Abstract
This study was performed to compare the therapeutic efficacy of cefuroxime with that of cefotaxime as initial antimicrobial therapies in women with complicated nonobstructive acute pyelonephritis (APN) caused by Enterobacteriaceae infections. The clinical characteristics and outcomes of a cefuroxime-treated group (n = 156) were compared with those of a cefotaxime-treated group (n = 166). Of these 322 women, 90 from each group were matched by propensity scores. The defervescence rates were not significantly different in the cefuroxime and cefotaxime groups at 72 h after the start of antimicrobial therapy (81.1% versus 78.9%, P = 0.709). The clinical and microbiological cure rates during the follow-up visits that were 4 to 14 days after the end of the antimicrobial therapies were not significantly different in the cefuroxime versus cefotaxime groups, which were 97.8% (87/89) versus 97.8% (87/89) (P > 0.999) and 89.5% (68/76) versus 90.7% (68/75) (P = 0.807), respectively. The median hospital stay duration and the median times to defervescence in the cefuroxime versus cefotaxime groups were 8 days (interquartile range [IQR], 7 to 10 days) versus 9 days (IQR, 7 to 11 days), respectively, and 55 h (IQR, 37 to 70 h) versus 55 h (IQR, 35 to 69 h), respectively. Bacteremia, extended-spectrum-β-lactamase-producing Enterobacteriaceae, C-reactive protein levels of ≥15 mg/dl, and white blood cell counts of ≥15,000/mm3 of blood had independent effects on the rates of early clinical failure. Our data suggest that the effects of cefuroxime are not different from those of cefotaxime when they are used as an initial antimicrobial treatments for community-onset complicated nonobstructive APN in women.
INTRODUCTION
Community-onset complicated acute pyelonephritis (APN) includes nonobstructive complicated APN and obstructive complicated APN, with the obstructive form requiring drainage and antimicrobial treatment. Extended-spectrum cephalosporin, penicillin with a β-lactamase inhibitor, an aminoglycoside, and a carbapenem are recommended as the initial antimicrobial agents for the treatment of complicated APN, which includes urosepsis, obstructive complicated APN, and health care-associated catheter-associated complicated APN (1–3).
However, we hypothesized that more step-down antibiotics, such as cefuroxime, can effectively be used for the treatment of community-onset nonobstructive complicated APN, the patient population of which is more homogeneous and the uropathogens are more susceptible to cephalosporin treatment than those for the obstructive form (4, 5). The antimicrobial susceptibilities of uropathogens in community-onset nonobstructive complicated APN may be more similar to those in uncomplicated APN rather than those in health care-associated or catheter-associated urinary tract infections (UTIs).
In the classification of UTIs and APNs, community-onset diabetic or elderly women with APN are usually included in the complicated APN subtype and may be treated initially with antimicrobial agents, which are recommended for complicated UTIs, although the antimicrobial susceptibilities of the uropathogens in community-onset nonobstructive complicated APNs are likely to be similar to those in uncomplicated APNs (2, 6). As multiresistant bacteria are recently on the increase, using step-down antibiotics is a necessity, both for the least ecological adverse effects and for the reduction in health costs.
Although cefuroxime has been used as a treatment for APN since the late 1970s, extended-spectrum cephalosporins, such as cefotaxime and ceftriaxone, have more commonly been recommended for the treatment of APN based on several guidelines and recent clinical trial results (7–9). To verify the effects of cefuroxime as a treatment for complicated APN, we need clinical studies that compare the efficacies of cefuroxime and cefotaxime in community-onset complicated APN cases.
The purpose of this study was to compare the therapeutic efficacies of intravenous cefuroxime and cefotaxime as an initial antimicrobial therapy for the treatment of women in the Republic of Korea with community-onset complicated nonobstructive APN due to Enterobacteriaceae infection.
MATERIALS AND METHODS
Study design.
We conducted a retrospective cohort study from 1 February 2011 to 30 June 2014 that included women with community-onset complicated nonobstructive APN due to Enterobacteriaceae infections. This study was conducted at Catholic University St. Vincent's Hospital, a teaching hospital in Suwon City, South Korea, according to the protocols approved by the St. Vincent's Hospital institutional review board (IRB). The IRB waived the requirement to obtain a patient written informed consent.
Patient population.
Hospitalized women (age ≥18 years) with community-onset nonobstructive complicated APN due to Enterobacteriaceae infections who required parenteral antibiotic therapy were analyzed following a review in the hospital database of the medical records of women with APN as a discharge diagnosis between 1 February 2011 and 30 June 2014. Finally, women with nonobstructive complicated APN who received cefuroxime or cefotaxime as an initial antimicrobial therapy were enrolled into the study. APN was defined as a fever of ≥38.0°C and the presence of pyuria and bacteriuria. Pyuria was defined as the presence of 5 to ≥9 leukocytes per high-power field of clean voided midstream urine. Bacteriuria was defined as the isolation of a single uropathogen (≥105 CFU/ml of urine) (10, 11). Patients were excluded if they had a urinary catheter-related infection or developed fever or urinary symptoms >48 h after hospital admission or needed a urological interventional procedure, such as catheterization, percutaneous drainage, or surgery to relieve the urinary obstruction. Patients who did not have a complicating factor or computerized tomography (CT) findings of APN were also excluded.
Complicated APN was defined as APN and the presence of any of the following: urinary tract abnormalities (e.g., vesicoureteral reflux, urolithiasis, neurogenic bladder, polycystic kidney disease, or anomaly of urinary tract), comorbid conditions (e.g., chronic liver disease, chronic kidney disease, congestive heart failure, diabetes mellitus, immunosuppression, kidney transplantation, malignancy, and pregnancy), or age > 65 years (2, 3, 12). Finally, women age ≥18 years with community-onset nonobstructive complicated APN who initially received either cefuroxime or cefotaxime were included in the study.
Data collection.
Demographic and clinical characteristics were collected by reviewing medical records retrospectively for all of the eligible patients. Age, history (of antibiotic use, urinary tract infection, hospitalization, and comorbid conditions), lower and upper urinary tract symptoms, physical manifestations, laboratory test results, intravenous and oral antibiotic administration durations, and microbiological laboratory results were analyzed. The hours to defervescence, hospitalization days, and mortality were included as variables to reveal clinical outcomes. Defervescence and clinical and microbiological cures were assessed at 72 h following the cefuroxime or cefotaxime treatments, at the 4- to 14-day follow-up, and at the 21- to 35-day follow-up visit after the end of antimicrobial therapy (EOT).
Clinical outcome measures and definitions.
Clinical outcomes were measured in terms of clinical success, hospitalization days, and time to defervescence after the start of the cefotaxime or cefuroxime therapies. Defervescence was defined as an afebrile state in which the (tympanic) body temperature was maintained at ≤37.0°C for ≥24 h. The time to defervescence was defined as the time from the start of intravenous cefotaxime or cefuroxime therapy to an afebrile state (11).
The early clinical outcome were assessed at 72 h after the initial administration of antibiotics. Early clinical success was defined as defervescence with an improvement in urinary tract symptoms or signs within 72 h after the initial administration of cefotaxime or cefuroxime therapy (13). Clinical cure was defined as defervescence and the absence of urinary tract symptoms or signs at the 4- to 14-day and 21- to 35-day follow-ups after the EOT, when available. The patients who did not satisfy the criteria for early clinical success or clinical cure were considered early clinical failures or clinical failures, respectively.
Microbiological outcome measures and definitions.
The species identification and in vitro antimicrobial susceptibility results from urine and blood culture tests at admission were assessed and analyzed. Species identification and susceptibility to antimicrobial agents were determined by means of either a semiautomated system (Vitek; bioMérieux, Hazelwood, MO) or disk diffusion susceptibility tests, according to the Clinical and Laboratory Standards Institute (CLSI) criteria (14, 15). Uropathogens were defined as those detected at ≥105 CFU/ml of identified organisms on urine culture, or the isolation of urinary pathogens from blood cultures. The urine culture results were also assessed at the 4- to 14-day follow-up and at the 21- to 35-day follow-up after the EOT, when available. A microbiological cure was defined as the eradication of all uropathogens or a reduction in the urine pathogen level from ≥105 CFU/ml to <103 CFU/ml, with no pathogen present in the blood. Those patients who did not satisfy the criteria for microbiological cure were regarded as microbiological failures.
Statistical methods.
Clinical data, laboratory data, and patient clinical outcomes were compared by using Fisher's exact test or the Pearson chi-square test for categorical variables and an independent t test or the Mann-Whitney test for continuous variables. A multiple logistic regression analysis was performed to evaluate the effects of independent variables on early clinical failure in the complicated nonobstructive APN patients who were treated with cefuroxime or cefotaxime. A retrospective propensity score-matched case-control analysis was performed in order to overcome confounding variables in the choice of initial antimicrobial agent. A P value of <0.05 (two-sided) was considered statistically significant. SPSS version 21.0 for Windows (SPSS, Inc., Chicago, IL) was used for the statistical analyses.
RESULTS
Demographic and clinical characteristics.
A total of 896 women were identified with community-onset APN as a discharge diagnosis. Of these, 322 cases who received cefuroxime or cefotaxime as initial antibiotics for community-onset complicated nonobstructive APN due to Enterobacteriaceae infection were enrolled and analyzed. Of the 322 patients, 156 (48.4%) received cefuroxime, and 166 (51.6%) received cefotaxime. Table 1 shows a comparison of the baseline demographics and clinical characteristics in the cefuroxime and cefotaxime groups. The median ages of the cefuroxime and cefotaxime groups were 71.0 years (interquartile range [IQR], 61.5 to 77.0 years) and 72.0 years (IQR, 63.0 to 77.0 years), respectively (P = 0.407) (Table 1). There were no significant differences in initial body temperature, lower urinary tract infection symptoms, nausea/vomiting, leukocyte counts, C-reactive protein (CRP) levels, hematuria, bacteremia, or numbers of postmenopausal women between the two groups. The frequency of costovertebral angle tenderness was significantly higher in the cefuroxime group than in the cefotaxime group (P = 0.001). No differences between the two groups were observed in the histories of UTI, antibiotic usage frequency, or history of hospitalization within 12 months before the hospital visit. There were also no significant differences in underlying diseases, except for cerebrovascular disorders, between the cefuroxime and cefotaxime groups. Additionally, the prevalences of cefotaxime-resistant Enterobacteriaceae, fluoroquinolone-resistant Enterobacteriaceae, and extended-spectrum β-lactamase [ESBL]-producing Enterobacteriaceae were also not significantly different between the two groups (Table 1).
TABLE 1.
Demographic and clinical characteristics in 322 women with community-onset complicated nonobstructive acute pyelonephritis due to Enterobacteriaceae, by treatment regimen
| Characteristica | Patients treated with: |
P value | |
|---|---|---|---|
| Cefuroxime (n = 156) | Cefotaxime (n = 166) | ||
| Age (median [1st quartile–3rd quartile]) (yr) | 71.0 (61.5–77.0) | 72.0 (63.0–77.0) | 0.407b |
| Clinical features | |||
| Body temp (median [IQR]) (°C) | 38.8 (38.2–39.4) | 38.7 (38.3–39.4) | 0.592b |
| Body temp ≥39.0°C | 60 (38.5) | 74 (44.6) | 0.266c |
| Costovertebral angle tenderness | 115 (73.7) | 81 (48.8) | 0.001c |
| Lower urinary tract infection symptoms | 93 (59.6) | 87 (52.4) | 0.193c |
| Nausea or vomiting | 63 (40.4) | 54 (32.5) | 0.143c |
| Enterobacteriaceae isolated from urine or blood | |||
| E. coli | 139 (89.1) | 146 (88.0) | |
| K. pneumoniae | 9 (5.8) | 14 (8.4) | |
| E. aerogenes | 2 (1.3) | 4 (2.4) | |
| Other | 6 (3.8) | 2 (1.2) | |
| Laboratory findings | |||
| Bacteremia | 66 (42.3) | 88 (53.0) | 0.055c |
| C-reactive protein level (median [IQR]) (mg/dl) | 11.3 (5.6–18.1) | 11.7 (5.7–19.7) | 0.455b |
| C-reactive protein level ≥15 mg/dl | 51 (32.7) | 64 (38.6) | 0.273c |
| Enterobacteriaceae resistant to cefazolin or cephradine | 39 (25.0) | 47 (28.3) | 0.502c |
| Enterobacteriaceae resistant to cefotaxime | 13 (8.3) | 23 (13.9) | 0.116c |
| Enterobacteriaceae resistant to fluoroquinolones | 31 (19.9) | 39 (23.5) | 0.431c |
| Extended-spectrum β-lactamase+ Enterobacteriaceae | 14 (9.0) | 25 (15.1) | 0.094c |
| Hematuria | 109 (69.9) | 111 (66.9) | 0.563c |
| White blood cell count (median [IQR]) (/mm3) | 11,985 (9,305–14,930) | 12,085 (8,905–14,758) | 0.623b |
| White blood cell count ≥15,000/mm3 of blood | 38 (24.4) | 39 (23.5) | 0.856c |
| Past history | |||
| Antibiotic use within 1 yr | 26/142 (18.3)d | 34/147 (19.4)d | 0.313c |
| Previous urinary tract infection history | 27/142 (19.0)d | 25/148 (16.9)d | 0.638c |
| Prior history of hospitalization within 1 yr | 27/143 (18.9)d | 34/149 (22.8)d | 0.408c |
| Underlying diseases | |||
| Cerebrovascular disorder | 6 (3.9) | 20 (12.1) | 0.007c |
| Chronic kidney disease | 7 (4.5) | 5 (3.0) | 0.485c |
| Chronic liver disease | 6 (3.9) | 9 (5.4) | 0.503c |
| Chronic pulmonary disease | 6 (3.9) | 5 (3.0) | 0.681c |
| Congestive heart failure | 5 (3.2) | 8 (4.8) | 0.462c |
| Connective tissue disorder | 4 (2.6) | 4 (2.4) | >0.999c |
| Diabetes mellitus | 68 (43.6) | 85 (51.2) | 0.172c |
| Hypertension | 62 (39.7) | 65 (39.2) | 0.914c |
| Malignancy | 9 (5.8) | 4 (2.4) | 0.126c |
| Neurogenic bladder | 6 (3.9) | 14 (8.4) | 0.088c |
| Polycystic kidney disease | 0 | 3 (1.8) | 0.248c |
| Urolithiasis | 6 (3.9) | 8 (4.8) | 0.669c |
| Vesicoureteral reflux | 1 (0.6) | 4 (2.4) | 0.372c |
Unless otherwise indicated, the data are shown as the number (%) of patients.
Mann-Whitney U test.
Pearson chi-square test or Fisher's exact test.
Denominators are the number of patients whose data were available in each group.
Microbiological data.
Of the 322 cases, Escherichia coli was the most common pathogen (285 patients [88.5%]), and non-E. coli Enterobacteriaceae were isolated from 37 patients (11.5%). The non-E. coli Enterobacteriaceae infections comprised 23 Klebsiella pneumoniae, 6 Enterobacter aerogenes, 3 Proteus mirabilis, and 2 Citrobacter koseri cases and 1 case each of Klebsiella oxytoca, Enterobacter cloacae, and Morganella morganii.
For the β-lactams, the 322 Enterobacteriaceae isolates had the highest rate of susceptibility to imipenem (98.8%), followed by piperacillin-tazobactam (94.1%), cefepime (93.5%), cefotaxime (88.8%), amoxicillin-clavulanate (80.0%), first-generation cephalosporins (73.5%), and ampicillin (34.0%). The rates of susceptibility for the 322 Enterobacteriaceae isolates to fluoroquinolone (FQ), trimethoprim-sulfamethoxazole (SXT), amikacin, and gentamicin were 78.3%, 72.7%, 99.4%, and 82.0%, respectively. There were no significant differences between the cefuroxime and cefotaxime groups in the rates of susceptibility to the β-lactam antibiotics, FQ, SXT, amikacin, and gentamicin.
Baseline characteristics and clinical outcomes of the women with complicated APN after propensity score matching.
Of the 322 patients, 90 patients in the cefuroxime-treated group were matched with 90 patients in the cefotaxime-treated group with the closest propensity scores. Following propensity score matching, the two groups were well matched, and no significant differences in demographic characteristics or underlying diseases were observed between the two groups (Table 2). In the unmatched analysis, no significant differences between the cefuroxime and cefotaxime groups were noted in the defervescence rates (84.6% [132/156] versus 77.7% [129/166], P = 0.114) at 72 h after the start of antimicrobial therapy, the clinical cure rates (98.1% [152/155] versus 98.2% [161/164], P = 0.944), and the microbiological cure rates (87.5% [119/136] versus 90.1% [118/131], P = 0.505) at the follow-up visit 4 to 14 days after the EOT. In the propensity score-matched case-control study, no significant differences between the cefuroxime and cefotaxime groups were noted in the defervescence rates (81.1% [73/90] versus 78.9% [71/90], P = 0.709) at 72 h after the start of antimicrobial therapy, the clinical cure rates (97.8% [87/89] versus 97.8% [87/89], P > 0.999), and the microbiological cure rates (89.5% [68/76] versus 90.7% [68/75], P = 0.807) at the follow-up visit 4 to 14 days after the EOT. The median fever clearance times were 55 h (95% confidence interval [CI], 52.5 to 65.3 h) for the cefuroxime group and 55 h (95% CI, 51.0 to 63.6 h) for the cefotaxime group (P = 0.710) (Fig. 1). Additionally, the clinical cure rates (95.0% [57/60] versus 98.3% [59/60], P = 0.333) and the microbiological cure rates (85.7% [18/21] versus 86.4% [19/22], P = 0.951) for the cefuroxime and cefotaxime groups, respectively, at the follow-up visit 21 to 35 days after the EOT were not significantly different (Table 3).
TABLE 2.
Demographic and clinical characteristics in 180 women with community-onset complicated nonobstructive acute pyelonephritis due to Enterobacteriaceae, by treatment regimen after propensity score matching
| Characteristica | Patients treated with: |
P value | |
|---|---|---|---|
| Cefuroxime (n = 90) | Cefotaxime (n = 90) | ||
| Age (median [1st quartile–3rd quartile]) (yr) | 71.5 (63.5–78.0) | 72.0 (63.0–77.3) | 0.881b |
| Clinical features | |||
| Body temp (median [IQR]) (°C) | 38.8 (38.3–39.4) | 38.6 (38.3–39.4) | 0.612b |
| Body temp ≥39.0°C | 38 (42.2) | 34 (37.8) | 0.543c |
| Costovertebral angle tenderness | 58 (64.4) | 62 (68.9) | 0.527c |
| Lower urinary tract infection symptoms | 53 (58.9) | 54 (60.0) | 0.879c |
| Nausea or vomiting | 37 (41.1) | 39 (43.3) | 0.763c |
| Enterobacteriaceae isolated from urine or blood | |||
| E. coli | 83 (92.2) | 83 (92.2) | |
| K. pneumoniae | 6 (6.7) | 5 (5.6) | |
| E. aerogenes | 0 | 2 (2.2) | |
| P. mirabilis | 1 (1.1) | 0 | |
| Laboratory findings | |||
| Bacteremia | 44 (48.9) | 46 (51.1) | 0.766c |
| C-reactive protein level (median [IQR]) (mg/dl) | 12.6 (6.0–20.3) | 11.9 (5.6–19.8) | 0.936b |
| C-reactive protein level ≥15 mg/dl | 35 (38.9) | 35 (38.9) | >0.999c |
| Enterobacteriaceae resistant to cefazolin or cephradine | 22 (24.4) | 23 (25.6) | 0.863c |
| Enterobacteriaceae resistant to cefotaxime | 8 (8.9) | 9 (10.0) | 0.799c |
| Enterobacteriaceae resistant to fluoroquinolones | 20 (22.2) | 16 (17.8) | 0.456c |
| Extended-spectrum β-lactamase+ Enterobacteriaceae | 9 (10.0) | 10 (11.1) | 0.808c |
| Hematuria | 64 (71.1) | 61 (67.8) | 0.627c |
| White blood cell count (median [IQR]) (/mm3) | 11,830 (9,153–14,960) | 12,620 (9,093–14,858) | 0.793c |
| White blood cell count ≥15,000/mm3 of blood | 22 (24.4) | 22 (24.4) | >0.999c |
| Past history | |||
| Antibiotic use within 1 yr | 14/81 (17.3)d | 14/79 (17.7)d | 0.942c |
| Previous urinary tract infection history | 9/79 (11.4)d | 9/77 (11.7)d | 0.954c |
| Prior history of hospitalization within 1 yr | 11/80 (13.8)d | 13/79 (16.5)d | 0.634c |
| Underlying diseases | |||
| Cerebrovascular disorder | 4 (4.4) | 6 (6.7) | 0.747c |
| Chronic kidney disease | 3 (3.3) | 3 (3.3) | >0.999c |
| Chronic liver disease | 4 (4.4) | 5 (5.6) | >0.999c |
| Chronic pulmonary disease | 4 (4.4) | 3 (3.3) | 0.722c |
| Congestive heart failure | 2 (2.2) | 5 (5.6) | 0.444c |
| Connective tissue disorder | 3 (3.3) | 3 (3.3) | >0.999c |
| Diabetes mellitus | 42 (46.7) | 41 (45.6) | 0.881c |
| Hypertension | 30 (33.3) | 35 (38.9) | 0.438c |
| Malignancy | 3 (3.3) | 3 (3.3) | >0.999c |
| Neurogenic bladder | 4 (4.4) | 4 (4.4) | >0.999c |
| Polycystic kidney disease | 0 | 0 | |
| Urolithiasis | 3 (3.3) | 4 (4.4) | 0.722c |
| Vesicoureteral reflux | 1 (1.1) | 0 | >0.999c |
Unless otherwise indicated, the data are shown as the number (%) of patients.
Mann-Whitney U test.
Pearson chi-square test or Fisher's exact test.
Denominators are the number of patients whose data were available in each group.
FIG 1.
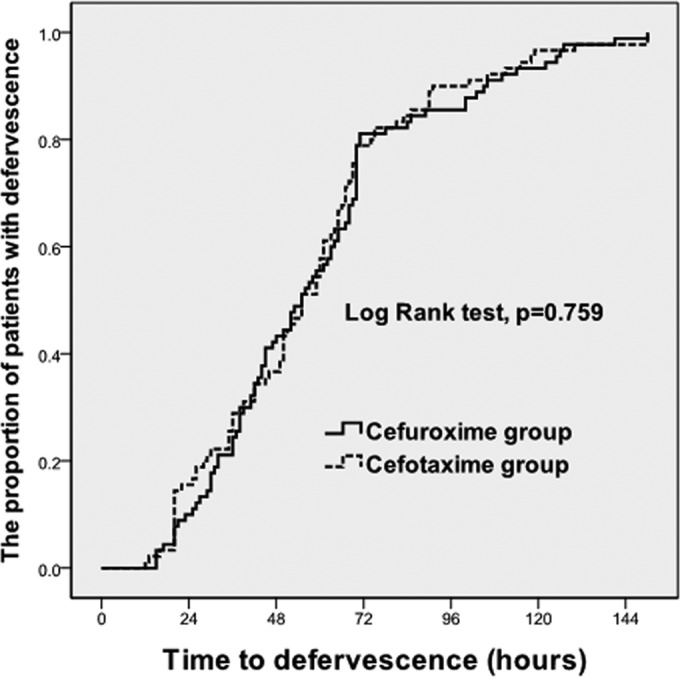
Kaplan-Meier curves of the time to defervescence for 180 patients who initially had fever and received cefuroxime or cefotaxime for the treatment of complicated APN. The time to fever clearance from the time of administration of the first hospital dose of cefuroxime or cefotaxime among hospitalized APN patients is shown. The estimated median fever clearance times were 55 h (95% CI, 52.5 to 65.3 h) for the cefuroxime group and 55 h (95% CI, 51.0 to 63.6 h) for the cefotaxime group (P = 0.710).
TABLE 3.
Comparison of clinical outcomes of 180 women with complicated acute pyelonephritis treated with intravenous cefuroxime or cefotaxime as initial empirical antibiotics after propensity score matching
| Characteristica | Patients treated with: |
P value | |
|---|---|---|---|
| Cefuroxime (n = 90) | Cefotaxime (n = 90) | ||
| Dose of cefuroxime or cefotaxime (mg/day) | 2,250 | 6,000 | |
| Dosing (mg) regimen of cefuroxime or cefotaxime | 750 at 8-h interval | 2,000 at 8-h interval | |
| Duration of cefuroxime or cefotaxime therapy (median [IQR]) (days) | 6.0 (5.0–7.0) | 6.0 (5.0–7.0) | 0.634b |
| Cases with alternative intravenous antibiotics | 3 (3.3) | 10 (11.1) | 0.081c |
| Alternative intravenous antibiotics used (no.) | |||
| Piperacillin-tazobactam | 1 | 8 | |
| Amikacin | 1 | 2 | |
| Ciprofloxacin | 1 | 0 | |
| Meropenem | 0 | 0 | |
| Switch to oral antibiotics (no. of cases) | |||
| Ciprofloxacin | 71 | 75 | |
| Cefditoren | 13 | 12 | |
| Amoxicillin-clavulanate | 3 | 2 | |
| Trimethoprim-sulfamethoxazole | 0 | 1 | |
| Other | 2 | 0 | |
| Duration of oral antimicrobial Tx (median [IQR]) (days) | 8.0 (7.0–9.0) | 7.0 (7.0–9.0) | 0.713b |
| Duration of total antimicrobial Tx (median [IQR]) (days) | 14.0 (14.0–14.0) | 14.0 (14.0–14.3) | 0.141b |
| Rate of defervescence occurring within: | |||
| 24 h | 8 (8.9) | 13 (14.4) | 0.246c |
| 48 h | 39 (43.3) | 32 (35.6) | 0.286c |
| 72 h | 73 (81.1) | 71 (78.9) | 0.709c |
| 96 h | 77 (85.6) | 81 (90.0) | 0.363c |
| 120 h | 83 (92.2) | 87 (96.7) | 0.330c |
| Time to defervescence (median [IQR]) (h) | 55.0 (36.8–70.0) | 55.0 (35.0–69.3) | 0.710b |
| Duration of hospital stay (median [IQR]) (days) | 8.0 (7.0–10.0) | 9.0 (7.0–11.0) | 0.285b |
| Clinical cure at 4–14 days after EOT | 87/89 (97.8)d | 87/89 (97.8)d | >0.999c |
| Microbiological cure at 4–14 days after EOT | 68/76 (89.5)d | 68/75 (90.7)d | 0.807c |
| Clinical cure at 21–35 days after EOT | 57/60 (95.0)d | 59/60 (98.3)d | 0.333c |
| Microbiological cure at 21–35 days after EOT | 18/21 (85.7)d | 19/22 (86.4)d | 0.951c |
| Overall mortality | 0 | 0 | |
Unless otherwise indicated, the data are shown as the number (%) of patients. Tx, treatment; EOT, end of therapy.
Mann-Whitney U test.
Pearson chi-square test or Fisher's exact test.
Denominators are the number of patients whose data were available in each group.
Factors related to early clinical failure in women with community-onset nonobstructive APN.
Among the 322 cases, 261 cases were placed in the early clinical success group (at 72 h) and 61 in the early clinical failure group. There were no significant differences in the mean age, initial body temperature, frequency of lower UTI symptoms, costovertebral angle tenderness, previous UTI history, antibiotic usage within 1 year, and history of hospital admission within 1 year. The proportion of postmenopausal women was significantly higher in the early clinical failure group than that in the early clinical success group (98.4% versus 88.9%, respectively; P = 0.025). The proportions of patients who had initial leukocyte counts of ≥15,000/mm3 of blood (39.3% versus 20.3%, P = 0.002), an initial serum CRP level of ≥15 mg/dl (62.3% versus 29.5%, P < 0.001), and bacteremia (65.6% versus 43.7%, P = 0.003) were significantly higher in the early clinical failure group than in the early clinical success group (Table 4). However, there were no significant differences between the early clinical failure and clinical success groups in the proportions of uropathogens that were resistant to ampicillin, amoxicillin-clavulanate, gentamicin, amikacin, cefazolin, cefotaxime, ceftazidime, cefepime, fluoroquinolone, trimethoprim-sulfamethoxazole, piperacillin-tazobactam, imipenem, cefoxitin, and ESBL-producing uropathogens. There were also no significant differences between the early clinical failure and clinical success groups in the frequencies of diabetes, hypertension, cerebrovascular diseases, congestive heart failure, chronic liver diseases, chronic lung diseases, malignancy, neurogenic bladder, and polycystic kidney disease. Although the median hospital stay durations (11 [IQR, 9 to 14] days versus 8 [IQR, 7 to 10] days; P < 0.001) were longer in the early clinical failure group, there were no significant differences in the overall clinical cure rates (96.7% versus 98.5% for the early clinical failure and success groups, respectively; P = 0.358) at the follow-up visit 4 to 14 days after the EOT. However, the microbiological cure rate at the follow-up visit 4 to 14 days after the EOT was significantly higher in the early clinical success group than in the early clinical failure group (91.7% versus 76.0%, respectively; P = 0.002) (Table 4).
TABLE 4.
Comparison of clinical characteristics of early clinical success and failure groups in the acute pyelonephritis patients treated with empirical cefuroxime or cefotaxime
| Characteristic | Patients in group at 72 h with: |
P value | |
|---|---|---|---|
| Early clinical failure (n = 61) | Early clinical success (n = 261) | ||
| Demographic data | |||
| Age (median [IQR]) (yr) | 70.0 (64.5–74.0) | 72.0 (62.0–77.0) | 0.523b |
| Postmenopausal women | 60 (98.4) | 232 (88.9) | 0.025c |
| Clinical features | |||
| Body temp (median [IQR]) (°C) | 38.9 (38.5–39.4) | 38.6 (38.2–39.4) | 0.082b |
| Costovertebral angle tenderness | 32 (52.5) | 164 (62.8) | 0.135c |
| Lower urinary tract infection symptoms | 29 (47.5) | 151 (57.9) | 0.144c |
| Nausea or vomiting | 28 (45.9) | 89 (34.1) | 0.084c |
| Pitt score of 1 | 30 (49.2) | 103 (39.5) | 0.165c |
| Pitt score of 2 to ∼4 | 6 (9.8) | 21 (8.0) | 0.650c |
| Use of cefuroxime | 24 (39.3) | 132 (50.6) | 0.114c |
| Laboratory findings | |||
| Bacteremia | 40 (65.6) | 114 (43.7) | 0.003c |
| C-reactive protein level (mg/dl) on admission (median [IQR]) | 17.3 (12.9–23.4) | 10.4 (5.0–17.4) | <0.001b |
| C-reactive protein level ≥15 mg/dl | 38 (62.3) | 77 (29.5) | <0.001c |
| ESBL-producing uropathogen | 11 (18.0) | 28 (10.7) | 0.115c |
| Fluoroquinolone-resistant uropathogen | 14 (23.0) | 56 (21.5) | 0.799c |
| Hematuria | 47 (77.0) | 173 (66.3) | 0.104c |
| White blood cell count (median [IQR]) (/mm3) | 13,800 (9,420–16,610) | 11,810 (9,140–14,285) | 0.055b |
| White blood cells ≥15,000/mm3 of blood | 24 (39.3) | 53 (20.3) | 0.002c |
| Past history | |||
| Antibiotic use within 1 yr | 10/54 (18.5)d | 50/235 (21.3)d | 0.652c |
| Previous urinary tract infection | 7/55 (12.7)d | 45/235 (19.1)d | 0.264c |
| Prior hospitalization within 1 yr | 13/54 (24.1)d | 48/238 (20.2)d | 0.524c |
| Clinical outcomes | |||
| Duration of hospital stay (median [IQR]) (days) | 11.0 (9.0–14.0) | 8.0 (7.0–10.0) | <0.001b |
| Clinical cure at 4–14 days after Tx | 58/60 (96.7)d | 255/259 (98.5)d | 0.358c |
| Microbiological cure at 4–14 days after Tx | 38/50 (76.0)d | 199/217 (91.7)d | 0.002c |
Unless otherwise indicated, the data are shown as the number (%) of patients. Tx, treatment.
Mann-Whitney U test.
Pearson chi-square test or Fisher's exact test.
Denominators are the number of patients whose data were available in each group.
Regarding the multiple logistic regression analysis, bacteremia, extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, initial serum C-reactive protein of ≥15 mg/dl, and white blood cell counts of ≥15,000/mm3 of blood were significantly associated with early clinical failure (P = 0.040, = 0.043, < 0.001, and = 0.010, respectively) (Table 5).
TABLE 5.
Related factors associated with early clinical failure in 322 women with complicated acute pyelonephritis due to Enterobacteriaceae in a final model of multiple logistic regression
| Factor | Odds ratio (95% confidence interval) | P value |
|---|---|---|
| Bacteremia | 1.928 (1.030–3.609) | 0.040 |
| C-reactive protein level ≥15 mg/dl | 3.185 (1.730–5.848) | <0.001 |
| ESBL-producing Enterobacteriaceae | 2.381 (1.026–5.528) | 0.043 |
| Hematuria | 1.508 (0.739–3.075) | 0.259 |
| Initial intravenous antibiotics (cefuroxime or cefotaxime) | 0.680 (0.365–1.265) | 0.223 |
| Menopause | 5.241 (0.667–41.207) | 0.115 |
| Nausea or vomiting | 1.608 (0.862–2.999) | 0.136 |
| White blood cell counts (/mm3) ≥15,000 | 2.353 (1.227–4.525) | 0.010 |
Clinical outcomes according to the presence of ESBL+ Enterobacteriaceae.
Of the 322 women with complicated APN, 39 patients had ESBL+ Enterobacteriaceae infections, and 283 patients had ESBL− Enterobacteriaceae infections. Of the 39 patients with ESBL+ Enterobacteriaceae infections, 14 patients received cefuroxime monotherapy, and 25 patients received cefotaxime monotherapy as an initial antibiotic. Sixteen (41.0%) of 39 patients with ESBL+ Enterobacteriaceae infections were switched to alternative intravenous therapy: 10 (25.6%), 3 (7.7%), 1 (2.6%), 1 (2.6%), and 1 (2.6%) were switched to piperacillin-tazobactam, amikacin, meropenem, cefepime, and intravenous ciprofloxacin, respectively, after 3 to 4 days of cefuroxime or cefotaxime monotherapy. Eight of these 16 patients reached defervescence before receiving alternative intravenous antibiotics. Twenty-three (59.0%) of 39 patients with ESBL+ Enterobacteriaceae infections received continuing cefuroxime (11 patients) or cefotaxime (12 patients) therapy before switching to oral antibiotic therapy: 20 (51.3%), 1 (2.6%), 1 (2.6%), and 1 (2.6%) reached defervescence within 72, 96, 120, and 144 h, respectively, of starting cefuroxime or cefotaxime therapy. The early clinical success rates were 71.8% (28/39) versus 82.3% (233/283) at 72 h in the ESBL+ versus ESBL− groups, respectively (P = 0.115). The clinical and microbiological cure rates in the ESBL+ versus ESBL− groups at the follow-up visit 4 to 14 days after the EOT were 97.4% (38/39) versus 98.2% (275/280) and 81.8% (27/33) versus 89.7% (210/234), respectively, which are not significantly different (P = 0.546 and 0.177). The median times to defervescence were 54 h (IQR, 35 to 70 h) and 55 h (IQR, 31 to 85 h) in the ESBL+ and ESBL− groups, respectively (P = 0.741). The median hospital stay durations in the ESBL+ and ESBL− groups were 11 days (IQR, 8 to 14 days) and 8 days (IQR, 7 to 10 days) days, respectively (P < 0.001). However, no complications, such as septic shock, renal abscess, or death, occurred in either the ESBL+ or ESBL− groups.
DISCUSSION
This study shows that the efficacy of cefuroxime, a second-generation cephalosporin, was similar to that of cefotaxime, a third-generation cephalosporin, as an initial antimicrobial therapy for community-onset complicated nonobstructive APN. In the propensity score-matched case-control analysis, the clinical cure and microbiological cure rates 4 to 14 days after the end of the antimicrobial therapies were 97.8% versus 97.8% and 89.5% versus 90.7% in the cefuroxime- or cefotaxime-treated women, respectively, with complicated nonobstructive APN. Additionally, the early clinical success rates at 72 h after the start of the cefuroxime or cefotaxime therapy were not significantly different (81.1% versus 78.9%, respectively).
Although complicated APN is a common disease entity that physicians have to manage, the efficacies of different antibiotics are not established for the treatment of complicated APN based on subclassifications, such as nonobstructive or obstructive or health care-associated complicated APN types, because most clinical evidence on antimicrobial therapy is from uncomplicated APN cases (2, 3, 16). In this study, we found that the cefuroxime efficacies were comparable to the cefotaxime efficacies, and these data suggest that cefuroxime and extended-spectrum cephalosporins, such as cefotaxime, can also be used as an initial antimicrobial agent for the treatment of community-onset complicated nonobstructive APN.
Antimicrobial therapy for complicated APN is recommended to be guided by urine culture results or the presence of other complicating factors, as complicated APN may occur in heterogeneous patient groups with a wide variety of urinary tract abnormalities (16, 17). Additionally, clinicians should consider complicating factors when making decisions for the treatment of complicated APN, especially in patients who have risk factors for poor clinical response (18–20). In this study, there were no significant differences between the early clinical failure and success groups in the proportions of uropathogens that were resistant to antimicrobial agents, such as ampicillin, cefazolin, cefoxitin, cefotaxime, cefepime, fluoroquinolone, and uropathogens that produce ESBL. In this study, the variables that were predictive of early clinical failure were parameters that reflected infection severity, such as bacteremia, serum CRP levels, and leukocyte counts in the blood, as well as ESBL-producing uropathogens; however, initial intravenous antibiotics, menopause, underlying diseases, and a history of antibiotic usage or prior hospitalization were not considered to be variables associated with early clinical failure.
As extended-spectrum cephalosporins, such as cefotaxime or ceftriaxone, are commonly recommended in clinical practice guidelines as empirical antibiotics for uncomplicated APN (8), many clinicians prefer to use the third-generation cephalosporins rather than second-generation cephalosporins. Furthermore, some clinicians may prefer to use fourth-generation cephalosporins, carbapenem, or piperacillin-tazobactam because of the greater likelihood of ESBL-producing uropathogens being present in women with complicated APN (21). As complicated APN includes more heterogeneous groups, such as obstructive APN, health care-associated APN, and community-onset complicated nonobstructive APN, it is difficult for clinicians to choose adequate and economic agents as the initial empirical antibiotics in the treatment of complicated APN. Therefore, we investigated and compared the therapeutic efficacies of cefuroxime and cefotaxime only in women with complicated nonobstructive APN.
This study has a few limitations. First, the therapeutic efficacies of cefuroxime and cefotaxime can be confounded, as the choice of initial antimicrobial agent may be influenced by underlying disease(s), initial laboratory findings, or other prognostic factors; therefore, we performed a propensity score-matched analysis to overcome this potential bias. Second, we excluded patients with obstructive APN that required urological interventions, and these exclusion criteria may have skewed the results of the study. Third, we considered that all of cases with microbiological failure in the follow-up microbiological assessment had recurrences due to bacterial relapse, as there were no changes in bacterial species; however, some cases with microbiological failure might be reinfected with a different serotype of the same species.
In conclusion, cefuroxime, the second-generation cephalosporin, was as effective as cefotaxime, the third-generation cephalosporin, in the initial empirical treatment of community-onset nonobstructive complicated APN in this study. Cefuroxime can be used as an economical, broad-spectrum cephalosporin-sparing, and fluoroquinolone-sparing antibiotic option in the treatment of complicated nonobstructive APN. Much larger prospective randomized trials comparing the therapeutic efficacies of second-generation cephalosporins with those of third-generation cephalosporins for the treatment of community-onset nonobstructive complicated APN are needed in the future.
ACKNOWLEDGMENTS
Statistical consultation was supported by the Department of Biostatistics of the Catholic Research Coordinating Center.
We acknowledge the financial support of St. Vincent's Hospital Research Institute of Medical Science (grant SVHR-2012-07). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We declare no conflicts of interest.
REFERENCES
- 1.Johansen TE, Botto H, Cek M, Grabe M, Tenke P, Wagenlehner FM, Naber KG. 2011. Critical review of current definitions of urinary tract infections and proposal of an EAU/ESIU classification system. Int J Antimicrob Agents 38S:64–70. doi: 10.1016/j.ijantimicag.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Bader MS, Hawboldt J, Brooks A. 2010. Management of complicated urinary tract infections in the era of antimicrobial resistance. Postgrad Med 122:7–15. doi: 10.3810/pgm.2010.11.2217. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi S, Kurimura Y, Takeyama K, Hashimoto K, Miyamoto S, Ichihara K, Igarashi M, Hashimoto J, Furuya R, Hotta H, Uchida K, Miyao N, Yanase M, Takagi Y, Tachiki H, Taguchi K, Tsukamoto T. 2009. Efficacy of treatment with carbapenems and third-generation cephalosporins for patients with febrile complicated pyelonephritis. J Infect Chemother 15:390–395. doi: 10.1007/s10156-009-0721-9. [DOI] [PubMed] [Google Scholar]
- 4.O'Callaghan CH, Sykes RB, Griffiths A, Thornton JE. 1976. Cefuroxime, a new cephalosporin antibiotic: activity in vitro. Antimicrob Agents Chemother 9:511–519. doi: 10.1128/AAC.9.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones RN, Fuchs PC, Gavan TL, Gerlach EH, Barry AL, Thornsberry C. 1977. Cefuroxime, a new parenteral cephalosporin: collaborative in vitro susceptibility comparison with cephalothin against 5,887 clinical bacterial isolates. Antimicrob Agents Chemother 12:47–50. doi: 10.1128/AAC.12.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermanides HS, Hulscher ME, Schouten JA, Prins JM, Geerlings SE. 2008. Development of quality indicators for the antibiotic treatment of complicated urinary tract infections: a first step to measure and improve care. Clin Infect Dis 46:703–711. doi: 10.1086/527384. [DOI] [PubMed] [Google Scholar]
- 7.The Korean Society of Infectious Diseases, The Korean Society for Chemotherapy, Korean Association of Urogenital Tract Infection and Inflammation, The Korean Society of Clinical Microbiology. 2011. Clinical guideline for the diagnosis and treatment of urinary tract infection: asymptomatic bacteriuria, uncomplicated & complicated urinary tract infections, bacterial prostatitis. Infect Chemother 43:1–25. doi: 10.3947/ic.2011.43.1.1. [DOI] [Google Scholar]
- 8.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE, Infectious Diseases Society of America, European Society for Microbiology and Infectious Diseases. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 9.Ramakrishnan K, Scheid DC. 2005. Diagnosis and management of acute pyelonephritis in adults. Am Fam Physician 71:933–942. [PubMed] [Google Scholar]
- 10.Wi YM, Kim SW, Chang HH, Jung SI, Kim YS, Cheong HS, Ki HK, Son JS, Kwon KT, Heo ST, Yeom JS, Ko KS, Kang CI, Chung DR, Peck KR, Song JH. 2014. Predictors of uropathogens other than Escherichia coli in patients with community-onset acute pyelonephritis. Int J Clin Pract 68:749–755. doi: 10.1111/ijcp.12368. [DOI] [PubMed] [Google Scholar]
- 11.Wie SH, Kim HW, Chang UI. 2013. Use of gentamicin for women with community-acquired uncomplicated acute pyelonephritis caused by gentamicin-susceptible or -resistant Escherichia coli: 10-year experience. Microb Drug Resist 19:316–322. doi: 10.1089/mdr.2012.0140. [DOI] [PubMed] [Google Scholar]
- 12.Lim SK, Park IW, Lee WG, Kim HK, Choi YH. 2012. Change of antimicrobial susceptibilities among Escherichia coli strains isolated from female patients with community-onset acute pyelonephritis. Yonsei Med J 53:164–171. doi: 10.3349/ymj.2012.53.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wie SH, Ki M, Kim J, Cho YK, Lim SK, Lee JS, Kwon KT, Lee H, Cheong HJ, Park DW, Ryu SY, Chung MH, Pai H. 2013. Clinical characteristics predicting early clinical failure after 72 h of antibiotic treatment in women with community-onset acute pyelonephritis: a prospective multicenter study. Clin Microbiol Infect 20:O721–O729. doi: 10.1111/1469-0691.12500. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. CLSI document M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.York MK. 2007. Aerobic bacteriology, p 3.12.1–3.12.15. In Isenberg HD. (ed), Clinical microbiology procedures handbook, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 16.Nicolle LE. 2011. Update in adult urinary tract infection. Curr Infect Dis Rep 13:552–560. doi: 10.1007/s11908-011-0212-x. [DOI] [PubMed] [Google Scholar]
- 17.Nicolle LE, AMMI Canada Guidelines Committee. 2005. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol 16:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronald AR, Harding GK. 1997. Complicated urinary tract infections. Infect Dis Clin North Am 11:583–592. doi: 10.1016/S0891-5520(05)70374-3. [DOI] [PubMed] [Google Scholar]
- 19.Pertel PE, Haverstock D. 2006. Risk factors for a poor outcome after therapy for acute pyelonephritis. BJU Int 98:141–147. doi: 10.1111/j.1464-410X.2006.06222.x. [DOI] [PubMed] [Google Scholar]
- 20.Efstathiou SP, Pefanis AV, Tsioulos DI, Zacharos ID, Tsiakou AG, Mitromaras AG, Mastorantonakis SE, Kanavaki SN, Mountokalakis TD. 2003. Acute pyelonephritis in adults: prediction of mortality and failure of treatment. Arch Intern Med 163:1206–1212. doi: 10.1001/archinte.163.10.1206. [DOI] [PubMed] [Google Scholar]
- 21.Naver KG, Savov O, Salmen HC. 2002. Piperacillin 2 g/tazobactam 0.5 g is as effective as imipenem 0.5 g/cilastatin 0.5 g for the treatment of acute uncomplicated pyelonephritis and complicated urinary tract infections. Int J Antimicrob Agents 19:95–103. doi: 10.1016/S0924-8579(01)00481-2. [DOI] [PubMed] [Google Scholar]


