Abstract
A nearly complete reversal of chloroquine (CQ) resistance in the CQ-resistant Plasmodium falciparum K-1 strain, with a significant decrease in the mean ± standard deviation (SD) 50% inhibitory concentration (IC50) from 1,050 ± 95 nM to 14 ± 2 nM, was achieved in vitro by the simultaneous administration of 2-aminoethyl diphenylborinate (2-APB). The CQ resistance-reversing activity of 2-APB, which showed the same efficacy as verapamil, was also observed in an in vivo mouse infection model with the CQ-resistant Plasmodium chabaudi AS(30CQ) strain.
TEXT
Malaria continues to be a worldwide public health problem, causing significant morbidity and mortality. In addition, the resistance of the causative parasite to existing antimalarial drugs has been a growing problem in countries where the disease is endemic (1). Thus, there is an urgent need to develop new antimalarial drugs (2–4) and find novel agents to reverse antimalarial resistance. Understanding the signaling pathways governing the blood-stage growth of the causative parasite may aid in the discovery of new therapeutic targets for antimalarial drugs. As such, the calcium ion (Ca2+) homeostasis and signaling pathways in the malaria parasite Plasmodium might be promising targets. Ca2+ is a ubiquitous intracellular signal responsible for controlling a wide range of cellular activities in eukaryotic cells (5). In protozoan parasites, Ca2+-mediated signaling controls various vital functions, such as protein secretion, motility, cell invasion, and differentiation (6–9). We recently, for the first time, demonstrated spontaneous Ca2+ oscillation in Plasmodium falciparum. Furthermore, we showed that the blockage of this oscillation in the trophozoite stage by 2-aminoethyl diphenylborinate (2-APB) inhibited 1,4,5-trisphosphate (IP3)-induced Ca2+ release (10–12), resulting in the death of the parasite (13). Chloroquine (CQ) is thought to exert its toxic effect in the intraerythrocytic parasite at the digestive vacuole (14). The compound was also found to induce Ca2+ release and disrupt Ca2+ and H+ homeostasis in the cytoplasm of Plasmodium chabaudi cells (15, 16). Therefore, with regard to the disruption of intracellular Ca2+ homeostasis, it was hypothesized that the potentiation of CQ activity could be achieved in the malaria parasite with the simultaneous administration of 2-APB.
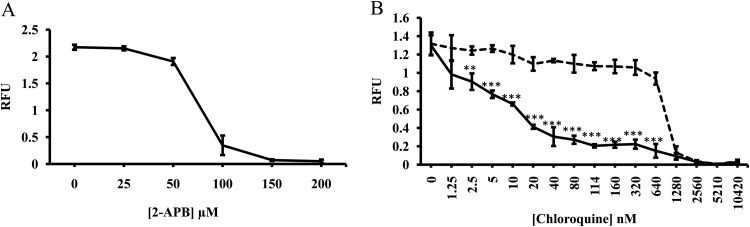
To evaluate the potential of 2-APB for reversing CQ resistance, a CQ-resistant K-1 strain of P. falciparum was cultured with the modified method of Trager and Jensen (17) in RPMI 1640 medium (Life Technologies Japan Co., Tokyo, Japan) supplemented with 0.5% AlbuMAX (Life Technologies Japan), 25 mM HEPES, 24 mM sodium bicarbonate, 0.5 g/liter l-glutamine, 50 mg/liter hypoxanthine, 25 μg/liter gentamicin, and human erythrocytes (from healthy Japanese volunteers) at a hematocrit level of 2%. Growth synchronization was achieved with 5% d-sorbitol (18). The outcome of the in vitro drug susceptibility test was assessed using the SYBR green I method (19). In brief, P. falciparum-infected erythrocytes were cultured with the standard method using a multigas incubator (5% O2 and 5% CO2). After reaching 1.5% ring-form parasitemia, the parasites were synchronized with 5% d-sorbitol for 30 min at room temperature and washed with RPMI 1640 medium twice by centrifugation at 1,000 × g for 5 min. Next, the erythrocytes were resuspended in the culture medium at 2% hematocrit. One hundred microliters of the erythrocyte suspension was then replaced in each well of a tissue culture plate (96-well flat-bottom plate; Corning Japan Co., Tokyo, Japan) in triplicate. For the CQ sensitivity test, chloroquine diphosphate (Sigma-Aldrich Japan Co., Tokyo, Japan) was added to the parasite culture (100 μl in total) in each well to give a series of dilutions from 1.25 to 10,240 nM. The sensitivity tests for 2-APB were performed as described for CQ. 2-APB (Sigma-Aldrich Japan Co.) was mixed with dimethyl sulfoxide (DMSO) (Sigma-Aldrich Japan Co.) before being added to the culture to give a series of dilutions from 25 to 200 μM. The simultaneous addition of the two compounds was performed by adding 50 μM 2-APB to the serially diluted CQ. After 72 h of incubation, each test plate was removed from the incubator, and 100 μl of lysis buffer (130.1 mM Tris-HCl [pH 7.5], 10 mM EDTA, 0.016 % [wt/vol] saponin, and 1.6 % [vol/vol] Triton X-100) containing SYBR green I (Life Technologies Japan) (2× final concentration) was added directly to each well in the plates and gently mixed. The plates were then covered with aluminum foil and incubated for another 24 h at room temperature in the dark. The relative fluorescent units per well were determined using a Fluoroskan Ascent (Thermo Fisher Scientific K.K., Yokohama, Japan), with the excitation and emission wavelength bands set at 485 and 530 nm, respectively. The concentration of antimalarial drug inhibiting parasite growth by 50% (IC50) was calculated using the probit method, as described previously (20). The mean ± standard deviation (SD) IC50s of 2-APB and CQ were determined to be 73.5 ± 3 μM and 1,050 ± 95 nM, respectively (Fig. 1A and B). The addition of the suboptimal dose of 2-APB (50 μM) to the series of CQ concentrations, which causes a minimum effect on the growth of the parasite in vitro (Fig. 1A), reversed CQ resistance and resulted in a significant decline in the IC50 (mean ± SD) from 1,050 ± 95 nM to 14 ± 2 nM (Fig. 1B). Similar experiments with the CQ-resistant P. falciparum Dd2 strain showed results comparable to those seen in P. falciparum K-1 (data not shown).
FIG 1.
Dose-dependent activities of 2-APB and chloroquine (CQ) on P. falciparum synchronized cultures of CQ-resistant K-1 strain. Shown are various concentrations of 2-APB (A) and CQ plus DMSO as solvent control (dashed line) and CQ plus 50 μM 2-APB (solid line) (B). The statistical significance of the differences between the treatments was assessed with Student's t test. **, P < 0.004, and ***, P < 0.0005. The results are presented as the mean ± SD from three independent experiments. RFU denotes relative fluorescence units in SYBR green I assay (see Materials and Methods).
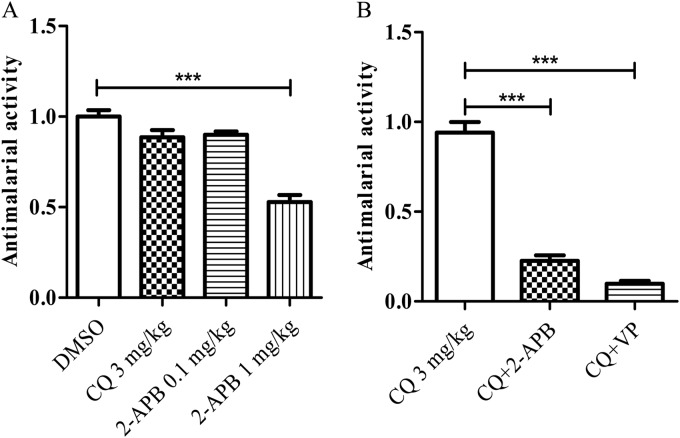
The strategy was then applied to the malaria mouse infection model, as previously described (21), with some modifications. Briefly, 6-week-old female ICR mice (CLEA Japan, Tokyo, Japan; 3 mice/group) were infected with the CQ-resistant P. chabaudi AS(30CQ) strain by an intraperitoneal injection of 5 × 106 parasitized erythrocytes. 2-APB (at 0.1 and 1 mg/kg of body weight) and CQ (3 mg/kg) were administered separately (via intraperitoneal injection) to different groups of mice to assess their antimalarial activities at days 0, 1, and 2. Thin blood films were prepared at days 1 to 4 and stained with Giemsa. The number of parasitized erythrocytes per 10,000 erythrocytes in each stained preparation was counted, with the mean values obtained from 3 preparations used as an index of parasitemia (%). Antimalarial activity was evaluated at day 4 as follows: antimalarial activity = (parasitemia in the compound-treated group)/(parasitemia in DMSO control group). The results showed that 1 mg/kg 2-APB exhibited an antimalarial effect but 0.1 mg/kg 2-APB did not (Fig. 2A). The potential of 2-APB to reverse CQ resistance was evaluated at day 4 as follows: antimalarial activity = (parasitemia in the two-compound-treated group)/(parasitemia in the CQ-treated group). The results showed that the simultaneous intraperitoneal injection of a lower concentration of 2-APB (0.1 mg/kg), which presented minimal antimalarial activity, produced a CQ resistance-reversing effect in the mice (Fig. 2B). It is noteworthy that the potency of 2-APB as a reverser of CQ resistance was equivalent to that of verapamil (Wako Chemical Co, Osaka, Japan) (at 20 mg/kg via intraperitoneal injection) (21). Verapamil is the first Ca2+ channel antagonist that has been reported to exhibit a resistance-reversing effect in CQ-resistant P. falciparum (22). These outcomes suggest the complete reversal of CQ resistance with 2-APB in vitro and in vivo; this is probably due to the disturbance of Ca2+ homeostasis in the parasite cell. To the best of our knowledge, this is the first observation of a CQ resistance-reversing effect induced by an IP3 receptor inhibitor in the malaria parasite.
FIG 2.
In vivo activities of chloroquine (CQ) and 2-APB on CQ-resistant P. chabaudi AS(30CQ) strain (A), and the potential of 2-APB in reversing CQ resistance (B). The antimalarial activity of each experimental group is shown as the ratio of parasitemia relative to the control (DMSO in panel A and CQ 3 mg/kg in panel B). (B) CQ plus 2-APB and CQ plus verapamil (VP) represent 3 mg/kg CQ plus 0.1 mg/kg 2-APB and 3 mg/kg CQ plus 20 mg/kg VP, respectively. The statistical significance of the differences between the groups (n = 3) was assessed with one-way analysis of variance (ANOVA), followed by Dunnett's test. ***, P < 0.0001 versus the control group.
The present study provides a novel strategy, which mainly targets Ca2+ homeostasis through the IP3 pathway for Ca2+ release, which is critical for the blood-stage development of the parasite (13). 2-APB and other functionally related compounds that block the IP3 pathway might be promising candidates as leads in the search for novel resistance-reversing agents. We anticipate that future studies will be undertaken to develop a 2-APB analogue that selectively affects Ca2+ homeostasis in the parasite cell.
ACKNOWLEDGMENTS
We thank S. Kano (National Center for Global Health and Medicine) for providing the P. falciparum strains and the Hokkaido Kushiro and Sapporo Red Cross Blood Centers for supplying human red blood cells. We also thank Jose M. Angeles for helpful discussions.
The following reagent was obtained through the MR4 as part of the BEI Resources Repository, NIAID, NIH: P. chabaudi, chabaudi AS(30CQ), MRA-744, deposited by D. Walliker.
REFERENCES
- 1.World Health Organization. 2011. World malaria report 2011. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/9789241564403/en/. [Google Scholar]
- 2.Kaur K, Jain M, Kaur T, Jain R. 2009. Antimalarials from nature. Bioorg Med Chem 17:3229–3256. doi: 10.1016/j.bmc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 3.Giha HA. 2010. Artemisinin derivatives for treatment of uncomplicated Plasmodium falciparum malaria in Sudan: too early for too much hope. Parasitol Res 106:549–552. doi: 10.1007/s00436-009-1700-x. [DOI] [PubMed] [Google Scholar]
- 4.Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S, Brown JR, Peishoff CE, Cardon LR, Garcia-Bustos JF. 2010. Thousands of chemical starting points for antimalarial lead identification. Nature 465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ, Lipp P, Bottman MD. 2000. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 6.Carruthers VB, Giddings OK, Sibley LD. 1999. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell Microbiol 1:225–235. doi: 10.1046/j.1462-5822.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 7.Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. 2008. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature 451:207–210. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sibley LD. 2004. Intracellular parasite invasion strategies. Science 304:248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- 9.Wetzel DM, Chen LA, Ruiz FA, Moreno SN, Sibley LD. 2004. Calcium-mediated protein secretion potentiates motility in Toxoplasma gondii. J Cell Sci 117:5739–5748. doi: 10.1242/jcs.01495. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 1997. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem 122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa S, Tanimura T, Miyawaki A, Nakamura M, Yuzaki M, Furuichi T, Mikoshiba K. 1992. Molecular cloning and characterization of the inositol 1,4,5-trisphosphate receptor in Drosophila melanogaster. J Biol Chem 267:16613–16619. [PubMed] [Google Scholar]
- 12.Zhou H, Iwasaki H, Nakamura T, Nakamura K, Maruyama T, Hamano S, Ozaki S, Mizutani A, Mikoshiba K. 2007. 2-Aminoethyl diphenylborinate analogues: selective inhibition for store-operated Ca2+ entry. Biochem Biophys Res Commun 352:277–282. doi: 10.1016/j.bbrc.2006.10.174. [DOI] [PubMed] [Google Scholar]
- 13.Enomoto M, Kawazu S, Kawai S, Furuyama W, Ikegami T, Watanabe J, Mikoshiba K. 2012. Blockage of spontaneous Ca2+ oscillation causes cell death in intraerythrocitic Plasmodium falciparum. PLoS One 7:e39499. doi: 10.1371/journal.pone.0039499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis SE, Sullivan DJ Jr, Goldberg DE. 1997. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu Rev Microbiol 51:97–123. doi: 10.1146/annurev.micro.51.1.97. [DOI] [PubMed] [Google Scholar]
- 15.Passos AP, Garcia CR. 1998. Inositol 1,4,5-trisphosphate induced Ca2+ release from chloroquine-sensitive and -insensitive intracellular stores in the intraerythrocytic stage of the malaria parasite P. chabaudi. Biochem Biophys Res Commun 245:155–160. doi: 10.1006/bbrc.1998.8338. [DOI] [PubMed] [Google Scholar]
- 16.Gazarini ML, Sigolo CA, Markus RP, Thomas AP, Garcia CR. 2007. Antimalarial drugs disrupt ion homeostasis in malarial parasites. Mem Inst Oswaldo Cruz 102:329–334. doi: 10.1590/S0074-02762007000300012. [DOI] [PubMed] [Google Scholar]
- 17.Trager W, Jensen JB. 1976. Human malaria parasite in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 18.Lambros C, Venderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420. doi: 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. 2007. Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother 51:1926–1933. doi: 10.1128/AAC.01607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taguchi N, Hatabu T, Yamaguchi H, Suzuki M, Sato K, Kano S. 2004. Plasmodium falciparum: selenium-induced cytotoxicity to P. falciparum. Exp Parasitol 106:50–55. doi: 10.1016/j.exppara.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Miyata Y, Fujii H, Osa Y, Kobayashi S, Takeuchi T, Nagase H. 2011. Opioid δ1 receptor antagonist 7-benzylidenenaltrexone as an effective resistance reverser for chloroquine-resistant Plasmodium chabaudi. Bioorg Med Chem Lett 21:4710–4712. doi: 10.1016/j.bmcl.2011.06.085. [DOI] [PubMed] [Google Scholar]
- 22.Martin SK, Oduola AMJ, Milhous WK. 1987. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science 235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]