed nonpathogenic bacteria (26–28). In agreement, we observed that cell pretreatment with sublethal AZM doses may also improve macrophage phagocytic function to ingest NTHI. These data should be considered in the context of the COPD patient, given that several studies have reported alterations in alveolar macrophage activity in COPD patients (66–69). These alterations include defective immune responsiveness in terms of diminished IL-8, tumor necrosis factor alpha (TNF-α), and IL-1β responses (69), impaired phagocytosis (66, 68), and dysfunctional innate responses associated with impaired Toll-like receptors (TLRs) (67).
AZM also displayed efficacy by reducing bacterial loads in lungs and BALF during mouse respiratory infection when administered before and after intranasal NTHI administration. This bacterial clearing effect was shown to correlate with the MIC of AZM of the infecting strain and with the number of AZM doses administered. To our knowledge, this is the first report of efficacy for AZM administered prior to infection, mimicking its prophylactic use. AZM's therapeutic potential has been proven previously in mouse and rat models by using different AZM-susceptible NTHI isolates and respiratory infection routes (43, 44, 46, 70). We showed here that under the conditions tested, AZM treatment did not reduce lung and BALF bacterial loads of the AZM-resistant isolate NTHI 353, indicating that AZM efficacy relates to the susceptibility of the infecting strain. Given the observed increasing carriage rates of macrolide-resistant bacteria after AZM administration, this observation should be taken into consideration by clinicians.
Besides their bactericidal efficacy, evidence suggests that macrolides present immunomodulatory properties in vivo. AZM prevents the progression of lung inflammation in Acinetobacter baumannii-infected mice, without having an antimicrobial effect (71), and alters the macrophage phenotype and pulmonary compartmentalization during P. aeruginosa lung infection (72). Moreover, CLR displays efficacy against an intubation model of induced pneumonia caused by a CLR-resistant NTHI strain in mice, with reductions of bacterial loads and inflammation in CLR-treated animals (45). Histopathological examination revealed a reduction of inflammatory lesions in NTHI-infected animals treated with AZM, to different extents, depending on the infecting strain. Differences between strains may relate to the differential bactericidal effect of the macrolide. AZM caused a reduction of NTHI 375 loads, which may have contributed to the observed reduced inflammatory lesions due to the lowering of the inflammatory stimulus. However, AZM did not reduce NTHI 353 loads, which may have contributed to keeping inflammatory lesions due to the maintenance of the inflammatory stimulus. Differences between strains may also relate to the observed slight inflammatory differences between them. NTHI 375-triggered lesions were more severe in the upper airways, and NTHI 353-triggered lesions were more severe in the lungs, which may relate to differences in their pathological origins, i.e., otitis and COPD, respectively. Even though NTHI isolates were chosen as representative of AZM resistance and sensitivity, we acknowledge that this inflammatory difference may be a limitation of the present study. Surprisingly, AZM increased the number of PMNs and macrophages in the alveoli of NTHI 375-infected animals. We speculate that AZM's bactericidal efficacy against NTHI 375 reduces lung and BALF bacterial loads, facilitating the exposure/release of pathogen-associated molecular patterns (PAMPs) and the subsequent recruitment of phagocytes. AZM also seems to increase phagocytic function by phagocytes, which would amplify bacterial clearance and, ultimately, further reduce bacterial counts. Together, the data show that AZM displays efficacy against mouse respiratory infection by an AZM-sensitive NTHI strain, causing reductions of bacterial loads and inflammation, which aligns with observations made for NTHI and CLR (45). Moreover, AZM mildly prevents the progression of lung inflammation in NTHI 353-infected mice, without having an antimicrobial effect, aligning with observations made for A. baumannii and AZM (71). AZM's anti-inflammatory effect on infected mice supports the notion of a potential benefit of this antibiotic on respiratory patients with inflammatory disorders, added to its bactericidal effect dependent on the MIC of AZM of the infecting strain.
Clinical studies have suggested that long-term low-dose AZM used to prevent and/or manage chronic inflammatory airway disorders, such as AECOPD, may be beneficial but also may increase the rate of macrolide resistance by colonizing opportunistic pathogens. In fact, a recent meta-analysis warns of the adverse effects of long-term AZM use in patients with chronic lung diseases (73). Interestingly, the use of AZM for AECOPD prevention is currently under debate, but without a comprehensive understanding of the effect of AZM on the progression of respiratory infection by bacterial pathogens frequently associated with AECOPD. The present study sheds light on this matter by evaluating the effect of AZM on infection by NTHI, considering the relationship between antibiotic dose and the MIC of the infecting strain, and shows that AZM's efficacy against NTHI respiratory infection very much relates to the MIC of the infecting strain. In conclusion, AZM has the potential to be used as an interference strategy to abolish NTHI-host interplay, which would limit NTHI adaptation, therefore preventing colonization and/or pathogenicity.
Supplementary Material
ACKNOWLEDGMENTS
J.M. was funded by Ph.D. studentship BES-2013-062644 from the Ministerio Economía y Competitividad (MINECO), Spain. This work was funded by grants from MINECO (grant SAF2012-31166) and the Departamento Salud Gobierno Navarra (grant 359/2012) to J.G. CIBERES is an initiative from ISCIII, Spain.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04447-14.
REFERENCES
- 1.Agrawal A, Murphy TF. 2011. Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. J Clin Microbiol 49:3728–3732. doi: 10.1128/JCM.05476-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agustí A, Barnes PJ. 2012. Update in chronic obstructive pulmonary disease 2011. Am J Respir Crit Care Med 185:1171–1176. doi: 10.1164/rccm.201203-0505UP. [DOI] [PubMed] [Google Scholar]
- 3.Cosio MG, Saetta M, Agustí A. 2009. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med 360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 4.Sethi S. 2011. Molecular diagnosis of respiratory tract infection in acute exacerbations of chronic obstructive pulmonary disease. Clin Infect Dis 52(Suppl 4):S290–S295. doi: 10.1093/cid/cir044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi S. 2010. Infection as a comorbidity of COPD. Eur Respir J 35:1209–1215. doi: 10.1183/09031936.00081409. [DOI] [PubMed] [Google Scholar]
- 6.Anzueto A. 2010. Impact of exacerbations on COPD. Eur Respir J 19:113–118. doi: 10.1183/09059180.00002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celli BR, Barnes PJ. 2007. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J 29:1224–1238. doi: 10.1183/09031936.00109906. [DOI] [PubMed] [Google Scholar]
- 8.Agusti A, Vestbo J. 2011. Current controversies and future perspectives in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 184:507–513. doi: 10.1164/rccm.201103-0405PP. [DOI] [PubMed] [Google Scholar]
- 9.Mammen MJ, Sethi S. 2012. Macrolide therapy for the prevention of acute exacerbations in chronic obstructive pulmonary disease. Pol Arch Med Wewn 122:54–59. [PubMed] [Google Scholar]
- 10.Matera MG, Calzetta L, Rinaldi B, Cazzola M. 2012. Treatment of COPD: moving beyond the lungs. Curr Opin Pharmacol 12:315–322. doi: 10.1016/j.coph.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Parnham MJ, Haber VE, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. 2014. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 143:225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Blumer JL. 2005. Evolution of a new drug formulation: the rationale for high-dose, short-course therapy with azithromycin. Int J Antimicrob Agents 26(Suppl 3):S143–S147. doi: 10.1016/S0924-8579(05)80320-6. [DOI] [PubMed] [Google Scholar]
- 13.Foulds G, Shepard RM, Johnson RB. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother 25(Suppl A):73–82. doi: 10.1093/jac/25.suppl_A.73. [DOI] [PubMed] [Google Scholar]
- 14.Girard AE, Girard D, English AR, Gootz TD, Cimochowski CR, Faiella JA, Haskell SL, Retsema JA. 1987. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother 31:1948–1954. doi: 10.1128/AAC.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Retsema J, Fu W. 2001. Macrolides: structures and microbial targets. Int J Antimicrob Agents 18(Suppl 1):S3–S10. doi: 10.1016/S0924-8579(01)00401-0. [DOI] [PubMed] [Google Scholar]
- 16.Blasi F, Mantero M, Aliberti S. 2012. Antibiotics as immunomodulant agents in COPD. Curr Opin Pharmacol 12:293–299. doi: 10.1016/j.coph.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Giamarellos-Bourboulis EJ. 2008. Macrolides beyond the conventional antimicrobials: a class of potent immunomodulators. Int J Antimicrob Agents 31:12–20. doi: 10.1016/j.ijantimicag.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Steel HC, Theron AJ, Cockeran R, Anderson R, Feldman C. 2012. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Med Inflamm 2012:584262. doi: 10.1155/2012/584262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asgrimsson V, Gudjonsson T, Gudmundsson GH, Baldursson O. 2006. Novel effects of azithromycin on tight junction proteins in human airway epithelia. Antimicrob Agents Chemother 50:1805–1812. doi: 10.1128/AAC.50.5.1805-1812.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aghai ZH, Kode A, Saslow JG, Nakhla T, Farhath S, Stahl GE, Eydelman R, Strande L, Leone P, Rahman I. 2007. Azithromycin suppresses activation of nuclear factor-κB and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr Res 62:483–488. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 21.Cigana C, Assael BM, Melotti P. 2007. Azithromycin selectively reduces TNF-α levels in cystic fibrosis airway epithelial cells. Antimicrob Agents Chemother 51:975–981. doi: 10.1128/AAC.01142-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desaki M, Okazaki H, Sunazuka T, Omura S, Yamamoto K, Takizawa H. 2004. Molecular mechanisms of anti-inflammatory action of erythromycin in human bronchial epithelial cells: possible role in the signaling pathway that regulates nuclear factor-κB activation. Antimicrob Agents Chemother 48:1581–1585. doi: 10.1128/AAC.48.5.1581-1585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desaki M, Takizawa H, Ohtoshi T, Kasama T, Kobayashi K, Sunazuka T, Omura S, Yamamoto K, Ito K. 2000. Erythromycin suppresses nuclear factor-κB and activator protein-1 activation in human bronchial epithelial cells. Biochem Biophys Res Commun 267:124–128. doi: 10.1006/bbrc.1999.1917. [DOI] [PubMed] [Google Scholar]
- 24.Shinkai M, Foster GH, Rubin BK. 2006. Macrolide antibiotics modulate ERK phosphorylation and IL-8 and GM-CSF production by human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 290:L75–L85. doi: 10.1152/ajplung.00093.2005. [DOI] [PubMed] [Google Scholar]
- 25.Takizawa H, Desaki M, Ohtoshi T, Kawasaki S, Kohyama T, Sato M, Tanaka M, Kasama T, Kobayashi K, Nakajima J, Ito K. 1997. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med 156:266–271. doi: 10.1164/ajrccm.156.1.9612065. [DOI] [PubMed] [Google Scholar]
- 26.Hodge S, Hodge G, Brozyna S, Jersmann H, Holmes M, Reynolds PN. 2006. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur Respir J 28:486–495. doi: 10.1183/09031936.06.00001506. [DOI] [PubMed] [Google Scholar]
- 27.Hodge S, Hodge G, Jersmann H, Matthews G, Ahern J, Holmes M, Reynolds PN. 2008. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178:139–148. doi: 10.1164/rccm.200711-1666OC. [DOI] [PubMed] [Google Scholar]
- 28.Hodge S, Reynolds PN. 2012. Low-dose azithromycin improves phagocytosis of bacteria by both alveolar and monocyte-derived macrophages in chronic obstructive pulmonary disease subjects. Respirology 17:802–807. doi: 10.1111/j.1440-1843.2012.02135.x. [DOI] [PubMed] [Google Scholar]
- 29.Khan AA, Slifer TR, Araujo FG, Remington JS. 1999. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int J Antimicrob Agents 11:121–132. doi: 10.1016/S0924-8579(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi T, Hagiwara K, Honda Y, Gomi K, Kobayashi T, Takahashi H, Tokue Y, Watanabe A, Nukiwa T. 2002. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-κB transcription factors. J Antimicrob Chemother 49:745–755. doi: 10.1093/jac/dkf008. [DOI] [PubMed] [Google Scholar]
- 31.Suzaki H, Asano K, Ohki S, Kanai K, Mizutani T, Hisamitsu T. 1999. Suppressive activity of a macrolide antibiotic, roxithromycin, on pro-inflammatory cytokine production in vitro and in vivo. Med Inflamm 8:199–204. doi: 10.1080/09629359990351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JAD, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, Make B, Marchetti N, Martinez FJ, Madinger NE, McEvoy C, Niewoehner DE, Porsasz J, Price CS, Reilly J, Scanlon PD, Sciurba FC, Scharf SM, Washko GR, Woodruff PG, Anthonisen NR. 2011. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berkhof FF, Doornewaard-ten Hertog NE, Uil SM, Kerstjens HA, van den Berg JW. 2013. Azithromycin and cough-specific health status in patients with chronic obstructive pulmonary disease and chronic cough: a randomised controlled trial. Respir Res 14:125. doi: 10.1186/1465-9921-14-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hare KM, Singleton RJ, Grimwood K, Valery PC, Cheng AC, Morris PS, Leach AJ, Smith-Vaughan HC, Chatfield M, Redding G, Reasonover AL, McCallum GB, Chikoyak L, McDonald MI, Brown N, Torzillo PJ, Chang AB. 2013. Longitudinal nasopharyngeal carriage and antibiotic resistance of respiratory bacteria in indigenous Australian and Alaska native children with bronchiectasis. PLoS One 8:e70478. doi: 10.1371/journal.pone.0070478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James GD, Petersen I, Nazareth I, Wedzicha JA, Donaldson GC. 2013. Use of long-term antibiotic treatment in COPD patients in the UK: a retrospective cohort study. Prim Care Respir J 22:271–277. doi: 10.4104/pcrj.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Reilly PJ, Jackson PL, Wells JM, Dransfield MT, Scanlon PD, Blalock JE. 2013. Sputum PGP is reduced by azithromycin treatment in patients with COPD and correlates with exacerbations. BMJ Open 3:e004140. doi: 10.1136/bmjopen-2013-004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uzun S, Djamin RS, Kluytmans J, Van't Veer NE, Ermens AA, Pelle AJ, Mulder P, van der Eerden MM, Aerts J. 2012. Influence of macrolide maintenance therapy and bacterial colonisation on exacerbation frequency and progression of COPD (COLUMBUS): study protocol for a randomised controlled trial. Trials 13:82. doi: 10.1186/1745-6215-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez FJ, Curtis JL, Albert R. 2008. Role of macrolide therapy in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 3:331–350. doi: 10.2147/COPD.S681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaya M, Azuma A, Takizawa H, Kadota J, Tamaoki J, Kudoh S. 2012. Macrolide effects on the prevention of COPD exacerbations. Eur Respir J 40:485–494. doi: 10.1183/09031936.00208011. [DOI] [PubMed] [Google Scholar]
- 40.Hotomi M, Arai J, Billal DS, Takei S, Ikeda Y, Ogami M, Kono M, Beder LB, Toya K, Kimura M, Yamanaka N. 2010. Nontypeable Haemophilus influenzae isolated from intractable acute otitis media internalized into cultured human epithelial cells. Auris Nasus Larynx 37:137–144. doi: 10.1016/j.anl.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Kratzer C, Graninger W, Macfelda K, Buxbaum A, Georgopoulos A. 2007. Comparative activities of antibiotics against intracellular non-typeable Haemophilus influenzae. Wien Klin Wochenschr 119:297–302. doi: 10.1007/s00508-007-0784-5. [DOI] [PubMed] [Google Scholar]
- 42.Sekiya Y, Eguchi M, Nakamura M, Ubukata K, Omura S, Matsui H. 2008. Comparative efficacies of different antibiotic treatments to eradicate nontypeable Haemophilus influenzae infection. BMC Infect Dis 8:15. doi: 10.1186/1471-2334-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girard D, Finegan SM, Dunne MW, Lame ME. 2005. Enhanced efficacy of single-dose versus multi-dose azithromycin regimens in preclinical infection models. J Antimicrob Chemother 56:365–371. doi: 10.1093/jac/dki241. [DOI] [PubMed] [Google Scholar]
- 44.Miyazaki S, Fujikawa T, Matsumoto T, Tateda K, Yamaguchi K. 2001. Efficacy of azithromycin, clarithromycin and β-lactam agents against experimentally induced bronchopneumonia caused by Haemophilus influenzae in mice. J Antimicrob Chemother 48:425–430. doi: 10.1093/jac/48.3.425. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura S, Yanagihara K, Araki N, Yamada K, Morinaga Y, Izumikawa K, Seki M, Kakeya H, Yamamoto Y, Kamihira S, Kohno S. 2010. Efficacy of clarithromycin against experimentally induced pneumonia caused by clarithromycin-resistant Haemophilus influenzae in mice. Antimicrob Agents Chemother 54:757–762. doi: 10.1128/AAC.00524-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallee E, Azoulay-Dupuis E, Pocidalo JJ, Bergogne-Berezin E. 1992. Activity and local delivery of azithromycin in a mouse model of Haemophilus influenzae lung infection. Antimicrob Agents Chemother 36:1412–1417. doi: 10.1128/AAC.36.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouchet V, Hood DW, Li J, Brisson JR, Randle GA, Martin A, Li Z, Goldstein R, Schweda EK, Pelton SI, Richards JC, Moxon ER. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc Natl Acad Sci U S A 100:8898–8903. doi: 10.1073/pnas.1432026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hood DW, Makepeace K, Deadman ME, Rest RF, Thibault P, Martin A, Richards JC, Moxon ER. 1999. Sialic acid in the lipopolysaccharide of Haemophilus influenzae: strain distribution, influence on serum resistance and structural characterization. Mol Microbiol 33:679–692. doi: 10.1046/j.1365-2958.1999.01509.x. [DOI] [PubMed] [Google Scholar]
- 49.CLSI. 2010. Performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI document M100-S20. Clinical Laboratory Standards Institute (CLSI), Wayne, PA. [Google Scholar]
- 50.CLSI. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6. Clinical Laboratory Standards Institute (CLSI), Wayne, PA. [Google Scholar]
- 51.Morey P, Cano V, Martí-Lliteras P, López-Gómez A, Regueiro V, Saus C, Bengoechea JA, Garmendia J. 2011. Evidence for a non-replicative intracellular stage of nontypable Haemophilus influenzae in epithelial cells. Microbiology 157:234–250. doi: 10.1099/mic.0.040451-0. [DOI] [PubMed] [Google Scholar]
- 52.Martí-Lliteras P, Regueiro V, Morey P, Hood DW, Saus C, Sauleda J, Agustí AG, Bengoechea JA, Garmendia J. 2009. Nontypeable Haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infect Immun 77:4232–4242. doi: 10.1128/IAI.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Araki N, Yanagihara K, Morinaga Y, Yamada K, Nakamura S, Yamada Y, Kohno S, Kamihira S. 2010. Azithromycin inhibits nontypeable Haemophilus influenzae-induced MUC5AC expression and secretion via inhibition of activator protein-1 in human airway epithelial cells. Eur J Pharmacol 644:209–214. doi: 10.1016/j.ejphar.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 54.Garmendia J, Viadas C, Calatayud L, Mell JC, Martí-Lliteras P, Euba B, Llobet E, Gil C, Bengoechea JA, Redfield RJ, Liñares J. 2014. Characterization of nontypable Haemophilus influenzae isolates recovered from adult patients with underlying chronic lung disease reveals genotypic and phenotypic traits associated with persistent infection. PLoS One 9:e97020. doi: 10.1371/journal.pone.0097020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morey P, Viadas C, Euba B, Hood DW, Barberán M, Gil C, Grilló MJ, Bengoechea JA, Garmendia J. 2013. Relative contributions of lipooligosaccharide inner and outer core modifications to nontypeable Haemophilus influenzae pathogenesis. Infect Immun 81:4100–4111. doi: 10.1128/IAI.00492-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.López-Gómez A, Cano V, Moranta D, Morey P, García del Portillo F, Bengoechea JA, Garmendia J. 2012. Host cell kinases, α5 and β1 integrins, and Rac1 signalling on the microtubule cytoskeleton are important for non-typable Haemophilus influenzae invasion of respiratory epithelial cells. Microbiology 158:2384–2398. doi: 10.1099/mic.0.059972-0. [DOI] [PubMed] [Google Scholar]
- 57.Clementi CF, Hakansson AP, Murphy TF. 2014. Internalization and trafficking of nontypeable Haemophilus influenzae in human respiratory epithelial cells and roles of IgA1 proteases for optimal invasion and persistence. Infect Immun 82:433–444. doi: 10.1128/IAI.00864-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clementi CF, Murphy TF. 2011. Non-typeable Haemophilus influenzae invasion and persistence in the human respiratory tract. Front Cell Infect Microbiol 1:1. doi: 10.3389/fcimb.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tyteca D, Van Der Smissen P, Mettlen M, Van Bambeke F, Tulkens PM, Mingeot-Leclercq MP, Courtoy PJ. 2002. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp Cell Res 281:86–100. doi: 10.1006/excr.2002.5613. [DOI] [PubMed] [Google Scholar]
- 60.Tyteca D, Van Der Smissen P, Van Bambeke F, Leys K, Tulkens PM, Courtoy PJ, Mingeot-Leclercq MP. 2001. Azithromycin, a lysosomotropic antibiotic, impairs fluid-phase pinocytosis in cultured fibroblasts. Eur J Cell Biol 80:466–478. doi: 10.1078/0171-9335-00180. [DOI] [PubMed] [Google Scholar]
- 61.Vanaudenaerde BM, Wuyts WA, Geudens N, Dupont LJ, Schoofs K, Smeets S, Van Raemdonck DE, Verleden GM. 2007. Macrolides inhibit IL17-induced IL8 and 8-isoprostane release from human airway smooth muscle cells. Am J Transplant 7:76–82. doi: 10.1111/j.1600-6143.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 62.Oishi K, Sonoda F, Kobayashi S, Iwagaki A, Nagatake T, Matsushima K, Matsumoto K. 1994. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect Immun 62:4145–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagaoka K, Yanagihara K, Harada Y, Yamada K, Migiyama Y, Morinaga Y, Hasegawa H, Izumikawa K, Kakeya H, Nishimura M, Kohno S. 2013. Macrolides inhibit Fusobacterium nucleatum-induced MUC5AC production in human airway epithelial cells. Antimicrob Agents Chemother 57:1844–1849. doi: 10.1128/AAC.02466-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reato G, Cuffini AM, Tullio V, Mandras N, Roana J, Banche G, Foa R, Carlone NA. 2004. Immunomodulating effect of antimicrobial agents on cytokine production by human polymorphonuclear neutrophils. Int J Antimicrob Agents 23:150–154. doi: 10.1016/j.ijantimicag.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 65.Khair OA, Devalia JL, Abdelaziz MM, Sapsford RJ, Davies RJ. 1995. Effect of erythromycin on Haemophilus influenzae endotoxin-induced release of IL-6, IL-8 and sICAM-1 by cultured human bronchial epithelial cells. Eur Respir J 8:1451–1457. [PubMed] [Google Scholar]
- 66.Berenson CS, Garlipp MA, Grove LJ, Maloney J, Sethi S. 2006. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J Infect Dis 194:1375–1384. doi: 10.1086/508428. [DOI] [PubMed] [Google Scholar]
- 67.Berenson CS, Kruzel RL, Eberhardt E, Dolnick R, Minderman H, Wallace PK, Sethi S. 2014. Impaired innate immune alveolar macrophage response and the predilection for COPD exacerbations. Thorax 69:811–818. doi: 10.1136/thoraxjnl-2013-203669. [DOI] [PubMed] [Google Scholar]
- 68.Berenson CS, Kruzel RL, Eberhardt E, Sethi S. 2013. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis 208:2036–2045. doi: 10.1093/infdis/jit400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berenson CS, Wrona CT, Grove LJ, Maloney J, Garlipp MA, Wallace PK, Stewart CC, Sethi S. 2006. Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease. Am J Respir Crit Care Med 174:31–40. doi: 10.1164/rccm.200509-1461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alder JD, Ewing PJ, Nilius AM, Mitten M, Tovcimak A, Oleksijew A, Jarvis K, Paige L, Tanaka SK. 1998. Dynamics of clarithromycin and azithromycin efficacies against experimental Haemophilus influenzae pulmonary infection. Antimicrob Agents Chemother 42:2385–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamada K, Yanagihara K, Kaku N, Harada Y, Migiyama Y, Nagaoka K, Morinaga Y, Nakamura S, Imamura Y, Miyazaki T, Izumikawa K, Kakeya H, Hasegawa H, Mikamo H, Kohno S. 2013. Azithromycin attenuates lung inflammation in a mouse model of ventilator-associated pneumonia by multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 57:3883–3888. doi: 10.1128/AAC.00457-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feola DJ, Garvy BA, Cory TJ, Birket SE, Hoy H, Hayes D Jr, Murphy BS. 2010. Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas. Antimicrob Agents Chemother 54:2437–2447. doi: 10.1128/AAC.01424-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li H, Liu DH, Chen LL, Zhao Q, Yu YZ, Ding JJ, Miao LY, Xiao YL, Cai HR, Zhang DP, Guo YB, Xie CM. 2014. Meta-analysis of the adverse effects of long-term azithromycin use in patients with chronic lung diseases. Antimicrob Agents Chemother 58:511–517. doi: 10.1128/AAC.02067-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.