Abstract
The objectives of this study were to investigate raltegravir transport across several blood-tissue barrier models and the potential interactions with drug efflux transporters. Raltegravir uptake, accumulation, and permeability were evaluated in vitro in (i) P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), multidrug resistance-associated protein 1 (MRP1), or MRP4-overexpressing MDA-MDR1 (P-gp), HEK-ABCG2, HeLa-MRP1, or HEK-MRP4 cells, respectively; (ii) cell culture systems of the human blood-brain (hCMEC/D3), mouse blood-testicular (TM4), and human blood-intestinal (Caco-2) barriers; and (iii) rat jejunum and ileum segments using an in situ single-pass intestinal perfusion model. [3H]Raltegravir accumulation by MDA-MDR1 (P-gp) and HEK-ABCG2-overexpressing cells was significantly enhanced in the presence of PSC833 {6-[(2S,4R,6E)-4-methyl-2-(methylamino)-3-oxo-6-octenoic acid]-7-l-valine-cyclosporine}, a P-gp inhibitor, or Ko143 [(3S,6S,12aS)-1,2,3,4,6,7,12,12a-octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino[1′,2′:1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester], a BCRP inhibitor, suggesting the inhibition of a P-gp- or BCRP-mediated efflux process, respectively. Furthermore, [3H]raltegravir accumulation by human cerebral microvessel endothelial hCMEC/D3 and mouse Sertoli TM4 cells was significantly increased by PSC833 and Ko143. In human intestinal Caco-2 cells grown on Transwell filters, PSC833, but not Ko143, significantly decreased the [3H]raltegravir efflux ratios. In rat intestinal segments, [3H]raltegravir in situ permeability was significantly enhanced by the concurrent administration of PSC833 and Ko143. In contrast, in the transporter inhibition assays, raltegravir (10 to 500 μM) did not increase the accumulation of substrate for P-gp (rhodamine-6G), BCRP ([3H]mitoxantrone), or MRP1 [2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF)] by MDA-MDR1 (P-gp)-, HEK-ABCG2-, or HeLa-MRP1-overexpressing cells, respectively. Our data suggest that raltegravir is a substrate but not an inhibitor of the drug efflux transporters P-gp and BCRP. These transporters might play a role in the restriction of raltegravir permeability across the blood-brain, blood-testicular, and blood-intestinal barriers, potentially contributing to its low tissue concentrations and/or low oral bioavailability observed in the clinic setting.
INTRODUCTION
Highly active antiretroviral therapy, which combines several classes of antiretroviral drugs (ARVs), has been proven effective in suppressing human immunodeficiency virus type 1 (HIV-1) replication and subsequently can reduce HIV-associated morbidity and mortality (1–3). Despite the success of ARVs in the treatment of HIV-1 infection, the emergence of resistant viral strains, nonadherence to therapy, interindividual variability in ARV pharmacokinetics/pharmacodynamics, drug-drug interactions, and adverse effects continue to pose major therapeutic challenges (4, 5). Furthermore, ARVs are also known to be substrates, inhibitors, and/or inducers of metabolic enzymes and/or membrane drug transporters, such as ATP-binding cassette (ABC) transporters, which actively efflux drugs from the cell, and/or solute carrier transporters, which mediate drug uptake and/or bidirectional transport across cell membranes (3). Overall, drug transporters and metabolic enzymes can regulate the intestinal absorption, brain and testis tissue distribution, renal excretion, and hepatobiliary elimination of ARVs and may also contribute to drug-drug interactions at these sites (3, 6).
The lack of an effective vaccine along with an increasing incidence of drug resistance has emphasized the need to develop new drugs with novel mechanisms of action (7). Raltegravir represents a new class of ARVs developed by Merck & Co., Inc., which functions as an inhibitor of HIV-1 integrase, a viral enzyme that catalyzes an essential process in the viral replication cycle, i.e., the insertion of HIV-1 proviral DNA into the host genome (8, 9). Clinical studies have shown that raltegravir has a sustained antiretroviral effect and good tolerability in both treatment-naive and -experienced HIV-1-infected patients (10, 11). In humans, raltegravir is not a substrate or an inhibitor of cytochrome P450 enzymes, and its primary route of metabolism and elimination is glucuronidation via UDP-glucuronosyltransferase 1A1 (12, 13).
Several anatomically protected sites, such as the central nervous system (CNS) and male genital tract, have been recognized as sanctuary sites for HIV-1 (14). Viral replication can occur in these sites even in the presence of therapeutic concentrations of ARVs in plasma (15). Clinical studies have shown significant variability in raltegravir penetration in such tissues compared to that in plasma (16–19). In addition, raltegravir is reported to have low oral bioavailability in humans (20–22). Studies from our group and those of others have shown the functional expression of several ABC drug efflux transporters, including P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance-associated proteins (MRPs), in blood-brain barrier, blood-testicular barrier, and blood-intestinal barrier models, which can collectively serve as a biochemical barrier to drug entry/distribution in these tissues (23–27). Raltegravir interactions with P-gp and BCRP transporters were recently investigated in a few studies; however, inconsistent results have been reported, depending on the methods used (28–30). To the best of our knowledge, raltegravir interactions with drug efflux transporters at critical blood-tissue barriers have not been addressed.
The aim of this study was to investigate raltegravir interactions with drug efflux transporters and identify the roles of these transporters in the permeability of raltegravir at blood-tissue barrier sites, such as the blood-brain barrier, blood-testicular barrier, and blood-intestinal barrier, using in vitro and in situ cell/organ systems.
MATERIALS AND METHODS
Materials.
Raltegravir and [3H]raltegravir (0.5 mCi/mmol) were kindly provided by Merck Canada, Inc. (Kirkland, Canada). [3H]Mitoxantrone (12.7 Ci/mmol) was purchased from Moravek Biochemicals (Brea, CA). Rhodamine-6G (R-6G) was purchased from Sigma-Aldrich (Oakville, Canada). PSC833/Valspodar (6-[(2S,4R,6E)-4-methyl-2-(methylamino)-3-oxo-6-octenoic acid]-7-l-valine-cyclosporine) and fumitremorgin C (FTC) were a gift from Novartis Pharma (Basel, Switzerland) and Susan Bates (Bethesda, MD), respectively. Cyclosporine (CsA), Ko143 [(3S,6S,12aS)-1,2,3,4,6,7,12,12a-octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino[1′,2′:1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester], and MK571 [(E)-3-[[[3-[(1E)-2-(7-chloro-2-quinolinyl) ethenyl]phenyl][[3-(dimethylamino)-3-oxopropyl]thio]methyl]thio]propanoic acid] were purchased from Tocris Biosciences (Ellisville, MO). All cell culture reagents and BCECF-AM [2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester] were purchased from Invitrogen (Carlsbad, CA).
Cell culture systems.
All cell lines were grown and maintained in a humidified incubator at 37°C, 5% CO2, and 95% air atmosphere with fresh medium replaced every 2 to 3 days. The human cell lines stably transfected with human cDNA for (i) MDR1/P-gp (MDA-MDR1), (ii) ABCC1/MRP1 (HeLa-MRP1), (iii) ABCC4/MRP4 (HEK-MRP4), and (iv) ABCG2/BCRP (HEK-ABCG2) were kindly provided by Robert Clarke (Georgetown University, Washington, DC), Susan Cole (Queen's University, Kingston, Canada), Piet Borst (Netherlands Cancer Institute, Amsterdam, the Netherlands), and Susan Bates (Bethesda, MD), respectively, and were cultured according to our previously published protocols (26). The immortalized human cerebral microvessel endothelial cell line (hCMEC/D3) was kindly provided by P. O. Couraud (Institut Cochin, Departement Biologie Cellulaire and INSERM, Paris, France). The mouse Sertoli testicular (TM4) and Caco-2 cell lines were obtained from the American Tissue Culture Collection (Manassas, VA). These cells were cultured as described in our previous publications and those of others (24, 26, 27).
Functional studies.
The cellular uptake/accumulation of fluorescent substrates of P-gp (1 μM R-6G) and MRP1 (5 μM BCECF-AM), radiolabeled substrate of BCRP [3H]mitoxantrone (20 nM, 0.1 μCi/ml) or [3H]raltegravir (1 μM, 0.1 μCi/ml) were determined by standard transport assay protocols that were well established in our laboratory (31, 32) in the absence or presence of P-gp inhibitors (25 μM CsA and/or 5 μM PSC833), BCRP (5 μM Ko143 and/or 10 μM FTC), or MRP1 (10 μM MK571), or in the presence of 25 to 500 μM raltegravir (33). Because BCECF-AM and mitoxantrone are also known substrates of P-gp, all assays involving these two substrates were performed in the presence of the P-gp inhibitor PSC833 (2 μM).
In situ single-pass perfusion of rat intestine.
Open-loop in situ perfusion studies were carried out in male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), according to our previously published method (27), in accordance with the study protocol approved by the University of Toronto animal ethics committee. The method was adapted from previously published protocols from our group (27). Briefly, each animal was anesthetized with the use of isoflurane inhalant and maintained at 37°C throughout the surgical and perfusion procedures. The abdominal cavity was opened by midline incision (3 to 4 cm) to expose the proximal jejunum (6 to 8 cm in length, starting 2 cm after the ligament of Treitz) and distal ileum (6 to 8 cm in length, ending 1 cm before the cecum) segments. These segments were selected for the perfusion studies because of their known importance in the absorption of drugs (34, 35). Each segment was connected to the inlet and outlet tubing via cannulas and perfused at a constant flow rate of 0.2 ml/min using a syringe infusion pump (Harvard Apparatus syringe pump, model 22) with a preincubation solution containing unlabeled raltegravir (10 μM) and d-mannitol (10 μM), a marker of paracellular permeability, for a 30-min period to reach steady state. To initiate the measurement of raltegravir permeability, the perfusion buffer containing [3H]raltegravir and [14C]d-mannitol, supplemented with unlabeled raltegravir and d-mannitol to achieve a concentration of 10 μM, was introduced into the segment (at time zero), and the perfusate exiting from each segment through the outlet tubing was collected into vials in consecutive 10-min intervals (control conditions). After 1 h, the perfusion buffer was replaced with an identical [3H]raltegravir (10 μM) and [14C]d-mannitol (10 μM) solution also containing 5 μM PSC833, 5 μM Ko143, or both PSC833 and Ko143 (5 μM each), and the perfusate was collected in consecutive 10-min intervals over a 60-min period. All the collected perfusate samples were weighed in order to determine the change in weight between the perfusion buffer entering and exiting each segment. The raltegravir and d-mannitol concentrations in the perfusate were quantified by liquid scintillation counting by measuring 3H/14C radioactivity. These concentrations were corrected for water reabsorption by the gravitational method (using the ratio of inflow/outflow weight). In each animal, the raltegravir steady-state effective permeability (Peff) value was determined during the first hour (0 to 60 min) in the absence of inhibitor (control) and compared to the Peff obtained in the same animal during the second hour (60 to 120 min), when 5 μM PSC833 or 5 μM Ko143 or both inhibitors (5 μM each) were present in the perfusate buffer, in order to use each animal as its own control and allow paired-data analysis.
Data analysis.
Each set of in vitro experiments was repeated at least three times in the cells pertaining to different passages. In an individual experiment, each experimental point was performed in triplicate. Statistical analysis was performed using GraphPad Prism (version 5.01 for Microsoft Windows; Graph Pad Software, San Diego, CA), and significance was assessed by applying the unpaired or paired two-tailed Student's t test for unpaired or paired experimental values, or the one-way analysis of variance (ANOVA) followed by Bonferroni's correction for a test of repeated measures, as appropriate. A P value of <0.05 was considered statistically significant.
To determine raltegravir permeability across the Caco-2 monolayers grown on Transwell inserts, the data for time-dependent raltegravir flux in the apical-to-basolateral or basolateral-to-apical direction were fitted into equation 1 (36):
| (1) |
where Papp is the apparent permeability coefficient (cm/s), ΔQ/Δt is the solute flux rate (mol/s) from the donor into the receiver compartment at steady state, A is the surface area of the filter insert (cm2), and C0 is the initial solute concentration (mol/cm3) in the donor compartment. To evaluate the role of active transport in raltegravir directional permeability, the efflux ratio was defined as the quotient of the secretory permeability, and the absorptive permeability was determined according to equation 2 (36):
| (2) |
where ER is the efflux ratio, Papp (A-B) is the apparent permeability coefficient measured for drug permeability in the apical-to-basolateral direction by introducing the drug into the donor compartment (apical) and measuring its appearance in the receiver compartment (basolateral), and Papp (B-A) is the apparent permeability coefficient measured in the basolateral-to-apical direction by introducing the drug into the donor compartment (basolateral) and measuring its appearance in the receiver compartment (apical).
The effective perfusion coefficient for raltegravir permeability across the rat intestinal segment at steady state (Peff) was measured according to equation 3 (37):
| (3) |
where Peff is the effective steady-state permeability coefficient (cm/s), Q is the flow rate (0.2 ml/min, converted to cm3/s), Cout/Cin is the ratio of the outlet raltegravir concentration (corrected for water reabsorption) and the inlet raltegravir concentration in the perfusion buffer (i.e., 10 μM), R is the radius of rat intestine (0.18 cm), and L is the length of the intestinal segment (approximately 8 cm). The data fitting into each model was performed by nonlinear least-squares analysis using GraphPad Prism.
RESULTS
ABC transporter inhibition assays.
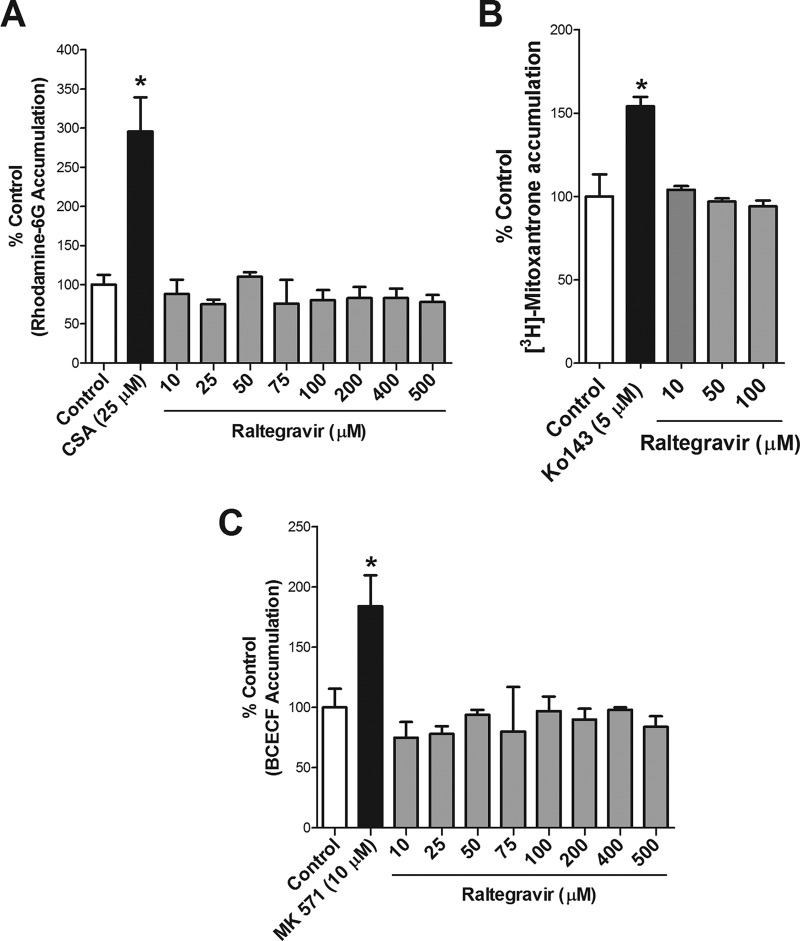
We investigated if raltegravir can serve as an inhibitor for the drug efflux transporters P-gp, BCRP, and MRP1. Initially, we examined the accumulation of the P-gp substrate R-6G by MDA-MDR1 (P-gp)-overexpressing cells in the presence of different concentrations of raltegravir. In contrast to the effect observed with the P-gp inhibitor CsA, no statistical significant differences were detected in R-6G accumulation in the presence of 10 to 500 μM raltegravir compared to that with the control. These data suggest that raltegravir does not inhibit P-gp-mediated transport of R-6G (Fig. 1A).
FIG 1.
Raltegravir interactions with ABC transporters. (A) Effect of raltegravir on R-6G accumulation by MDA-MDR1 (P-gp) monolayer cells. The cellular accumulation of R-6G (1 μM) was determined at 60 min by exposing confluent MDA-MDR1 (P-gp) cells to R-6G in assay buffer with or without the P-gp inhibitor CsA (25 μM) or raltegravir (10 to 500 μM). (B) Effect of raltegravir on [3H]mitoxantrone accumulation by HEK-ABCG2-overexpressing cells. The accumulation of [3H]mitoxantrone (20 nM) at 120 min was measured in the absence (control) or presence of BCRP inhibitor (5 μM Ko143) or raltegravir (10 to 100 μM), all in the presence of 2 μM PSC833. (C) Effect of raltegravir on BCECF accumulation by HeLa-MRP1 monolayer cells. The cellular accumulation (at 120 min) of BCECF was determined by exposing confluent HeLa-MRP1 cells to 5 μM BCECF-AM, with or without the MRP inhibitor MK571 (10 μM) or raltegravir (10 to 500 μM), all in the presence of 2 μM PSC833. The results are expressed as the mean ± standard error of the mean (SEM) from at least three independent experiments, with each data point in an individual experiment representing triplicate measurements. Statistical significance was assessed by applying the unpaired two-tailed Student's t test analysis. *, P < 0.05.
We then examined the accumulation of the BCRP substrate [3H]mitoxantrone by HEK-ABCG2-overexpressing cells in the absence or presence of the BCRP inhibitor Ko143 or with several concentrations of raltegravir (10 to 100 μM). [3H]Mitoxantrone accumulation was significantly enhanced in the presence of Ko143 but not in the presence of raltegravir, suggesting that raltegravir does not interfere with BCRP-mediated transport processes (Fig. 1B).
In order to investigate if raltegravir is an inhibitor of MRP1, we determined the accumulation of the MRP substrate BCECF in HeLa-MRP1-overexpressing cells in the presence or absence of the MRP inhibitor MK571 (10 μM) or raltegravir (10 to 500 μM). MK571 significantly enhanced the accumulation of BCECF, whereas raltegravir did not exert any effect, suggesting that raltegravir does not serve as an inhibitor of this efflux transporter (Fig. 1C).
ABC transporter substrate assays.
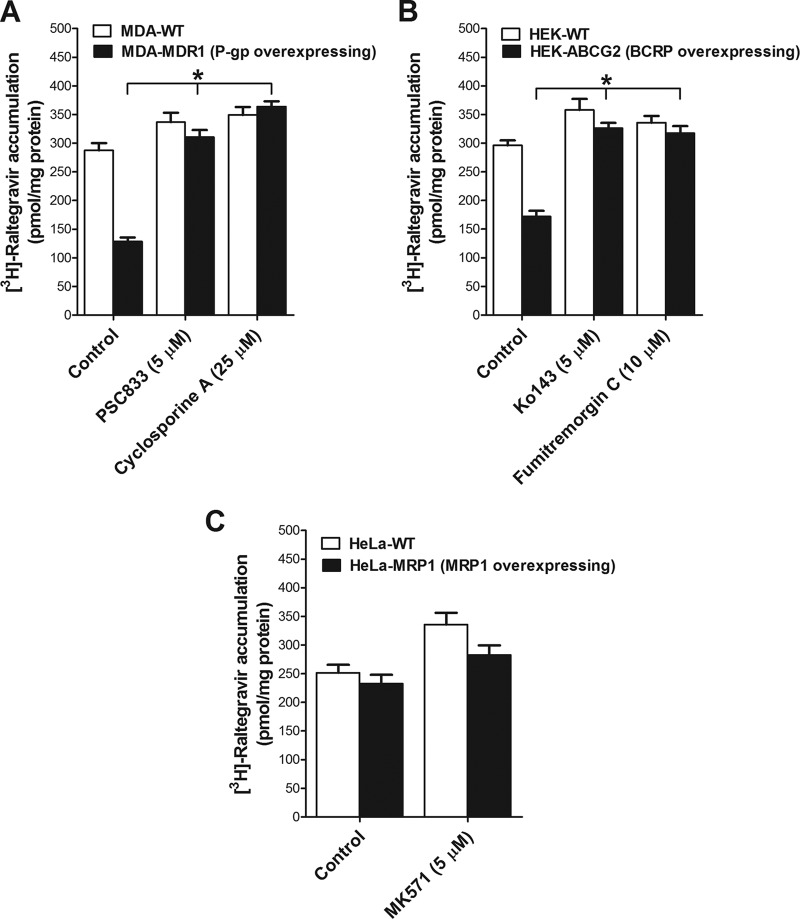
We investigated if raltegravir can serve as a substrate for the drug efflux transporters P-gp, BCRP, MRP1, and MRP4. We characterized the accumulation of [3H]raltegravir by MDA-wild-type (WT) and MDA-MDR1 (P-gp)-overexpressing cells in the absence or presence of the P-gp inhibitors PSC833 and CsA. [3H]Raltegravir accumulation was significantly lower in the P-gp-overexpressing MDA-MDR1 (P-gp) cells than in the MDA-WT cells. In addition, its accumulation was significantly enhanced in the presence of the two inhibitors in MDA-MDR1 (P-gp) but not in MDA-WT cells. Together, these data suggest that raltegravir is a P-gp substrate (Fig. 2A).
FIG 2.
Interactions of raltegravir with ABC transporters in overexpressing cell lines. Shown is the accumulation of [3H]raltegravir by MDA-WT/MDA-MDR1 (P-gp) cells (A), HEK-WT/HEK-ABCG2 cells (B), and HeLa-WT/HeLa-MRP1 cells (C). The accumulation of 1 μM [3H]raltegravir (at 120 min) was measured in cell monolayers in the absence or presence of the inhibitors for P-gp (5 μM PSC833 and 25 μM CsA), BCRP (5 μM Ko143 and 10 μM FTC), or MRP1 (5 μM MK571). The results are expressed as mean ± SEM from at least three independent experiments, with each data point in an individual experiment representing triplicate measurements. Statistical significance was assessed applying one-way analysis of variance (ANOVA), followed by Bonferroni's correction for a test of repeated measures. *, P < 0.05.
[3H]Raltegravir accumulation was also measured in both HEK-WT and HEK-ABCG2-overexpressing cells in the absence or presence of the BCRP inhibitors Ko143 and FTC. Compared to that with the HEK-WT cells, [3H]raltegravir accumulation was significantly lower in HEK-ABCG2 cells. In addition, [3H]raltegravir accumulation was significantly enhanced in the presence of Ko143 or FTC but remained unchanged in HEK-WT cells. These data suggest that [3H]raltegravir can serve as a BCRP substrate (Fig. 2B).
We further determined [3H]raltegravir accumulation in HeLa-WT and HeLa-MRP1-overexpressing cells in the absence or presence of the MRP inhibitor MK571. No difference in [3H]raltegravir accumulation was observed between the HeLa-WT and HeLa-MRP1 cells, suggesting that [3H]raltegravir is not a substrate for MRP1 (Fig. 2C). We also investigated if raltegravir can serve as a substrate for MRP4 using HEK-WT and HEK-MRP4-overexpressing cells. However, [3H]raltegravir did not interact with this transporter (data not shown).
Raltegravir accumulation in hCMEC/D3 and TM4 cells.
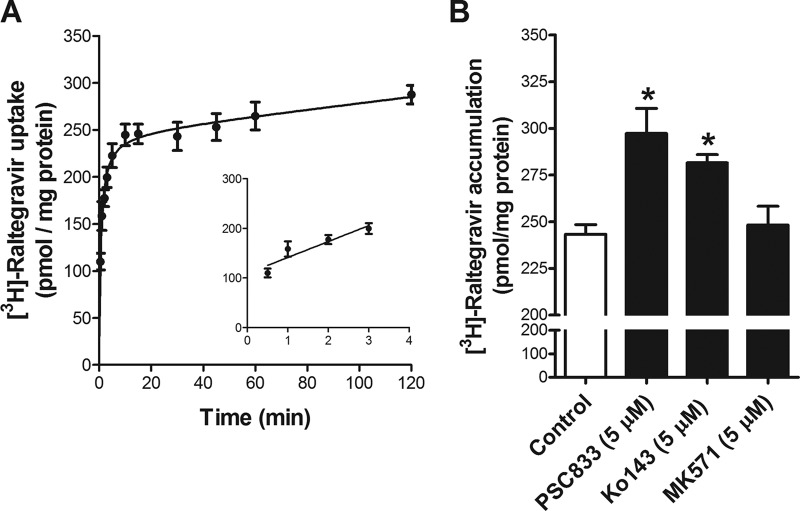
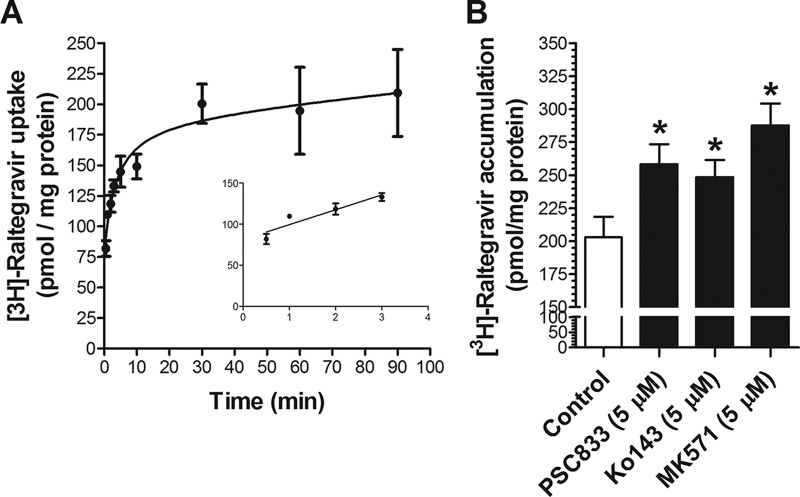
We investigated the time-dependent uptake of [3H]raltegravir in hCMEC/D3 and TM4 cells, two cell systems that are representative of human brain microvessel endothelial cells and rodent testicular Sertoli cells, respectively. As shown in Fig. 3A and 4A, [3H]raltegravir cellular uptake at 37°C gradually increased over time until a plateau was reached at approximately 60 min.
FIG 3.
(A) Time profile of [3H]raltegravir in hCMEC/D3 cells. The uptake of 1 μM [3H]raltegravir by the hCMEC/D3 confluent monolayer cells was measured over time at 37°C. The inset shows the linearity of initial uptake up to 3 min. (B) Accumulation of 1 μM [3H]raltegravir (for 120 min) by hCMEC/D3 cells in the absence or presence of inhibitors of P-gp (5 μM PSC833), BCRP (5 μM Ko143), or MRPs (5 μM MK571). The results are expressed as mean ± SEM from at least three independent experiments, with each data point in an individual experiment representing triplicate measurements. *, P < 0.05.
FIG 4.
(A) Time profile of [3H]raltegravir in TM4 cells. The uptake of 1 μM [3H]raltegravir by the TM4 confluent monolayer cells was measured over time at 37°C. The inset shows the linearity of initial uptake up to 3 min. (B) Accumulation of 1 μM [3H]raltegravir (120 min) by TM4 cells in the absence or presence of inhibitors of P-gp (5 μM PSC833), Bcrp (5 μM Ko143) or Mrps (5 μM MK571). The results are expressed as mean ± SEM from at least three independent experiments, with each data point in an individual experiment representing triplicate measurements. *, P < 0.05.
In order to determine if P-gp, BCRP/Bcrp, or MRPs/Mrps can recognize [3H]raltegravir as a substrate in hCMEC/D3 and TM4 cells, we performed [3H]raltegravir accumulation assays in the absence or presence of the P-gp, BCRP/Bcrp, and MRP/Mrp selective inhibitors PSC833, Ko143, and MK571, respectively. The accumulation of [3H]raltegravir was significantly increased when cells were incubated in the presence of PSC833 or Ko143 in both hCMEC/D3 cells (Fig. 3B) and TM4 cells (Fig. 4B). Taken together, these data suggest that [3H]raltegravir permeability can be regulated by P-gp- and/or BCRP/Bcrp-mediated efflux processes in in vitro human blood-brain barrier and mouse blood-testicular barrier cell culture systems. [3H]Raltegravir accumulation in TM4 cells was also significantly enhanced by MK571; however, in hCMEC/D3 cells, the addition of MK571 did not exert any effect.
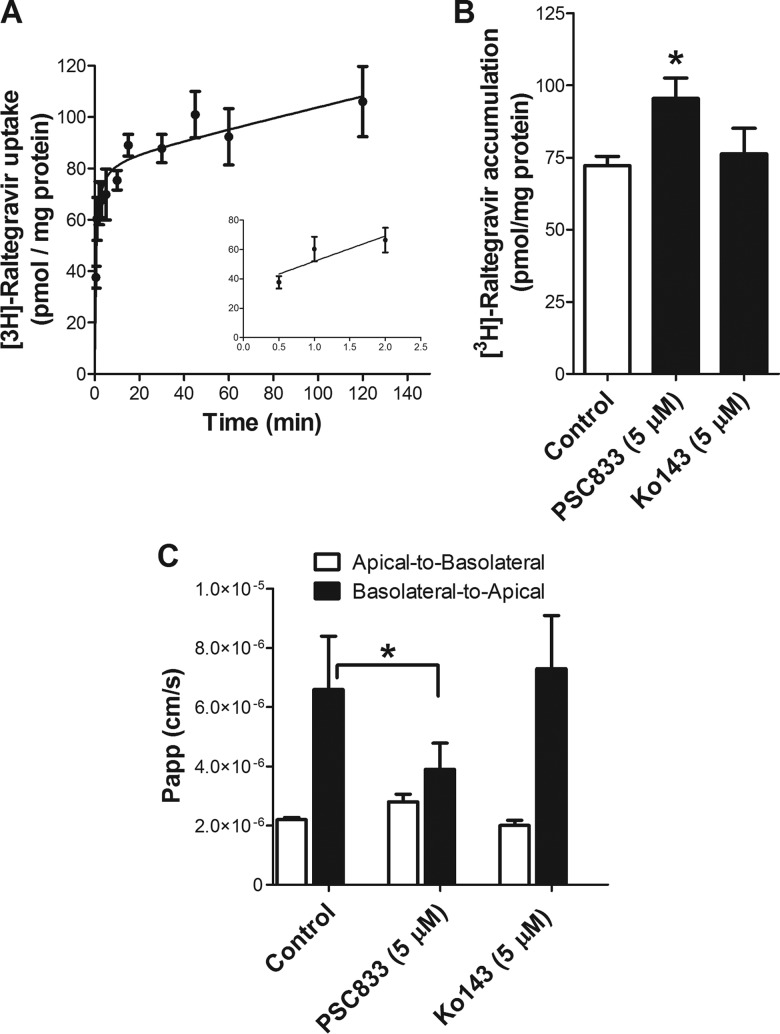
Raltegravir permeability across Caco-2 monolayers.
We also investigated the time-dependent accumulation of [3H]raltegravir by the human intestinal Caco-2 monolayer cells. [3H]Raltegravir uptake gradually increased over time until a plateau was reached at approximately 60 min (Fig. 5A). In addition, [3H]raltegravir accumulation (60 min) by Caco-2 monolayer cells was significantly enhanced in the presence of the P-gp inhibitor PSC833 but not the BCRP inhibitor Ko143 (Fig. 5B). We further evaluated in vitro raltegravir permeability across Caco-2 cell monolayers grown on Transwell filters (27). In the absence of inhibitors, [3H]raltegravir permeability in the apical-to-basolateral direction [Papp (A-B)] was significantly lower than its basolateral-to-apical permeability [Papp (B-A)] (Fig. 5C), with an efflux ratio of 3.1. The addition of the P-gp inhibitor PSC833 resulted in decreased basolateral-to-apical raltegravir Papp, with an efflux ratio approaching 1.43. In contrast, [3H]raltegravir permeability remained unchanged in the presence of the BCRP inhibitor Ko143 (Fig. 5C). This might be attributed to the fact that BCRP expression is undetectable in Caco-2 cells (38). Together, these results suggest that raltegravir efflux at the apical membrane of Caco-2 cells is mediated by P-gp.
FIG 5.
Raltegravir accumulation and permeability in Caco-2 cells: interactions with ABC transporters. (A) Time profile of [3H]raltegravir in Caco-2 cells. The uptake of 1 μM [3H]raltegravir by the Caco-2 confluent monolayer cells was measured over time at 37°C. The inset shows the linearity of initial uptake up to 2 min. (B) Accumulation of 1 μM [3H]raltegravir (120 min) by Caco-2 cells in the absence or presence of inhibitors of P-gp (5 μM PSC833), BCRP (5 μM Ko143), or MRPs (5 μM MK571). (C) Raltegravir intestinal permeability across Caco-2 monolayer cells in the apical-to-basolateral and basolateral-to-apical directions. The flux of raltegravir was determined by introducing 1 μM [3H]raltegravir into the donor compartment (apical or basolateral) and measuring its appearance over time in the receiver compartment (basolateral or apical, respectively) in the absence or presence of 5 μM PSC833 or 5 μM Ko143 to calculate the apparent permeability coefficients (Papp). The results are expressed as the mean ± SEM from at least three independent experiments, with each data point in an individual experiment representing triplicate measurements. Statistical significance was assessed applying the one-way ANOVA, followed by Bonferroni's correction for a test of repeated measures. *, P < 0.05.
Effect of P-gp and/or Bcrp inhibitors on raltegravir in situ intestinal permeability in rat intestinal segments.
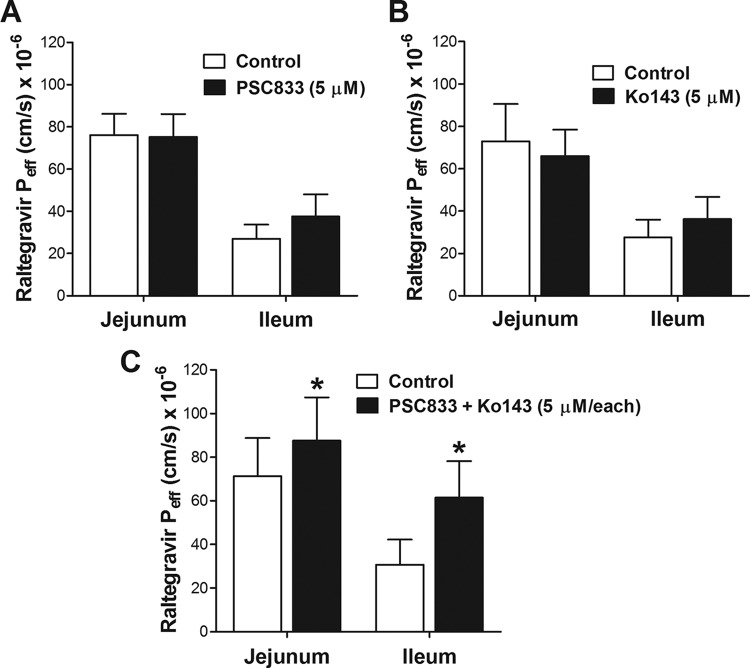
The effects of the P-gp and Bcrp inhibitors PSC833 and Ko143, respectively, on raltegravir in situ intestinal permeability were investigated by single-pass intestinal perfusion of proximal jejunum and distal ileum segments of rat intestine. These segments were selected because of their significant roles in drug absorption (34, 35). Raltegravir steady-state permeability (Peff) was measured over a 2-h period (at 0 to 60 min without inhibitors and at 60 to 120 min in the presence of PSC833, Ko143, or both inhibitors), and the Peff values obtained during the two periods in each animal were compared by paired Student's t test using five animals per treatment group. The inhibition of P-gp or Bcrp alone did not increase the raltegravir Peff in the jejunum or ileum (Fig. 6A and B). However, when both the PSC833 and Ko143 inhibitors were used together, significant increases in raltegravir permeability were observed in both the jejunum (average increase, 78% [47% to 153%], P < 0.0001) and ileum (average increase, 79% [46% to 127%], P < 0.0001) segments (Fig. 6C). In a set of control experiments, the raltegravir Peff was measured in the absence of inhibitors during both periods or in the presence of PSC833, Ko143, or both inhibitors during both perfusion periods (data not shown). No significant differences were observed between raltegravir permeability measurements during the 0- to t60-min and 60- to 120-min periods when the experimental conditions were maintained.
FIG 6.
Raltegravir in situ intestinal permeability and interactions with ABC transporters. Effect of P-gp inhibitor (PSC833) (A), Bcrp inhibitor (Ko143) (B), or both P-gp and Bcrp inhibitors (PSC833 plus Ko143) (C) on raltegravir intestinal permeability, as measured by in situ single-pass perfusion of Sprague-Dawley rat intestinal segments, was quantified. The effective permeability (Peff) of raltegravir was measured in the jejunum or ileum segment of rat intestine in each animal by in situ single-pass perfusion. The intestinal segment was perfused with 10 μM [3H]raltegravir at pH 6.5, first without inhibitor (first hour, control) and then in the presence of 5 μM PSC833, 5 μM Ko143, or both (second hour) to determine the effect of these inhibitors on raltegravir permeability. The raltegravir Peff values measured in the presence of the P-gp inhibitor PSC833, the Bcrp inhibitor Ko143, or both the P-gp and the Bcrp inhibitors, PSC833 + Ko143, were compared to those of the control period in the same animal by paired two-tailed Student's t test analysis using five Sprague-Dawley rats per group.
DISCUSSION
Raltegravir is a preferred drug regimen used along with other ARVs for the treatment of HIV-1 infection in HIV-naive and -experienced patients (39). It is known that ARVs can be substrates, inhibitors, and/or inducers of drug efflux transporters, which can play an important role in ARV permeability in tissues having barrier functions, such as those in the blood-brain barrier, blood-testicular barrier, and blood-intestinal barrier (3). Although several recent studies have examined raltegravir interactions with drug transporters, these data remain limited and provide contradicting outcomes, depending on the methods used (28–30, 40–42). Furthermore, the role that these drug transporters play in raltegravir permeability across blood-tissue barriers and its distribution into sanctuary sites of HIV infection (e.g., CNS and the male genital tract) is not fully understood.
We initially investigated if raltegravir can serve as an inhibitor of drug efflux transporters. The results obtained from our R-6G accumulation assay using P-gp-overexpressing cells suggest that raltegravir (up to 500 μM) is not an inhibitor of P-gp. Our observation is consistent with findings reported in other studies showing no inhibition of P-gp function by raltegravir (29, 30, 42). In our mitoxantrone accumulation assay using BCRP overexpressing cells, we were unable to detect any functional inhibition of BCRP by raltegravir (up to 100 μM). These findings are in agreement with other studies reporting that raltegravir does not inhibit the BCRP-mediated efflux of pheophorbide A in MDCKII-BCRP-overexpressing cells (29), and it only slightly inhibits the BCRP-mediated transport of [3H]mitoxantrone in membrane vesicles expressing human BCRP. This slight inhibition reported by Rizk et al. (40) may be due to the different method used to study BCRP function (i.e., membrane vesicles) and/or the interaction of raltegravir with P-gp-mediated transport of [3H]mitoxantrone in these vesicles, since mitoxantrone is a known substrate of P-gp. In our experiments, PSC833 was used to inhibit P-gp activity in order to evaluate only the raltegravir interactions with the BCRP-mediated transport of mitoxantrone. In a BCECF accumulation assay using MRP1-overexpressing cells, we did not detect MRP1 inhibition by raltegravir (up to 500 μM). To the best of our knowledge, this is the first study reporting that raltegravir does not inhibit MRP1-mediated transport. Collectively, our results suggest that raltegravir does not inhibit the transport function of P-gp, BCRP, or MRP1 and most likely will not exert drug-drug interactions with other concurrently administered drugs that are known to be substrates for these transporters.
We further evaluated raltegravir transport in ABC transporter-overexpressing cell lines and found that raltegravir serves as a substrate of P-gp and BCRP but not of MRP1 and MRP4. Our results are in agreement with those of a recent study in which raltegravir was reported to be a substrate of both P-gp and BCRP using overexpressed LLC-PK1 (L-MDR1) and HEK293 (ABCG2) cells, respectively (30). Similarly, Zembruski et al. (29) reported that raltegravir is a substrate of P-gp, but not of MRP1 or other MRP isoforms (MRP2 and MRP3), using cell proliferation assays in MDCKII-overexpressing cell lines for each ABC transporter; however, they did not detect raltegravir transport by BCRP in the MDCKII/BCRP cell line system (29). Raltegravir transport by P-gp is also controversial. Most previous studies are in agreement with our findings that raltegravir is a substrate for P-gp (28–30, 41). However, a recent study by Dupuis et al. (42) reported that raltegravir is a weak P-gp substrate in a P-gp-overexpressing cell line and Caco-2 monolayers, but at the same time, it shows no interactions with P-gp in an UIC2 shift assay, highlighting that different assays and methods used to investigate transporter interactions can provide contradictory results (42). Based on our results, raltegravir tissue distribution into the sanctuary sites of HIV infection (e.g., CNS and the male genital tract) and its oral bioavailability might be regulated by drug transporters (e.g., P-gp and BCRP) expressed at these blood-tissue barriers.
We examined the role of P-gp and BCRP in raltegravir permeability across the blood-brain barrier using hCMEC/D3 cells, a well-characterized in vitro cell line known to display morphological and biochemical properties of human brain microvessel endothelial cells representative of the blood-brain barrier. We found that P-gp and BCRP inhibitors, PSC833 and Ko143, respectively, significantly increased raltegravir accumulation by hCMEC/D3 cells. However, raltegravir accumulation was not enhanced in the presence of the MRP inhibitor MK571. To the best of our knowledge, these are the first observations showing the roles of P-gp and BCRP in raltegravir transport in an in vitro human blood-brain barrier model. These data suggest that P-gp and BCRP localized at the blood-brain barrier might play a role in limiting raltegravir permeability in the brain. Our data support the recent observation of Yilmaz et al. (16), who found a significantly lower raltegravir concentration in cerebrospinal fluid (2.0 to 126 ng/ml; median, 18.4 ng/ml) than that in plasma (37 to 5,180 ng/ml; median, 448 ng/ml) in 16 HIV-1-infected individuals receiving raltegravir at the recommended therapeutic dose (400 mg twice daily) (16). Furthermore, the raltegravir cerebrospinal fluid-to-plasma concentration ratio varies significantly between individuals (16–18), suggesting that its permeability across the blood-brain barrier may be dependent on the expression/activity of drug efflux transporters, such as P-gp or BCRP, at this blood-tissue barrier. Poor drug penetration into the CNS, a known sanctuary site for HIV-1 replication, has been linked to a suboptimal clinical response to ARV therapy and a decline in neurocognitive function (43, 44). Although a recent study by Johnson et al. (18) did not demonstrate a link between raltegravir cerebrospinal fluid concentrations and ABCB1 (P-gp) polymorphisms in healthy volunteers, this observation might be due to compensatory efflux mechanisms at the blood-brain barrier (e.g., BCRP-mediated efflux) or other interindividual differences that were not accounted for in this sample population (18). Further studies are needed to elucidate the roles of P-gp and BCRP in limiting raltegravir penetration into the CNS.
The male genital tract is another recognized HIV sanctuary site that is protected by the blood-testicular barrier, which is primarily composed of Sertoli epithelial cells that form tight junctions near the basement membrane. Raltegravir accumulation by a mouse Sertoli cell line (TM4), an in vitro cell culture model of blood-testicular barrier, was significantly enhanced by the P-gp, Bcrp, and Mrp inhibitors PSC833, Ko143, and MK571, respectively, demonstrating that raltegravir permeability across the blood-testicular barrier might be regulated by drug efflux transporters. Using human in vitro cell culture systems overexpressing MRP1 and MRP4, we did not detect an increased accumulation of [3H]raltegravir in the presence of the MRP-established inhibitor MK571, suggesting that raltegravir is not a substrate of MRP1 or MRP4. However, in a recent publication from our group, we demonstrated that BCECF (a fluorescent substrate of several Mrp isoforms, including Mrp1 and Mrp4) accumulation in TM4 cells was significantly enhanced in the presence of MK571, showing that Mrp efflux transporters are functional in Sertoli cells (26). These results suggest that raltegravir might interact with other MRP isoforms (e.g., MRP2, MRP3, or MRP5) at the blood-testicular barrier. While Zembruski et al. (29) reported that raltegravir is not a substrate of human MRP1, MRP2, or MRP3 using growth inhibition assays in MDCKII cells overexpressing these transporters, Hashiguchi et al. (30) provided contradictory evidence that raltegravir is a substrate of MRP2 in HEK293-MRP2-overexpressing cells. Hence, further studies are needed to explore this hypothesis.
The intestinal epithelium is another blood-tissue barrier characterized by the expression of tight junctions that restrict the paracellular transport of drugs, cytochrome P450 enzymes, and drug transporters, which collectively act as a biochemical barrier restricting the transcellular passage of drugs into the circulation. In humans, raltegravir is reported to have low oral bioavailability (approximately 30%), which suggests that its permeability across the intestinal epithelium is low (21, 22). Since raltegravir is not metabolized by cytochrome P450 enzymes, drug transporters localized to the intestinal barrier may restrict raltegravir oral absorption. Using Caco-2 cell monolayers grown on Transwell filters in an in vitro model for the human intestinal epithelium, we found that PSC833, a P-gp inhibitor, significantly reduced raltegravir basolateral-to-apical permeability, demonstrating that P-gp mediates raltegravir efflux at the apical membrane of Caco-2 cells, in agreement with findings from other studies (28, 30, 41). However, we did not observe similar effects in the presence of Ko143, the BCRP inhibitor. This is likely due to the low BCRP protein expression in this cell system (38). These results demonstrate that P-gp is the primary efflux transporter mediating raltegravir efflux from Caco-2 cells and restricting its apical membrane permeability. We further investigated the roles of P-gp and Bcrp in raltegravir permeability across the rat intestinal barrier using an in situ perfusion technique. The raltegravir Peff was not changed in the presence of the P-gp or Bcrp inhibitors, PSC833 or Ko143, respectively, when used individually. However, in the presence of both the P-gp and Bcrp inhibitors, the raltegravir Peff increased significantly in both the jejunum and ileum segments, suggesting a cooperative activity of these transporters in restricting raltegravir intestinal permeability. The cooperative function of P-gp and Bcrp transporters in the brain accumulation of the anticancer drug sunitinib was reported previously (45). In addition, previous studies have reported that raltegravir accumulation and permeability in Caco-2 cells are dependent on intestinal lumen pH (41, 46), suggesting that the oral absorption of raltegravir might be altered by changes in luminal pH, as well as the expression and/or activity of the drug efflux transporters at the blood-intestinal barrier.
Both P-gp and BCRP are known to play a significant role in the permeability of several drugs, including ARVs in HIV sanctuary sites, such as the brain and testis (3, 6). Here, we show that raltegravir can serve as a substrate of both P-gp and BCRP in respective transporter-overexpressing cells and in in vitro cell culture models of the human blood-brain barrier (hCMEC/D3), blood-testicular barrier (TM4), and blood-intestinal barrier (Caco-2), and in an in situ intestinal perfusion rat model. Together, our results suggest that P-gp and/or BCRP may play a significant role in restricting the permeability of raltegravir in these tissues.
ACKNOWLEDGMENTS
This work was supported by a research grant from the Investigator-Initiated Studies Program of Merck Canada, Inc.
The opinions expressed in this paper are those of the authors and do not necessarily represent the opinion of Merck Canada, Inc.
Reina Bendayan is a recipient of the Ontario HIV Treatment Network (OHTN) Career Scientist Award.
REFERENCES
- 1.Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338:853–860. [DOI] [PubMed] [Google Scholar]
- 2.Gange SJ, Barron Y, Greenblatt RM, Anastos K, Minkoff H, Young M, Kovacs A, Cohen M, Meyer WA III, Munoz A, Women's Interagency HIV Study Collaborative Study Group. 2002. Effectiveness of highly active antiretroviral therapy among HIV-1 infected women. J Epidemiol Community Health 56:153–159. doi: 10.1136/jech.56.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kis O, Robillard K, Chan GN, Bendayan R. 2010. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci 31:22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Chen TK, Aldrovandi GM. 2008. Review of HIV antiretroviral drug resistance. Pediatr Infect Dis J 27:749–752. doi: 10.1097/INF.0b013e3181846e2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickinson L, Khoo S, Back D. 2010. Pharmacokinetics and drug-drug interactions of antiretrovirals: an update. Antiviral Res 85:176–189. doi: 10.1016/j.antiviral.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Robillard KR, Chan GN, Zhang G, la Porte C, Cameron W, Bendayan R. 2014. Role of P-glycoprotein in the distribution of the HIV protease inhibitor atazanavir in the brain and male genital tract. Antimicrob Agents Chemother 58:1713–1722. doi: 10.1128/AAC.02031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iaccino E, Schiavone M, Fiume G, Quinto I, Scala G. 2008. The aftermath of the Merck's HIV vaccine trial. Retrovirology 5:56. doi: 10.1186/1742-4690-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirch LM, Morrison S, Steigbigel RT. 2009. Treatment of HIV infection with raltegravir. Expert Opin Pharmacother 10:1203–1211. doi: 10.1517/14656560902911488. [DOI] [PubMed] [Google Scholar]
- 9.Espeseth AS, Felock P, Wolfe A, Witmer M, Grobler J, Anthony N, Egbertson M, Melamed JY, Young S, Hamill T, Cole JL, Hazuda DJ. 2000. HIV-1 integrase inhibitors that compete with the target DNA substrate define a unique strand transfer conformation for integrase. Proc Natl Acad Sci U S A 97:11244–11249. doi: 10.1073/pnas.200139397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steigbigel RT, Cooper DA, Teppler H, Eron JJ, Gatell JM, Kumar PN, Rockstroh JK, Schechter M, Katlama C, Markowitz M, Yeni P, Loutfy MR, Lazzarin A, Lennox JL, Clotet B, Zhao J, Wan H, Rhodes RR, Strohmaier KM, Barnard RJ, Isaacs RD, Nguyen BY, BENCHMRK Study Teams. 2010. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 phase III trials. Clin Infect Dis 50:605–612. doi: 10.1086/650002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temesgen Z, Siraj DS. 2008. Raltegravir: first in class HIV integrase inhibitor. Ther Clin Risk Manag 4:493–500. doi: 10.2147/TCRM.S2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kassahun K, McIntosh I, Cui D, Hreniuk D, Merschman S, Lasseter K, Azrolan N, Iwamoto M, Wagner JA, Wenning LA. 2007. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab Dispos 35:1657–1663. doi: 10.1124/dmd.107.016196. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto M, Kassahun K, Troyer MD, Hanley WD, Lu P, Rhoton A, Petry AS, Ghosh K, Mangin E, DeNoia EP, Wenning LA, Stone JA, Gottesdiener KM, Wagner JA. 2008. Lack of a pharmacokinetic effect of raltegravir on midazolam: in vitro/in vivo correlation. J Clin Pharmacol 48:209–214. doi: 10.1177/0091270007310382. [DOI] [PubMed] [Google Scholar]
- 14.Dahl V, Josefsson L, Palmer S. 2010. HIV reservoirs, latency, and reactivation: prospects for eradication. Antiviral Res 85:286–294. doi: 10.1016/j.antiviral.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Solas C, Lafeuillade A, Halfon P, Chadapaud S, Hittinger G, Lacarelle B. 2003. Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 47:238–243. doi: 10.1128/AAC.47.1.238-243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yilmaz A, Gisslen M, Spudich S, Lee E, Jayewardene A, Aweeka F, Price RW. 2009. Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS One 4:e6877. doi: 10.1371/journal.pone.0006877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croteau D, Letendre S, Best BM, Ellis RJ, Breidinger S, Clifford D, Collier A, Gelman B, Marra C, Mbeo G, McCutchan A, Morgello S, Simpson D, Way L, Vaida F, Ueland S, Capparelli E, Grant I, CHARTER Group. 2010. Total raltegravir concentrations in cerebrospinal fluid exceed the 50-percent inhibitory concentration for wild-type HIV-1. Antimicrob Agents Chemother 54:5156–5160. doi: 10.1128/AAC.00507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson DH, Sutherland D, Acosta EP, Erdem H, Richardson D, Haas DW. 2013. Genetic and non-genetic determinants of raltegravir penetration into cerebrospinal fluid: a single arm pharmacokinetic study. PLoS One 8:e82672. doi: 10.1371/journal.pone.0082672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barau C, Delaugerre C, Braun J, de Castro N, Furlan V, Charreau I, Gerard L, Lascoux-Combe C, Molina JM, Taburet AM. 2010. High concentration of raltegravir in semen of HIV-infected men: results from a substudy of the EASIER-ANRS 138 trial. Antimicrob Agents Chemother 54:937–939. doi: 10.1128/AAC.01261-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summa V, Petrocchi A, Bonelli F, Crescenzi B, Donghi M, Ferrara M, Fiore F, Gardelli C, Gonzalez Paz O, Hazuda DJ, Jones P, Kinzel O, Laufer R, Monteagudo E, Muraglia E, Nizi E, Orvieto F, Pace P, Pescatore G, Scarpelli R, Stillmock K, Witmer MV, Rowley M. 2008. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J Med Chem 51:5843–5855. doi: 10.1021/jm800245z. [DOI] [PubMed] [Google Scholar]
- 21.Merck Research Laboratories. 2007. FDA Antiviral Drugs Advisory Committee Meeting: ISENTRESS (raltegravir) 400 mg for treatment of HIV (NDA 22-145). September 5, 2007: briefing document (background package) Merck & Co., Inc., Whitehouse Station, NJ: http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4314b1-01-Merck.pdf. [Google Scholar]
- 22.Brainard DM, Wenning LA, Stone JA, Wagner JA, Iwamoto M. 2011. Clinical pharmacology profile of raltegravir, an HIV-1 integrase strand transfer inhibitor. J Clin Pharmacol 51:1376–1402. doi: 10.1177/0091270010387428. [DOI] [PubMed] [Google Scholar]
- 23.Bruyère A, Declèves X, Bouzom F, Ball K, Marques C, Treton X, Pocard M, Valleur P, Bouhnik Y, Panis Y, Scherrmann JM, Mouly S. 2010. Effect of variations in the amounts of P-glycoprotein (ABCB1), BCRP (ABCG2) and CYP3A4 along the human small intestine on PBPK models for predicting intestinal first pass. Mol Pharm 7:1596–1607. doi: 10.1021/mp100015x. [DOI] [PubMed] [Google Scholar]
- 24.Hoque MT, Robillard KR, Bendayan R. 2012. Regulation of breast cancer resistant protein by peroxisome proliferator-activated receptor αin human brain microvessel endothelial cells. Mol Pharmacol 81:598–609. doi: 10.1124/mol.111.076745. [DOI] [PubMed] [Google Scholar]
- 25.Chan GN, Hoque MT, Cummins CL, Bendayan R. 2011. Regulation of P-glycoprotein by orphan nuclear receptors in human brain microvessel endothelial cells. J Neurochem 118:163–175. doi: 10.1111/j.1471-4159.2011.07288.x. [DOI] [PubMed] [Google Scholar]
- 26.Robillard KR, Hoque T, Bendayan R. 2012. Expression of ATP-binding cassette membrane transporters in rodent and human sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier. J Pharmacol Exp Ther 340:96–108. doi: 10.1124/jpet.111.186916. [DOI] [PubMed] [Google Scholar]
- 27.Kis O, Zastre JA, Hoque MT, Walmsley SL, Bendayan R. 2013. Role of drug efflux and uptake transporters in atazanavir intestinal permeability and drug-drug interactions. Pharm Res 30:1050–1064. doi: 10.1007/s11095-012-0942-y. [DOI] [PubMed] [Google Scholar]
- 28.Moss DM, Kwan WS, Liptrott NJ, Smith DL, Siccardi M, Khoo SH, Back DJ, Owen A. 2011. Raltegravir is a substrate for SLC22A6: a putative mechanism for the interaction between raltegravir and tenofovir. Antimicrob Agents Chemother 55:879–887. doi: 10.1128/AAC.00623-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zembruski NC, Buchel G, Jodicke L, Herzog M, Haefeli WE, Weiss J. 2011. Potential of novel antiretrovirals to modulate expression and function of drug transporters in vitro. J Antimicrob Chemother 66:802–812. doi: 10.1093/jac/dkq501. [DOI] [PubMed] [Google Scholar]
- 30.Hashiguchi Y, Hamada A, Shinohara T, Tsuchiya K, Jono H, Saito H. 2013. Role of P-glycoprotein in the efflux of raltegravir from human intestinal cells and CD4+ T-cells as an interaction target for anti-HIV agents. Biochem Biophys Res Commun 439:221–227. doi: 10.1016/j.bbrc.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 31.Zastre JA, Chan GN, Ronaldson PT, Ramaswamy M, Couraud PO, Romero IA, Weksler B, Bendayan M, Bendayan R. 2009. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J Neurosci Res 87:1023–1036. doi: 10.1002/jnr.21898. [DOI] [PubMed] [Google Scholar]
- 32.Ronaldson PT, Ashraf T, Bendayan R. 2010. Regulation of multidrug resistance protein 1 by tumor necrosis factor alpha in cultured glial cells: involvement of nuclear factor-kappaB and c-Jun N-terminal kinase signaling pathways. Mol Pharmacol 77:644–659. doi: 10.1124/mol.109.059410. [DOI] [PubMed] [Google Scholar]
- 33.Lee G, Babakhanian K, Ramaswamy M, Prat A, Wosik K, Bendayan R. 2007. Expression of the ATP-binding cassette membrane transporter, ABCG2, in human and rodent brain microvessel endothelial and glial cell culture systems. Pharm Res 24:1262–1274. doi: 10.1007/s11095-007-9244-1. [DOI] [PubMed] [Google Scholar]
- 34.Adachi Y, Suzuki H, Sugiyama Y. 2003. Quantitative evaluation of the function of small intestinal P-glycoprotein: comparative studies between in situ and in vitro. Pharm Res 20:1163–1169. doi: 10.1023/A:1025088628787. [DOI] [PubMed] [Google Scholar]
- 35.Masaoka Y, Tanaka Y, Kataoka M, Sakuma S, Yamashita S. 2006. Site of drug absorption after oral administration: assessment of membrane permeability and luminal concentration of drugs in each segment of gastrointestinal tract. Eur J Pharm Sci 29:240–250. doi: 10.1016/j.ejps.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Hubatsch I, Ragnarsson EG, Artursson P. 2007. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc 2:2111–2119. doi: 10.1038/nprot.2007.303. [DOI] [PubMed] [Google Scholar]
- 37.Dahan A, Amidon GL. 2009. Segmental dependent transport of low permeability compounds along the small intestine due to P-glycoprotein: the role of efflux transport in the oral absorption of BCS class III drugs. Mol Pharm 6:19–28. doi: 10.1021/mp800088f. [DOI] [PubMed] [Google Scholar]
- 38.Crowe A. 2011. The role of P-glycoprotein and breast cancer resistance protein (BCRP) in bacterial attachment to human gastrointestinal cells. J Crohns Colitis 5:531–542. doi: 10.1016/j.crohns.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Adams JL, Greener BN, Kashuba AD. 2012. Pharmacology of HIV integrase inhibitors. Curr Opin HIV AIDS 7:390–400. doi: 10.1097/COH.0b013e328356e91c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizk ML, Houle R, Chan GH, Hafey M, Rhee EG, Chu X. 2014. Raltegravir has a low propensity to cause clinical drug interactions through inhibition of major drug transporters: an in vitro evaluation. Antimicrob Agents Chemother 58:1294–1301. doi: 10.1128/AAC.02049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moss DM, Siccardi M, Murphy M, Piperakis MM, Khoo SH, Back DJ, Owen A. 2012. Divalent metals and pH alter raltegravir disposition in vitro. Antimicrob Agents Chemother 56:3020–3026. doi: 10.1128/AAC.06407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dupuis ML, Ascione A, Palmisano L, Vella S, Cianfriglia M. 2013. Raltegravir does not revert efflux activity of MDR1-P-glycoprotein in human MDR cells. BMC Pharmacol Toxicol 14:47. doi: 10.1186/2050-6511-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, Evans S, Collier AC, Taylor M, Ellis R. 2011. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS 25:357–365. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ, CHARTER Group. 2008. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oberoi RK, Mittapalli RK, Elmquist WF. 2013. Pharmacokinetic assessment of efflux transport in sunitinib distribution to the brain. J Pharmacol Exp Ther 347:755–764. doi: 10.1124/jpet.113.208959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moss DM, Curley P, Shone A, Siccardi M, Owen A. 2014. A multisystem investigation of raltegravir association with intestinal tissue: implications for pre-exposure prophylaxis and eradication. J Antimicrob Chemother 69:3275–3281. doi: 10.1093/jac/dku312. [DOI] [PubMed] [Google Scholar]