Abstract
Between September 2013 and July 2014, 2,482 influenza 2009 pandemic A(H1N1) [A(H1N1)pdm09] viruses were screened in Japan for the H275Y substitution in their neuraminidase (NA) protein, which confers cross-resistance to oseltamivir and peramivir. We found that a large cluster of the H275Y mutant virus was present prior to the main influenza season in Sapporo/Hokkaido, with the detection rate for this mutant virus reaching 29% in this area. Phylogenetic analysis suggested the clonal expansion of a single mutant virus in Sapporo/Hokkaido. To understand the reason for this large cluster, we examined the in vitro and in vivo properties of the mutant virus. We found that it grew well in cell culture, with growth comparable to that of the wild-type virus. The cluster virus also replicated well in the upper respiratory tract of ferrets and was transmitted efficiently between ferrets by way of respiratory droplets. Almost all recently circulating A(H1N1)pdm09 viruses, including the cluster virus, possessed two substitutions in NA, V241I and N369K, which are known to increase replication and transmission fitness. A structural analysis of NA predicted that a third substitution (N386K) in the NA of the cluster virus destabilized the mutant NA structure in the presence of the V241I and N369K substitutions. Our results suggest that the cluster virus retained viral fitness to spread among humans and, accordingly, caused the large cluster in Sapporo/Hokkaido. However, the mutant NA structure was less stable than that of the wild-type virus. Therefore, once the wild-type virus began to circulate in the community, the mutant virus could not compete and faded out.
INTRODUCTION
The neuraminidase (NA) inhibitors oseltamivir and zanamivir are recommended by the World Health Organization (WHO) for the treatment of influenza patients, as well as for chemoprophylaxis (1). In Japan, four NA inhibitors, oseltamivir, zanamivir, peramivir, and laninamivir, are approved and prescribed at the highest frequency in the world. Therefore, the surveillance of NA inhibitor-resistant viruses is important for public health authorities and clinicians to control influenza. We have been conducting such surveillance throughout Japan since 1999 (2–6).
During the 2006-2007 influenza season, an oseltamivir-resistant former seasonal influenza A(H1N1) virus was first reported in Norway; this virus then spread rapidly and predominated globally until it was replaced by the pandemic A(H1N1) [A(H1N1)pdm09] virus in 2009 (7). The resistant A(H1N1) virus possessed a histidine-to-tyrosine substitution at position 275 (N1 numbering, H275Y) in its NA protein, which was responsible for its drug resistance (7). Four additional NA substitutions, R222Q, V234M, D344N, and D354G, were shown to compensate for the detrimental effect of the H275Y substitution on viral fitness, thereby making the mutant virus more transmissible in the community than the wild-type virus (8–11). However, since the A(H1N1)pdm09 virus began circulating globally in 2009, the H275Y mutant A(H1N1)pdm09 virus has been detected in various regions of the world only sporadically. In 2011, a widespread cluster of the H275Y mutant A(H1N1)pdm09 virus occurred in Newcastle, Australia (12). The V241I and N369K substitutions in the NA of this mutant virus were reported to increase its replication and transmission fitness, contributing to its efficient transmission (13, 14).
Recently, we reported a community cluster of A(H1N1)pdm09 viruses exhibiting cross-resistance to oseltamivir and peramivir in Sapporo, the capital of Hokkaido, Japan (15). In November and December 2013, all six A(H1N1)pdm09 viruses isolated in Sapporo were resistant to oseltamivir and peramivir and possessed the H275Y substitution (15). We subsequently increased nationwide monitoring for NA inhibitor-resistant viruses and observed a further spread of this resistant virus to other areas of Hokkaido in January and February 2014. However, after the wild-type A(H1N1)pdm09 virus began to spread in Hokkaido, the resistant virus was replaced by the wild-type virus and disappeared.
Here, we report our assessment of the in vitro and in vivo properties of the cluster virus to understand the basis for the epidemic in Hokkaido.
MATERIALS AND METHODS
Viruses.
Clinical specimens and the corresponding patient records were collected in 500 sentinel clinics and hospitals as part of the National Epidemiological Surveillance of Infectious Diseases in Japan. Influenza A(H1N1)pdm09 viruses, isolated using MDCK or Caco-2 cells, were propagated in MDCK cells for further analysis. Strain A/Chiba/1017/2009 served as an early H275Y mutant A(H1N1)pdm09 virus from a sporadic case in Japan (5). The strain A/Shizuoka-c/99/2013 represented wild-type A(H1N1)pdm09 viruses detected at the end of the previous influenza season in Japan.
Allelic discrimination assay.
To detect the H275Y substitution, the allelic discrimination assay was performed as previously described (6, 16). A culture supernatant of virus-infected cells was applied, without RNA extraction, to the one-step duplex reverse transcription-PCR using the QuantiTect virus kit (Qiagen, Düsseldorf, Germany). This assay can distinguish between the H275Y mutant and wild-type virus in a mixed virus population (16).
Phylogenetic analysis.
The nucleotide sequences of the NA and hemagglutinin (HA) genes were subjected to phylogenetic analysis. Phylogenetic trees were constructed using the MEGA 6 software (17) with the neighbor-joining method. The nucleotide sequences used in this study are available from the EpiFlu database of the Global Initiative on Sharing All Influenza Data (GISAID).
NA inhibition assay.
The susceptibilities of the viruses to four NA inhibitors, oseltamivir, peramivir, zanamivir, and laninamivir, were determined by using a fluorescent NA inhibition assay with the NA-Fluor influenza neuraminidase assay kit (Applied Biosystems, Foster City, CA, USA). Oseltamivir carboxylate, peramivir, and zanamivir were purchased from Sequoia Research Products (Pangbourne, United Kingdom), and laninamivir was kindly provided by Daiichi Sankyo Co. Ltd. (Tokyo, Japan). The results are expressed as the drug concentration required to inhibit NA activity by 50% (IC50). To interpret NA inhibitor susceptibility, we used the WHO criteria, which are based on the fold change in the IC50 compared with the median IC50 of the same type/subtype/lineage (18). For influenza A viruses, normal inhibition (NI) (<10-fold increase), reduced inhibition (RI) (10- to 100-fold increase), and highly reduced inhibition (HRI) (>100-fold increase) were defined.
Experimental infection of ferrets.
To explore the replication of the H275Y mutant virus from the Sapporo/Hokkaido cluster (Sapporo/Hokkaido cluster virus) in respiratory organs, we experimentally infected ferrets, a commonly used animal model of human influenza, as described previously (19). Under anesthesia, six 5- to 6-month-old female ferrets per group, which were serologically negative for influenza viruses, were infected intranasally with 0.5 ml of 106 PFU A/Sapporo/114/2013, a representative Sapporo/Hokkaido cluster virus (15), or A/Shizuoka-c/99/2013, a representative wild-type virus detected at the end of the previous influenza season. Three ferrets from each group were euthanized on days 3 and 6 postinfection (p.i.), and various organs were subjected to virus titration by using plaque assays in MDCK cells. All ferret experiments were performed in accordance with the University of Tokyo Regulations for Animal Care and Use and approved by the animal experiment committee of the Institute of Medical Science at the University of Tokyo (approval no. PA11-13).
Ferret transmission study.
To assess the transmissibility of the Sapporo/Hokkaido cluster virus, we performed a ferret transmission study, as described previously (19). Pairs of 5- to 6-month-old female ferrets were housed separately in wire-frame cages 5 cm apart, such that direct contact between the ferrets was prevented but virus spread could occur via respiratory droplets. Under anesthesia, three ferrets per group were infected intranasally with 0.5 ml of 106 PFU A/Sapporo/114/2013 from the cluster or wild-type A/Shizuoka-c/99/2013 (inoculated ferrets). The next day, three naive ferrets (contact ferrets) were each placed in a cage adjacent to that of an inoculated ferret. Nasal washes were collected from the inoculated ferrets on day 1 after inoculation and from the contact ferrets on day 1 after cohousing, and then every other day for virus titration by using plaque assays in the MDCK cells. Serum samples were collected at 20 days p.i. Seroconversion was evaluated by using hemagglutination inhibition assays with A/Sapporo/114/2013 virus and turkey red blood cells.
Competitive virus replication in cell culture.
To compare the concomitant growth capability of the Sapporo/Hokkaido cluster virus with that of the wild-type virus, either A/Sapporo/114/2013 from the cluster or strain A/Chiba/1017/2009, an early H275Y mutant virus from a sporadic case, six-well plates were coinfected with wild-type A/Shizuoka-c/99/2013 at a multiplicity of infection (MOI) of 0.01 PFU/cell in triplicate. For this experiment, MDCK-AX4 cells were used, which overexpress the β-galactoside α2,6-sialyltransferase I gene (20), to mimic the receptor environment of the epithelia of human upper respiratory airways. At 2 days p.i., the culture fluids were subjected to virus titration by using plaque assays in MDCK-AX4 cells and to the allelic discrimination assay to determine the relative proportion of each genotype. The viruses were serially passaged three times at an MOI of 0.01 PFU/cell.
Structural analysis of the NA protein of the H275Y mutant virus.
A structure model of the NA protein of the H275Y mutant virus was constructed by use of homology modeling and was refined by using MOE (Chemical Computing Group, Inc., Quebec, Canada). The crystal structure of the A(H1N1)pdm09 NA protein (PDB ID 4B7R; resolution, 1.9 Å) (21) served as the modeling template. Single-point substitutions were generated on the NA model. Ensembles of the protein conformations were generated by using the LowMode MD module in MOE. The average stability changes of the ensembles were calculated by using the Boltzmann distribution. The stability scores (ΔΔGs) of the structures were obtained through the stability scoring function of the Protein Design application, as previously described (22).
Statistical analysis.
All statistical analyses were performed using GraphPad Prism version 5.04 for Windows (GraphPad Software, San Diego, CA, USA). Statistically significant differences between groups were determined by using Fisher's exact test, Welch's t test, or Student's t test on the result of the F-test. P values of <0.05 were considered statistically significant.
Nucleotide sequence accession numbers.
The accession numbers of the viruses used in the trees are shown in Table S1 in the supplemental material. The amino acids are described with the N1 numbering. The accession numbers for A/California/07/2009, A/Chiba/1017/2009, and A/Hong Kong/5008/2013 are listed in Table 1.
TABLE 1.
Characteristic amino acids of the H275Y mutant influenza A(H1N1)pdm09 viruses detected in Japan, China, and the United States in 2013/2014 and in Australia in 2011
| Strain (type) | Amino acid by position in protein: |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB2 |
PB1 |
HA |
NP |
NA |
M1 |
M2 |
|||||||||||
| 627 | 191 | 261 | 327 | 652 | 15 | 222 | 223 | 283 | 202 | 241 | 275 | 369 | 386 | 30 | 227 | 31 | |
| A/California/07/2009 (reference wild type)a | E | V | S | R | A | T | D | Q | K | N | V | H | N | N | S | A | N |
| A/Chiba/1017/2009 (early sporadic H275Y mutant)a | E | V | S | R | A | T | D | Q | K | N | V | Y | N | N | S | A | N |
| Newcastle cluster, Australia, 2011 (H275Y mutant) | E | V | S | R | A | T | D | Q | K | N | I | Y | K | S | S | A | N |
| A/Shizuoka-c/99/2013 (wild type) | E | V | S | R | A | T | D | Q | E | N | I | H | K | N | S | A | N |
| United States, 2013/2014 (H275Y mutant) | E | V | S | R | A | T | D | Q | E | N | I | Y | K | N/K | S | A | N |
| China, 2013/2014 (H275Y mutant) | —b | — | — | — | — | T | D | Q | E/D | — | I | Y | K | K | S | A/T | N |
| A/Hong Kong/5008/2013 (wild type)a | E | V | N | K | T | T | D | Q | D | N | I | H | K | K | S | T | N |
| Sapporo/Hokkaido cluster, Japan, 2013/2014 (H275Y mutant)c | E | I | N | K | T | S | D | Q | D | S | I | Y | K | K | N | T | N |
The accession numbers of A/California/07/2009, A/Chiba/1017/2009, and A/Hong Kong/5008/2013 are EPI_ISL_31158, EPI_ISL_65885, and EPI_ISL_150295, respectively.
Sequences were not available in the GISAID EpiFlu database.
Substitutions specific to the H275Y mutant viruses in the Sapporo/Hokkaido cluster are shown in bold type.
RESULTS
Detection of influenza A(H1N1)pdm09 viruses with the H275Y substitution.
In the 2013-2014 influenza season, the A(H1N1)pdm09 virus predominated in Japan (Fig. 1A and B). Between September 2013 and July 2014, 2,482 A(H1N1)pdm09 viruses were screened for the H275Y substitution by using the allelic discrimination assay and/or NA gene sequencing (Fig. 1C and D). In contrast to the low global average (approximately 1%), 104 (4.2%) of the viruses tested possessed the H275Y mutation, 39 of which were isolated in Hokkaido. In Sapporo, the capital of Hokkaido, a community cluster of the H275Y mutant virus occurred between November and December 2013 (15) prior to the main influenza epidemic in Japan. This mutant virus predominated in Sapporo and spread to neighboring areas in Hokkaido (Fig. 2) in January and February 2014. When the wild-type A(H1N1)pdm09 virus invaded Hokkaido, it replaced the mutant virus, which then disappeared (Fig. 1C). However, in wide areas outside Hokkaido, the H275Y mutant virus continued to appear sporadically (Fig. 1D). The overall detection rates of the mutant virus within and outside Hokkaido were 29% and 2.8%, respectively.
FIG 1.
Detection of influenza viruses between September 2013 and May 2014 in Japan. (A and B) Weekly reports of influenza virus isolation/detection from local public health institutes as part of the National Epidemiological Surveillance of Infectious Diseases. (C and D) Number of A(H1N1)pdm09 viruses with the H275Y substitution. The viruses in Hokkaido were reported by the Sapporo City Institute of Public Health and the Hokkaido Institute of Public Health.
FIG 2.

Distribution of the H275Y mutant influenza A(H1N1)pdm09 viruses in Hokkaido. The cities and towns where the mutant viruses were detected are shown in black.
Genetic analysis of the H275Y mutant viruses.
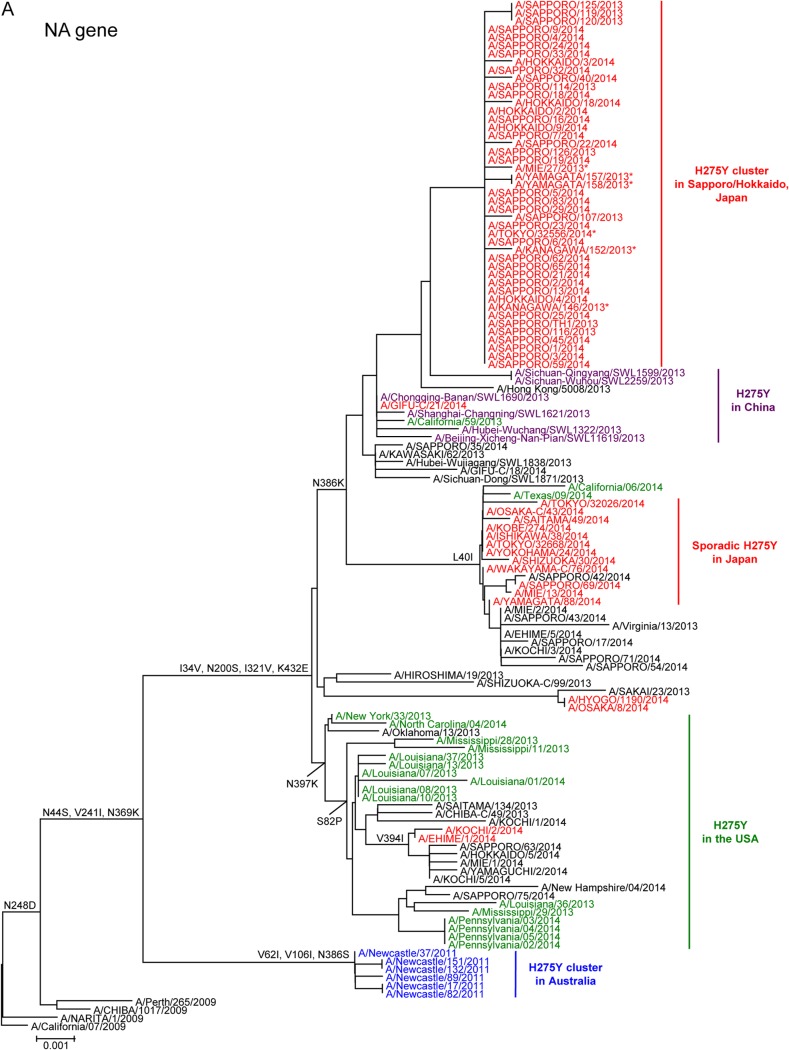
Representative A(H1N1)pdm09 viruses isolated during the 2013-2014 influenza season were analyzed phylogenetically. The nucleotide sequences of the NA (Fig. 3A) and HA (Fig. 3B) genes of the Sapporo/Hokkaido cluster viruses were closely related to one another and formed a distinct subgroup in both phylogenetic trees. These results suggest the clonal expansion of a single mutant virus in Sapporo and subsequently to neighboring areas in Hokkaido. Six out of 65 mutant viruses detected outside Hokkaido belonged to the same subgroup of the Sapporo/Hokkaido cluster. Two viruses (A/Mie/27/2013 and A/Tokyo/32556/2014) were isolated from patients who had visited Sapporo a few days before the onset of symptoms. The other four viruses (A/Kanagawa/146/2013, A/Kanagawa/152/2013, A/Yamagata/157/2013, and A/Yamagata/158/2013) were detected in November 2013, almost the same time as the first case of the Sapporo cluster. However, these four patients had no known epidemiological link to Sapporo/Hokkaido. None of the six mutant viruses spread further in their local communities, where wild-type viruses had been circulating. These findings suggest that the original mutant virus was seeded to several different areas in Japan at around the same time in early November, but only the virus in Sapporo continued to spread locally.
FIG 3.
Phylogenetic analysis of the neuraminidase (A) and hemagglutinin (B) genes of the H275Y mutant influenza A(H1N1)pdm09 viruses detected in Japan, China, and the United States in 2013/2014 and in the Australian cluster in 2011. The gene sequences were downloaded from the GISAID EpiFlu database (www.gisaid.org). The phylogenetic trees were constructed using the MEGA 6 software with the neighbor-joining method. The mutant viruses isolated in Japan, China, the United States, and Australia are shown in red, purple, green, and blue, respectively. Wild-type viruses are shown in black. The amino acid substitutions relative to the A/California/07/2009 virus are shown to the left of the nodes. The scale bar indicates the nucleotide substitutions per site. *, Sapporo/Hokkaido cluster viruses detected outside Hokkaido.
During the 2013-2014 influenza season, H275Y mutant viruses were also reported sporadically in other areas of the world (23, 24). The vast majority of the recently circulating A(H1N1)pdm09 viruses possessed two amino acid substitutions, V241I and N369K, in NA, which compensate for the detrimental effect of the H275Y substitution on viral fitness (18). All of the Sapporo/Hokkaido cluster viruses possessed an additional NA substitution, N386K (Table 1 and Fig. 3A). The NA and HA genes of the mutant viruses of the Sapporo/Hokkaido cluster were distinct from those of the United States, as well as from those of the Newcastle cluster in 2011 (Table 1 and Fig. 3). However, the mutant viruses of the Sapporo/Hokkaido cluster were genetically similar to those detected in northern China between September and December 2013, sharing the N386K substitution in NA (Table 1 and Fig. 3A). Therefore, the mutant viruses of the Sapporo/Hokkaido cluster and those of China may derive from a common ancestor. However, N386K also occurred in both the sporadic mutant and wild-type viruses in Japan, China, and the United States (Table 1 and Fig. 3A), although most (>89%) H275Y mutant viruses in the United States did not possess the N386K substitution (23).
A comparison of the entire genome sequences of the A(H1N1)pdm09 viruses demonstrated that four amino acid substitutions, V191I in polymerase basic 1 (PB1), N202S in nucleoprotein (NP), T15S in HA, and S30N in matrix 1 (M1), were unique to the Sapporo/Hokkaido cluster virus (Table 1).
Patients infected with the H275Y mutant viruses.
The characteristics of the patients infected with the H275Y mutant virus of the Sapporo/Hokkaido cluster were compared with those of patients infected in sporadic cases with the mutant virus (Table 2). In the cluster subgroup, 27 (60%) of the 45 patients were male and 26 (58%) were children <10 years of age. No epidemiological links were identified among the patients except for one family infection, and almost all of the patients had had no exposure to NA inhibitors before the specimen collection. These results suggest that sustained transmissions of the mutant virus occurred in Sapporo/Hokkaido. Except for a young and otherwise healthy woman who was hospitalized for severe pneumonia (15), all patients in the cluster showed mild symptoms and received outpatient care only.
TABLE 2.
Characteristics of patients infected with the H275Y mutant influenza A(H1N1)pdm09 viruses
| Patient characteristic | No. (%) with characteristic among: |
P value | |
|---|---|---|---|
| Sapporo/Hokkaido cluster (n = 45) | Sporadic cases (n = 59) | ||
| Male sex | 27 (60) | 32 (54) | 0.69 |
| Age <10 yr | 26 (58) | 49 (83) | 0.008 |
| Hospitalized | 1 (2) | 12 (20) | 0.006 |
| No exposure to NA inhibitors | 44 (98) | 19 (32) | <0.0001 |
Among 59 sporadic cases that included 12 hospitalized patients, 32 (54%) were male and 49 (83%) were children. Most of the sporadic cases were associated with oseltamivir and/or peramivir treatment, suggesting that the emergence of the sporadic mutant viruses was attributable to the selective pressure of the NA inhibitors.
Antiviral susceptibility of the H275Y mutant viruses.
We compared the susceptibilities of the Sapporo/Hokkaido cluster viruses and the sporadic H275Y mutant viruses to four NA inhibitors, oseltamivir, peramivir, zanamivir, and laninamivir (Fig. 4). The median IC50s to oseltamivir and peramivir of the cluster viruses were 780- and 370-fold higher, respectively, than those of the wild-type viruses isolated during the same influenza season in Japan. The sporadic mutant viruses also exhibited 830- and 380-fold higher median IC50s to oseltamivir and peramivir, respectively, than those of the wild-type viruses. In contrast, the median IC50s to zanamivir and laninamivir of both mutant virus groups were comparable to those of wild-type viruses. These results demonstrate that both sources of mutant virus showed HRI against oseltamivir and peramivir but were susceptible to zanamivir and laninamivir, according to the WHO criteria (18).
FIG 4.

Susceptibilities of the H275Y mutant influenza A(H1N1)pdm09 viruses to neuraminidase inhibitors. The IC50s of the mutant viruses from the Sapporo/Hokkaido cluster and other sporadic cases to oseltamivir, peramivir, zanamivir, and laninamivir were determined by the use of a fluorescent neuraminidase inhibition assay. Box-and-whisker plots of the IC50s (medians and interquartile ranges) are shown. The numbers at the bottom of each box-and-whisker plot indicate the fold change in IC50 compared with the median IC50 of wild-type viruses isolated in Japan during the same period.
Replication of the H275Y mutant virus in the respiratory organs of ferrets.
Because the Sapporo/Hokkaido cluster virus emerged ahead of the wild-type virus in the 2013-2014 influenza season, we compared the replication of a representative cluster virus and a representative wild-type virus that was isolated at the end of the previous influenza season in the respiratory organs of ferrets. The ferrets were infected intranasally with A/Sapporo/114/2013 from the cluster or with wild-type A/Shizuoka-c/99/2013. Both viruses replicated more efficiently in the nasal turbinates than in the trachea and lungs, and the virus titers at 3 days p.i. were higher than those at 6 days p.i. for both viruses (Table 3). At 6 days p.i., the cluster virus was detected in the lower respiratory tract of one of six infected ferrets, whereas the wild-type virus was not, consistent with our previous study using a wild-type A(H1N1)pdm09 virus in Japan (19). These results suggest that the Sapporo/Hokkaido cluster virus replicated preferentially in the upper respiratory tract of ferrets as efficiently as did the wild-type virus, but less efficiently in the lower respiratory tract.
TABLE 3.
Virus titers in organs of infected ferretsa
| Virus (type) | Day | Virus titer of ferrets infected in: |
||
|---|---|---|---|---|
| Nasal turbinates | Trachea | Lung | ||
| A/Sapporo/114/2013 (H275Y mutant) | 3 | 6.0 ± 1.6 | 4.1 | 2.0 |
| 6 | 2.2, 5.0 | 2.5 | —b | |
| A/Shizuoka-c/99/2013 (wild type) | 3 | 6.7 ± 0.9 | 2.7, 3.4 | 3.2 ± 0.3 |
| 6 | 2.0 ± 0.2 | — | — | |
Ferrets were intranasally infected with 0.5 ml of 106 PFU virus. Three ferrets from each group were euthanized on days 3 and 6 postinfection for viral titration. Virus replication in the liver, duodenum, spleen, and kidneys was also examined; no virus was detected in these organs. The values are presented as the mean log10 PFU ± SD/g or as individual titers when virus was not recovered from all three ferrets.
—, virus not detected.
Transmissibility of the H275Y mutant virus in ferrets.
To assess the droplet transmission of the Sapporo/Hokkaido cluster virus, wire-frame cages housing naive ferrets were placed next to cages containing ferrets that had been infected on the previous day with A/Sapporo/114/2013 from the cluster or with wild-type A/Shizuoka-c/99/2013. We recovered the cluster virus from the nasal washes of all of the contact ferrets and the wild-type virus from the nasal washes of one of the three contact ferrets (Table 4). Seroconversion against A/Sapporo/114/2013 virus and the wild-type virus supported droplet transmission (Table 4). These results suggest that the Sapporo/Hokkaido cluster virus underwent droplet transmission among ferrets similarly to or somewhat better than the wild-type virus.
TABLE 4.
Virus titers in nasal washes of inoculated and contact ferrets and seroconversion titers
| Virus (type) | Pair | Ferret groupa | Virus titer (log10 PFU/ml) in nasal wash on day: |
Seroconversion (HI titer)b | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 9 | 11 | ||||
| A/Sapporo/114/2013 (H275Y mutant) | 1 | I | 7.7 | 4.6 | 3.1 | —c | — | — | 1,280 |
| C | 4.5 | 5.1 | 5.3 | — | — | — | 1,280 | ||
| 2 | I | 7.6 | 3.7 | 3.4 | — | — | — | 1,280 | |
| C | — | — | — | 6.0 | 3.8 | — | 1,280 | ||
| 3 | I | 7.7 | 3.3 | 4.7 | 1.0 | — | — | 640 | |
| C | — | — | 6.5 | 3.6 | — | — | 1,280 | ||
| A/Shizuoka-c/99/2013 (wild type) | 1 | I | 7.3 | 5.3 | 4.3 | — | — | — | 2,560 |
| C | — | — | — | — | — | — | <10 | ||
| 2 | I | 7.3 | 5.4 | 5.3 | — | 2,560 | |||
| C | — | — | — | — | — | — | <10 | ||
| 3 | I | 7.8 | 5.3 | 4.1 | — | — | — | 2,560 | |
| C | — | 5.7 | 4.6 | 2.1 | — | — | 1,280 | ||
For each pair of ferrets, one animal was intranasally inoculated with 0.5 ml of 106 PFU virus (inoculated ferret [I]) and 24 h later, a naive ferret was placed in an adjacent cage (contact ferret [C]). Nasal washes were collected from inoculated and contact ferrets every other day for virus titration.
Hemagglutination inhibition (HI) assays were carried out with A/Sapporo/114/2013 virus and turkey red blood cells.
—, virus not detected (detection limit, 1.3 log10 PFU/ml).
Competitive growth capability of the H275Y mutant viruses with that of wild-type virus.
Previous reports suggested that the introduction of the V241I and N369K substitutions to the NA protein of the H275Y mutant A(H1N1)pdm09 virus resulted in greater viral fitness (13, 14). Because all circulating viruses in Japan, including the cluster viruses, possessed the V241I and N369K substitutions (Table 1 and Fig. 3A), we used H275Y mutant A/Chiba/1017/2009 virus from an early sporadic case, which has neither V241I nor N369K, to compare the competitive growth of A/Sapporo/114/2013 from the Sapporo/Hokkaido cluster or the early mutant A/Chiba/1017/2009 with that of the wild-type virus. MDCK-AX4 cells were coinfected with either A/Sapporo/114/2013 or A/Chiba/1017/2009 virus and with wild-type A/Shizuoka-c/99/2013. The culture fluids were harvested at 2 days p.i. and passaged serially to other cell cultures three times. After each passage, the relative proportions of each virus were determined (Fig. 5). Both mixed virus populations replicated efficiently. However, the proportion of the early mutant A/Chiba/1017/2009 to that of wild-type virus decreased significantly, whereas the proportion of the mutant A/Sapporo/114/2013 from the cluster to that of wild-type virus was 84% at passage 1, decreasing to 69% and 39% at passages 2 and 3, respectively, indicating that the mutant A/Sapporo/114/2013 virus continued to replicate efficiently, competing with the wild-type virus at the initial cycle of infection. These results suggest that the Sapporo/Hokkaido cluster virus retained the ability to grow comparably to the wild-type virus and grew significantly better than did the early mutant virus, consistent with previous reports (13, 14).
FIG 5.

Competitive growth capabilities of the H275Y mutant influenza A(H1N1)pdm09 virus and the wild-type virus. MDCK-AX4 cells overexpressing the β-galactoside α2,6-sialyltransferase I gene were coinfected in triplicate with mutant A/Sapporo/114/2013 or A/Chiba/1017/2009, and with wild-type A/Shizuoka-c/99/2013 at a multiplicity of infection of 0.01 PFU/cell for each virus. At 2 days postinfection, the culture supernatant was harvested and serially passaged three times at a multiplicity of infection of 0.01 PFU/cell. Each culture supernatant was subjected to the allelic discrimination assay to determine the relative proportion of each genotype. The error bars indicate the standard deviations.
Effects of amino acid substitutions on the stability of the NA protein.
The H275Y mutant viruses from the community cluster in Newcastle, Australia, in 2011 possessed three additional amino acid substitutions, V241I, N369K, and N386S, in their NA (12). The H275Y substitution caused a detrimental effect on viral fitness by decreasing the stability of the NA protein (12). Three additional changes were thought to improve the stability of the mutant NA, thereby compensating for the negative effects of the H275Y substitution on the growth and transmissibility of the mutant virus (13, 14). The Sapporo/Hokkaido cluster viruses also possessed three substitutions (Table 1 and Fig. 3A and 6), two of which, V241I and N369K, were identical to those of the Newcastle cluster virus. The third substitution, N386K, occurred at the same position but with a different amino acid (N386S in the Newcastle cluster virus). Both of these substitutions at residue 386 resulted in the loss of a glycosylation site.
FIG 6.
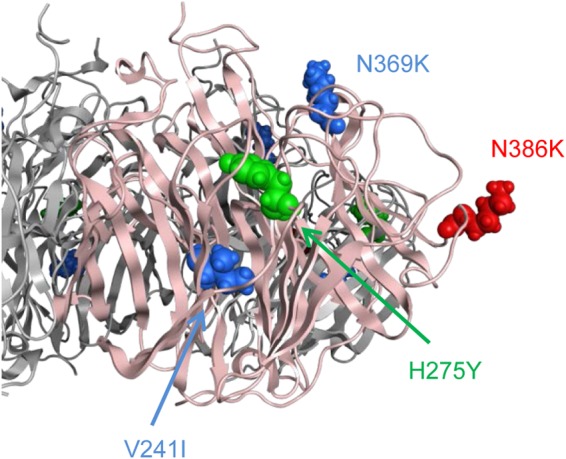
Three-dimensional structure of mutant A/Sapporo/144/2013 NA. A molecular model of the NA protein of the mutant virus was constructed by means of homology modeling and refined by using MOE. Single-point substitutions were generated on the NA model.
To assess the potential effect of the substitution at residue 386 on mutant NA stability, we conducted an in silico mutagenesis study. In the presence of two additional substitutions, V241I and N369K, stability changes by the reversion K386N (to wild type) or K386S (to Newcastle cluster virus) in A/Sapporo/114/2013 from the Sapporo/Hokkaido cluster were −0.90 ± 0.37 and −1.25 ± 0.21 kcal/mol (means ± standard deviations), respectively, whereas those by the reversion S386N (to wild type) or S386K (to Sapporo/Hokkaido cluster virus) in A/Newcastle/37/2011 from the Newcastle cluster were 0.20 ± 0.53 and 0.91 ± 0.27 kcal/mol, respectively. These results suggest that the N386K substitution destabilized the mutant NA structure in the presence of the V241I and N369K substitutions, in contrast to the effect of the N386S substitution in the Newcastle cluster virus, negatively affecting the transmission of the mutant virus.
DISCUSSION
Between November 2013 and February 2014, Japan experienced a large cluster of infections with influenza A(H1N1)pdm09 virus that was cross-resistant to oseltamivir and peramivir. This resistant virus, which possessed the H275Y substitution in its NA, was first detected in November 2013 at four sentinel clinics and hospitals in Sapporo, the capital of Hokkaido (15), and then spread predominantly in the community and to other areas of Hokkaido. By 7 January 2014, all of the A(H1N1)pdm09 viruses detected in Sapporo were the H275Y mutant virus. The mutant virus exhibited high IC50s to oseltamivir and peramivir but remained sensitive to zanamivir and laninamivir. The finding that most patients had not taken an NA inhibitor before specimen collection, together with a phylogenetic analysis of the viral genes, suggests that the clonal expansion of the mutant virus occurred in the community.
In Japan outside Hokkaido, two distinct subgroups of A(H1N1)pdm09 virus cocirculated during the 2013-2014 influenza season. One possessed the signature amino acid L40I in its NA, and the other shared the S82P and N397K substitutions with the H275Y mutant viruses of the United States (Fig. 3A). Both of these subgroups belong to lineages distinct from those of the Sapporo/Hokkaido cluster viruses. The H275Y mutant viruses appeared in the two subgroups but at a low level of 2.8%. The first wild-type A(H1N1)pdm09 virus detected in Hokkaido on 7 January 2014 belonged to the L40I subgroup, whereas the other wild-type virus of the S82P and N397K subgroup was first detected on 14 January 2014. Both wild-type viruses spread rapidly into Hokkaido and became predominant by week 5 of 2014. Meanwhile, the Sapporo/Hokkaido cluster virus became a minor population and disappeared by mid-February 2014. No significant antigenic differences were found among these viruses (data not shown), excluding possible immune selection via antigenic drift. Six Sapporo/Hokkaido cluster viruses were detected outside Hokkaido, where wild-type viruses had been circulating, but the cluster viruses did not spread further. Based on these observations, we hypothesized that the Sapporo/Hokkaido cluster viruses had acquired better replication and transmission fitness than did the H275Y mutant viruses of the sporadic cases but retained a disadvantage in competing with wild-type virus. To assess this hypothesis, we conducted a comparative study of the in vitro and in vivo properties of the viruses.
In the ferret model, the Sapporo/Hokkaido cluster virus replicated more efficiently in the upper respiratory tract than in the lower respiratory tract, indicating a favorable condition for droplet transmission. Indeed, the virus transmitted among ferrets by droplets as efficiently as did the wild-type virus. In cultured cells coinfected with the H275Y mutant virus and wild-type virus, the Sapporo/Hokkaido cluster virus replicated to a similar extent as the wild-type virus and better than an early sporadic H275Y mutant virus. These results suggest that the Sapporo/Hokkaido cluster virus possessed viral fitness comparable to that of wild-type virus and better than that of an early sporadic H275Y mutant virus and thus had the potential to transmit efficiently among humans.
During the 2007-2008 influenza season, an oseltamivir-resistant former seasonal A(H1N1) virus emerged in Europe and spread rapidly worldwide, replacing the wild-type virus and becoming the major seasonal A(H1N1) virus within a year (7). This resistant virus possessed, in addition to the H275Y substitution, permissive mutations in its NA (8–11) and HA (25) proteins. Some of these substitutions likely restored its viral fitness and enhanced its replication and transmission capabilities, thereby conferring advantageous characteristics to outcompete the parent wild-type virus and predominate in the community. After the (H1N1)2009 pandemic started, a widespread community cluster of infections with an oseltamivir-resistant A(H1N1)pdm09 virus occurred in 2011 in Newcastle, Australia (12). This resistant virus was found to possess the H275Y substitution and three additional substitutions, V241I, N369K, and N386S, in its NA. Two of the substitutions, V241I and N369K, were reported to confer robust viral fitness on the H275Y mutant virus (13, 14). However, the third substitution, N386S, decreased the enzymatic activity and surface expression of NA in infected cells, suggesting a negative effect on viral fitness (13). Similarly, the Sapporo/Hokkaido cluster viruses were found to possess the V241I, N369K, and N386K substitutions. V241I and N369K were present in the Newcastle cluster virus, strongly suggesting the same advantageous effects on viral fitness as those reported previously (13, 14). The third substitution, N386K, also occurred in the Newcastle cluster virus at the same position but with a different amino acid. Our structure analysis predicted that N386K destabilizes the mutant NA structure in the presence of the V241I and N369K substitutions, causing a negative effect on the fitness of the Sapporo/Hokkaido cluster viruses. The N386K also occurred in the sporadic H275Y mutant and wild-type viruses (Fig. 3A). However, most of the viruses possessed the L40I substitution in their NA in addition to the N386K substitution. The effect of the combination of these two substitutions on NA stability remains unknown.
A comparative analysis of the entire genome sequences of A(H1N1)pdm09 viruses revealed that four amino acid substitutions, V191I in PB1, N202S in NP, T15S in HA, and S30N in M1, were specific for the Sapporo/Hokkaido cluster virus. The effect of these substitutions on the viral fitness of the H275Y mutant viruses remains to be clarified.
In summary, our results suggest that the Sapporo/Hokkaido cluster virus retained replication and transmission capabilities to some extent, due to the permissive substitutions V241I and N369K in NA. Accordingly, the cluster virus was able to spread and predominate in the community as long as no competitor virus circulated simultaneously. However, the structure of the mutant NA molecule was less stable than that of the wild-type virus, presumably because of the N386K substitution. Therefore, once the wild-type virus began to circulate in the community, the cluster virus was not able to compete with the wild-type virus and faded out.
The H275Y mutant viruses are currently resistant to oseltamivir and peramivir but sensitive to zanamivir and laninamivir, and their transmission remains restricted, due to impaired viral fitness. However, given that oseltamivir- and peramivir-cross-resistant viruses in the community have the potential to be readily transmitted among humans, the surveillance of these viruses must continue in order to protect public health and aid in clinical management.
Supplementary Material
ACKNOWLEDGMENTS
We thank Miho Ejima, Hiromi Sugawara, Aya Sato, Reiko Itoh, Mai Miura, Teruko Doi, Reina Yamaji, Naomi Fujimoto, Kiyoko Iwatsuki-Horimoto, Seiya Yamayoshi, and Shinya Yamada for technical assistance. We also thank Susan Watson for scientific editing. We also thank the laboratories for originating and submitting the sequences to the GISAID EpiFlu database that we used in this paper.
This work was supported in part by grants-in-aid for emerging and reemerging infectious diseases from the Ministry of Health, Labor, and Welfare, Japan, by JSPS KAKENHI grant 26460816, by the Japan Initiative for the Global Research Network on Infectious Diseases from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, by ERATO (Japan Science and Technology Agency), by Strategic Basic Research Programs, Japan Science and Technology Agency, by the National Institute of Allergy and Infectious Diseases Public Health Service research grants, and by an NIAID-funded Center for Research on Influenza Pathogenesis (CRIP) grant (HHSN266200700010C).
The members of the Influenza Virus Surveillance Group of Japan are Rika Komagome, Masahiro Miyoshi, and Hideki Nagano (Hokkaido Institute of Public Health), Asami Ohnishi (Sapporo City Institute of Public Health), Rika Tsutsui (Aomori Prefectural Institute of Public Health and Environment), Masaki Takahashi (Research Institute for Environmental Sciences and Public Health of Iwate Prefecture), Yuko Suzuki (Miyagi Prefectural Institute of Public Health and Environment), Ayumi Nakata (Sendai City Institute of Public Health), Chihiro Shibata (Akita Prefectural Research Center for Public Health and Environment), Yoko Aoki (Yamagata Prefectural Institute of Public Health), Kaduhiro Kitakawa (Fukushima Prefectural Institute of Public Health), Chika Hirokawa (Niigata Prefectural Institute of Public Health and Environmental Sciences), Kazunari Yamamoto (Niigata City Institute of Public Health and Environment), Ikuko Doi (Ibaraki Prefectural Institute of Public Health), Fuminori Mizukoshi (Tochigi Prefectural Institute of Public Health and Environmental Sciences), Chikako Nagashima (Utsunomiya City Institute of Public Health and Environment Science), Hiroyuki Tsukagoshi (Gunma Prefectural Institute of Public Health and Environmental Sciences), Noriko Suzuki (Saitama Institute of Public Health), Yuka Uno (Saitama City Institute of Health Science and Research), Atsushi Ogura (Chiba Prefectural Institute of Public Health), Ayano Mizumura (Chiba City Institute of Health and Environment), Sachiko Harada (Tokyo Metropolitan Institute of Public Health), Sumi Watanabe (Kanagawa Prefectural Institute of Public Health), Chiharu Kawakami (Yokohama City Institute of Health), Hideaki Shimizu (Kawasaki City Institute of Public Health), Junko Yamaguchi (Yokosuka Institute of Public Health), Kyoko Mochizuki (Sagamihara City Laboratory of Public Health), Masayuki Oonuma (Yamanashi Institute for Public Health), Hiroki Kobayashi (Nagano Environmental Conservation Research Institute), Yuichiro Okamura (Nagano City Health Center), Toshihiro Yamada (Shizuoka Institute of Environment and Hygiene), Nona Shibahara (Shizuoka City Institute of Environmental Sciences and Public Health), Tatsuya Jimbo (Hamamatsu City Health Environment Research Center), Masatsugu Obuchi (Toyama Institute of Health), Hiroe Kodama (Ishikawa Prefectural Institute of Public Health and Environmental Science), Nozomi Noda (Fukui Prefectural Institute of Public Health and Environmental Science), Tsuyoshi Kuzuguchi (Gifu Prefectural Research Institute for Health and Environmental Sciences), Yasunori Tanaka (Gifu Municipal Institute of Public Health), Yoshihiro Yasui (Aichi Prefectural Institute of Public Health), Takuya Yano (Mie Prefecture Health and Environment Research Institute), Hiromi Kodama (Shiga Prefectural Institute of Public Health), Masayoshi Watanabe (Kyoto City Institute of Health and Environmental Sciences), Satoshi Hiroi (Osaka Prefectural Institute of Public Health), Hideyuki Kubo (Osaka City Institute of Public Health and Environmental Sciences), Kiyoko Uchino (Sakai City Institute of Public Health), Tomohiro Oshibe (Hyogo Prefectural Institute of Public Health and Consumer Sciences), Ai Mori (Kobe Institute of Health), Masaki Yoneda (Nara Prefecture Institute of Health), Fumio Terasoma (Wakayama Prefectural Research Center of Environment and Public Health), Hidenobu Ekawa (Wakayama City Institute of Public Health), Nobuyuki Kato (Tottori Prefectural Institute of Public Health and Environmental Science), Hirokazu Takimoto (Shimane Prefectural Institute of Public Health and Environmental Science), Masako Hamano (Okayama Prefectural Institute for Environmental Science and Public Health), Shinichi Takao (Center for Public Health and Environment, Hiroshima Prefectural Technology Research Institute), Miwako Yamamoto (Hiroshima City Institute of Public Health), Shoichi Toda (Yamaguchi Prefectural Institute of Public Health and Environment), Miyuki Katayama (Tokushima Prefectural Public Health, Pharmaceutical and Environmental Sciences Center), Yukari Terajima (Kagawa Prefectural Research Institute for Environmental Sciences and Public Health), Miki Kan (Ehime Prefecture Institute of Public Health and Environmental Science), Tae Taniwaki (Kochi Public Health and Sanitation Institute), Hideaki Yoshitomi (Fukuoka Institute of Health and Environmental Sciences), Keiko Kajiyama (Fukuoka City Institute for Hygiene and the Environment), Wakako Sakata (Kitakyushu City Institute of Environmental Sciences), Katsuyuki Ando (Saga Prefectural Institute of Public Health and Pharmaceutical Research), Yumika Kitagawa (Nagasaki Prefectural Institute for Environment Research and Public Health), Junko Toda (Kumamoto Prefectural Institute of Public Health and Environmental Science), Kaori Nishizawa (Kumamoto City Environmental Research Center), Miki Kato (Oita Prefectural Institute of Health and Environment), Miho Miura (Miyazaki Prefectural Institute for Public Health and Environment), Gokuden Mutsuyo (Kagoshima Prefectural Institute for Environmental Research and Public Health), and Hisako Kyan (Okinawa Prefectural Institute of Health and Environment).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04836-14.
REFERENCES
- 1.World Health Organization. 2010. WHO guidelines for pharmacological management of pandemic influenza A(H1N1) 2009 and other influenza viruses. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/resources/publications/swineflu/h1n1_use_antivirals_20090820/en/. [PubMed] [Google Scholar]
- 2.Monto AS, McKimm-Breschkin JL, Macken C, Hampson AW, Hay A, Klimov A, Tashiro M, Webster RG, Aymard M, Hayden FG, Zambon M. 2006. Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob Agents Chemother 50:2395–2402. doi: 10.1128/AAC.01339-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tashiro M, McKimm-Breschkin JL, Saito T, Klimov A, Macken C, Zambon M, Hayden FG, Neuraminidase Inhibitor Susceptibility Network. 2009. Surveillance for neuraminidase-inhibitor-resistant influenza viruses in Japan, 1996–2007. Antivir Ther 14:751–761. doi: 10.3851/IMP1194. [DOI] [PubMed] [Google Scholar]
- 4.Ujike M, Shimabukuro K, Mochizuki K, Obuchi M, Kageyama T, Shirakura M, Kishida N, Yamashita K, Horikawa H, Kato Y, Fujita N, Tashiro M, Odagiri T, Working Group for Influenza Virus Surveillance in Japan. 2010. Oseltamivir-resistant influenza viruses A(H1N1) during 2007-2009 influenza seasons, Japan. Emerg Infect Dis 16:926–935. doi: 10.3201/eid1606.091623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ujike M, Ejima M, Anraku A, Shimabukuro K, Obuchi M, Kishida N, Hong X, Takashita E, Fujisaki S, Yamashita K, Horikawa H, Kato Y, Oguchi A, Fujita N, Tashiro M, Odagiri T, Influenza Virus Surveillance Group of Japan. 2011. Monitoring and characterization of oseltamivir-resistant pandemic (H1N1) 2009 virus, Japan, 2009–2010. Emerg Infect Dis 17:470–479. doi: 10.3201/eid1703.101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takashita E, Fujisaki S, Kishida N, Xu H, Imai M, Tashiro M, Odagiri T, Influenza Virus Surveillance Group of Japan. 2013. Characterization of neuraminidase inhibitor-resistant influenza A(H1N1)pdm09 viruses isolated in four seasons during pandemic and post-pandemic periods in Japan. Influenza Other Respir Viruses 7:1390–1399. doi: 10.1111/irv.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lackenby A, Hungnes O, Dudman SG, Meijer A, Paget WJ, Hay AJ, Zambon MC. 2008. Emergence of resistance to oseltamivir among influenza A(H1N1) viruses in Europe. Euro Surveill 13:pii=8026 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=8026. [DOI] [PubMed] [Google Scholar]
- 8.Bloom JD, Gong LI, Baltimore D. 2010. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 328:1272–1275. doi: 10.1126/science.1187816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rameix-Welti MA, Munier S, Le Gal S, Cuvelier F, Agou F, Enouf V, Naffakh N, van der Werf S. 2011. Neuraminidase of 2007-2008 influenza A(H1N1) viruses shows increased affinity for sialic acids due to the D344N substitution. Antivir Ther 16:597–603. doi: 10.3851/IMP1804. [DOI] [PubMed] [Google Scholar]
- 10.Abed Y, Pizzorno A, Bouhy X, Boivin G. 2011. Role of permissive neuraminidase mutations in influenza A/Brisbane/59/2007-like (H1N1) viruses. PLoS Pathog 7:e1002431. doi: 10.1371/journal.ppat.1002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouvier NM, Rahmat S, Pica N. 2012. Enhanced mammalian transmissibility of seasonal influenza A/H1N1 viruses encoding an oseltamivir-resistant neuraminidase. J Virol 86:7268–7279. doi: 10.1128/JVI.07242-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurt AC, Hardie K, Wilson NJ, Deng YM, Osbourn M, Leang SK, Lee RT, Iannello P, Gehrig N, Shaw R, Wark P, Caldwell N, Givney RC, Xue L, Maurer-Stroh S, Dwyer DE, Wang B, Smith DW, Levy A, Booy R, Dixit R, Merritt T, Kelso A, Dalton C, Durrheim D, Barr IG. 2012. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 206:148–157. doi: 10.1093/infdis/jis337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler J, Hooper KA, Petrie S, Lee R, Maurer-Stroh S, Reh L, Guarnaccia T, Baas C, Xue L, Vitesnik S, Leang SK, McVernon J, Kelso A, Barr IG, McCaw JM, Bloom JD, Hurt AC. 2014. Estimating the fitness advantage conferred by permissive neuraminidase mutations in recent oseltamivir-resistant A(H1N1)pdm09 influenza viruses. PLoS Pathog 10:e1004065. doi: 10.1371/journal.ppat.1004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abed Y, Pizzorno A, Bouhy X, Rhéaume C, Boivin G. 2014. Impact of potential permissive neuraminidase mutations on viral fitness of the H275Y oseltamivir-resistant influenza A(H1N1)pdm09 virus in vitro, in mice and in ferrets. J Virol 88:1652–1658. doi: 10.1128/JVI.02681-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takashita E, Ejima M, Itoh R, Miura M, Ohnishi A, Nishimura H, Odagiri T, Tashiro M. 2014. A community cluster of influenza A(H1N1)pdm09 virus exhibiting cross-resistance to oseltamivir and peramivir in Japan, November to December 2013. Euro Surveill 19:pii=20666 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20666. [DOI] [PubMed] [Google Scholar]
- 16.Nakauchi M, Ujike M, Obuchi M, Takashita E, Takayama I, Ejima M, Oba K, Konomi N, Odagiri T, Tashiro M, Kageyama T, Influenza Virus Surveillance Group of Japan. 2011. Rapid discrimination of oseltamivir-resistant 275Y and -susceptible 275H substitutions in the neuraminidase gene of pandemic influenza A/H1N1 2009 virus by duplex one-step RT-PCR assay. J Med Virol 83:1121–1127. doi: 10.1002/jmv.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meijer A, Rebelo-de-Andrade H, Correia V, Besselaar T, Drager-Dayal R, Fry A, Gregory V, Gubareva L, Kageyama T, Lackenby A, Lo J, Odagiri T, Pereyaslov D, Siqueira MM, Takashita E, Tashiro M, Wang D, Wong S, Zhang W, Daniels RS, Hurt AC. 2014. Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2012–2013. Antiviral Res 110:31–41. doi: 10.1016/j.antiviral.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki-Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Takahashi K, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, et al. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatakeyama S, Sakai-Tagawa Y, Kiso M, Goto H, Kawakami C, Mitamura K, Sugaya N, Suzuki Y, Kawaoka Y. 2005. Enhanced expression of an α2,6-linked sialic acid on MDCK cells improves isolation of human influenza viruses and evaluation of their sensitivity to a neuraminidase inhibitor. J Clin Microbiol 43:4139–4146. doi: 10.1128/JCM.43.8.4139-4146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Vries E, Collins PJ, Vachieri SG, Xiong X, Liu J, Walker PA, Haire LF, Hay AJ, Schutten M, Osterhaus AD, Martin SR, Boucher CA, Skehel JJ, Gamblin SJ. 2012. H1N1 2009 pandemic influenza virus: resistance of the I223R neuraminidase mutant explained by kinetic and structural analysis. PLoS Pathog 8:e1002914. doi: 10.1371/journal.ppat.1002914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nomaguchi M, Yokoyama M, Kono K, Nakayama EE, Shioda T, Doi N, Fujiwara S, Saito A, Akari H, Miyakawa K, Ryo A, Ode H, Iwatani Y, Miura T, Igarashi T, Sato H, Adachi A. 2013. Generation of rhesus macaque-tropic HIV-1 clones that are resistant to major anti-HIV-1 restriction factors. J Virol 87:11447–11461. doi: 10.1128/JVI.01549-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okomo-Adhiambo M, Fry AM, Su S, Nguyen HT, Elal AA, Negron E, Hand J, Garten RJ, Barnes J, Xiyan X, Villanueva JM, Gubareva LV, 2013-14 U.S. Influenza Antiviral Working Group. 2015. Oseltamivir-resistant influenza A(H1N1)pdm09 viruses, United States, 2013–14. Emerg Infect Dis 21:136–141. doi: 10.3201/eid2101/141006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takashita E, Meijer A, Lackenby A, Gubareva L, Rebelo-de-Andrade H, Besselaar T, Fry A, Gregory V, Leang SK, Huang W, Lo J, Pereyaslov D, Siqueira MM, Wang D, Mak GC, Zhang W, Daniels RS, Hurt AC, Tashiro M. 2015. Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2013–2014. Antiviral Res 117:27–38. doi: 10.1016/j.antiviral.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ginting TE, Shinya K, Kyan Y, Makino A, Matsumoto N, Kaneda S, Kawaoka Y. 2012. Amino acid changes in hemagglutinin contribute to the replication of oseltamivir-resistant H1N1 influenza viruses. J Virol 86:121–127. doi: 10.1128/JVI.06085-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.