Abstract
ASP9726 is an investigational echinocandin with in vitro activity against Aspergillus species. We evaluated the pharmacokinetics and efficacy of this agent in an established guinea pig model of invasive pulmonary aspergillosis. ASP9726 plasma concentrations were measured in guinea pigs administered either a single dose or multiple doses of this agent at 2.5, 5, and 10 mg/kg of body weight/day by subcutaneous injection. Immunosuppressed guinea pigs were inoculated with A. fumigatus AF293, and ASP9726 (2.5, 5, and 10 mg/kg/day), voriconazole (10 mg/kg by oral gavage twice daily), or caspofungin (3 mg/kg/day by intraperitoneal injection) was administered for 8 days. Changes in fungal burden were measured by enumerating CFU and by quantitative PCR of specimens from within the lungs, as well as by analysis of serum (1→3)-β-d-glucan and galactomannan. Lung histopathology was also evaluated. ASP9726 plasma concentrations increased in a dose-proportional manner, and the drug was well tolerated at each dose. Each dose of ASP9726, voriconazole, and caspofungin significantly reduced pulmonary fungal burden as measured by quantitative PCR and by determining (1→3)-β-d-glucan and galactomannan levels, but only voriconazole significantly reduced numbers of CFU. ASP9726 at 5 mg/kg also significantly improved survival. Histopathology demonstrated morphological changes in hyphae in animals exposed to ASP9726 and caspofungin, consistent with the activities of the echinocandins. These results suggest that ASP9726 may be efficacious for the treatment of invasive pulmonary aspergillosis.
INTRODUCTION
Invasive aspergillosis is a leading cause of morbidity and mortality in immunocompromised patients (1). In addition, increasing rates of this infection in critically ill patients not traditionally considered at high risk, including those with chronic obstructive pulmonary disease and those receiving corticosteroids, has been reported (2, 3). Despite the availability of newer broad-spectrum antifungal agents (e.g., voriconazole, posaconazole, and lipid amphotericin B formulations), rates of response to treatment are suboptimal, and significant issues with toxicity and drug interactions further limit clinical efficacy. Thus, the development of new therapeutic strategies for invasive aspergillosis is of paramount importance.
The echinocandins have been a welcome addition to the antifungal armamentarium due to their activity against a fungus-specific target, (1→3)-β-d-glucan synthase (4). Inhibition of this enzyme leads to reductions in (1→3)-β-d-glucan, a key component of the fungal cell wall. Against Aspergillus species, this leads to short, stubby hyphae with abnormal branching and growth (5). As (1→3)-β-d-glucan synthase is specific to fungi, the adverse effects and drug-drug interactions associated with the polyenes and azoles are avoided with the use of the echinocandins (6). Although clinical studies have indicated that the currently available echinocandins are effective as salvage therapy (either alone or in combination) against invasive aspergillosis (7–9), questions remain regarding their utility as monotherapy for the primary treatment of this opportunistic infection.
ASP9726 is an investigational echinocandin discovered by Astellas Pharmaceuticals (Fig. 1) (10). This agent has been shown to have potent activity against fungi that cause invasive disease in immunocompromised hosts, including Candida and Aspergillus species (11, 12). Our objective was to determine the pharmacokinetic profile and in vivo efficacy of ASP9726 as a treatment in an established guinea pig model of invasive pulmonary aspergillosis. We hypothesized that ASP9726 would achieve favorable concentrations in plasma and would be effective at improving survival and reducing tissue burden and other markers of disease burden.
FIG 1.
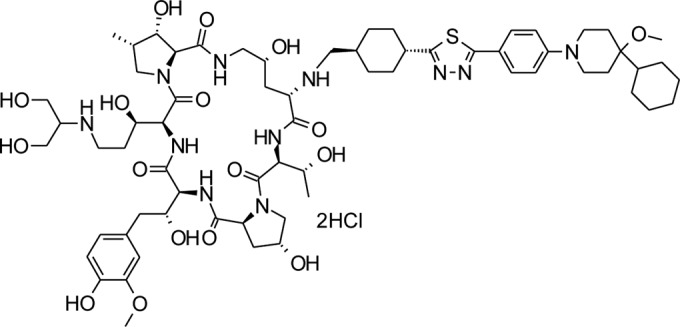
Chemical structure of ASP9726.
MATERIALS AND METHODS
Isolate.
Aspergillus fumigatus clinical isolate 293 (AF293) was grown on potato dextrose agar (PDA) at 37°C for 7 days. This isolate has been utilized by our laboratory in numerous studies and results in consistent infections in animal models (13–16). This isolate was recovered from lung tissue at autopsy from a patient with fatal invasive pulmonary aspergillosis, is also extensively used by other researchers, and was the isolate used in the A. fumigatus genome sequencing project (17). Conidia were harvested by washing and scraping agar surfaces with 0.1% Tween 80 in sterile physiological saline. Conidia were then concentrated through centrifugation and resuspended to give a final working concentration of ∼1 × 108 conidia/ml, which was measured with a hemocytometer and confirmed by enumeration of CFU. The ASP9726 minimum effective concentration against this isolate was 0.5 μg/ml, as measured by the Clinical and Laboratory Standards Institute M38-A2 guidelines (18). The minimum effective concentration of caspofungin and the MIC of voriconazole against this isolate were 0.25 μg/ml and 1 μg/ml, respectively.
Animal model.
Two days prior to infection, male Hartley guinea pigs (0.5 kg; Charles River Laboratories, Wilmington, MA) were rendered immunosuppressed with cyclophosphamide (250 mg/kg of body weight intraperitoneally; Mead Johnson, Princeton, NJ) and cortisone acetate (250 mg/kg subcutaneously; Sigma, St. Louis, MO). Additional doses of cyclophosphamide (200 mg/kg) and cortisone acetate (250 mg/kg) were administered on day 3 postinoculation (19). Enrofloxacin (7.5 mg/kg subcutaneously) was administered daily for prevention of bacterial infections. In the in vivo efficacy experiments, guinea pigs were exposed to AF293 conidia at 1 × 108 conidia/ml for 1 h in an aerosol chamber (14, 19). Guinea pigs were monitored multiple times per day for signs and symptoms of morbidity, including weight loss, hypothermia, inactivity, hyper- or hypoventilation, and hunched posture. Any animal that appeared moribund by prespecified criteria was humanely euthanized, and death was recorded as occurring the next day. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio, TX, and animals were maintained in accordance with the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care (20).
Pharmacokinetic determination.
Three doses of ASP9726 (2.5, 5, and 10 mg/kg) were evaluated in the single-dose and multiple-dose pharmacokinetic experiments. ASP9726 (in 4% hydroxypropyl-β-cyclodextrin [HPβCD] plus 0.1 M lactate buffer, pH 4.5) was administered by subcutaneous injection. In the single-dose pharmacokinetic study, blood was collected under anesthesia by cardiac puncture from two animals per dose group at various time points postadministration (1, 2, 4, 8, and 24 h). In multiple-dose studies, guinea pigs were administered ASP9726 at the same doses listed above daily for 5 days. After administration of the last dose on day 5, blood was collected by cardiac puncture from two anesthetized animals per dose group at the same time points described for the single-dose studies. Ketamine and xylazine were used to anesthetize the animals. Plasma was collected following centrifugation of the blood samples and immediately frozen. The plasma samples were sent to Astellas for measurement of ASP9726 concentrations, which was performed using a liquid chromatography tandem mass spectrometry (LC/MS/MS) assay.
In vivo efficacy.
Guinea pigs were divided into one of six regimens beginning 1 day postinoculation (n = 8 to 16 per group): (i) placebo control (4% HPβCD plus 0.1 M lactate buffer, pH 4.5), (ii) ASP9726 at 2.5 mg/kg/day, (iii) ASP9726 at 5 mg/kg/day, (iv) ASP9726 at 10 mg/kg/day, (v) voriconazole at 10 mg/kg twice daily by oral gavage, and (vi) caspofungin at 3 mg/kg/day by intraperitoneal injection. Treatment was continued through day 8, and animals were monitored off therapy until day 12. Each group consisted of at least 8 guinea pigs, and the doses for some groups were repeated on a separate occasion to ensure the reproducibility of the results. Uninfected immunosuppressed controls were also included in each experiment. At the study endpoint, day 12, the remaining animals were humanely euthanized by intramuscular administration of ketamine and xylazine followed by exsanguination and thoracotomy.
Pulmonary fungal burden.
Tissue fungal burden was measured using enumeration of CFU in lungs from animals that succumbed to infection prior the study endpoint (days 6 through 11) and at the prespecified endpoint for survival (day 12 postinoculation). Lungs from each animal were weighed, placed into sterile saline containing gentamicin and chloramphenicol, and homogenized, and serial dilutions were prepared in sterile saline and plated onto PDA plates. The number of CFU/gram of lung tissue was determined following incubation at 37°C for 48 h (13, 19).
In addition, real-time PCR was used to measure pulmonary fungal burden, as previous studies have shown this to be an appropriate assay for quantifying fungal burden following echinocandin therapy against invasive pulmonary aspergillosis (21, 22). For quantitative real-time PCR, DNA was extracted from the lung homogenates using DNA minikits (Qiagen, Valencia, CA). Five hundred microliters of each homogenate was bead beaten at 3,200 rpm for 90 s using a bead beater homogenizer and 0.5-mm glass beads (Mini Beadbeater-16; Biospec Products, Inc., Bartlesville, OK). One hundred microliters was removed and incubated with buffer ATL and proteinase K at 55°C overnight. The samples were then processed according to the manufacturer's instructions. Quantitative reverse transcription (RT)-PCRs were performed in triplicate using an ABI Prism 7900 sequence detector system (ABI) to detect minor-groove binder probe binding. FKS1 (VIC) (Applied Biosystems/Life Technologies, Grand Island, NY) was used as a reference for comparison to the 18S ribosomal DNA (rDNA) 6-carboxyfluorescein (FAM) probe from each strain (AFKS; GenBank accession number U79728) (19, 23). Six serial 1:2 dilutions (20.0, 10.0, 5.0, 2.5, 1.25, and 0.625 ng/μl) of genomic DNA from A. fumigatus AF293 were used to generate standard curves of threshold cycle (CT) values against the log DNA concentration on each PCR plate for the FKS1 and 18S rDNA genes. CT values were determined and then converted into template quantity. After the creation of standard curves, the AF293 DNA concentration for each lung homogenate sample was determined by DNA quantification using TaqMan technology. PCR cycle numbers were plotted against the value of the 5′ fluorescence signal, and then threshold values were plotted against the number of copies of the template DNA that was used to generate standard curves (24).
(1→3)-β-d-Glucan and galactomannan.
Changes in (1→3)-β-d-glucan and galactomannan levels were also measured, as these assays are approved for the clinical diagnosis of invasive fungal infections and are accurate surrogate markers of fungal burden and survival in animal models of invasive fungal infections (15, 19). On day 8 postinoculation between 2 and 4 h after the last dose, 4 guinea pigs from each treatment group were randomly chosen and blood was collected from the saphenous vein. The serum was separated, and the surrogate markers were measured using commercially available kits (Fungitell [Associates of Cape Cod, East Falmouth, MA] and Platelia Aspergillus enzyme immunoassay [EIA] [Bio-Rad Laboratories, Hercules, CA]). For (1→3)-β-d-glucan, the mean rate of change in optical density over time was measured and the unknowns were interpolated from a standard curve. For the galactomannan assay, the optical density of each sample, positive control, negative control, and cutoff control were measured and the galactomannan index was calculated as the optical density of each sample divided by the mean cutoff for the control supplied by the manufacturer. Sera from uninfected immunosuppressed controls were used to ensure that the samples were free of contamination.
Histopathology.
Pulmonary fungal burden was also qualitatively assessed by histopathology of guinea pigs in the control group and of those administered ASP9726 and caspofungin. Lungs were collected as the animals became moribund or reached the predefined study endpoint (day 12). One-gram samples of the lungs from each of these animals were processed for determining pulmonary fungal burden, and the remainder was placed into 10% (vol/vol) neutral buffered formaldehyde. The lungs were then processed and embedded into paraffin wax. Sections of embedding tissue were stained with Gomori methamine silver (GMS) stain in order to visualize the fungal elements within the lungs.
Statistical analysis.
Survival was plotted by Kaplan-Meier analysis, and differences in the median survival times and percentages of survival between the groups were analyzed by the log rank test and the Fisher exact test, respectively. Differences in pulmonary fungal burdens and serum biomarkers among the treatment groups were assessed by analysis of variance (ANOVA) with Tukey's posttest for multiple comparisons. A P value of ≤0.05 was considered statistically significant for all comparisons. Noncompartmental pharmacokinetic analysis was used for the ASP9726 plasma concentrations.
RESULTS
Pharmacokinetics.
Results from the determination of single-dose and multiple-dose plasma ASP9726 concentrations demonstrated linear pharmacokinetics over the dosages studied. As shown in Table 1, increases in the Cmax and area under the concentration-time curve from 0 to 24 h (AUC0–24) values for ASP9726 were dose proportional. The half-lives of ASP9726 in animals that received multiple doses ranged between 25 and 32 h. Similar values were also observed for guinea pigs that received single doses of ASP9726 at 2.5 and 5 mg/kg (18.3 to 25.2 h). Interestingly, the half-lives in animals that received a single 10-mg/kg dose of this agent were markedly lower (6.7 h). However, plasma was available only from one guinea pig that received this dose at the 24-h time point. Thus, this most likely represents an outlier. ASP9726 was well tolerated at each dose level without signs or symptoms of intolerance following single or multiple doses of the agent.
TABLE 1.
Single-dose and multiple-dose plasma pharmacokinetic parameters of ASP9726a
| ASP9726 dose (mg/kg) | Single-dose PK value |
Multiple-dose PK value |
||||
|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Half-life (h) | AUC0–24 (μg · h/ml) | Cmax (μg/ml) | Half-life (h) | AUC0–24 (μg · h/ml) | |
| 2.5 | 1.33 | 18.3 | 19.1 | 1.83 | 27.5 | 27.5 |
| 5 | 2.90 | 25.2 | 48.2 | 2.80 | 25 | 50.8 |
| 10 | 5.90 | 6.7 | 78.8 | 6.82 | 32.1 | 111 |
ASP9726 was prepared in 4% HPβCD plus 0.1 M lactate buffer (pH 4.5). Single and daily doses (5 days) were administered by subcutaneous injection to immunosuppressed guinea pigs. Following the last dose, blood was collected by cardiac puncture from two animals per dose group at various time points postadministration (1, 2, 4, 8, and 24 h). Plasma was collected following centrifugation, and ASP9726 concentrations were measured by LC/MS/MS.
Survival.
Treatment with ASP9726 led to a survival advantage in this guinea pig model of invasive pulmonary aspergillosis. Median survival with daily doses of 2.5 and 5 mg/kg of ASP9726 (11 and >12 days, respectively) was significantly longer than that observed in animals that received the placebo (8.5 days; P < 0.05) (Fig. 2). In addition, the percentage of animals that survived to the study endpoint was significantly higher in the group administered ASP9726 at 5 mg/kg than in the placebo group (68.75% versus 18.75%; P = 0.0113). Neither median survival (9.5 days) nor the percent survival (31.25%) in the ASP9726 10-mg/kg group was significantly different from that of the placebo group. Similarly, no survival advantage was observed in guinea pigs that were treated with caspofungin (10 days and 50%) or voriconazole (10 days and 43.75%). There were no significant differences in survival between groups that received ASP9726 and either voriconazole or caspofungin. All guinea pigs that survived to the day 12 study endpoint appeared healthy.
FIG 2.
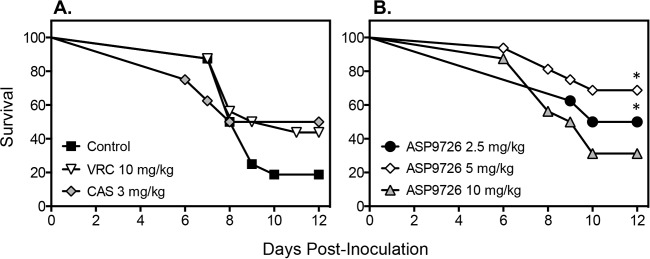
Survival in guinea pigs with invasive pulmonary aspergillosis that were treated with a placebo control (4% HPβCD plus 0.1 M lactate buffer, pH 4.5), voriconazole (VRC; 10 mg/kg twice daily by oral gavage), caspofungin (CAS; 3 mg/kg/day by intraperitoneal injection), and ASP9726 (2.5, 5, and 10 mg/kg/day by subcutaneous injection). Immunosuppressed guinea pigs were inoculated via an aerosol chamber. Treatment began 1 day postchallenge and continued until day 8. Animals were then followed off therapy until day 12. Sixteen guinea pigs were included in the groups receiving the placebo, voriconazole, and ASP9726 at 5 and 10 mg/kg, and 8 were included in the groups receiving caspofungin and ASP9726 at 2.5 mg/kg. ∗, P value < 0.05 versus the value for the placebo group.
Fungal burden and serum surrogate markers.
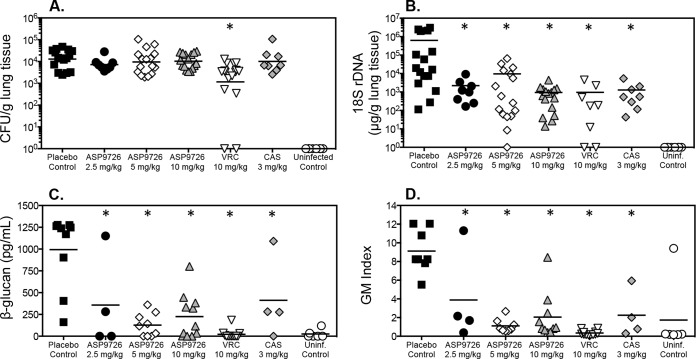
As in previous studies that evaluated echinocandin efficacy against invasive pulmonary aspergillosis, treatment with ASP9726 or caspofungin did not reduce numbers of CFU within the lungs (Fig. 3A). Mean fungal burdens with these agents ranged between 3.86 and 4.01 log10 CFU/g, compared to 4.11 log10 CFU/g in the placebo group. In contrast, pulmonary fungal burden was significantly reduced in guinea pigs treated with voriconazole (3.06 log10 CFU/g; P < 0.001). All treatments lead to significant reductions in pulmonary fungal burden when measured by quantitative real-time PCR (Fig. 3B).
FIG 3.
Pulmonary fungal burden and serum surrogate marker concentrations in guinea pigs with invasive pulmonary aspergillosis treated with the placebo control (4% HPβCD plus 0.1 M lactate buffer, pH 4.5), voriconazole (VRC; 10 mg/kg twice daily by oral gavage), caspofungin (CAS; 3 mg/kg/day by intraperitoneal injection), and ASP9726 (2.5, 5, and 10 mg/kg/day by subcutaneous injection). In vivo efficacy was measured by CFU enumeration (CFU/g) within the lungs (A), quantitative real-time PCR (18S rDNA μg/g of lung homogenate) (B), serum (1→3)-β-d-glucan (pg/ml) determination (C), and serum galactomannan determination (GM index) (D). Sixteen guinea pigs were included in the groups administered the placebo, voriconazole, and ASP9726 at 5 and 10 mg/kg, and 8 were included in the groups administered caspofungin and ASP9726 at 2.5 mg/kg. ∗, P value < 0.05 versus the value for the placebo control.
In vivo efficacy was also measured by changes in the serum surrogate markers galactomannan and (1→3)-β-d-glucan. As shown in Fig. 3C and D, serum galactomannan was significantly reduced with ASP9726. The mean galactomannan index in guinea pigs treated with this echinocandin ranged between 1.11 and 3.88, and these values were significantly lower than that measured in the placebo group (9.12; P < 0.05). Caspofungin also resulted in a similar reduction in the galactomannan index (2.25; P < 0.001). As with the pulmonary fungal burden as measured by determining the number of CFU, the lowest serum galactomannan levels were observed with voriconazole (0.34; P < 0.001). Serum (1→3)-β-d-glucan concentrations were also reduced in animals treated with ASP9726 (mean range, 127 to 358 pg/ml), caspofungin (412 pg/ml), and voriconazole (22 pg/ml) compared to concentrations in the placebo group (994 ± 424 pg/ml; P < 0.01). Serum galactomannan and (1→3)-β-d-glucan were below the thresholds for positivity [galactomannan index, 0.5, and (1→3)-β-d-glucan at 80 pg/ml] in all uninfected guinea pigs, except for one, in which the values were elevated. However, fungal burden was undetectable in this animal by enumerating CFU and by quantitative real-time PCR.
Histopathology.
Representative histopathology slides for animals administered the placebo, ASP9726 at 5 and 10 mg/kg, and caspofungin are presented in Fig. 4. In the placebo group, dichotomously branching septate hyphae characteristic of invasive tissue growth were observed. In contrast, increased fragmentation of the hyphal elements and spherical structures, representing damage to the apical hyphal tips, were observed in the lung tissues of guinea pigs treated with ASP9726 and caspofungin.
FIG 4.
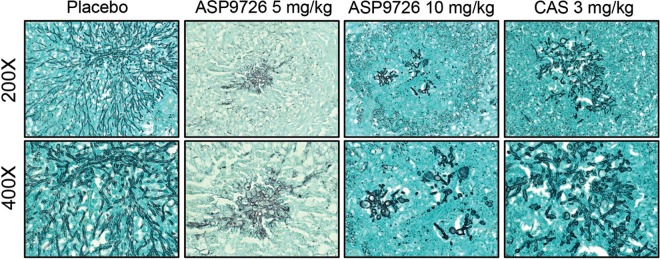
Representative lung histopathology sections in immunosuppressed guinea pigs treated with a placebo control (4% HPβCD plus 0.1 M lactate buffer, pH 4.5), ASP9726 (5 and 10 mg/kg/day by subcutaneous injection), and caspofungin (CAS; 3 mg/kg/day by intraperitoneal injection). Lung sections were stained with Gomori methamine silver stain and viewed by light microscopy in order to visualize the fungal elements within the lungs.
DISCUSSION
The echinocandins have been shown in clinical studies to be safe and effective for the treatment of invasive fungal infections. Several large clinical studies have demonstrated that members of this class are efficacious in patients with invasive candidiasis (25–28). Data for invasive aspergillosis from clinical trials regarding the use of members of this class are somewhat limited. These smaller studies have included primarily patients who received an echinocandin, either alone or in combination, as salvage therapy due to refractory infection or the inability to tolerate other antifungal agents (7, 8, 29). However, the use of these agents against invasive aspergillosis is still of interest, as the echinocandins are relatively safe and without clinically significant drug interactions compared to other antifungals (i.e., amphotericin B and the triazoles).
Our objective was to assess the in vivo efficacy of the investigational echinocandin ASP9726, a novel echinocandin with potent in vitro activity against Aspergillus spp., in an experimental model of invasive pulmonary aspergillosis. Our results demonstrate that this echinocandin was effective as monotherapy in an established guinea pig model of this invasive mycosis. Dose-proportional changes in pharmacokinetic parameters were observed following subcutaneous administration of this antifungal, and treatment with this echinocandin resulted in improvements in survival and reductions in markers of infection, including fungal burden (as measured by quantitative real-time PCR) and levels of serum galactomannan and (1→3)-β-d-glucan. Changes in fungal morphology characteristic of echinocandin exposure were also observed within the lung tissues of guinea pigs treated with ASP9726. Others have also reported improvements in survival and reductions in markers of infection in different models of invasive aspergillosis. Using a persistently neutropenic rabbit model, Petraitis et al. demonstrated improvements in survival and reductions in organism-mediated pulmonary injury and lung weights with ASP9726 treatment (11). Similarly, improvements in survival that were associated with reductions in pulmonary lesion size and hyphal invasion have been observed in a murine model of invasive aspergillosis (12).
Overall, these results are consistent with those of previous animal model studies that have used multiple outcome measures to evaluate echinocandin therapy against invasive pulmonary aspergillosis. Improvements in survival and reductions in markers of infection, including galactomannan, (1→3)-β-d-glucan, and pulmonary infarct scores, have been reported for each of the clinically available members of this class in a well-established neutropenic rabbit model of invasive pulmonary aspergillosis (30, 31). As with our results, increased hyphal fragmentation and the presence of spherical structures near the apical tips, traits consistent with the mechanisms of action of the echinocandins, have also been observed upon histopathology (22, 31).
Despite elevated plasma concentrations and significant reductions in fungal burden as measured by quantitative real-time PCR and serum galactomannan and (1→3)-β-d-glucan concentrations, treatment with the highest dose of ASP9726, 10 mg/kg, did not result in a survival advantage, as the differences in median survival times and percentages of survival were not significant compared to those of the placebo group. In contrast, significant improvements in median survival were observed in animals treated with the lower doses of ASP9726 (2.5 and 5 mg/kg). The exact reason for this is unknown, and one may speculate that this result is similar to the paradoxical effect that has been reported for other echinocandins in animal models of invasive pulmonary aspergillosis (32, 33). The lack of a survival benefit with ASP9726 at 10 mg/kg was reproducible, as this dosage group was evaluated in two separate experiments, which achieved similar results. Similarly, the survival advantage observed with ASP9726 at 5 mg/kg was also consistent in these two separate experiments. It should be noted that in this study, treatment with caspofungin also did not result in a survival advantage over that of the placebo group despite reductions in fungal burdens and serum galactomannan and (1→3)-β-d-glucan levels. However, only one dose of caspofungin was evaluated. It is also important to remember that the significance of the paradoxical effect observed in animal models is unknown, as this phenomenon has not been reported clinically. Interestingly, a survival advantage was also not observed with voriconazole despite significant reductions in all markers of infection, including fungal burden as measured by enumerating CFU. Although we did not measure voriconazole plasma concentrations or assess for toxicity, one potential explanation is a drug interaction between this triazole and the cyclophosphamide used to induce neutropenia in this model. Studies have demonstrated that a highly significant drug interaction exists between itraconazole and cyclophosphamide, resulting in renal toxicity and hepatotoxicity, although such an interaction with voriconazole has not been reported in the literature (34, 35).
Overall, our results demonstrate that the investigational echinocandin ASP9726 is efficacious against invasive pulmonary aspergillosis. The agent was well tolerated, and significant improvements in survival and reductions in various measures of infection were observed in guinea pigs that received monotherapy with this echinocandin. Additional work is warranted to further determine its potential clinical utility. These include pharmacokinetic/pharmacodynamic studies to determine the dose that maximizes in vivo outcomes as well as studies to determine the effectiveness against infections caused by other Aspergillus species.
ACKNOWLEDGMENTS
We thank Marcos Olivo for his assistance with the animal models.
This work was supported by an investigator-initiated research grant from Astellas Pharmaceuticals to T. F. Patterson and N. P. Wiederhold. N.P.W. has received research support from Astellas, Dow, F2G, Merck, Merz, and Viamet and has served on advisory boards for Merck, Astellas, Toyama, and Viamet. T.F.P. has benefited from research grants to the UT Health Science Center, San Antonio, from Astellas and Merck and has served as a consultant for Astellas, Merck, Toyama, Viamet, and Scynexis. L.K.N. has received travel support from Viamet Pharmaceuticals, Inc. S.M. is a former employee of Astellas Pharmaceuticals, Inc., and is currently an employee of LSI Medience Corporation. All other authors have no conflicts of interest to declare.
REFERENCES
- 1.Lin SJ, Schranz J, Teutsch SM. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis 32:358–366. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 2.Meersseman W, Vandecasteele SJ, Wilmer A, Verbeken E, Peetermans WE, Van Wijngaerden E. 2004. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med 170:621–625. doi: 10.1164/rccm.200401-093OC. [DOI] [PubMed] [Google Scholar]
- 3.Garnacho-Montero J, Amaya-Villar R, Ortiz-Leyba C, Leon C, Alvarez-Lerma F, Nolla-Salas J, Iruretagoyena JR, Barcenilla F. 2005. Isolation of Aspergillus spp. from the respiratory tract in critically ill patients: risk factors, clinical presentation and outcome. Crit Care 9:R191–R199. doi: 10.1186/cc3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douglas CM, D'Ippolito JA, Shei GJ, Meinz M, Onishi J, Marrinan JA, Li W, Abruzzo GK, Flattery A, Bartizal K, Mitchell A, Kurtz MB. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-beta-d-glucan synthase inhibitors. Antimicrob Agents Chemother 41:2471–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman JC, Hicks PS, Kurtz MB, Rosen H, Schmatz DM, Liberator PA, Douglas CM. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob Agents Chemother 46:3001–3012. doi: 10.1128/AAC.46.9.3001-3012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiederhold NP, Lewis RE. 2003. The echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacy. Expert Opin Invest Drugs 12:1313–1333. doi: 10.1517/13543784.12.8.1313. [DOI] [PubMed] [Google Scholar]
- 7.Marr KA, Boeckh M, Carter RA, Kim HW, Corey L. 2004. Combination antifungal therapy for invasive aspergillosis. Clin Infect Dis 39:797–802. doi: 10.1086/423380. [DOI] [PubMed] [Google Scholar]
- 8.Maertens J, Raad I, Petrikkos G, Boogaerts M, Selleslag D, Petersen FB, Sable CA, Kartsonis NA, Ngai A, Taylor A, Patterson TF, Denning DW, Walsh TJ. 2004. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis 39:1563–1571. doi: 10.1086/423381. [DOI] [PubMed] [Google Scholar]
- 9.Denning DW, Marr KA, Lau WM, Facklam DP, Ratanatharathorn V, Becker C, Ullmann AJ, Seibel NL, Flynn PM, van Burik JA, Buell DN, Patterson TF. 2006. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect 53:337–349. doi: 10.1016/j.jinf.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morikawa H, Tomishima M, Kayakiri N, Araki T, Barrett D, Akamatsu S, Matsumoto S, Uchida S, Nakai T, Takeda S, Maki K. 2014. Synthesis and antifungal activity of ASP9726, a novel echinocandin with potent Aspergillus hyphal growth inhibition. Bioorg Med Chem Lett 24:1172–1175. doi: 10.1016/j.bmcl.2013.12.116. [DOI] [PubMed] [Google Scholar]
- 11.Petraitis V, Petraitiene R, Such KA, Moradi PW, Strauss GE, Petraityte E, Fleener S, Akamatsu S, Matsumoto S, Walsh TJ. 2012. Antifungal activity, plasma pharmacokinetics and safety of second-generation echinocandin ASP9726 in experimental pulmonary aspergillosis in persistently neutropenic rabbits, abstr M-981. 52nd Intersci Conf Antimicrob Agents Chemother, San Francisco, CA. [Google Scholar]
- 12.Paderu P, Jimenez-Ortigosa C, Akamatsu S, Matsumoto S, Perlin D. 2012. Evaluation of ASP9726 on glucan synthase inhibition from echinocandin susceptible and resistant fungi (abstr F-822). 52nd Intersci Conf Antimicrob Agents Chemother, San Francisco, CA. [Google Scholar]
- 13.Sheppard DC, Graybill JR, Najvar LK, Chiang LY, Doedt T, Kirkpatrick WR, Bocanegra R, Vallor AC, Patterson TF, Filler SG. 2006. Standardization of an experimental murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 50:3501–3503. doi: 10.1128/AAC.00787-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheppard DC, Rieg G, Chiang LY, Filler SG, Edwards JE Jr, Ibrahim AS. 2004. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 48:1908–1911. doi: 10.1128/AAC.48.5.1908-1911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiederhold NP, Najvar LK, Vallor AC, Kirkpatrick WR, Bocanegra R, Molina D, Olivo M, Graybill JR, Patterson TF. 2008. Assessment of serum (1→3)-beta-d-glucan concentration as a measure of disease burden in a murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 52:1176–1178. doi: 10.1128/AAC.01425-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiederhold NP, Najvar LK, Bocanegra R, Kirkpatrick WR, Patterson TF, Thornton CR. 2013. Interlaboratory and interstudy reproducibility of a novel lateral-flow device and influence of antifungal therapy on detection of invasive pulmonary aspergillosis. J Clin Microbiol 51:459–465. doi: 10.1128/JCM.02142-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, Berriman M, Abe K, Archer DB, Bermejo C, Bennett J, Bowyer P, Chen D, Collins M, Coulsen R, Davies R, Dyer PS, Farman M, Fedorova N, Fedorova N, Feldblyum TV, Fischer R, Fosker N, Fraser A, Garcia JL, Garcia MJ, Goble A, Goldman GH, Gomi K, Griffith-Jones S, Gwilliam R, Haas B, Haas H, Harris D, Horiuchi H, Huang J, Humphray S, Jimenez J, Keller N, Khouri H, Kitamoto K, Kobayashi T, Konzack S, Kulkarni R, Kumagai T, Lafon A, Latge JP, Li W, Lord A, Lu C, Majoros WH, May GS, Miller BL, Mohamoud Y, Molina M, Monod M, Mouyna I, Mulligan S, Murphy L, O'Neil S, Paulsen I, Penalva MA, Pertea M, Price C, Pritchard BL, Quail MA, Rabbinowitsch E, Rawlins N, Rajandream MA, Reichard U, Renauld H, Robson GD, Rodriguez de Cordoba S, Rodriguez-Pena JM, Ronning CM, Rutter S, Salzberg SL, Sanchez M, Sanchez-Ferrero JC, Saunders D, Seeger K, Squares R, Squares S, Takeuchi M, Tekaia F, Turner G, Vazquez de Aldana CR, Weidman J, White O, Woodward J, Yu JH, Fraser C, Galagan JE, Asai K, Machida M, Hall N, Barrell B, Denning DW. 2005. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151–1156. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- 18.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard, 2nd ed Document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Vallor AC, Kirkpatrick WR, Najvar LK, Bocanegra R, Kinney MC, Fothergill AW, Herrera ML, Wickes BL, Graybill JR, Patterson TF. 2008. Assessment of Aspergillus fumigatus burden in pulmonary tissue of guinea pigs by quantitative PCR, galactomannan enzyme immunoassay, and quantitative culture. Antimicrob Agents Chemother 52:2593–2598. doi: 10.1128/AAC.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC. [Google Scholar]
- 21.Bowman JC, Abruzzo GK, Anderson JW, Flattery AM, Gill CJ, Pikounis VB, Schmatz DM, Liberator PA, Douglas CM. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob Agents Chemother 45:3474–3481. doi: 10.1128/AAC.45.12.3474-3481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiederhold NP, Kontoyiannis DP, Chi J, Prince RA, Tam VH, Lewis RE. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J Infect Dis 190:1464–1471. doi: 10.1086/424465. [DOI] [PubMed] [Google Scholar]
- 23.Costa C, Vidaud D, Olivi M, Bart-Delabesse E, Vidaud M, Bretagne S. 2001. Development of two real-time quantitative TaqMan PCR assays to detect circulating Aspergillus fumigatus DNA in serum. J Microbiol Methods 44:263–269. doi: 10.1016/S0167-7012(01)00212-3. [DOI] [PubMed] [Google Scholar]
- 24.Herrera ML, Vallor AC, Gelfond JA, Patterson TF, Wickes BL. 2009. Strain-dependent variation in 18S ribosomal DNA copy numbers in Aspergillus fumigatus. J Clin Microbiol 47:1325–1332. doi: 10.1128/JCM.02073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora-Duarte J, Betts R, Rotstein C, Colombo AL, Thompson-Moya L, Smietana J, Lupinacci R, Sable C, Kartsonis N, Perfect J, Caspofungin Invasive Candidiasis Study Group. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 347:2020–2029. doi: 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- 26.Kuse ER, Chetchotisakd P, da Cunha CA, Ruhnke M, Barrios C, Raghunadharao D, Sekhon JS, Freire A, Ramasubramanian V, Demeyer I, Nucci M, Leelarasamee A, Jacobs F, Decruyenaere J, Pittet D, Ullmann AJ, Ostrosky-Zeichner L, Lortholary O, Koblinger S, Diekmann-Berndt H, Cornely OA, Micafungin Invasive Candidiasis Working Group. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 369:1519–1527. doi: 10.1016/S0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- 27.Pappas PG, Rotstein CM, Betts RF, Nucci M, Talwar D, De Waele JJ, Vazquez JA, Dupont BF, Horn DL, Ostrosky-Zeichner L, Reboli AC, Suh B, Digumarti R, Wu C, Kovanda LL, Arnold LJ, Buell DN. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis 45:883–893. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]
- 28.Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D, Betts R, Wible M, Goldstein BP, Schranz J, Krause DS, Walsh TJ. 2007. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 356:2472–2482. doi: 10.1056/NEJMoa066906. [DOI] [PubMed] [Google Scholar]
- 29.Kontoyiannis DP, Ratanatharathorn V, Young JA, Raymond J, Laverdiere M, Denning DW, Patterson TF, Facklam D, Kovanda L, Arnold L, Lau W, Buell D, Marr KA. 2009. Micafungin alone or in combination with other systemic antifungal therapies in hematopoietic stem cell transplant recipients with invasive aspergillosis. Transpl Infect Dis 11:89–93. doi: 10.1111/j.1399-3062.2008.00349.x. [DOI] [PubMed] [Google Scholar]
- 30.Petraitiene R, Petraitis V, Groll AH, Sein T, Schaufele RL, Francesconi A, Bacher J, Avila NA, Walsh TJ. 2002. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob Agents Chemother 46:12–23. doi: 10.1128/AAC.46.1.12-23.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petraitis V, Petraitiene R, Groll AH, Bell A, Callender DP, Sein T, Schaufele RL, McMillian CL, Bacher J, Walsh TJ. 1998. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY303366, a novel echinocandin B, in experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother 42:2898–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiederhold NP. 2007. Attenuation of echinocandin activity at elevated concentrations: a review of the paradoxical effect. Curr Opin Infect Dis 20:574–578. doi: 10.1097/QCO.0b013e3282f1be7f. [DOI] [PubMed] [Google Scholar]
- 33.Wiederhold NP. 2009. Paradoxical echinocandin activity: a limited in vitro phenomenon? Med Mycol 47(Suppl 1):S369–S375. doi: 10.1080/13693780802428542. [DOI] [PubMed] [Google Scholar]
- 34.Marr KA, Crippa F, Leisenring W, Hoyle M, Boeckh M, Balajee SA, Nichols WG, Musher B, Corey L. 2004. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood 103:1527–1533. doi: 10.1182/blood-2003-08-2644. [DOI] [PubMed] [Google Scholar]
- 35.Marr KA, Leisenring W, Crippa F, Slattery JT, Corey L, Boeckh M, McDonald GB. 2004. Cyclophosphamide metabolism is affected by azole antifungals. Blood 103:1557–1559. doi: 10.1182/blood-2003-07-2512. [DOI] [PubMed] [Google Scholar]