Abstract
Proteases and peptidases in Trypanosoma cruzi are considered potential targets for antichagasic chemotherapy. We monitored changes in low-mass metabolites in T. cruzi epimastigotes treated with bestatin, a dipeptide metalloaminopeptidase inhibitor. After treatment, multiple dipeptides were shown to be increased, confirming in situ inhibition of the leucine aminopeptidase of T. cruzi (LAPTc) and probably other peptidases.
TEXT
Peptidases are considered attractive targets for drug development in various parasitic protozoa, including Trypanosoma cruzi, which possesses a highly active proteolytic metabolism (1–3). Proteolytic enzymes are key regulators of a number of important biological processes in virtually all organisms, including invasion and host-cell rupture in parasites. They also are viable drug targets; there are many protease-directed small-molecule therapeutics currently in use for the treatment of human diseases ranging from hypertension to cancer (4). T. cruzi causes Chagas disease in humans, a neglected disease with only two drugs available for treatment and no immediate prospects for vaccines (5, 6). Metabolomics is a powerful tool for unveiling the modes of action of drugs and has been successfully used in trypanosomatids (7–13). To evaluate the metabolite changes occurring after exposure to a peptidase inhibitor, we performed an untargeted metabolomics analysis of bestatin treated T. cruzi. Bestatin is a natural-product broad-spectrum metalloaminopeptidase inhibitor (14). It has been proposed to inhibit the M17 leucine aminopeptidase of T. cruzi (LAPTc) (15).
We used a platform consisting of Zic hydrophilic interaction liquid chromatography (HILIC) coupled to high-accuracy mass spectrometry as previously described (9). T. cruzi epimastigotes were treated with 300 μM bestatin for 6 h (3 biological replicates). After quenching and before metabolite extraction, bestatin or the nonrelated anti-Chagas drug benznidazole was added to control cells (4 biological replicates). Metabolite identities were assigned based on the predicted formula associated with the measured exact mass (within 3 ppm) and retention time (RT). Where authentic standards were available, these identifications were tier 1-confirmed annotations according to the metabolomics standards initiative; otherwise, they remained putative based on the exact mass and predicted RT screened against the IDEOM database. This database contains all metabolites from the KEGG, MetaCyc, and Lipid Maps databases, and all possible dipeptides, tripeptides, and tetrapeptides derived from proteinogenic amino acids (16, 17). In cases where metabolite identification was ambiguous, the most likely metabolite was assigned based on available biochemical knowledge, and lists of alternative identifications, i.e., isomeric compounds with the same mass, were accessible in the IDEOM file (see File S1 [intranet.pasteur.edu.uy/publico/robello/FileS1.xlsb]).
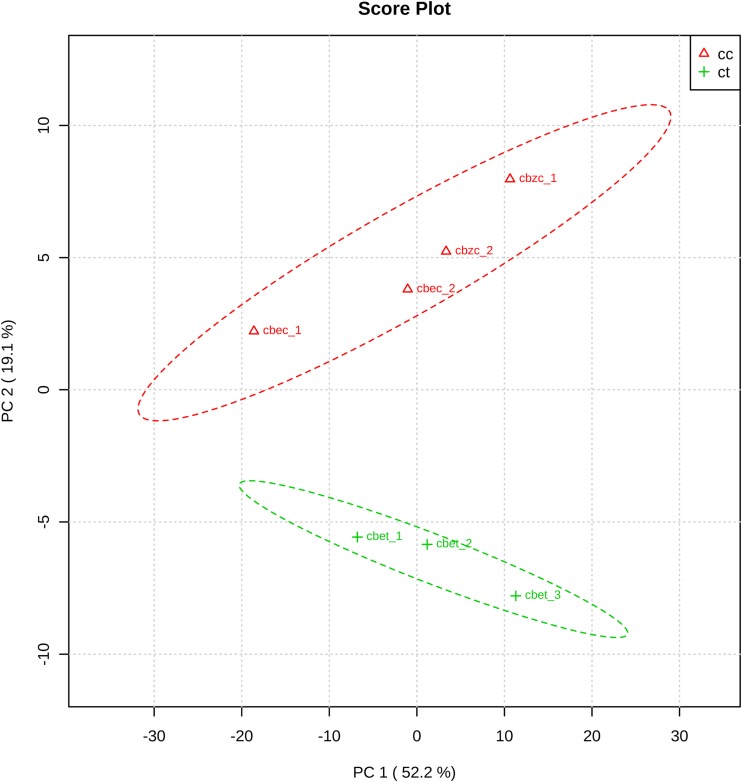
After removing mass spectrometry (MS) artifacts, automatic identification yielded 1,076 metabolites in all the samples (see File S1 [intranet.pasteur.edu.uy/publico/robello/FileS1.xlsb]). As previously reported using the same methodology (9), a wide range of different metabolites was identified, including peptides, nucleotides, lipids, and sugars. Principal components analysis (PCA) showed significant differences between treated and nontreated samples (Fig. 1). The most striking differences were observed among dipeptides, with several detected with higher intensities after bestatin treatment (Table 1; Fig. 2). Leucine and/or isoleucine, valine, threonine, and histidine were highly represented in the significantly upregulated dipeptides (Table 1; Fig. 2 and 3). The observed phenomenon is consistent with a bestatin-mediated inhibition of the peptidases involved in the conversion of these dipeptides to single amino acids.
FIG 1.
Principal components analysis of metabolomics data. PCA score plot was generated with normalized MS peak intensity data using MetaboAnalyst (20, 21). Samples: cbzc, control of nontreated parasites (20 μM bestatin added after quenching of metabolism); cbec, control of nontreated parasites (300 μM bestatin added after quenching of metabolism); cbet, 300 μM bestatin-treated parasites; cc,cbec + cbzc; ct, cbet.
TABLE 1.
Metabolites with significant changes after bestatin treatmenta
| Mass (m/z)b | RT (min)c | Formulad | Isomere | Putative metabolitef | PPMg | Fold change | P valueh |
|---|---|---|---|---|---|---|---|
| 216.1474 | 12.8 | C10H20N2O3 | 1 | Val-Val | 0.1 | 17.4 | 1.6E-05 |
| 230.1631 | 11.8 | C11H22N2O3 | 2 | Leu-Val, Ile-Val | 0.2 | 13.7 | 0.012 |
| 317.1734 | 11.4 | C17H23N3O3 | 2 | Leu-Trp, Ile-Trp | −1.8 | 12.8 | 7.5E-05 |
| 244.1787 | 11.0 | C12H24N2O3 | 4* | Leu-Leu, Ile-Ile, Ile-Leu | −0.034 | 10.7 | 0.018 |
| 254.1379 | 25.4 | C11H18N4O3 | 2* | Val-His | −0.1 | 10.3 | 0.009 |
| 134.0766 | 24.2 | C12H20N4O3 | 2 | Ile-His, His-Leu | −1.1 | 8.5 | 3.4E-04 |
| 268.1533 | 23.0 | C12H20N4O3 | 2 | Ile-His, His-Leu | −1.0 | 4.1 | 0.033 |
| 218.1266 | 15.1 | C9H18N2O4 | 5* | Leu-ser, Thr-Val, Ile-Val | −0.5 | 6.7 | 0.002 |
| 250.0986 | 14.5 | C9H18N2O4S | 1 | Met-Thr | −0.3 | 6.5 | 0.023 |
| 248.1195 | 12.8 | C10H20N2O3S | 1 | Met-Val | 0.2 | 6.4 | 1.0E-04 |
| 264.1473 | 11.6 | C14H20N2O3 | 2* | Phe-Val | −0.5 | 5.7 | 0.016 |
| 232.1423 | 13.5 | C10H20N2O4 | 3* | Leu-Thr, Ile-Thr | 0.03 | 4.9 | 0.000 |
| 269.1124 | 27.4 | C10H15N5O4 | 3* | Asn-His | −0.2 | 4.4 | 0.047 |
| 266.1267 | 13.4 | C13H18N2O4 | 1 | Phe-Thr | 0.3 | 4.2 | 0.010 |
| 278.1631 | 10.8 | C15H22N2O3 | 2 | Leu-Phe, Ile-Phe | 0.1 | 3.7 | 0.002 |
| 214.1319 | 13.0 | C10H18N2O3 | 2* | Val-Pro | 0.9 | 3.7 | 4.5E-04 |
| 286.1101 | 25.1 | C11H18N4O3S | 1 | Met-His | 0.3 | 3.4 | 0.010 |
| 228.1476 | 12.2 | C11H20N2O3 | 2 | Leu-Pro, Ile-Pro | 0.8 | 3.3 | 0.008 |
| 190.0954 | 17.0 | C7H14N2O4 | 5* | Thr-Ala | 0.4 | 3.2 | 4.9E-04 |
| 128.0585 | 27.0 | C5H8N2O2 | 3* | γ-Amino-gamma-cyanobutanoate | −0.5 | 3.2 | 4.7E-04 |
| 244.1060 | 15.3 | C10H16N2O5 | 2* | Glu-Pro | 0.1 | 3.0 | 0.003 |
| 246.1217 | 14.9 | C10H18N2O5 | 6* | Glu-Val, Leu-Asp, Ile-Asp, γ-Glu-Val, β-Asp-Leu, β-Asp-Ile | 0.4 | 3.0 | 0.024 |
| 174.1004 | 15.0 | C7H14N2O3 | 5* | Val-Gly | −0.1 | 2.9 | 0.001 |
| 286.1893 | 5.5 | C14H26N2O4 | 1 | N-Acetyl-leucyl-leucine | 0.1 | 2.7 | 0.007 |
| 260.1372 | 14.3 | C11H20N2O5 | 4 | Glu-Leu, Ile-Glu, γ-Glu-Ile, γ-Glu-Leu | −0.03 | 2.7 | 0.017 |
| 136.5901 | 26.3 | C11H23N5O3 | 1 | Val-Arg | 0.1 | 2.7 | 0.002 |
| 217.1062 | 18.9 | C8H15N3O4 | 3* | Ala-Gln | −0.3 | 2.6 | 3.8E-05 |
| 137.5796 | 28.2 | C10H21N5O4 | 1 | Thr-Arg | −0.4 | 2.6 | 0.004 |
| 220.1059 | 18.2 | C8H16N2O5 | 3* | Thr-Thr | 0.1 | 2.6 | 0.033 |
| 232.1060 | 15.5 | C9H16N2O5 | 5* | Val-Asp | 0.3 | 2.6 | 0.008 |
| 233.1011 | 18.9 | C8H15N3O5 | 4* | Gln-Ser, Thr-Asn | −0.4 | 2.4 | 1.0E-04 |
| 202.1319 | 13.1 | C9H18N2O3 | 3* | Leu-Ala, Ile-Ala | 0.6 | 2.3 | 0.018 |
| 256.1170 | 26.7 | C10H16N4O4 | 2* | Thr-His | −0.5 | 2.3 | 0.028 |
| 189.0748 | 20.5 | C6H11N3O4 | 3* | Asn-Gly | −1.0 | 2.2 | 0.009 |
| 206.0902 | 19.0 | C7H14N2O5 | 5* | Thr-Ser | −0.6 | 2.1 | 0.006 |
Metabolites with significantly different abundances in control (cc) and bestatin-treated (ct) samples are listed (fold change > 2 and P < 0.05, unpaired t test).
Mass, m/z values corrected for proton gain or loss.
RT, retention time.
Predicted formulae using m/z data with IDEOM.
Number of putative isomers matching the same molecular formula. For a given dipeptide, only one of the two possible sequences is shown.
Proposed metabolites for each ion are listed. For dipeptides, all the alternative compositions are listed. If a nonpeptide alternative identification is possible (indicated by an asterisk in the “isomer” column), those metabolites are listed in the macro-enabled IDEOM file (see File S1 [intranet.pasteur.edu.uy/publico/robello/FileS1.xlsb]).
Mass error, [(m/zobserved − m/ztheoretical)/(m/ztheoretical)]*1E+6, represents the mass error between the observed mass for each metabolite and the theoretical mass of its corresponding proposed formula.
Unpaired t test, cc versus ct.
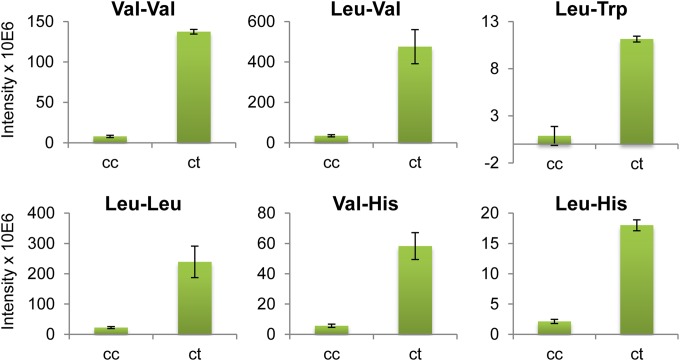
FIG 2.
Most affected metabolites after bestatin treatment in Trypanosoma cruzi. Shown are metabolites displaying significant differences between control samples (cc) and 300 μM bestatin-treated samples (ct) (P < 0.05 [unpaired t test]; fold change, >8).
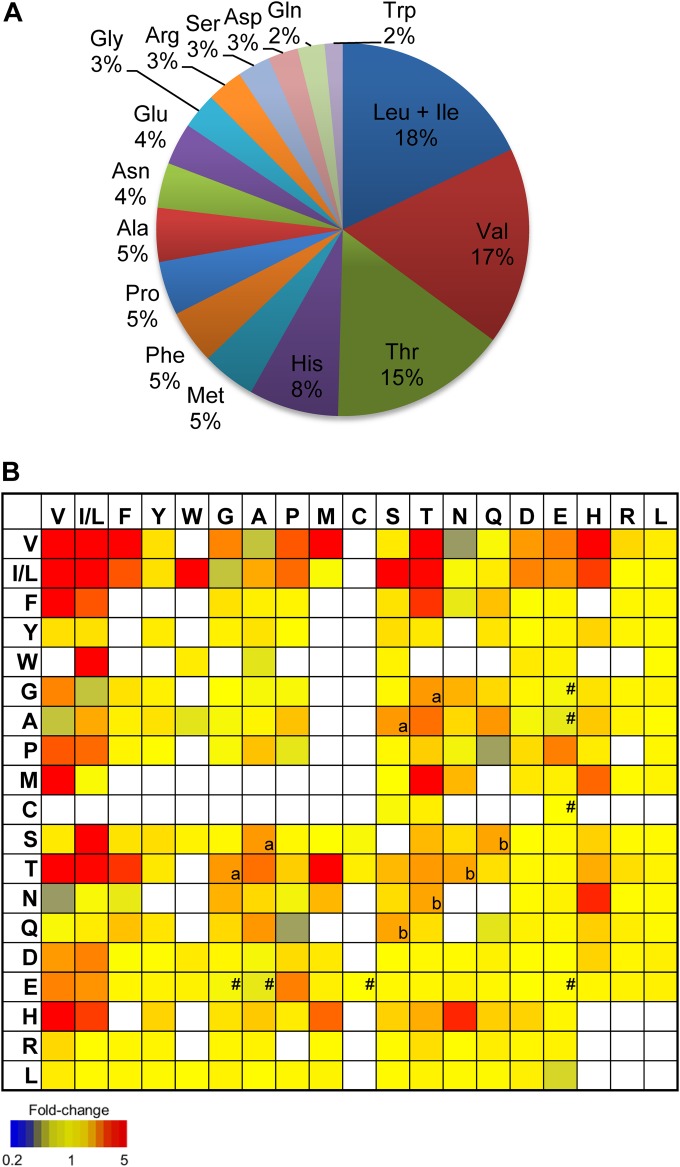
FIG 3.
Composition of dipeptides affected by bestatin in T. cruzi. (A) Amino acid composition in the 32 dipeptides with augmented levels after bestatin treatment (P < 0.05 unpaired t test; fold change, >2). In cases where more than one dipeptide may be assigned to a detected mass (i.e., isomers), the values of amino acid presence were calculated assigning equal probability to each of the alternative dipeptides for a unique formula. (B) Heat map of relative abundance of all detected dipeptides from semitargeted analysis in bestatin-treated parasites compared to those of the untreated parasites. White boxes, not detected. For isomeric peptides, the same relative abundance was entered for all isomers. a, Ser-Ala is isomeric with Thr-Gly; b, Ser-Gln is isomeric with Thr-Asn; #, peptides found upregulated after parasites were treated with benznidazole (9).
The activity seen in bestatin-treated T. cruzi was highly specific. In addition to those metabolites putatively annotated as dipeptides, only two other metabolites demonstrated significant differences. One is an N-acetylated dipeptide (N-acetyl leucyl-leucine), while the other, putatively annotated as gamma-amino-gamma-cyanobutanoate, has a retention time (27 min) and degree of unsaturation (C5H8N2O2) more consistent with being an MS-derived fragment of another basic dipeptide (see File S1 [intranet.pasteur.edu.uy/publico/robello/FileS1.xlsb]). When T. cruzi was treated with benznidazole, by contrast, alterations were related primarily to depletion and turnover of low-molecular-weight thiols due to the formation of drug-thiol conjugates (9). Some gamma-glutamyl-containing dipeptides were raised (see Fig. 3B), and these metabolic alterations were associated with augmented recycling of the observed conjugates (9). No such changes were identified in this experiment, indicating that the changes associated with treatment with bestatin are specific and exclusively related to a failure to degrade dipeptides.
Although it is clear that the effect of bestatin on trypanosomes are restricted to specific inhibition of peptidases, we cannot pinpoint a single molecular target using this approach. Nevertheless, the substrate specificities of the peptidase target(s) were evident from the liquid chromatography-mass spectrometry (LC-MS) data. Nearly 90% of significantly upregulated peptides contained leucine and/or isoleucine, valine, threonine, and/or histidine. In order to confirm the substrate specificities of the bestatin-inhibited enzymes, the LC-MS signals from all possible dipeptide combinations of the 20 proteinogenic amino acids were extracted. It was not possible to distinguish isomeric peptides such as those containing leucine and isoleucine or the sequence of each peptide, and the single most abundant peak for each accurate mass (within the predicted retention time window) was used to represent each of the isomeric dipeptides. Approximately three-quarters of all theoretical dipeptides were observed with this semitargeted profiling approach, which confirmed the selective accumulation of dipeptides containing Leu and/or Ile, Val, Thr, and His residues (Fig. 3B).
Significant accumulation of leucine and/or isoleucine dipeptides is consistent with the expected activity of bestatin as an inhibitor of T. cruzi leucine aminopeptidase LAPTc, which was previously demonstrated in vitro (14). The additional inhibition of the peptidase activities observed here against Val-, Thr-, and His-containing dipeptides suggests that either LAPTc has diverse substrate specificity or bestatin inhibits multiple peptidase enzymes. T. cruzi has at least three genes encoding M17 aminopeptidases, including the leucine aminopeptidase LAPTc, which demonstrated activity against Leu-7-amido-4-methylcoumarin (AMC), but not Pro-AMC or Asp-AMC in vitro (15). Other leucine aminopeptidases from three pathogenic Leishmania species were identified and are susceptible to inhibition by bestatin. In addition to the Leu-AMC fluorogenic substrate, these enzymes demonstrated partial activities against Cys-AMC, Met-AMC, Ala-AMC, Ile-AMC, and Trp-AMC (18). A recent analysis of Plasmodium falciparum A1-M17 aminopeptidase substrate specificity showed activities against Phe, Met, Thr, and Tyr, in addition to activities against the preferred S1 substrates Leu and Trp (19). Additional molecular studies are necessary to determine whether LAPTc demonstrates specificity toward the unique array of substrates observed here (Leu and/or Ile, Val, Thr, and His dipeptides) or if bestatin exerts its effects by the inhibition of multiple> aminopeptidase targets within the parasite. The richness of the medium in which these cells are grown, with highly abundant supplies of all amino acids, means that the loss of production of single amino acids through peptidase inhibition is not lethal at the doses evaluated here.
In summary, under the tested conditions, bestatin had no toxic effects on the parasites, but it had a substantial effect on the dipeptide pool, giving confirmation of its action as an inhibitor of enzymes acting as dipeptidases. Thus, the metabolomics platform used here shows great potential for the in situ analysis of enzyme inhibition by pharmacological agents. Further tandem mass spectrometry (MS/MS) analysis of the ions of interest would be adequate for resolving ambiguities in peptide identification. The approach can be directly combined with enzyme knockout, knockdown, or overexpression in parasites to reveal perturbations to endogenous peptides that demonstrate the physiological function of specific peptidase enzymes in situ.
ACKNOWLEDGMENTS
We thank Karl Burgess at the Glasgow Polyomics Facility for technical assistance.
This work was funded by the European Union (grant 223238) and research fellowships from CONICET-Argentina (A.T.) and ANII-Uruguay (P.F.-T.).
REFERENCES
- 1.Perez B, Teixeira C, Gomes JR, Gomes P. 2013. Development of Plasmodium falciparum protease inhibitors in the past decade (2002–2012). Curr Med Chem 20:3049–3068. doi: 10.2174/0929867311320250003. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez VE, Niemirowicz GT, Cazzulo JJ. 2013. Metacaspases, autophagins and metallocarboxypeptidases: potential new targets for chemotherapy of the trypanosomiases. Curr Med Chem 20:3069–3077. doi: 10.2174/0929867311320250004. [DOI] [PubMed] [Google Scholar]
- 3.de Souza W, Sant'Anna C, Cunha-e-Silva NL. 2009. Electron microscopy and cytochemistry analysis of the endocytic pathway of pathogenic protozoa. Prog Histochem Cytochem 44:67–124. doi: 10.1016/j.proghi.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Arastu-Kapur S, Ponder EL, Fonovic UP, Yeoh S, Yuan F, Fonovic M, Grainger M, Phillips CI, Powers JC, Bogyo M. 2008. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nat Chem Biol 4:203–213. doi: 10.1038/nchembio.70. [DOI] [PubMed] [Google Scholar]
- 5.Schofield CJ, Jannin J, Salvatella R. 2006. The future of Chagas disease control. Trends Parasitol 22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Remme JHF, Feenstra P, Lever PR, Medici AC, Morel CM, Noma M, Ramaiah KD, Richards F, Seketeli A, Schmunis G, van Brakel WH, Vassall A. 2006. Tropical diseases targeted for elimination: Chagas disease, lymphatic filariasis, onchocerciasis, and leprosy. In Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P (ed), Disease control priorities in developing countries, 2nd ed World Bank, Washington DC. [PubMed] [Google Scholar]
- 7.Ali JA, Creek DJ, Burgess K, Allison HC, Field MC, Maser P, De Koning HP. 2013. Pyrimidine salvage in Trypanosoma brucei bloodstream forms and the trypanocidal action of halogenated pyrimidines. Mol Pharmacol 83:439–453. doi: 10.1124/mol.112.082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent IM, Creek DJ, Burgess K, Woods DJ, Burchmore RJ, Barrett MP. 2012. Untargeted metabolomics reveals a lack of synergy between nifurtimox and eflornithine against Trypanosoma brucei. PLoS Negl Trop Dis 6:e1618. doi: 10.1371/journal.pntd.0001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trochine A, Creek DJ, Faral-Tello P, Barrett MP, Robello C. 2014. Benznidazole biotransformation and multiple targets in Trypanosoma cruzi revealed by metabolomics. PLoS Negl Trop Dis 8:e2844. doi: 10.1371/journal.pntd.0002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canuto GA, Castilho-Martins EA, Tavares MF, Rivas L, Barbas C, Lopez-Gonzalvez A. 2014. Multi-analytical platform metabolomic approach to study miltefosine mechanism of action and resistance in Leishmania. Anal Bioanal Chem 406:3459–3476. doi: 10.1007/s00216-014-7772-1. [DOI] [PubMed] [Google Scholar]
- 11.Canuto GA, Castilho-Martins EA, Tavares M, Lopez-Gonzalvez A, Rivas L, Barbas C. 2012. CE-ESI-MS metabolic fingerprinting of Leishmania resistance to antimony treatment. Electrophoresis 33:1901–1910. doi: 10.1002/elps.201200007. [DOI] [PubMed] [Google Scholar]
- 12.Creek DJ, Barrett MP. 2014. Determination of antiprotozoal drug mechanisms by metabolomics approaches. Parasitology 141:83–92. doi: 10.1017/S0031182013000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent IM, Barrett MP. 2015. Metabolomic-based strategies for anti-parasite drug discovery. J Biomol Screen 20:44–55. doi: 10.1177/1087057114551519. [DOI] [PubMed] [Google Scholar]
- 14.Umezawa H, Aoyagi T, Suda H, Hamada M, Takeuchi T. 1976. Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J Antibiot (Tokyo) 29:97–99. doi: 10.7164/antibiotics.29.97. [DOI] [PubMed] [Google Scholar]
- 15.Cadavid-Restrepo G, Gastardelo TS, Faudry E, de Almeida H, Bastos IM, Negreiros RS, Lima MM, Assumpcao TC, Almeida KC, Ragno M, Ebel C, Ribeiro BM, Felix CR, Santana JM. 2011. The major leucyl aminopeptidase of Trypanosoma cruzi (LAPTc) assembles into a homohexamer and belongs to the M17 family of metallopeptidases. BMC Biochem 12:46. doi: 10.1186/1471-2091-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creek DJ, Jankevics A, Breitling R, Watson DG, Barrett MP, Burgess KE. 2011. Toward global metabolomics analysis with hydrophilic interaction liquid chromatography-mass spectrometry: improved metabolite identification by retention time prediction. Anal Chem 83:8703–8710. doi: 10.1021/ac2021823. [DOI] [PubMed] [Google Scholar]
- 17.Creek DJ, Jankevics A, Burgess KE, Breitling R, Barrett MP. 2012. IDEOM: an Excel interface for analysis of LC-MS-based metabolomics data. Bioinformatics 28:1048–1049. doi: 10.1093/bioinformatics/bts069. [DOI] [PubMed] [Google Scholar]
- 18.Morty RE, Morehead J. 2002. Cloning and characterization of a leucyl aminopeptidase from three pathogenic Leishmania species. J Biol Chem 277:26057–26065. doi: 10.1074/jbc.M202779200. [DOI] [PubMed] [Google Scholar]
- 19.Poreba M, McGowan S, Skinner-Adams TS, Trenholme KR, Gardiner DL, Whisstock JC, To J, Salvesen GS, Dalton JP, Drag M. 2012. Fingerprinting the substrate specificity of M1 and M17 aminopeptidases of human malaria, Plasmodium falciparum. PLoS One 7:e31938. doi: 10.1371/journal.pone.0031938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. 2012. MetaboAnalyst 2.0–a comprehensive server for metabolomic data analysis. Nucleic Acids Res 40:W127–W133. doi: 10.1093/nar/gks374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia J, Psychogios N, Young N, Wishart DS. 2009. MetaboAnalyst: a Web server for metabolomic data analysis and interpretation. Nucleic Acids Res 37:W652–W660. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]