Abstract
U.S. surveillance for Neisseria gonorrhoeae antimicrobial susceptibilities is based exclusively on male urethral isolates. These data inform gonorrhea treatment guidelines, including recommendations for the treatment of extragenital infections, but data on the susceptibilities of extragenital isolates are limited. We compared the antimicrobial susceptibilities of pharyngeal, rectal, and urethral gonococcal isolates collected from men who have sex with men (MSM), at five sentinel sites throughout the United States. MICs were determined by the agar dilution method. Generalized linear models were used to compare (i) the proportions of isolates with elevated MICs and (ii) geometric mean MICs according to anatomic site, adjusted for city. In December 2011 to September 2013, totals of 205 pharyngeal, 261 rectal, and 976 urethral isolates were obtained. The proportions of isolates with elevated ceftriaxone MICs (≥0.125 μg/ml) did not differ according to anatomic site (0.5% of pharyngeal isolates, 1.5% of rectal isolates, and 1.7% of urethral isolates, with a city-adjusted odds ratio [aOR] of 0.4 [95% confidence interval {CI}, 0.0 to 3.9] for pharyngeal versus urethral isolates and an aOR of 0.9 [95% CI, 0.2 to 4.2] for rectal versus urethral isolates). The city-adjusted geometric mean ceftriaxone MICs of pharyngeal (0.0153 μg/ml) and rectal (0.0157 μg/ml) isolates did not differ from that of urethral isolates (0.0150 μg/ml) (ratios of geometric mean MICs of 1.02 [95% CI, 0.90 to 1.17] and 1.05 [95% CI, 0.93 to 1.19], respectively). Similar results were observed for other antimicrobials, including cefixime and azithromycin. These findings suggest that, at the population level, gonococcal antimicrobial susceptibility surveillance based on urethral isolates from MSM adequately reflects the susceptibilities of N. gonorrhoeae strains circulating among MSM.
INTRODUCTION
Neisseria gonorrhoeae has developed resistance to multiple antimicrobials that were previously recommended for treatment, including penicillin, tetracycline, and ciprofloxacin (1). Currently, the only recommended treatment for gonorrhea in Europe and the United States is dual treatment with ceftriaxone and azithromycin (2, 3). However, decreases in susceptibilities to cephalosporins (4–7) and reports of cefixime and ceftriaxone treatment failures worldwide (8–10) indicate that the emergence of cephalosporin-resistant gonorrhea could be imminent. Because there are limited remaining effective therapeutic options for gonorrhea (11, 12), ceftriaxone resistance in N. gonorrhoeae would present significant challenges for individual case management and gonorrhea control in the community. As a result, surveillance of antimicrobial susceptibilities among N. gonorrhoeae strains will be increasingly critical to inform gonorrhea treatment recommendations.
N. gonorrhoeae can infect the urogenital tract, rectum, and pharynx through sexual contact, and infection at extragenital sites may facilitate the acquisition or development of resistance mutations. Rectal infection and exposure to fecal lipids provide selective pressure for mutations such as those in the mtr locus, which confer resistance to hydrophobic molecules and drugs (13–16). Pharyngeal infection supplies the opportunity for genetic reassortment between N. gonorrhoeae and other Neisseria species that colonize the pharynx (17, 18). Specifically, the mosaic penA allele, a key genetic determinant associated with decreased cephalosporin susceptibility, appears to have evolved through recombination with penA genes from commensal Neisseria species (18–20). In addition, pharyngeal infections are more difficult to eradicate than urogenital or rectal infections (21, 22) and are typically asymptomatic, which may provide an optimal setting for selection of resistance mutations. It is notable that the first identified N. gonorrhoeae strain with high-level resistance to ceftriaxone was isolated from the pharynx (8). Furthermore, high rates of resistance and elevated MICs are frequently observed among gay men, bisexual men, and other men who have sex with men (MSM) (7, 23), among whom extragenital infections are common (24–26).
Globally, surveillance for N. gonorrhoeae antimicrobial susceptibilities is conducted mostly with urogenital isolates (5, 6, 27), and there are few published data comparing the susceptibilities of urogenital, rectal, and pharyngeal isolates. Although some reports suggested that rectal isolates or pharyngeal isolates are less susceptible than urethral isolates (14, 28, 29), those evaluations did not control for the sex of sex partners, which potentially confounded the association of anatomic site with antimicrobial susceptibility. In the United States, the Centers for Disease Control and Prevention (CDC) Gonococcal Isolate Surveillance Project (GISP) monitors susceptibility trends exclusively among urethral isolates obtained from symptomatic men (30). Because the CDC relies on GISP data in producing gonorrhea treatment guidelines, including treatment recommendations for extragenital infections, it is important to ascertain whether the susceptibilities of urethral isolates differ from those of isolates from pharyngeal or rectal infections. The objectives of this analysis were to describe the antimicrobial susceptibilities of urethral, rectal, and pharyngeal isolates obtained from MSM and to determine whether antimicrobial susceptibilities differed according to anatomic site.
MATERIALS AND METHODS
Study group and isolate collection.
Demographic data and N. gonorrhoeae isolates were collected from MSM attending five sexually transmitted disease (STD)/sexual health clinics throughout the United States. Clinics were located in five jurisdictions (state or local health departments) that participated in the STD Surveillance Network (SSuN), i.e., Chicago, Illinois; Los Angeles, California; New York, New York; Philadelphia, Pennsylvania; and Seattle, Washington. The SSuN is a sentinel site surveillance network that collects demographic, behavioral, and clinical data from persons attending STD/sexual health clinics. For this evaluation, MSM were defined as men who reported having had sex with at least one male partner or who self-identified as gay, bisexual, or homosexual. Between December 2011 and September 2013, specimens for N. gonorrhoeae culture and antimicrobial susceptibility testing were collected from (i) MSM who presented with urethral symptoms consistent with N. gonorrhoeae infections, (ii) MSM who reported sexual contact with a N. gonorrhoeae-infected partner, and (iii) MSM with N. gonorrhoeae infections that had been identified by screening using a nonculture test (e.g., nucleic acid amplification test [NAAT]) who had not yet received treatment. Among MSM who met at least one of these criteria, pharyngeal and rectal cultures were collected from individuals who reported exposure at those sites. Urethral cultures were collected only from MSM with urethral symptoms. Because the data were collected through disease surveillance activity, this activity was determined not to be human subjects research and was approved by the CDC National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Clinics that had implemented NAAT-based screening for N. gonorrhoeae continued to screen and to test for N. gonorrhoeae according to clinic protocols. MSM diagnosed with N. gonorrhoeae infections were treated according to routine standards of care.
Laboratory procedures.
At local clinics and/or laboratories, specimens for N. gonorrhoeae isolation were inoculated onto selective media and incubated at 35°C in 5% CO2. Presumptive N. gonorrhoeae isolates were confirmed by local laboratories according to their standard protocols. Gonococcal isolates were subcultured on supplemented chocolate medium, frozen in trypticase soy broth with 20% glycerol, and shipped monthly to one of two participating GISP reference laboratories (30).
Reference laboratories performed confirmatory tests on all isolates, to verify the isolation of N. gonorrhoeae, and determined the antimicrobial susceptibilities of all isolates using agar dilution methods. Standardized bacterial suspensions were inoculated on Difco GC medium base supplemented with 1% IsoVitalex Enrichment supplement (Becton Dickinson Diagnostic Systems, Sparks, MD). Antimicrobials tested included azithromycin (in the range of 0.03 μg/ml to 2 μg/ml at one laboratory and the range of 0.03 μg/ml to 16 μg/ml at the second laboratory), cefixime (0.015 μg/ml to 2 μg/ml), ceftriaxone (0.008 μg/ml to 2 μg/ml), ciprofloxacin (0.015 μg/ml to 16 μg/ml), penicillin (0.25 μg/ml to 16 μg/ml), and tetracycline (0.25 μg/ml to 16 μg/ml). Isolates found to have cefixime or ceftriaxone MICs of ≥0.5 μg/ml were tested to the endpoint at the reference laboratories. One laboratory shipped isolates with azithromycin MICs of ≥2 μg/ml to the CDC, and the MIC endpoints obtained by the CDC were used for analysis. At the second laboratory, isolates found to have azithromycin MICs of ≥16 μg/ml were tested to the endpoint at that laboratory. All isolates were tested for β-lactamase activity using the nitrocefin test. As described by the GISP protocol (30), isolates with azithromycin MICs of ≥2 μg/ml, cefixime MICs of ≥0.25 μg/ml, or ceftriaxone MICs of ≥0.125 μg/ml were shipped to the CDC for confirmation. To ensure the accuracy of susceptibility results, control N. gonorrhoeae strains with known MICs were included with each susceptibility test run. For additional quality assurance, reference laboratories tested a panel of 15 unidentified N. gonorrhoeae strains (provided by the CDC) twice yearly.
Interpretation of susceptibility results.
MIC data were interpreted according to criteria recommended by the Clinical and Laboratory Standards Institute (CLSI) for ciprofloxacin resistance (MIC of ≥1 μg/ml), penicillin resistance (MIC of ≥2 μg/ml or β-lactamase positive), and tetracycline resistance (MIC of ≥2 μg/ml) (31). Because CLSI has not established criteria for resistance to azithromycin, cefixime, or ceftriaxone, we used breakpoints described in the GISP protocol (30) (azithromycin MIC of ≥2 μg/ml, cefixime MIC of ≥0.25 μg/ml, and ceftriaxone MIC of ≥0.125 μg/ml) as the definitions of “elevated MICs” for these antimicrobials.
Statistical analyses.
We used the chi-square statistic to compare frequency distributions of categorical variables (city, age group, race/ethnicity, and HIV status) according to anatomic site. To assess antimicrobial susceptibilities according to anatomic site of infection, we compared (i) the proportions of isolates with elevated MICs or resistance and (ii) the geometric mean MIC for each antimicrobial according to anatomic site. Because of differences in susceptibilities according to geographic location (23, 27), geographic location was treated as a confounder and all analyses were adjusted for city as an independent variable. To calculate geometric mean MICs, we converted MICs from an exponential scale to a linear scale by taking the logarithm of the MICs. Our analytic sample included isolates obtained from MSM with concurrent infections at multiple anatomic sites. MICs for isolates from different anatomic sites from the same person could be correlated, resulting in a lack of independence among observations. To take this correlation into account, we applied generalized linear models to the log-transformed data by using the generalized estimating equations method to test for differences between anatomical sites. The proportions of isolates with elevated MICs or resistance were also compared according to anatomic site using these models. Adjustments for multiple comparisons between sites were made with the Tukey-Kramer method. P values of <0.05 were used to indicate significant differences. All statistical analyses were conducted using SAS (version 9.3; SAS Institute, Cary, NC).
Analysis of paired isolates from MSM with concurrent infections.
We identified paired isolates that were obtained from MSM with concurrent pharyngeal and urethral infections or concurrent rectal and urethral infections. For these pairs, we compared urethral isolate MICs and extragenital isolate MICs and described the proportions of pairs with discordant MICs (differing by ≥2 doubling dilutions). For cases with discordant MICs, we assessed the proportion with a higher urethral MIC than extragenital MIC and the proportion with a higher extragenital MIC than urethral MIC.
RESULTS
Sources of tested isolates.
In total, 205 pharyngeal, 261 rectal, and 976 urethral isolates were obtained from MSM during the study period of December 2011 through September 2013. The geographic distributions of isolates varied according to anatomic site (Table 1). Almost one-half of pharyngeal isolates were collected in Seattle (49.3%), and the majority of rectal isolates were collected in either Seattle (41.0%) or New York City (34.1%). In contrast, the geographic distribution of urethral isolates was more even, with the greatest proportion of isolates collected in New York City (37.2%). The ages and races/ethnicities of MSM from whom isolates were collected also differed according to anatomic site. MSM in the youngest age category (ages of 16 to 25 years) accounted for a greater proportion of rectal isolates (51.0%) than pharyngeal (39.0%) or urethral (32.9%) isolates. White MSM accounted for the majority of pharyngeal (60.2%) and rectal (55.5%) isolates but a smaller proportion of urethral isolates (46.7%). MSM HIV status did not differ significantly according to anatomic site of infection.
TABLE 1.
Descriptive characteristics of MSM from whom isolates were collected, according to anatomic site of infection
| Parameter | No./total no. (%) |
P | ||
|---|---|---|---|---|
| Pharyngeal (n = 205) | Rectal (n = 261) | Urethral (n = 976) | ||
| City | <0.0001 | |||
| Chicago | 8/205 (3.9) | 9/261 (3.5) | 29/976 (3.0) | |
| Los Angeles | 27/205 (13.2) | 28/261 (10.7) | 168/976 (17.2) | |
| New York | 21/205 (10.2) | 89/261 (34.1) | 363/976 (37.2) | |
| Philadelphia | 48/205 (23.4) | 28/261 (10.7) | 201/976 (20.6) | |
| Seattle | 101/205 (49.3) | 107/261 (41.0) | 215/976 (22.0) | |
| Age | <0.0001 | |||
| 16–25 yr | 80/205 (39.0) | 133/261 (51.0) | 319/971 (32.9) | |
| 26–35 yr | 78/205(38.1) | 84/261 (32.2) | 416/971 (42.8) | |
| 36–45 yr | 29/205 (14.1) | 27/261 (10.3) | 138/971 (14.2) | |
| >45 yr | 18/205 (8.8) | 17/261 (6.5) | 98/971 (10.1) | |
| Race/ethnicity | <0.0001 | |||
| Asian | 8/191 (4.2) | 13/254 (5.1) | 36/939 (3.8) | |
| Black | 30/191 (15.7) | 27/254 (10.6) | 267/939 (28.4) | |
| Hispanic | 30/191 (15.7) | 60/254 (23.6) | 175/939 (18.6) | |
| Othera | 8/191 (4.2) | 13/254 (5.1) | 23/939 (2.5) | |
| White | 115/191 (60.2) | 141/254 (55.5) | 438/939 (46.7) | |
| HIV status | 0.09 | |||
| Positive | 34/196 (17.3) | 64/251 (25.5) | 225/941 (23.9) | |
| Negative | 162/196 (82.7) | 187/251 (74.5) | 716/941 (76.1) | |
Includes American Indian/Alaska Native, Pacific Islander/Hawaiian, and other race/ethnicity.
Comparison of antimicrobial susceptibilities according to anatomic site.
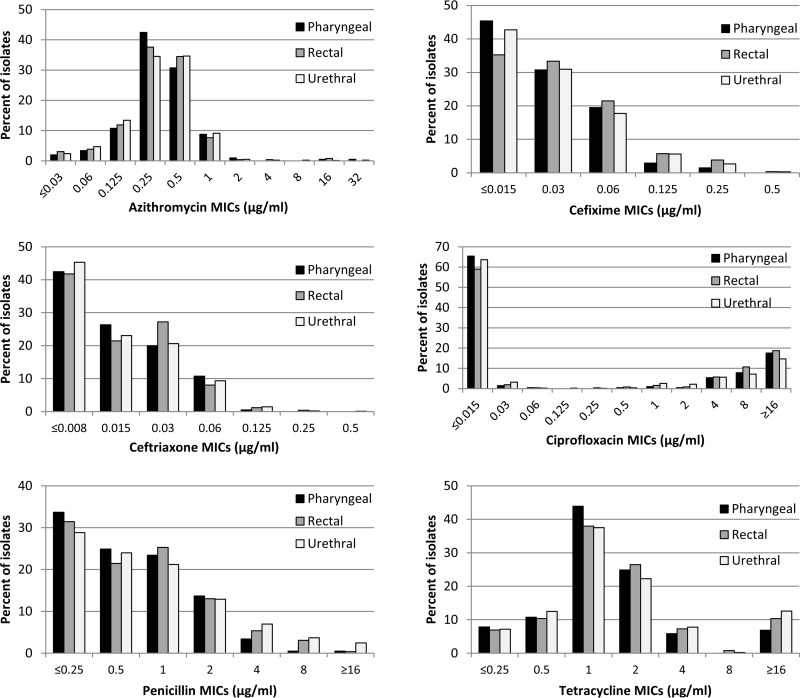
The MIC distributions for pharyngeal, rectal, and urethral isolates for each antimicrobial are presented in Fig. 1. The proportions of pharyngeal and rectal isolates with elevated MICs did not differ from the proportions of urethral isolates with elevated MICs for azithromycin, cefixime, or ceftriaxone (Table 2). Overall, 1.2% of urethral isolates had elevated azithromycin MICs (≥2 μg/ml), compared with 2.0% of pharyngeal isolates (city-adjusted odds ratio [aOR], 2.2 [95% confidence interval {CI}, 0.6 to 8.5]) and 1.5% of rectal isolates (aOR, 1.6 [95% CI, 0.4 to 6.0]). For cefixime, 3.0% of urethral isolates had elevated MICs (≥0.25 μg/ml), compared with 1.5% of pharyngeal isolates (aOR, 0.8 [95% CI, 0.2 to 3.2]) and 4.2% of rectal isolates (aOR, 1.6 [95% CI, 0.7 to 3.9]). For ceftriaxone, 1.7% of urethral isolates had elevated MICs (≥0.125 μg/ml), compared with 0.5% of pharyngeal isolates (aOR, 0.4 [95% CI, 0.0 to 3.9]) and 1.5% of rectal isolates (aOR, 0.9 [95% CI, 0.2 to 4.2]). The proportions of pharyngeal isolates with elevated azithromycin, cefixime, or ceftriaxone MICs did not differ from the proportions of rectal isolates with elevated MICs (data not shown). Similarly, there were no significant differences in the proportions of isolates resistant to ciprofloxacin, penicillin, or tetracycline according to anatomic site in the city-adjusted analysis.
FIG 1.
Distributions of antimicrobial MICs for pharyngeal (n = 205), rectal (n = 261), and urethral (n = 976) isolates.
TABLE 2.
Comparison of MICs according to antimicrobial and anatomic site of infection
| Antimicrobial and measured parametera | Pharyngeal (n = 205) | Rectal (n = 261) | Urethral (n = 976) |
|---|---|---|---|
| Azithromycin | |||
| MIC50 (μg/ml) | 0.25 | 0.25 | 0.25 |
| MIC90 (μg/ml) | 1 | 0.5 | 1 |
| MIC range (μg/ml) | ≤0.03-32 | ≤0.03-16 | ≤0.03-32 |
| No. (%) with elevated MIC (≥2 μg/ml) | 4 (2.0) | 4 (1.5) | 12 (1.2) |
| Crude OR (95% CI) | 1.6 (0.4–6.3) | 1.3 (0.3–4.9) | Reference |
| City-adjusted OR (95% CI) | 2.2 (0.6–8.5) | 1.6 (0.4–6.0) | Reference |
| Cefixime | |||
| MIC50 (μg/ml) | 0.03 | 0.03 | 0.03 |
| MIC90 (μg/ml) | 0.06 | 0.06 | 0.06 |
| MIC range (μg/ml) | ≤0.015-0.25 | ≤0.015-0.5 | ≤0.015-0.5 |
| No. (%) with elevated MIC (≥0.25 μg/ml) | 3 (1.5) | 11 (4.2) | 29 (3.0) |
| Crude OR (95% CI) | 0.5 (0.1–2.0) | 1.4 (0.6–3.3) | Reference |
| City-adjusted OR (95% CI) | 0.8 (0.2–3.2) | 1.6 (0.7–3.9) | Reference |
| Ceftriaxone | |||
| MIC50 (μg/ml) | 0.015 | 0.015 | 0.015 |
| MIC90 (μg/ml) | 0.06 | 0.03 | 0.06 |
| MIC range (μg/ml) | ≤0.008-0.125 | ≤0.008-0.25 | ≤0.008-0.5 |
| No. (%) with elevated MIC (≥0.125 μg/ml) | 1 (0.5) | 4 (1.5) | 17 (1.7) |
| Crude OR (95% CI) | 0.3 (0.0–3.1) | 0.9 (0.2–3.3) | Reference |
| City-adjusted OR (95% CI) | 0.4 (0.0–3.9) | 0.9 (0.2–4.2) | Reference |
| Ciprofloxacin | |||
| MIC50 (μg/ml) | 0.015 | 0.015 | 0.015 |
| MIC90 (μg/ml) | 16 | 16 | 16 |
| MIC range (μg/ml) | ≤0.015 to ≥16 | ≤0.015 to ≥16 | ≤0.015 to ≥16 |
| No. (%) resistant (MIC of ≥1 μg/ml) | 69 (32.2) | 98 (37.6) | 314 (32.2) |
| Crude OR (95% CI) | 1.0 (0.7–1.5) | 1.3 (0.9–1.8) | Reference |
| City-adjusted OR (95% CI) | 0.9 (0.6–1.3) | 1.2 (0.9–1.7) | Reference |
| Penicillin | |||
| MIC50 (μg/ml) | 0.5 | 0.5 | 0.5 |
| MIC90 (μg/ml) | 2 | 2 | 4 |
| MIC range (μg/ml) | ≤0.25 to ≥16 | ≤0.25 to ≥16 | ≤0.25 to ≥16 |
| No. (%) resistant (β-lactamase positive or MIC of ≥2 μg/ml) | 37 (18.1) | 57 (21.8) | 255 (26.1) |
| Crude OR (95% CI) | 0.6 (0.4–1.0)b | 0.8 (0.5–1.2) | Reference |
| City-adjusted OR (95% CI) | 0.8 (0.5–1.3) | 1.0 (0.6–1.4) | Reference |
| Tetracycline | |||
| MIC50 (μg/ml) | 1 | 1 | 1 |
| MIC90 (μg/ml) | 4 | 16 | 16 |
| MIC range (μg/ml) | ≤0.25 to ≥16 | ≤0.25 to ≥16 | ≤0.25 to ≥16 |
| No. (%) resistant (MIC of ≥2 μg/ml) | 77 (37.6) | 117 (44.8) | 418 (42.8) |
| Crude OR (95% CI) | 0.8 (0.6–1.2) | 1.1 (0.8–1.5) | Reference |
| City-adjusted OR (95% CI) | 0.8 (0.6–1.2) | 1.1 (0.8–1.6) | Reference |
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by employing generalized linear models.
P < 0.05.
City-adjusted geometric mean MICs for azithromycin, ceftriaxone, ciprofloxacin, penicillin, or tetracycline did not differ according to anatomic site (Table 3). For cefixime, the city-adjusted geometric mean MIC for rectal isolates was significantly higher than that for pharyngeal isolates (ratio of city-adjusted geometric means, 1.17 [95% CI, 1.01 to 1.37]). However, neither the pharyngeal mean MIC nor the rectal mean MIC differed significantly from the urethral geometric mean MIC.
TABLE 3.
Comparison of geometric mean MICs according to anatomic site of infection
| Antimicrobial and MIC type | Geometric mean MIC (μg/ml)a |
Ratio of geometric mean MICs (95% CI) |
||||
|---|---|---|---|---|---|---|
| Pharyngeal (n = 205) | Rectal (n = 261) | Urethral (n = 976) | Pharyngeal vs urethral | Rectal vs urethral | Rectal vs pharyngeal | |
| Azithromycin | ||||||
| Geometric mean MIC | 0.3129 | 0.3034 | 0.3035 | 1.03 (0.90–1.19) | 1.00 (0.87–1.15) | 0.97 (0.81–1.16) |
| City-adjusted geometric mean MIC | 0.3369 | 0.3166 | 0.2954 | 1.14 (0.98–1.33) | 1.07 (0.94–1.23) | 0.94 (0.78–1.12) |
| Cefixime | ||||||
| Geometric mean MIC | 0.0270 | 0.0325 | 0.0292 | 0.92 (0.82–1.04) | 1.11 (0.98–1.26) | 1.20 (1.03–1.40)b |
| City-adjusted geometric mean MIC | 0.0275 | 0.0323 | 0.0287 | 0.96 (0.85–1.09) | 1.13 (0.99–1.28) | 1.17 (1.01–1.37)b |
| Ceftriaxone | ||||||
| Geometric mean MIC | 0.0155 | 0.0161 | 0.0154 | 1.00 (0.88–1.14) | 1.05 (0.93–1.18) | 1.04 (0.89–1.22) |
| City-adjusted geometric mean MIC | 0.0153 | 0.0157 | 0.0150 | 1.02 (0.90–1.17) | 1.05 (0.93–1.19) | 1.03 (0.88–1.20) |
| Ciprofloxacin | ||||||
| Geometric mean MIC | 0.1241 | 0.1759 | 0.1160 | 1.07 (0.62–1.84) | 1.52 (0.92–2.51) | 1.41 (0.73–2.77) |
| City-adjusted geometric mean MIC | 0.1053 | 0.1599 | 0.1132 | 0.93 (0.54–1.60) | 1.41 (0.85–2.34) | 1.52 (0.78–2.95) |
| Penicillin | ||||||
| Geometric mean MIC | 0.6229 | 0.7080 | 0.7911 | 0.79 (0.67–0.93)b | 0.90 (0.76–1.05) | 1.14 (0.93–1.39) |
| City-adjusted geometric mean MIC | 0.6660 | 0.7492 | 0.7813 | 0.85 (0.72–1.00) | 0.96 (0.82–1.12) | 1.13 (0.93–1.37) |
| Tetracycline | ||||||
| Geometric mean MIC | 1.2974 | 1.5214 | 1.5367 | 0.84 (0.71–1.01) | 0.99 (0.83–1.18) | 1.17 (0.94–1.46) |
| City-adjusted geometric mean MIC | 1.3684 | 1.5763 | 1.5187 | 0.90 (0.75–1.08) | 1.04 (0.87–1.24) | 1.15 (0.93–1.43) |
Geometric mean MICs, ratios of geometric mean MICs, and 95% confidence intervals (CIs) were calculated by employing generalized linear models.
P < 0.05 (CI does not contain 1).
Analysis of paired isolates from MSM with concurrent infections.
Paired pharyngeal and urethral isolates were obtained from 55 MSM with concurrent pharyngeal and urethral gonococcal infections (Table 4). Paired rectal and urethral isolates were obtained from 45 MSM with concurrent rectal and urethral infections (Table 5). These pairs included isolates from 12 MSM with concurrent infections at all three anatomic sites. Overall, >90% of pharyngeal-urethral pairs and >88% of rectal-urethral pairs had concordant MICs (identical MICs or MICs within 1 dilution of each other) for each antimicrobial tested. Although the absolute numbers of pairs with discordant MICs (≥2-dilution differences) were small, the proportion of pairs with a higher urethral MIC than extragenital MIC was similar to the proportion with a higher extragenital MIC than urethral MIC when discordance was observed. There was no trend toward either pharyngeal or rectal isolates having higher MICs than urethral MICs among MSM with concurrent pharyngeal and urethral or rectal and urethral infections.
TABLE 4.
Numbers and proportions of discordant MIC results among paired isolates obtained from patients with concurrent pharyngeal and urethral infections (n = 55)
| Discordant resultsa | No. (%) |
|---|---|
| Discordant azithromycin MIC results | 3 (5.5) |
| Urethral MIC higher than pharyngeal MIC | 2 (3.6) |
| Pharyngeal MIC higher than urethral MIC | 1 (1.8) |
| Discordant cefixime MIC results | 1 (1.8) |
| Urethral MIC higher than pharyngeal MIC | 1 (1.8) |
| Pharyngeal MIC higher than urethral MIC | 0 |
| Discordant ceftriaxone MIC results | 3 (5.5) |
| Urethral MIC higher than pharyngeal MIC | 3 (5.5) |
| Pharyngeal MIC higher than urethral MIC | 0 |
| Discordant ciprofloxacin MIC results | 4 (7.3) |
| Urethral MIC higher than pharyngeal MIC | 3 (5.5) |
| Pharyngeal MIC higher than urethral MIC | 1 (1.8) |
| Discordant penicillin MIC results | 5 (9.1) |
| Urethral MIC higher than pharyngeal MIC | 4 (7.3) |
| Pharyngeal MIC higher than urethral MIC | 1 (1.8) |
| Discordant tetracycline MIC results | 3 (5.5) |
| Urethral MIC higher than pharyngeal MIC | 2 (3.6) |
| Pharyngeal MIC higher than urethral MIC | 1 (1.8) |
Discordant indicates that the difference between the pharyngeal MIC and the urethral MIC was ≥2 doubling dilutions.
TABLE 5.
Numbers and proportions of discordant MIC results among paired isolates obtained from patients with concurrent rectal and urethral infections (n = 45)
| Discordant resultsa | No. (%) |
|---|---|
| Discordant azithromycin MIC results | 2 (4.4) |
| Urethral MIC higher than rectal MIC | 0 |
| Rectal MIC higher than urethral MIC | 2 (4.4) |
| Discordant cefixime MIC results | 3 (6.7) |
| Urethral MIC higher than rectal MIC | 2 (4.4) |
| Rectal MIC higher than urethral MIC | 1 (2.2) |
| Discordant ceftriaxone MIC results | 2 (4.4) |
| Urethral MIC higher than rectal MIC | 2 (4.4) |
| Rectal MIC higher than urethral MIC | 0 |
| Discordant ciprofloxacin MIC results | 5 (11.1) |
| Urethral MIC higher than rectal MIC | 4 (8.9) |
| Rectal MIC higher than urethral MIC | 1 (2.2) |
| Discordant penicillin MIC results | 5 (11.1) |
| Urethral MIC higher than rectal MIC | 3 (6.7) |
| Rectal MIC higher than urethral MIC | 2 (4.4) |
| Discordant tetracycline MIC results | 3 (6.7) |
| Urethral MIC higher than rectal MIC | 3 (6.7) |
| Rectal MIC higher than urethral MIC | 0 |
Discordant indicates that the difference between the rectal MIC and the urethral MIC was ≥2 doubling dilutions.
DISCUSSION
In this multicity analysis of gonococcal isolates collected from MSM, we found no evidence that MICs of isolates obtained from extragenital sites were higher than those of isolates obtained from the urethral site. We found relatively high prevalences of elevated azithromycin MICs (1.2% to 2.0%), elevated cefixime MICs (1.5% to 4.2%), and elevated ceftriaxone MICs (0.5% to 1.7%). We also found high prevalences of resistance to antimicrobials previously recommended for gonorrhea treatment, i.e., ciprofloxacin (32.2% to 37.6%), penicillin (18.1% to 26.1%), and tetracycline (37.6% to 44.8%). These data are consistent with GISP reports of gonococcal susceptibilities among MSM during the same time period (27, 32) and underscore the importance of identifying new antimicrobial options for gonorrhea treatment, given the potential threat of resistance to cephalosporins.
The proportions of isolates with elevated azithromycin, cefixime, or ceftriaxone MICs or with resistance to ciprofloxacin, penicillin, or tetracycline did not differ according to anatomic site. In addition, neither pharyngeal nor rectal isolate geometric mean MICs differed significantly from those of urethral isolates. For each antimicrobial tested, pharyngeal, rectal, and urethral geometric mean MICs were within 1 dilution of each other. These findings differ from the recent report by Hottes et al. (28) that the prevalences of elevated azithromycin, cefixime, and ceftriaxone MICs were consistently higher among rectal and pharyngeal isolates than among urethral isolates. As Hottes et al. noted, however, they were unable to control for the sex of sex partners, which likely confounded the results. GISP isolates from MSM are more likely than isolates from men who have sex only with women to exhibit elevated MICs and resistance (23). Consequently, the higher prevalences of elevated MICs among extragenital isolates versus urethral isolates in the report by Hottes et al. (28) likely reflected the higher prevalences among N. gonorrhoeae strains circulating within MSM sexual networks versus heterosexual networks. Our data indicate that, among MSM, extragenital isolates are not more likely than urethral isolates to have elevated MICs or resistance.
This finding has implications for the surveillance of N. gonorrhoeae antimicrobial susceptibilities, as well as the use of surveillance data to inform treatment guidelines, including treatment recommendations for pharyngeal and rectal infections. The U.S. surveillance system for N. gonorrhoeae antimicrobial susceptibilities, GISP, monitors susceptibility trends exclusively among urethral isolates obtained from symptomatic men (30). This sampling strategy is an efficient means of conducting surveillance of gonococcal susceptibilities in settings in which gonococcal culture, which is required for antimicrobial susceptibility testing, has largely been replaced by NAATs for routine diagnosis of gonorrhea. Male urogenital N. gonorrhoeae infections are easier to identify because they are typically symptomatic, whereas pharyngeal, rectal, and female urogenital infections are typically asymptomatic. Additionally, the sensitivity of gonococcal culture is higher for the urethral site than for other sites (33–35). Men with urethral symptoms also represent a consistent population likely to seek clinical evaluation and less likely to be affected by changes in screening practices, which provides additional advantages for surveillance systems that monitor susceptibility trends over time. Our results suggest that surveillance of gonococcal antimicrobial susceptibilities based on urethral isolates from MSM adequately represents the susceptibilities of N. gonorrhoeae strains circulating among MSM, supporting the GISP sampling strategy.
Our analysis also compared the MICs of paired isolates collected from MSM with concurrent urethral and extragenital gonococcal infections, and it found that, depending on the antimicrobial, 2% to 11% of pairs had discordant MICs that differed by ≥2 dilutions. Within these discordant pairs, there was no apparent association between anatomic site and the higher MIC. Concurrent infections at multiple anatomic sites with different strains of N. gonorrhoeae that exhibited different antimicrobial susceptibilities were reported previously (36, 37). These cases could represent separate infection events (i.e., infections at different anatomic sites with different strains), simultaneous infections with the same strain at different anatomic sites but subsequent mutation, or simultaneous infections with multiple strains at different anatomic sites but isolation of different strains in the laboratory. Regardless of etiology, clinicians should be aware that individuals can be infected with different N. gonorrhoeae strains at different anatomic sites. If there is clinical concern regarding possible treatment failure or resistance, then specimens for culture and antimicrobial susceptibility testing should be collected from all exposed anatomic sites and tested separately.
This analysis has several limitations. These data do not exclude the possibility that pharyngeal or rectal gonococcal infections facilitate development of antimicrobial resistance. It is possible that gonococcal resistance emerges first at extragenital sites but is rapidly transmitted to other anatomic sites within sexual networks, so that antimicrobial susceptibilities do not differ according to anatomic site at the population level. A second limitation is that the sensitivity of gonococcal culture is lower for extragenital sites than for the urethra (33–35), possibly due to the presence of other bacteria and differences in bacterial loads (38). As a result, we obtained fewer pharyngeal and rectal isolates than urethral isolates. Additionally, in order to minimize discomfort, we limited the collection of urethral cultures to symptomatic men only, whereas extragenital cultures were obtained from eligible MSM regardless of symptoms. These two factors could potentially bias our findings, if growth in cultures or the presence of symptoms was associated with antimicrobial susceptibility. It is also possible that our study was insufficiently powered to detect small differences between anatomic sites; however, we observed no trend toward one site having consistently higher MICs than another. Finally, our data were limited to MSM, and it is possible that MICs differ according to anatomic site among other populations, such as women.
In conclusion, we found no evidence that extragenital N. gonorrhoeae isolates were less susceptible to antimicrobials than urethral isolates. At the population level, MICs for each antimicrobial were similar across anatomic sites, and the proportions of isolates with elevated MICs or resistance did not differ according to anatomic site. For individuals with concurrent N. gonorrhoeae infections at multiple anatomic sites, N. gonorrhoeae susceptibilities may be different at different sites, but no anatomic site was consistently less susceptible than another. Given the threat of cephalosporin resistance and the limited number of treatment options currently available, local surveillance of N. gonorrhoeae susceptibilities will become increasingly important for gonorrhea control programs. Our findings support the GISP strategy of monitoring susceptibilities among male urethral isolates to inform gonorrhea treatment guidelines.
ACKNOWLEDGMENTS
We thank all of the clinic, laboratory, and public health staff members who collaborated on this project, including staff members at the Howard Brown Health Center (Chicago, IL); staff members at the Illinois Department of Public Health; Matthew Beymer and the clinic staff members at the Los Angeles Gay and Lesbian Center; staff members at the Los Angeles County Public Health Laboratory; Ellen Klingler at the New York City Department of Health and Mental Hygiene (DOHMH); Thomas Cherneskie, Pei-Chi Chung, Eric Friedenberg, Eliana Hecht, and Elsie Lee and staff members at the New York City DOHMH Chelsea Clinic; Inessa Rubinstein and Lillian Lee at the New York City DOHMH Public Health Laboratory; clinicians and staff members at the Health Center 1 clinic (Philadelphia, PA); Greta Anschuetz at the Philadelphia Department of Public Health (PDPH); Barbara Lucy at the PDPH Public Health Laboratory; Cheryl Malinski and other staff members and clinicians at the Public Health-Seattle and King County STD Clinic and Public Health Laboratory; and Jim Braxton at the Centers for Disease Control and Prevention (Atlanta, GA).
This project, as well as the SSuN and the GISP, was funded by the CDC, an agency of the U.S. Department of Health and Human Services. Staff members from the CDC were involved in the design and conduct of this surveillance activity and the collection, management, analysis, and interpretation of the data.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the CDC.
REFERENCES
- 1.Lewis DA. 2014. Global resistance of Neisseria gonorrhoeae: when theory becomes reality. Curr Opin Infect Dis 27:62–67. doi: 10.1097/QCO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 2.Bignell C, Unemo M. 2013. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 24:85–92. doi: 10.1177/0956462412472837. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep, in press. [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2012. Update to CDC's sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep 61:590–594. [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control. 2013. Gonococcal antimicrobial susceptibility surveillance in Europe, 2011. European Centre for Disease Prevention and Control, Stockholm, Sweden. [Google Scholar]
- 6.Lahra MM. 2013. Australian Gonococcal Surveillance Programme annual report, 2012. Commun Dis Intell Q Rep 37:E233–E239. [DOI] [PubMed] [Google Scholar]
- 7.Ison CA, Town K, Obi C, Chisholm S, Hughes G, Livermore DM, Lowndes CM. 2013. Decreased susceptibility to cephalosporins among gonococci: data from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) in England and Wales, 2007–2011. Lancet Infect Dis 13:762–768. doi: 10.1016/S1473-3099(13)70143-9. [DOI] [PubMed] [Google Scholar]
- 8.Ohnishi M, Saika T, Hoshina S, Iwasaku K, Nakayama S, Watanabe H, Kitawaki J. 2011. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis 17:148–149. doi: 10.3201/eid1701.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. doi: 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen VG, Mitterni L, Seah C, Rebbapragada A, Martin IE, Lee C, Siebert H, Towns L, Melano RG, Low DE. 2013. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA 309:163–170. doi: 10.1001/jama.2012.176575. [DOI] [PubMed] [Google Scholar]
- 11.Unemo M, Shafer W. 2011. Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann N Y Acad Sci 1230:E19–E28. doi: 10.1111/j.1749-6632.2011.06215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 13.Shafer WM, Balthazar JT, Hagman KE, Morse SA. 1995. Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology 141:907–911. [DOI] [PubMed] [Google Scholar]
- 14.Fagan D. 1985. Comparison of Neisseria gonorrhoeae isolates from homosexual and heterosexual men. Genitourin Med 61:363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morse SA, Lysko PG, McFarland L, Knapp JS, Sandstrom E, Critchlow C, Holmes KK. 1982. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect Immun 37:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McFarland L, Mietzner TA, Knapp JS, Sandstrom E, Holmes KK, Morse SA. 1983. Gonococcal sensitivity to fecal lipids can be mediated by an Mtr-independent mechanism. J Clin Microbiol 18:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuya R, Onoye Y, Kanayama A, Saika T, Iyoda T, Tatewaki M, Matsuzaki K, Kobayashi I, Tanaka M. 2007. Antimicrobial resistance in clinical isolates of Neisseria subflava from the oral cavities of a Japanese population. J Infect Chemother 13:302–304. doi: 10.1007/s10156-007-0541-8. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M, Nakayama H, Huruya K, Konomi I, Irie S, Kanayama A, Saika T, Kobayashi I. 2006. Analysis of mutations within multiple genes associated with resistance in a clinical isolate of Neisseria gonorrhoeae with reduced ceftriaxone susceptibility that shows a multidrug-resistant phenotype. Int J Antimicrob Agents 27:20–26. doi: 10.1016/j.ijantimicag.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Ameyama S, Onodera S, Takahata M, Minami S, Maki N, Endo K, Goto H, Suzuki H, Oishi Y. 2002. Mosaic-like structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob Agents Chemother 46:3744–3749. doi: 10.1128/AAC.46.12.3744-3749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito M, Deguchi T, Mizutani KS, Yasuda M, Yokoi S, Ito S, Takahashi Y, Ishihara S, Kawamura Y, Ezaki T. 2005. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in central Japan. Antimicrob Agents Chemother 49:137–143. doi: 10.1128/AAC.49.1.137-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran JS. 2007. Gonorrhoea. Clin Evid 2007:1604. [PMC free article] [PubMed] [Google Scholar]
- 22.Moran JS. 1995. Treating uncomplicated Neisseria gonorrhoeae infections: is the anatomic site of infection important? Sex Transm Dis 22:39–47. doi: 10.1097/00007435-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Kirkcaldy RD, Zaidi A, Hook EW, Holmes KK, Soge O, del Rio C, Hall G, Papp J, Bolan G, Weinstock HS. 2013. Neisseria gonorrhoeae antimicrobial resistance among men who have sex with men and men who have sex exclusively with women: the Gonococcal Isolate Surveillance Project, 2005–2010. Ann Intern Med 158:321–328. doi: 10.7326/0003-4819-158-5-201303050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patton ME, Kidd S, Llata E, Stenger M, Braxton J, Asbel L, Bernstein K, Gratzer B, Jespersen M, Kerani R, Mettenbrink C, Mohamed M, Pathela P, Schumacher C, Stirland A, Stover J, Tabidze I, Kirkcaldy RD, Weinstock H. 2014. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men–STD Surveillance Network, United States, 2010–2012. Clin Infect Dis 58:1564–1570. doi: 10.1093/cid/ciu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kent CK, Chaw JK, Wong W, Liska S, Gibson S, Hubbard G, Klausner JD. 2005. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis 41:67–74. doi: 10.1086/430704. [DOI] [PubMed] [Google Scholar]
- 26.Marcus JL, Bernstein KT, Kohn RP, Liska S, Philip SS. 2011. Infections missed by urethral-only screening for chlamydia or gonorrhea detection among men who have sex with men. Sex Transm Dis 38:922–924. doi: 10.1097/OLQ.0b013e31822a2b2e. [DOI] [PubMed] [Google Scholar]
- 27.Kirkcaldy RD, Kidd S, Weinstock HS, Papp JR, Bolan GA. 2013. Trends in antimicrobial resistance in Neisseria gonorrhoeae in the USA: the Gonococcal Isolate Surveillance Project (GISP), January 2006-June 2012. Sex Transm Infect 89(Suppl 4):iv5–iv10. doi: 10.1136/sextrans-2013-051162. [DOI] [PubMed] [Google Scholar]
- 28.Hottes TS, Lester RT, Hoang LM, McKay R, Imperial M, Gilbert M, Patrick D, Wong T, Martin I, Ogilvie G. 2013. Cephalosporin and azithromycin susceptibility in Neisseria gonorrhoeae isolates by site of infection, British Columbia, 2006 to 2011. Sex Transm Dis 40:46–51. doi: 10.1097/OLQ.0b013e31827bd64c. [DOI] [PubMed] [Google Scholar]
- 29.Ansink-Schipper MC, van Klingeren B, Huikeshoven H, Woudstra R, Dessens-Kroon M. 1985. Relation between nutritional requirements and susceptibilities to antibiotics of strains of Neisseria gonorrhoeae from pharyngeal and anogenital sites. Genitourin Med 61:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. 2014. Gonococcal Isolate Surveillance Project (GISP) protocol. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/std/gisp/gisp-protocol-may-2014.pdf. [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. CLSI document M100-S24 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 32.Kirkcaldy R, Papp J, Soge O, Hook EW, del Rio C, Harrington S, Kubin G, Weinstock HS. 2014. Cephalosporin antimicrobial susceptibility of Neisseria gonorrhoeae in the United States, 2009–2013. Sex Transm Dis 41:S35–S36. [Google Scholar]
- 33.Bachmann LH, Johnson RE, Cheng H, Markowitz L, Papp JR, Palella FJ Jr, Hook EW III. 2010. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 48:1827–1832. doi: 10.1128/JCM.02398-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachmann LH, Johnson RE, Cheng H, Markowitz LE, Papp JR, Hook EW III. 2009. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae oropharyngeal infections. J Clin Microbiol 47:902–907. doi: 10.1128/JCM.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harryman L, Scofield S, Macleod J, Carrington D, Williams OM, Fernandes A, Horner P. 2012. Comparative performance of culture using swabs transported in Amies medium and the Aptima Combo 2 nucleic acid amplification test in detection of Neisseria gonorrhoeae from genital and extra-genital sites: a retrospective study. Sex Transm Infect 88:27–31. doi: 10.1136/sextrans-2011-050075. [DOI] [PubMed] [Google Scholar]
- 36.Noble RC. 1980. Characterisation of Neisseria gonorrhoeae from women with simultaneous infections at two sites. Br J Vener Dis 56:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saika T, Nishiyama T, Kanayama A, Kobayashi I, Nakayama H, Tanaka M, Naito S. 2001. Comparison of Neisseria gonorrhoeae isolates from the genital tract and pharynx of two gonorrhea patients. J Infect Chemother 7:175–179. doi: 10.1007/s101560100031. [DOI] [PubMed] [Google Scholar]
- 38.Bissessor M, Tabrizi SN, Fairley CK, Danielewski J, Whitton B, Bird S, Garland S, Chen MY. 2011. Differing Neisseria gonorrhoeae bacterial loads in the pharynx and rectum in men who have sex with men: implications for gonococcal detection, transmission, and control. J Clin Microbiol 49:4304–4306. doi: 10.1128/JCM.05341-11. [DOI] [PMC free article] [PubMed] [Google Scholar]