Abstract
Eravacycline is a novel broad-spectrum fluorocycline antibiotic being developed for a wide range of serious infections. Eravacycline was efficacious in mouse septicemia models, demonstrating 50% protective dose (PD50) values of ≤1 mg/kg of body weight once a day (q.d.) against Staphylococcus aureus, including tetracycline-resistant isolates of methicillin-resistant S. aureus (MRSA), and Streptococcus pyogenes. The PD50 values against Escherichia coli isolates were 1.2 to 4.4 mg/kg q.d. In neutropenic mouse thigh infection models with methicillin-sensitive S. aureus (MSSA) and S. pyogenes, eravacycline produced 2 log10 reductions in CFU at single intravenous (i.v.) doses ranging from 0.2 to 9.5 mg/kg. In a neutropenic mouse lung infection model, eravacycline administered i.v. at 10 mg/kg twice a day (b.i.d.) reduced the level of tetracycline-resistant MRSA in the lung equivalent to that of linezolid given orally (p.o.) at 30 mg/kg b.i.d. At i.v. doses of 3 to 12 mg/kg b.i.d., eravacycline was more efficacious against tetracycline-resistant Streptococcus pneumoniae in a neutropenic lung infection model than linezolid p.o. at 30 mg/kg b.i.d. Eravacycline showed good efficacy at 2 to 10 mg/kg i.v. b.i.d., producing up to a 4.6 log10 CFU reduction in kidney bacterial burden in a model challenged with a uropathogenic E. coli isolate. Eravacycline was active in multiple murine models of infection against clinically important Gram-positive and Gram-negative pathogens.
INTRODUCTION
The rising number of infections caused by multidrug-resistant bacteria is a serious public health threat (1). A recent study concluded that the spread of resistant pathogens has resulted in higher health care and societal costs and longer hospital stays (2). Current therapies often fail to completely eradicate the bacterial infection, leading to higher levels of resistance development as well as increased morbidity and mortality. Further, the lack of appropriate antibiotic treatment could compromise successful outcomes in the growing number of elderly patients and patients undergoing surgery, transplantation, and chemotherapy (3). The Infectious Diseases Society of America (IDSA) has singled out a set of “ESKAPE” pathogens, Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species, which currently cause the majority of U.S. hospital infections and effectively “escape” the effects of antibacterial drugs (3, 4). In a campaign to stoke the current pipeline with new antibacterial agents targeting ESKAPE pathogens, the IDSA recently proposed the 10 × ′20 initiative to encourage global political, scientific, industry, economic, intellectual property, policy, medical, and philanthropic leaders to develop creative incentives to stimulate antibacterial research and development, with a goal to develop 10 effective antibiotics by 2020 (5).
Eravacycline is a new broad-spectrum fluorocycline antibiotic with in vitro potency against emerging multidrug-resistant Gram-negative pathogens, including carbapenem-resistant Enterobacteriaceae (CRE) and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae and Acinetobacter baumannii (6). Eravacycline maintains activity in clinical isolates expressing major antibiotic resistance mechanisms, including tetracycline-specific efflux and ribosomal protection mechanisms (6, 7). When the less prevalent tetracycline-specific mechanism tet(X) is recombinantly overexpressed in Escherichia coli, the MIC of eravacycline increases, similar to that of other tetracyclines (7). In a phase 2 trial in complicated intraabdominal infections (cIAI), an intravenous (i.v.) formulation of eravacycline was (i) clinically effective against a broad spectrum of pathogens, including ESBL-positive and carbapenem-resistant Gram-negative isolates (test of cure, 92.9% for eravacycline at 1.5 mg/kg of body weight every 24 h or 100% for eravacycline at 1.0 mg/kg every 12 h in the microbiologically evaluable population), (ii) safe and well-tolerated, and (iii) capable of being administered in a convenient dosing regimen (8). Phase 1 studies have shown that eravacycline is orally bioavailable, is safe and well tolerated, and has potential to be used as an oral step-down therapy (9). Eravacycline is currently in phase 3 clinical studies for the i.v./oral treatment of complicated urinary tract infections (cUTI) and the i.v. treatment of cIAI.
Eravacycline was evaluated in multiple preclinical efficacy models of serious hospital- and community-acquired infections. Eravacycline has ∼30% oral bioavailability in humans (9); however, because of low oral bioavailability in preclinical species used for toxicology and efficacy models (10), preclinical evaluations of eravacycline required intravenous administration. The objective of these animal infection studies was to demonstrate the efficacy of eravacycline in mouse models of infection to support its ongoing clinical development.
MATERIALS AND METHODS
Bacterial isolates.
S. aureus ATCC 13709, Streptococcus pyogenes ATCC 19615, S. pyogenes ATCC 8668, E. coli ATCC 25922, and E. coli ATCC BAA-1161 [EC200, tet(B), uropathogenic] were obtained from the American Type Culture Collection, Manassas, VA. S. aureus SA161 is a tet(M) methicillin-resistant S. aureus (MRSA) strain from Micromyx, LLC, Kalamazoo, MI. S. aureus SA191 (NRS123) and SA192 (NRS384) are hospital-acquired and community-acquired (USA300) MRSA isolates, respectively, obtained from ViviSource, Waltham, MA; SA191 is tet(M) and SA192 is tet(K). Streptococcus pneumoniae SP160 is a tet(M) strain obtained from Marilyn Roberts at the University of Washington. E. coli EC133 is a tet(B), tet(D), ESBL+ (blaSHV) isolate from Clinical Microbiology Institute, Wilsonville, OR.
Antibiotics and media.
Eravacycline was synthesized at Tetraphase Pharmaceuticals as described in Xiao et al. (11). Tigecycline for injection (Pfizer, Groton, CT) was purchased from Skenderian Apothecary, Cambridge, MA. Linezolid, vancomycin, imipenem, meropenem, cilastatin, tetracycline, and levofloxacin were from Henry Schein Veterinary Supply (Melville, NY) or Sigma-Aldrich (St. Louis, MO). Trypticase soy agar (TSA), TSA II agar plates, and brain heart infusion broth (BHI) were from BBL (Franklin Lakes, NJ). Cyclophosphamide and type III hog gastric mucin were obtained from Sigma-Aldrich.
In vitro susceptibility testing.
MIC microdilution assays were performed according to Clinical and Laboratory Standards Institute methodology (12, 13).
Mice.
All studies were performed at ViviSource, an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facility, under approved institutional animal care and use committee (IACUC) protocols, and conformed to the Office of Laboratory Animal Welfare (OLAW) standards. Pathogen-free female mice, CD-1 or BALB/c, purchased from Charles River Laboratories, Inc. (Wilmington, MA), were acclimated for a minimum of 5 days prior to the start of the studies. The animals had free access to food and water throughout the study.
Preparation of inocula. (i) Systemic infection model.
S. aureus ATCC 13709 log-phase bacteria grown at 37°C in BHI were diluted in 5% hog gastric mucin. For S. aureus SA161 and SA192, S. pyogenes ATCC 8668 and ATCC 19615, and E. coli ATCC 25922 and EC133 inocula, bacteria grown overnight on TSA at 37°C under 5% CO2 were resuspended in saline to an optical density of 0.1 at 625 nm. Bacterial suspensions were then diluted in BHI and further diluted in 8% hog gastric mucin to achieve the infecting inocula.
(ii) Thigh infection, lung infection, and pyelonephritis models.
Bacteria were grown at 37°C on TSA in 5% CO2 overnight. The optimized bacterial concentration was prepared by suspending a portion of the overnight growth in sterile saline to achieve an optical density of 0.1 at 625 nm. The culture was subsequently diluted in BHI (thigh and lung models) or saline (pyelonephritis model) to achieve the infecting inocula.
Data analysis.
All data were analyzed using GraphPad Prism (version 4.03; GraphPad Software). For systemic infection studies, at the termination of the study (48 h postinfection), percent survival was calculated, and the dose (mg/kg) affecting 50% survival, the 50% protective dose (PD50), was reported along with 95% confidence intervals (95% CI) as calculated by probit analysis. For thigh infection models, the amount of compound required to achieve 1, 2, and 3 log10 reductions relative to the value for untreated mice 24 h posttreatment was calculated using a linear line plot of the mean log10 CFU per gram of thigh versus compound dose. For lung and pyelonephritis models, log10 CFU per gram of lung or kidney was graphed, and statistically significant differences between groups were calculated by nonparametric Mann-Whitney analysis.
Mouse systemic infection model.
Inocula were optimized to achieve a bacterial inoculum that resulted in 100% mortality in CD-1 mice (18 to 20 g, n = 6 per group) infected by the intraperitoneal (i.p.) route (0.5 ml) within 48 h postinfection. Mice received a single treatment with drug formulated in sterile 0.9% saline ranging from 0.05 to 10 mg/kg in a dose volume of 10 ml/kg via tail vein intravenous injection 1 h after i.p. infection. Inocula were serially diluted, plated on TSA, and incubated overnight to determine the bacterial concentration. Inocula used in these experiments were as follows (in CFU per mouse): S. aureus ATCC 13709, 2.6 × 106; S. aureus SA161, 1.13 × 108; S. aureus SA192, 1.38 × 107; S. pyogenes ATCC 8668, 3.15 × 104; S. pyogenes ATCC 19615, 1.23 × 107; E. coli ATCC 25922, 1.43 × 107; and E. coli EC133, 6.25 × 106.
Neutropenia induction.
For the lung and thigh infection studies, mice were rendered neutropenic through two consecutive i.p. injections of cyclophosphamide of 150 and 100 mg/kg on days −4 and −1, respectively.
Mouse thigh infection model.
CD-1 mice (18 to 20 g, n = 4 per group) were infected with approximately 5 × 105 CFU/ml of bacteria in a 0.1-ml volume by intramuscular injection into the right thigh. At 1.5 h postinfection, mice received a single dose of drug formulated in sterile 0.9% saline ranging from 0.3 to 30 mg/kg in a dose volume of 10 ml/kg via a tail vein i.v. injection. At the time of bacterial burden determination (pretreatment and 24 h posttreatment), mice were euthanized by CO2 inhalation, their right thighs were aseptically removed, weighed, and homogenized in sterile saline, and the homogenate was serially diluted and plated on TSA. CFU per gram of thigh were calculated after overnight incubation of TSA plates at 37°C in 5% CO2. Inocula used in these experiments were as follows (in CFU per mouse): S. aureus ATCC 13709, 2.99 × 105; S. aureus SA161, 2.6 × 105; S. aureus SA192, 2.83 × 105; S. aureus SA158, 1.5 × 105; and S. pyogenes ATCC 8668, 1.9 × 105.
Mouse lung infection model.
BALB/c mice (18 to 20 g, n = 4 to 6 per group) were inoculated with 50 μl of the prepared bacterial inoculum via intranasal inhalation under light anesthesia (4.5% isoflurane with 1.5 liters/min O2). Mice received drug formulated in sterile 0.9% saline in 10 ml/kg via tail vein i.v. injection or via oral gavage in water (linezolid only) at 2 and 12 h postinfection. At the time of bacterial burden determination (pretreatment and 24 h after initiation of treatment), mice were euthanized by CO2 inhalation, the lungs of the mice were aseptically removed, weighed, and homogenized in sterile saline, and the homogenate was serially diluted and plated on TSA. CFU per gram of lung were calculated after overnight incubation of TSA plates at 37°C in 5% CO2. Inocula used in these experiments were as follows (in CFU per mouse): S. aureus SA191, 7.5 × 107; and S. pneumoniae SP160, 7.0 × 106.
Mouse pyelonephritis model with E. coli EC200.
BALB/c mice (18 to 20 g, n = 4 to 6 per group) were inoculated with 0.2 ml of prepared bacterial inoculum via intravenous injection to seed the kidney. Animals were administered antibiotics formulated in sterile 0.9% saline at 10 ml/kg i.v. via the tail vein 12 and 24 h postinfection. At the time of bacterial burden determination (initiation of treatment and 12 h after treatment termination), mice were euthanized by CO2 inhalation, the kidneys of the mice were aseptically removed, weighed, and homogenized in sterile saline, and the homogenate was serially diluted and plated on TSA. CFU per gram of kidney were calculated after overnight incubation at 37°C in 5% CO2. The log10 CFU per gram of kidney was graphed using GraphPad Prism (version 4.03). The inoculum was 1.3 × 108 CFU per mouse.
RESULTS
Antibacterial activity of eravacycline and comparators against isolates used in infection models.
Eravacycline showed potent antibacterial activity (Table 1) ranging from ≤0.016 to 0.5 μg/ml. The presence of tetracycline-specific resistance genes such as tet(M) (strains SA161, SA191, and SP160), tet(K) (strains SA158 and SA192), and tet(B) (strains EC133 and EC200) has been shown in previous work to have minimal or no effect on the antibacterial potency of eravacycline (6, 7).
TABLE 1.
Susceptibilities of strains used in mouse infection models
| Organism | MIC (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| Eravacycline | Tigecycline | Tetracycline | Vancomycin | Linezolid | Levofloxacin | Imipenem | |
| S. aureus ATCC 13709 | 0.016 | 0.063 | 0.25 | 1 | 4 | 0.25 | NTa |
| S. aureus SA158 [tet(K)] | 0.063 | 0.13 | 64 | 2 | 4 | 0.25 | NT |
| MRSA SA161 [tet(M)] | 0.063 | 0.25 | 64 | 1 | 4 | 16 | NT |
| MRSA SA192 [tet(K) USA300] | 0.25 | 0.13 | 64 | 1 | 4 | 1 | NT |
| MRSA SA191 [tet(M)] | 0.5 | 0.5 | >64 | 1 | 2 | 0.25 | NT |
| S. pyogenes ATCC 8668 | 0.016 | ≤0.016 | 0.25 | 0.5 | 2 | 0.5 | NT |
| S. pyogenes ATCC 19615 | 0.016 | ≤0.016 | 0.13 | 0.5 | 1 | NT | NT |
| S. pneumoniae SP160 [tet(M)] | ≤0.016 | 0.03 | 32 | 0.25 | 0.5 | 1 | NT |
| E. coli ATCC 25922 | 0.063 | 0.13 | 1 | NT | NT | 0.03 | 0.25 |
| E. coli EC133 [ESBL+ tet(B) tet(D)] | 0.13 | 0.13 | >32 | NT | NT | >32 | 0.13 |
| E. coli EC200 [tet(B)] | 0.13 | 0.13 | >64 | NT | NT | 0.063 | 0.25 |
NT, not tested.
Eravacycline protection in a mouse septicemia model with susceptible and tetracycline-resistant isolates.
In mouse systemic infection models, eravacycline exhibited potent efficacy when administered as a single i.v. dose, producing PD50 values ranging from 0.05 to 4.4 mg/kg (Table 2). Eravacycline PD50 values of 0.30, 1.0, and 0.30 mg/kg were similar to those of tigecycline against S. aureus ATCC 13709 and MRSA strains SA161 and SA192, respectively, regardless of tetracycline resistance. Tetracycline was found to be relatively inactive (PD50 of >10 mg/kg) against SA161 and SA192, and vancomycin had a PD50 value of 0.30 mg/kg against SA192. Eravacycline was more potent than tigecycline against both S. pyogenes isolates, ATCC 8668 and ATCC 19615, with eravacycline PD50 values of 1.0 and ∼0.05 mg/kg versus tigecycline PD50 values of 2.5 and 0.30 mg/kg, respectively. Linezolid was relatively inactive against S. pyogenes ATCC 8668 (PD50 of >10 mg/kg) but had a PD50 value of 0.63 mg/kg against S. pyogenes ATCC 19615. For E. coli ATCC 25922, the PD50 value of eravacycline (4.4 mg/kg) was 2.5-fold higher than that of tigecycline (1.7 mg/kg); however, the 95% confidence values were broad for eravacycline. For E. coli EC133, the PD50 value of eravacycline was ∼3-fold lower than that of tigecycline (1.2 versus 3.5 mg/kg, respectively), and the PD50 value for imipenem was <0.3 mg/kg.
TABLE 2.
Efficacies of eravacycline and comparators in a mouse septicemia model with intraperitoneal challenge
| Organism | PD50, mg/kg i.v. (95% CI) |
|||||
|---|---|---|---|---|---|---|
| Eravacycline | Tigecycline | Tetracycline | Vancomycin | Linezolid | Imipenem | |
| S. aureus ATCC 13709 | 0.30 (0.29 to 0.31) | 0.32 ± 0.07a | NTb | NT | NT | NT |
| MRSA SA161 [tet(M)] | 1.0 (0.56 to 1.4) | 1.0 (0.78 to 1.2) | >10 | NT | NT | NT |
| MRSA SA192 [tet(K) USA300] | 0.30 (0.13 to 0.47) | 0.35 (0.34 to 0.37) | >10 | 0.30 (0.15 to 0.45) | NT | NT |
| S. pyogenes ATCC 8668 | 1.0 (0.78 to 1.2) | 2.5 (1.7 to 3.4) | NT | NT | >10 | NT |
| S. pyogenes ATCC 19615 | ∼0.05 (—c) | 0.30 (0.04 to 0.56) | NT | NT | 0.63 (0.06 to 1.2) | NT |
| E. coli ATCC 25922 | 4.4 (−0.01 to 8.7) | 1.7 (0.91 to 2.6) | NT | NT | NT | NT |
| E. coli EC133 [ESBL+ (blaSHV) tet(B) tet(D)] | 1.2 (0.84 to 1.6) | 3.5 (2.4 to 4.7) | NT | NT | NT | <0.3 |
Mean and standard deviation (SD) from three experiments.
NT, not tested.
—, PD50 is estimated and a 95% CI could not be determined due to the significant activity of eravacycline against this strain.
Efficacy of eravacycline in a mouse thigh infection model with susceptible and tetracycline-resistant isolates.
Single doses of eravacycline and comparator antibiotics were evaluated in mouse thigh infection models with S. aureus and S. pyogenes infections (Table 3). All treatment-related CFU reductions in each thigh model were compared to the values for the corresponding 24-hour untreated controls. Against S. aureus ATCC 13709, eravacycline produced 1, 2, and 3 log10 CFU reductions at 5- to 7.5-fold lower doses (0.2, 0.2, and 0.4 mg/kg, respectively) than tigecycline (1.2, 1.5, and 2 mg/kg, respectively). For MRSA SA161, eravacycline showed 5- to 12.5-fold greater potency than tigecycline, producing 1, 2, and 3 log10 CFU reductions at 0.6, 1, and 3 mg/kg, respectively, versus 3, 12.5, and 17.3 mg/kg, respectively, for tigecycline. For SA161, vancomycin showed 1, 2, and 3 log10 CFU reductions at 0.8, 2.9, and 10 mg/kg. Against MRSA SA192, S. aureus SA158, and S. pyogenes ATCC 8668, eravacycline produced similar log10 CFU reductions at doses within <2-fold of those of tigecycline (Table 3). For SA158, vancomycin showed 1 and 2 log10 CFU reductions at 2.8 and >20 mg/kg, and linezolid was relatively inactive against S. pyogenes ATCC 8668, requiring >20 mg/kg to produce a 1 log10 CFU reduction.
TABLE 3.
Efficacies of eravacycline and comparators in a neutropenic mouse thigh model
| Strain | Antibiotic | i.v. dose (mg/kg) providing the following CFU reduction from untreated controls at 24 h posttreatment: |
Maximum log10 CFU reduction (dose in mg/kg) | ||
|---|---|---|---|---|---|
| 1 log10 | 2 log10 | 3 log10 | |||
| S. aureus ATCC 13709 | Eravacycline | 0.2 | 0.2 | 0.4 | 5.1 (10) |
| Tigecycline | 1.2 | 1.5 | 2 | 4.8 (30) | |
| S. aureus SA161 MRSA [tet(M)] | Eravacycline | 0.6 | 1 | 3 | 3.5 (10) |
| Tigecycline | 3 | 12.5 | 17.3 | 3.6 (20) | |
| Vancomycin | 0.8 | 2.9 | 10 | 3.5 (30) | |
| S. aureus SA192 MRSA [tet(M), USA300] | Eravacycline | 3.5 | 9.5 | NAa | 2.1 (10) |
| Tigecycline | 3.8 | 8 | NA | 2.9 (20) | |
| Vancomycin | 2.1 | 19.2 | NA | 2.8 (30) | |
| S. aureus SA158 [tet(K)] | Eravacycline | 2.3 | 8.2 | 16.2 | 3.5 (20) |
| Tigecycline | 2.1 | 5.4 | 9.7 | 3.2 (20) | |
| Vancomycin | 2.8 | >20 | >20 | 1.7 (20) | |
| S. pyogenes ATCC 8668 | Eravacycline | 5.3 | 9 | NA | 2.3 (10) |
| Tigecycline | 6 | 15.8 | NA | 2.3 (20) | |
| Linezolid | >20 | >20 | NA | 0.7 (20) | |
NA, not applicable.
Efficacy of eravacycline in mouse lung infection models with tetracycline-resistant S. pneumoniae and MRSA isolates.
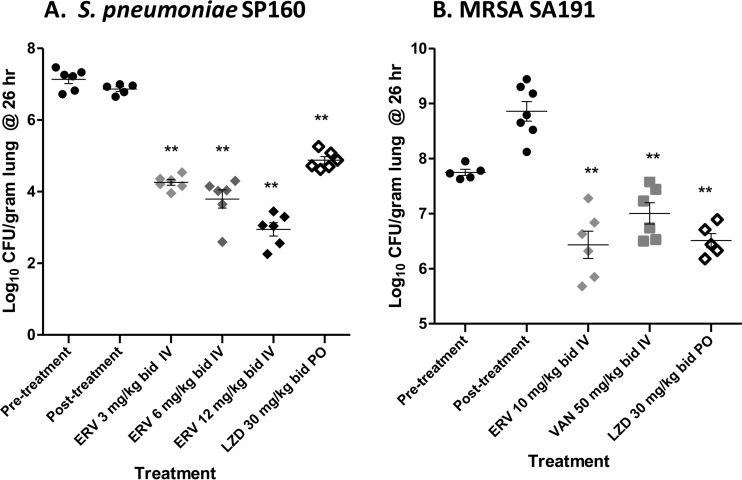
In a lung infection model with S. pneumoniae tet(M) isolate SP160, two doses of eravacycline given i.v., at 2 and 12 h postinfection, produced log10 CFU reductions in the lung bacterial burden of 2.6, 3.1, and 3.9 at 3, 6, and 12 mg/kg, respectively, versus the values for 24-hour untreated controls (Fig. 1A). All eravacycline dosages exceeded the efficacy of linezolid given orally at 30 mg/kg, producing a 2.0 log10 CFU reduction. For lung infections with MRSA SA191, when dosed at 2 and 12 h postinfection, eravacycline given at 10 mg/kg i.v. produced a 2.4 log10 CFU reduction versus the values for the 24-hour untreated controls. This reduction was similar to that for linezolid given at 30 mg/kg orally and exceeded the efficacy of vancomycin given at 50 mg/kg i.v., which produced a 1.4 log10 CFU reduction versus the value for the 24-hour untreated controls (Fig. 1B).
FIG 1.
Efficacy of eravacycline and comparators in murine lung infection models. Lung infection models were conducted as described in Materials and Methods. Each symbol represents an individual mouse, horizontal lines indicate the mean, and error bars indicate the standard error of the mean. (A) SP160 model. Closed circles, untreated controls at 0 and 24 h, relative to the start of treatment; light gray diamonds, 3 mg/kg i.v. eravacycline (ERV); medium gray diamonds, 6 mg/kg i.v. ERV; black diamonds, 12 mg/kg i.v. ERV; open diamonds, 30 mg/kg oral (p.o.) linezolid (LZD). (B) SA191 model. Closed circles, untreated controls at 0 and 24 h, relative to the start of treatment; light gray diamonds, 10 mg/kg i.v. ERV; dark gray squares, 50 mg/kg i.v. vancomycin (VAN); open diamonds, 30 mg/kg p.o. LZD.; **, statistically significant reduction versus 24 h untreated control, P < 0.01.
Efficacy of eravacycline in mouse pyelonephritis models with uropathogenic tetracycline-resistant E. coli.
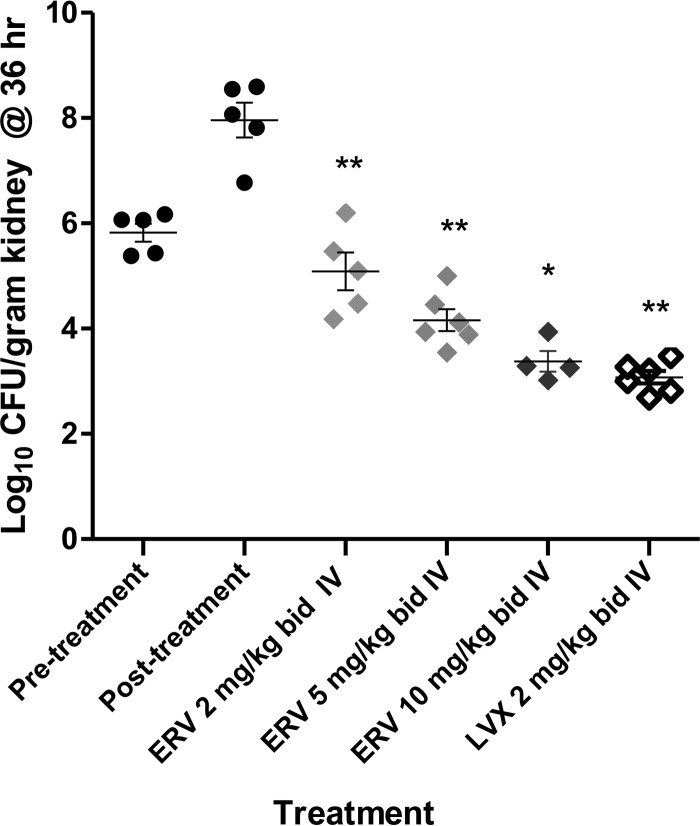
At 2, 5, and 10 mg/kg administered i.v. at 12 and 24 h postinfection, eravacycline produced statistically significant log10 CFU reductions of 1.3, 3.8, and 4.6 in the kidneys versus the values for the 36-hour untreated controls in a mouse pyelonephritis model with E. coli EC200 (Fig. 2). In the same model, levofloxacin at 2 mg/kg given i.v. produced a 4.9 log10 CFU reduction.
FIG 2.
Efficacy of eravacycline and comparators in a mouse pyelonephritis model with uropathogenic, tet(B) tetracycline-resistant E. coli EC200. The pyelonephritis infection model was conducted as described in Materials and Methods. Each symbol represents an individual mouse, and the horizontal line indicates the mean. Error bars indicate the standard error of the mean. Closed circles, untreated controls at 0 and 24 h, relative to the start of treatment; light gray diamonds, 2 mg/kg i.v. ERV; medium gray diamonds, 5 mg/kg i.v. ERV; black diamonds, 10 mg/kg i.v. ERV; open diamonds, 2 mg/kg i.v. levofloxacin (LVX). Statistically significant reduction versus 24 h untreated control: *, P < 0.05; **, P < 0.01.
DISCUSSION
Eravacycline was efficacious in mouse models of serious hospital- and community-acquired infections. Eravacycline provided significant protection against Gram-positive pathogens in neutropenic thigh infection models, against septicemia following peritoneal challenge, and in pneumonia models when challenge bacteria were hospital- and community-acquired MRSA, S. pneumoniae, or S. pyogenes. Against the Gram-negative pathogen E. coli, the highest-incidence pathogen in cIAI and cUTI, eravacycline showed potent efficacy in septicemia and pyelonephritis models. In agreement with the preclinical efficacy demonstrated in the present studies, eravacycline was shown to be efficacious in a phase 2 study for complicated intraabdominal infections (8). Together, these data support the advancement of eravacycline into pivotal phase 3 trials under way for the treatment of cIAI and cUTI.
Eravacycline was active in mouse infection models against isolates expressing both tetracycline-specific efflux [tet(K) and tet(B)] and ribosomal protection [tet(M)] mechanisms. In earlier work, the in vitro antibacterial activity of eravacycline was shown to be minimally affected by expression of tetracycline-specific mechanisms in clinical isolates or resistance to other major antibiotic classes, including fluoroquinolones, macrolides, aminoglycosides, β-lactams, cephalosporins, and carbapenems (6, 7). The management of cIAI and cUTI and other complicated infections has become increasingly difficult due to emerging resistance in Gram-negative bacteria to all of these cornerstone antibiotic classes (14, 15, 16). Its demonstrated preclinical and clinical efficacies continue to support eravacycline as a promising new i.v. and oral antibiotic option for the treatment of cIAI, cUTI, and other serious infections with a high incidence of drug-resistant Gram-negative and Gram-positive pathogens.
ACKNOWLEDGMENT
We acknowledge the contributions from the Tetraphase Chemistry Department for the synthesis of eravacycline for these studies.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 2.Roberts RR, Hota B, Ahmad I, Scott RD II, Foster SD, Abbasi F, Schabowski S, Kampe LM, Ciavarella GG, Supino M, Naples J, Cordell R, Levy SB, Weinstein RA. 2009. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 49:1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 3.Boucher HW, Talbot GH, Bradley JS, Edwards JE Jr, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 4.Rice L. 2008. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 197:1079–1181. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 5.Infectious Diseases Society of America. 2010. The 10 × ′20 initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis 50:1081–1083. doi: 10.1086/652237. [DOI] [PubMed] [Google Scholar]
- 6.Sutcliffe JA, O'Brien W, Fyfe C, Grossman TH. 2013. Antibacterial activity of ERV (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother 57:5548–5558. doi: 10.1128/AAC.01288-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grossman TH, Starosta AL, Fyfe C, O'Brien W, Rothstein DM, Mikolajka A, Wilson DN, Sutcliffe JA. 2012. Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob Agents Chemother 56:2559–2564. doi: 10.1128/AAC.06187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomkin JS, Ramesh MK, Cesnauskas G, Novikovs N, Sutcliffe JA, Walpole SM, Horn PT. 2014. Phase 2, randomized, double-blind study of the efficacy and safety of two dose regimens of eravacycline versus ertapenem for adult community-acquired complicated intra-abdominal infections. Antimicrob Agents Chemother 58:1847–1854. doi: 10.1128/AAC.01614-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horn PT, Sutcliffe JA, Walpole SM, Leighton A. 2011. Pharmacokinetics, safety and tolerability of a novel fluorocycline, TP-434, following multiple dose oral administration with and without food, abstr 603. Abstr 49th Annu Meet Infect Dis Am Annu Meeting, Boston, MA, 20 to 23 October 2011. [Google Scholar]
- 10.Ronn M, Dunwoody N, Sutcliffe J. 2010. Pharmacokinetics of TP-434 in mouse, rat, dog, monkey and chimpanzee, abstr F1-2163. Abstr 50th Annu Intersci Conf Antimicrob Agents Chemother, 12 to 15 September 2010. [Google Scholar]
- 11.Xiao X-Y, Hunt DK, Zhou J, Clark RB, Dunwoody N, Fyfe C, Grossman TH, O'Brien WJ, Plamondon L, Rönn M, Sun C, Zhang W-Y, Sutcliffe JA. 2012. Fluorocyclines. 1. 7-Fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline: a potent, broad spectrum antibacterial agent. J Med Chem 55:597–605. doi: 10.1021/jm201465w. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI document M07-A9 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. CLSI document M100-S22 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. 2010. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 50:133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 15.Talan DA, Krishnadasan A, Abrahamian FM, Stamm WE, Moran GJ. 2008. Prevalence and risk factor analysis of trimethoprim-sulfamethoxazole- and fluoroquinolone-resistant Escherichia coli infection among emergency department patients with pyelonephritis. Clin Infect Dis 47:1150–1158. doi: 10.1086/592250. [DOI] [PubMed] [Google Scholar]
- 16.Lautenbach E. 2008. Finding the path of least antimicrobial resistance in pyelonephritis. Clin Infect Dis 47:1159–1161. doi: 10.1086/592251. [DOI] [PubMed] [Google Scholar]