Abstract
Here, we report that the genetic structure of Tn1331 remained conserved in Argentina from 1989 to 2013 (72 of 73 isolates), with the exception being the plasmid-borne Tn1331-like transposon Tn6238 containing a new aac(6′)-Ib-cr allele recovered from a colistin-resistant Klebsiella pneumoniae clinical isolate. A bioinformatic analysis of aac(6′)-Ib-like gene cassettes suggests that this new aac(6′)-Ib-cr allele emerged through mutation or homologous recombination in the Tn1331 genetic platform. Tn6238 is a novel platform for the dissemination of aminoglycoside and fluoroquinolone resistance determinants.
TEXT
The Tn1331 transposon was the first member of the Tn3 subfamily reported to contain a structure of integron gene cassettes, with the attI1*-aac(6′)-Ib-attCaac(6′)-Ib-aadA1-attI1*-blaOXA-9-attCblaOXA-9 array (1–5). The basic structure of a gene cassette consists of a gene and a recombination site (called attC), which can be targeted by integrases of integrons. The attC recombination sites are 57 to 141 bp long and are composed of 2 short regions of sequence similarity at their boundaries (1R to 2R and 1L to 2L) separated by a stretch (20 to 104 bp) of imperfect internal dyad symmetry (6). attI1* in Tn1331 shows identity with 8 bp of the attI1 recombination site from class 1 integrons (2).
It was previously shown that two point mutations (W87R and D164Y) (Fig. 1) within the aac(6′)-Ib gene confer the capability of the gene product to acetylate not only aminoglycosides but also the fluoroquinolones norfloxacin and ciprofloxacin (7). This gene, named aac(6′)-Ib-cr, also known as aacA4-cr, is the first that encodes an enzyme able to inactivate two families of antibiotics (7). Currently, this gene and the qnrB alleles are the prevalent plasmid-mediated quinolone resistance genes (8).
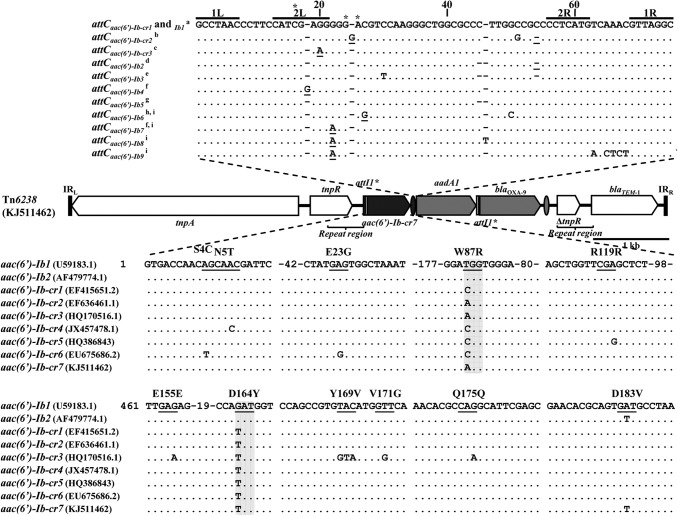
FIG 1.
Structure of Tn6238 (GenBank accession no. KJ511462). The transposon number was assigned by the Tn number registry Web page (http://www.ucl.ac.uk/eastman/research/departments/microbial-diseases/tn). In the central diagram, the horizontal bars represent inverted repeats, the arrows represent ORFs, the white bars represent attI1*, and the gray arrows and ovals represent the ORFs and attC sites of the gene cassettes, respectively. The top strands of the 11 different attC sites found in the aac(6′)-Ib-cr and aac(6′)-Ib gene cassettes are shown above the Tn6238 structure. The dots indicate identities and the dashes indicate insertions. The 1R, 2R, 1L, and 2L sites are marked with horizontal bars, and the extrahelical bases are marked with an asterisk. The most important mutations related to their potential impact on the attC functionality are underlined. The ORFs of the aac(6′)-Ib-cr alleles are shown below the Tn6238 structure. They are compared with the AAC(6′)-Ib protein encoded by aac(6′)-Ib1 [aac(6′)-Ib allele of the reference, GenBank accession no. U59183.1, bases 301 to 859]. The dots indicate identities. The numbers between dashes in the aac(6′)-Ib1 ORF indicate the number of bases not shown. The codons with mutations are underlined, and above them are shown the codified amino acids, numbered from the GTG start codon. The aac(6′)-Ib2 allele found in Tn1331 is shown (GenBank accession no. AF479774.1). The key mutations that make the protein capable of modifying quinolones are highlighted with gray boxes. All the aac(6′)-Ib-cr variants share the mutation GAT to TAT at position 490 of the aac(6′)-Ib1 ORF (D164Y). Position 259, also responsible for the ciprofloxacin-resistant phenotype, exhibits the mutation TGG to CGG [variant C, called here aac(6′)-Ib-cr1] or to AGG [variant A, called here aac(6′)-Ib-cr2], both generating W87R (13). aMost frequent are attCaac(6′)-Ib-cr1 and attCaac(6′)-Ib1, which are found in all alleles of the aac(6′)-Ib-cr gene cassettes other than aac(6′)-Ib-cr3 (e.g., GenBank accession no. KJ511462, EF636461.1, and EF415651.2), and they are also found in the prevalent aac(6′)-Ib gene cassette in integrons, the Tn1331, Tn1331.2, and Tn1332 transposons, and all the AAC(6′)-Ib protein variants except 3 and 4 [e.g., GenBank accession no. U59183.1 used as reference of aac(6′)-Ib gene cassette] (14); battCaac(6′)-Ib-cr2 is linked to aac(6′)-Ib-cr2 (e.g., GenBank accession no. HM998988.1); cattCaac(6′)-Ib-cr3 is associated with aac(6′)-Ib-cr3, found only in GenBank accession no. HQ170516.1; dattCaac(6′)-Ib2 is found in Tn1331 of pJHCMW1 (GenBank accession no. AF479774.1); eattCaac(6′)-Ib3 is found in gene cassettes, with the constant region of the ORF identical to the aac(6′)-Ib of reference, but whose complete ORF encodes variants 4 and 39 of the AAC(6′)-Ib protein (GenBank accession no. AY370764.1 and X60321.1, respectively) (14); fattCaac(6′)-Ib4 and attCaac(6′)-Ib7 are found in gene cassettes in which the complete ORF encodes variants 8 and 14 of the AAC(6′)-Ib protein, respectively (GenBank accession no. DQ767903.1 and GQ293499.1) (14); gattCaac(6′)-Ib5 is found in the aac(6′)-Ib gene cassette in a Tn1331-like structure in which the complete ORF encodes variant 3 of the AAC(6′)-Ib protein (GenBank accession no. M23634.1) (14); hThis is the second attCaac(6′)-Ib in terms of frequency (e.g., GenBank accession no. AF458080.1); iattCaac(6′)-Ib6, attCaac(6′)-Ib7, attCaac(6′)-Ib8, and attCaac(6′)-Ib9 are found in gene cassettes, with the constant region of the ORF identical to the aac(6′)-Ib of reference, in which the complete ORF encodes variants 13, 14, and 17 of AAC(6′)-Ib protein (e.g., GenBank accession no. AF458080.1, AJ313334.1, JF262177.1, and FO203354.1).
Currently, six alleles of aac(6′)-Ib-cr have been identified, all as gene cassettes, with 5 of them generating amino acid changes at the protein level (7, 9–12) (Fig. 1). They have been found mainly in classical class 1 integrons (13, 14) and sporadically in gene cassette arrays with IS26 in the structures IS26-aac(6′)-Ib-cr1-blaOXA-30-catB3-IS26 (GenBank accession no. AY458016), aac(3)-II-IS26-aac(6′)-Ib-cr2-blaOXA-1 (GenBank accession no. GQ438247), and aac(6′)-Ib-cr2-blaOXA-1-ΔcatB3-IS26-aac(3)-II (GenBank accession no. GQ438248) (15, 16). In the plasmid pMdT1, the allele is not associated with other gene cassettes or integron-related sequences and appears to have inserted in a secondary site (12).
Taking into account that the first isolation of gene cassettes embedded in Tn3, including aac(6′)-Ib, occurred in Argentina in the 1980s (GenBank accession no. AF479774.1) (1, 2, 4, 17–19), we decided to evaluate the evolution of Tn1331, focusing on the nucleotide sequence of aac(6′)-Ib to study the emergence of aac(6′)-Ib-cr within this genetic structure, due to its clinical importance.
We performed a retrospective study over 24 years in which we included 331 clinical isolates resistant to at least three families of antibiotics. The isolates belonged to 8 species from 5 hospitals in Argentina recovered since 1989. According to PCR mapping (Table 1), Tn1331 was found in 65% of Klebsiella pneumoniae (39/60), 14% of Serratia marcescens (4/28), 17% of Enterobacter cloacae (2/12), 17% of Citrobacter freundii (1/6), 60% of Proteus mirabilis (24/40), and 10% of Escherichia coli (3/30) isolates, and it was not detected in Acinetobacter baumannii (0/80) or in Pseudomonas aeruginosa (0/75) isolates. This finding shows that Tn1331 is frequently found and stably maintained in clinical isolates from Buenos Aires analyzed over the 24 years. Furthermore, this shows a different dissemination of Tn1331 among fermenting and nonfermenting bacilli. A sequence analysis of the 73 Tn1331-like-positive isolates showed that all but one contained the typical gene cassette array of the transposon, as found previously in isolates from Argentina (19, 20). The colistin-resistant K. pneumoniae KF7 isolate contained a novel allele of aac(6′)-Ib-cr, named here aac(6′)-Ib-cr7, instead of aac(6′)-Ib [Fig. 1, aac(6′)-Ib2]. KF7 was isolated in 2008 from a urine sample from a 48-year-old female patient with a nosocomial infection who was at the intensive care unit and treated with different antibiotics but not fluoroquinolones. The multidrug resistance profile of the isolate (21, 22) is shown in Table 2. This new gene cassette array gives rise to the transposon Tn6238 (GenBank accession no. KJ511462). This new aac(6′)-Ib-cr allele is like variant A, as defined by Partridge et al. (13), plus a new additional point mutation (GAT to GTT at position 548) that encodes a valine as the penultimate amino acid, as found in Tn1331, instead of the aspartic acid encoded by aac(6′)-Ib-cr and most of the aac(6′)-Ib alleles (30/35) (7, 9–11, 13, 18) (Fig. 1). Like the aac(6′)-Ib allele in Tn1331, aac(6′)-Ib-cr7 also contains a unique structure at the 5′ end, consisting of attI1* and the nucleotides that encode the last six amino acids of blaTEM-1 (23) instead of the previously reported 5′ aac(6′)-Ib-cr gene cassette variations (13). To determine if Tn6238 was located in a transferable plasmid, we performed a biparental conjugation, as described before (24), with E. coli J53-AZr (AZr, azide resistant) as the recipient strain. The transconjugants were selected on Mueller-Hinton agar supplemented with sodium azide (100 μg/ml) and ampicillin (25 μg/ml). The transconjugant E. coli strain J53 KF7-TC6 showed the aminoglycoside and quinolone resistance profiles for aac(6′)-Ib-cr (Table 2), with a difference of 6 mm between the inhibition zones of levofloxacin (LVX) and ciprofloxacin (CIP) in the disk diffusion method (ΔLVX-CIP ≥ 5), as described before (25). Although the plasmid incompatibility group was not determined by replicon typing (26), the presence of Tn6238 in the transconjugant was confirmed by PCR mapping. By sequencing this transconjugant with outward primers targeting the tnpA and blaTEM-1 genes, we determined that the inverted repeats (IRs) of Tn6238 were 100% identical to the IRs of Tn3 and Tn1331 (4, 27). The In197 class 1 integron, which harbors the dfrA16c gene cassette (Integrall [http://integrall.bio.ua.pt/]), was also detected in the transconjugant by PCR mapping. The fact that this plasmid contains a Tn1331 derivative and a complete class 1 integron potentiates the exchange of gene cassettes between the two genetic platforms.
TABLE 1.
Primers used in the study
| Primers by targeta | Sequence (5′–3′) | Location in Tn6238 (GenBank accession no. KJ511462)b | Reference |
|---|---|---|---|
| Tn1331 and Tn6238 | |||
| IRs Tn3 inside | GGGGTCTGACGCTCAGTGG | Fw 1–19 Rev 7977–7995 | This study |
| TnpA F3′ | CTCTCCCCGCTTTGCCACG | Rev 139–159 | This study |
| TnpA R | TCTGACTGGCGTAACAAAGC | Fw 946–965 | This study |
| TnpA Rc | TACTGCTCCACCATTTCGTC | Fw 1874–1893 | This study |
| TnpR | AAGTTCATCGGGTTCGC | Fw 2968–2984 | 29 |
| TnpA F | AGGTTGAGAGTTATGGCAGG | Rev 2992–3011 | This study |
| aac(6′)-F | GAAGAAGCACGCCCGAC | Fw 4100–4116 | This study |
| aac(6′)-R | GTGTTCGCTCGAATGCC | Rev 4516–4532 | This study |
| aadA1 | TCGATGACGCCAACTAC | Rev 4661–4677 | 30 |
| aadA1F | TTGCTGGCCGTACATTTG | Fw 4697–4714 | 9 |
| aadA1R | TCATTGCGCTGCCATTC | Rev 4946–4962 | 9 |
| Oxa9-fb | GAACACCAACATATGCA | Rev 5483–5499 | 29 |
| Oxa9r | GGGACAATAACGGCAAG | Fw 6101–6117 | 29 |
| blaTEM1F | GCTCACCCAGAAACGCTGGTGAAAG | Fw 7054–7078 | This study |
| blaTEM1R | CACCCAACTGATCTTCAGCATC | Rev 7084–7105 | This study |
| blaTEM F3′ | GGGAGTCAGGCAACTATGG | Fw 7774–7792 | This study |
| Class 1 integrons | |||
| Inti1F | CGAGGCATAGACTGTAC | 29 | |
| Inti1R | TTCGAATGTCGTAACCGC | 29 | |
| sulpro3 | GCCTGACGATGCGTGGA | 30 | |
| 3′CsNew | AAGCAGACTTGACCTGATAG | Rev 6412–6431 | 31 |
PCR mapping was performed targeting first a portion of the tnpA gene and the gene cassette array of the transposon using the TnpR and 3′CsNew primer pair. Subsequently, we used different combinations of the primers listed to amplify and sequence the complete transposon.
Fw, forward primer; Rev, reverse primer.
TABLE 2.
Relevant characteristics of the clinical isolate harboring aac(6′)Ib-cr7 and transconjugants from this study
| Isolate or strain | Relevant resistance phenotypea | MIC (mg/liter)b |
|
|---|---|---|---|
| AMK | CIP | ||
| K. pneumoniae KF7 | AMPr CEFr SXTr AMKr NALr CIPr LVXr TZPr SAMr AMCr CSTr STRr KANr TOBr NORr | 6 | >32 |
| E. coli J53 | AZr | 0.25 | 0.01 |
| E. coli J53 KF7-TC6 | AMPr CEFr SXTr AMKr NALr CIPr SAMr AMCr STRr KANr TOBr | 2 | 0.5 |
Antimicrobial resistance and reduced susceptibility were tested by the disk diffusion method (19). AMP, ampicillin; CEF, cephalothin; SXT; trimethoprim-sulfamethoxazole; AMK, amikacin; NAL, nalidixic acid; CIP, ciprofloxacin; LVX, levofloxacin; TZP, piperacillin-tazobactam; SAM, ampicillin-sulbactam; AMC, amoxicillin-clavulanic acid; CST, colistin; STR, streptomycin; KAN, kanamycin; TOB, tobramycin; NOR, norfloxacin; AZ, azide.
MIC determinations were performed according to CLSI recommendations (19).
In order to further analyze the origin of aac(6′)-Ib-cr7, we performed a bioinformatic analysis of the aac(6′)-Ib-cr7 gene cassette and related sequences from GenBank (Fig. 1). To perform the comparison, we selected the conserved region of the open reading frame (ORF) of each aac(6′)-Ib-cr allele, discarding the heterogeneous 5′ end that generates N-terminal extensions of the encoded proteins (13), and subsequently compared their attC sites. We found 3 attCaac(6′)-Ib-cr sites among 61 complete aac(6′)-Ib-cr gene cassettes (Fig. 1). One of them, attCaac(6′)-Ib-cr1, occurred very frequently. It was present in 57/61 sequences from all the aac(6′)-Ib-cr alleles other than aac(6′)-Ib-cr3, which showed a unique attC site called attCaac(6′)-Ib-cr3 (1/61). The remaining attCaac(6′)-Ib-cr2 sites (3/61) were associated with aac(6′)-Ib-cr2. In addition, we identified 9 attC sites in the aac(6′)-Ib gene cassettes (Fig. 1). The attCaac(6′)-Ib-cr1 site described above was identical to the most common attCaac(6′)-Ib site [attCaac(6′)-Ib1] among the aac(6′)-Ib gene cassettes (586/634), including those in class 1 integrons, Tn1331, Tn1331.2, or Tn1332, and it was associated with almost all the aac(6′)-Ib alleles. Among the aac(6′)-Ib gene cassettes from the 15 Tn1331 transposons found in GenBank, only Tn1331 from pJHCMW1 showed the different and unique attC site attCaac(6′)-Ib2 (Fig. 1). As expected, the attCaac(6′)-Ib-cr and attCaac(6′)-Ib sites are highly related, and there is one prevalent attC site, while the remaining are associated with only a few alleles. Nevertheless, some of the mutations in the attC sites may have an effect on the recombination efficiencies, since they disrupt the 2R/2L complementarity or generate an additional extrahelical base, thus affecting their dissemination (Fig. 1). In regard to aac(6′)-Ib-cr7 in Tn6238 and aac(6′)-Ib in the other Tn1331-related transposons, the 8-bp AAACAAAG motif of the attI1* site located at the 5′ end has a crucial effect in minimizing IntI1-mediated recombination (28).
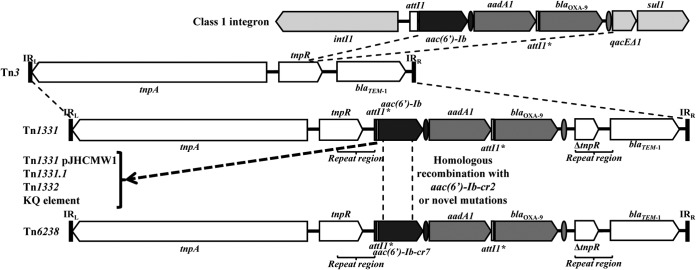
The stability of the attI1*-aac(6′)-Ib-attCaac(6′)-Ib-aadA1-attI1*-blaOXA-9-attCblaOXA-9 array in Tn1331 found in our isolates and others (14, 19, 20) reveals that the event that created Tn1331 is not very common. Based on this and the analyses showing that (i) the mutation that determines the D183V change in the AAC(6′)-Ib protein encoded in Tn1331 is not very frequent in aac(6′)-Ib found in class 1 integrons from GenBank or in Argentinian isolates (data not shown), (ii) the attC site is the same as the most frequent variant found in both Tn1331 from pMET and in class 1 integrons, (iii) the aac(6′)-Ib-cr variant found in Tn6238 has only the two mutations that lead to fluoroquinolone resistance, (iv) the excision of aac(6′)-Ib from Tn1331 by IntI1 is null or very low (28), and (v) we could not discard homologous recombination with a transient aac(6′)-Ib-cr that would include only the region that contains both crucial mutations for the fluoroquinolone resistance phenotype, then our subsequent proposal is that the simplest explanation for the creation of Tn6238 that requires the minimum amount of events of mobilization and/or mutation and hence is the most likely is that both mutations leading to amino acid changes D164Y and W87R or even homologous recombination with a transient aac(6′)-Ib-cr2 have happened in the genetic platform of Tn1331 (Fig. 2).
FIG 2.
Evolutionary scheme of Tn6238. The horizontal bars represent inverted repeats, the arrows represent ORFs, the white bars represent attI1*, and the gray arrows and ovals represent the ORFs and attC sites of gene cassettes, respectively.
In conclusion, Tn1331 is frequent and stably maintained among fermenting bacilli in clinical isolates analyzed from Buenos Aires over 24 years, but it has the potential to increase its antimicrobial resistance background, as shown by the emergence of Tn6238. There are now seven alleles of aac(6′)-Ib-cr reported around the world. These alleles are spreading in two successful genetic platforms, class 1 integrons and Tn3-derivative transposons, in clinical bacterial isolates from Argentina (9, 25). The emergence of Tn6238 shows how bacteria are able to acquire new resistance determinants and novel platforms that enhance the dissemination of antimicrobial resistance.
Nucleotide sequence accession number.
The nucleotide sequences determined in this work have been submitted to the GenBank database and assigned accession no. KJ511462. The bioinformatics analysis has been performed using sequences from GenBank, National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/).
ACKNOWLEDGMENTS
M.P.Q. is a recipient of a CONICET fellowship. This study was supported by a grant from BID/OC ANPCyT (0034) and by UBACYT (20020100100417) Programación 2011–2014 given to D.C., Buenos Aires, Argentina.
D.C. is a member of the Carrera del Investigador Científico, CONICET, Argentina.
REFERENCES
- 1.Tolmasky ME, Crosa JH. 1987. Tn1331, a novel multiresistance transposon encoding resistance to amikacin and ampicillin in Klebsiella pneumoniae. Antimicrob Agents Chemother 31:1955–1960. doi: 10.1128/AAC.31.12.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tolmasky ME. 1990. Sequencing and expression of aadA, bla, and tnpR from the multiresistance transposon Tn1331. Plasmid 24:218–226. doi: 10.1016/0147-619X(90)90005-W. [DOI] [PubMed] [Google Scholar]
- 3.Stokes HW, Hall RM. 1989. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol 3:1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 4.Tolmasky ME, Crosa JH. 1993. Genetic organization of antibiotic resistance genes (aac(6′)-Ib, aadA, and oxa9) in the multiresistance transposon Tn1331. Plasmid 29:31–40. doi: 10.1006/plas.1993.1004. [DOI] [PubMed] [Google Scholar]
- 5.Sarno R, McGillivary G, Sherratt DJ, Actis LA, Tolmasky ME. 2002. Complete nucleotide sequence of Klebsiella pneumoniae multiresistance plasmid pJHCMW1. Antimicrob Agents Chemother 46:3422–3427. doi: 10.1128/AAC.46.11.3422-3427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stokes HW, O'Gorman DB, Recchia GD, Parsekhian M, Hall RM. 1997. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol Microbiol 26:731–745. doi: 10.1046/j.1365-2958.1997.6091980.x. [DOI] [PubMed] [Google Scholar]
- 7.Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Park CH, Bush K, Hooper DC. 2006. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med 12:83–88. doi: 10.1038/nm1347. [DOI] [PubMed] [Google Scholar]
- 8.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. 2009. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev 22:664–689. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quiroga MP, Andrés P, Petroni A, Soler Bistué AJ, Guerriero L, Vargas LJ, Zorreguieta A, Tokumoto M, Quiroga C, Tolmasky ME, Galas M, Centrón D. 2007. Complex class 1 integrons with diverse variable regions, including aac(6′)-Ib-cr, and a novel allele, qnrB10, associated with ISCR1 in clinical enterobacterial isolates from Argentina. Antimicrob Agents Chemother 51:4466–4470. doi: 10.1128/AAC.00726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Q, Jiang X, Yang Z, Chen N, Chen X, Li G, Lu Y. 2009. dfrA27, a new integron-associated trimethoprim resistance gene from Escherichia coli. J Antimicrob Chemother 63:405–406. doi: 10.1093/jac/dkn474. [DOI] [PubMed] [Google Scholar]
- 11.Moura A, Pereira C, Henriques I, Correia A. 2012. Novel gene cassettes and integrons in antibiotic-resistant bacteria isolated from urban wastewaters. Res Microbiol 163:92–100. doi: 10.1016/j.resmic.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 12.de Toro M, Rodriguez I, Rojo-Bezares B, Helmuth R, Torres C, Guerra B, Saenz Y. 2013. pMdT1, a small ColE1-like plasmid mobilizing a new variant of the aac(6′)-Ib-cr gene in Salmonella enterica serovar Typhimurium. J Antimicrob Chemother 68:1277–1280. doi: 10.1093/jac/dkt001. [DOI] [PubMed] [Google Scholar]
- 13.Partridge SR, Tsafnat G, Coiera E, Iredell JR. 2009. Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol Rev 33:757–784. doi: 10.1111/j.1574-6976.2009.00175.x. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez MS, Nikolaidis N, Tolmasky ME. 2013. Rise and dissemination of aminoglycoside resistance: the aac(6′)-Ib paradigm. Front Microbiol 4:121. doi: 10.3389/fmicb.2013.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd DA, Tyler S, Christianson S, McGeer A, Muller MP, Willey BM, Bryce E, Gardam M, Nordmann P, Mulvey MR. 2004. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob Agents Chemother 48:3758–3764. doi: 10.1128/AAC.48.10.3758-3764.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz E, Rojo-Bezares B, Saenz Y, Olarte I, Esteban I, Rocha-Gracia R, Zarazaga M, Torres C. 2010. Outbreak caused by a multi-resistant Klebsiella pneumoniae strain of new sequence type ST341 carrying new genetic environments of aac(6′)-Ib-cr and qnrS1 genes in a neonatal intensive care unit in Spain. Int J Med Microbiol 300:464–469. doi: 10.1016/j.ijmm.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Woloj M, Tolmasky ME, Roberts MC, Crosa JH. 1986. Plasmid-encoded amikacin resistance in multiresistant strains of Klebsiella pneumoniae isolated from neonates with meningitis. Antimicrob Agents Chemother 29:315–319. doi: 10.1128/AAC.29.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nobuta K, Tolmasky ME, Crosa LM, Crosa JH. 1988. Sequencing and expression of the 6′-N-acetyltransferase gene of transposon Tn1331 from Klebsiella pneumoniae. J Bacteriol 170:3769–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia DC, Woloj M, Kaufman S, Sordelli DO, Piñeiro S. 1995. Sequences related to Tn1331 associated with multiple antimicrobial resistance in different Salmonella serovars. Int J Antimicrob Agents 5:199–202. doi: 10.1016/0924-8579(95)00005-S. [DOI] [PubMed] [Google Scholar]
- 20.Chamorro RM, Actis LA, Crosa JH, Tolmasky ME. 1990. Dissemination of plasmid-mediated amikacin resistance among pathogenic Klebsiella pneumoniae. Medicina (B Aires) 50:543–547. [PubMed] [Google Scholar]
- 21.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement, vol 33 CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.Dery KJ, Soballe B, Witherspoon MS, Bui D, Koch R, Sherratt DJ, Tolmasky ME. 2003. The aminoglycoside 6′-N-acetyltransferase type Ib encoded by Tn1331 is evenly distributed within the cell's cytoplasm. Antimicrob Agents Chemother 47:2897–2902. doi: 10.1128/AAC.47.9.2897-2902.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melano R, Corso A, Petroni A, Centrón D, Orman B, Pereyra A, Moreno N, Galas M. 2003. Multiple antibiotic-resistance mechanisms including a novel combination of extended-spectrum beta-lactamases in a Klebsiella pneumoniae clinical strain isolated in Argentina. J Antimicrob Chemother 52:36–42. doi: 10.1093/jac/dkg281. [DOI] [PubMed] [Google Scholar]
- 25.Andrés P, Lucero C, Soler-Bistué A, Guerriero L, Albornoz E, Tran T, Zorreguieta A, PMQR Group, Galas M, Corso A, Tolmasky ME, Petroni A. 2013. Differential distribution of plasmid-mediated quinolone resistance genes in clinical enterobacteria with unusual phenotypes of quinolone susceptibility from Argentina. Antimicrob Agents Chemother 57:2467–2475. doi: 10.1128/AAC.01615-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Heffron F, McCarthy BJ, Ohtsubo H, Ohtsubo E. 1979. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell 18:1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez MS, Parenteau TR, Centrón D, Tolmasky ME. 2008. Functional characterization of Tn1331 gene cassettes. J Antimicrob Chemother 62:669–673. doi: 10.1093/jac/dkn279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orman BE, Piñeiro SA, Arduino S, Galas M, Melano R, Caffer MI, Sordelli DO, Centrón D. 2002. Evolution of multiresistance in nontyphoid Salmonella serovars from 1984 to 1998 in Argentina. Antimicrob Agents Chemother 46:3963–3970. doi: 10.1128/AAC.46.12.3963-3970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lévesque C, Piche L, Larose C, Roy PH. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother 39:185–191. doi: 10.1128/AAC.39.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quiroga MP, Arduino SM, Merkier AK, Quiroga C, Petroni A, Argentinian Integron Study Group, Roy PH, Centrón D. 2013. Distribution and functional identification of complex class 1 integrons. Infect Genet Evol 19:88–96. doi: 10.1016/j.meegid.2013.06.029. [DOI] [PubMed] [Google Scholar]