Abstract
Perianal fistula is a clinical entity with multiple surgical treatment options. Recently, magnetic resonance imaging (MRI) has emerged as an important imaging modality in the management of perianal fistulas. It provides accurate description of the fistula within the anal canal in relation to the sphincter complex and other pelvic floor structures as well as the associated complications such as abscess. By understanding the surgical viewpoint, the appearance of perianal fistulas, associated complications, and post-treatment findings of commonly used surgical interventions can more accurately be interpreted to aid clinicians. The objective of the article is to review MRI indications and findings, radiological versus surgical classification schemes, and surgical treatment options for perianal fistulas.
Keywords: Perianal fistula, magnetic resonance imaging, classification, Crohn’ disease
Introduction
Fistula-in-ano is a heterogeneous affliction with significant patient morbidity and challenging treatment. Perianal fistulization can result from inflammation due to inflammatory bowel disease, sequelae of perianal abscess, or other conditions such as anal or rectal cancer and trauma. The incidence of perianal fistula ranges from approximately 1 to 2 per 10,000 individuals with an approximate 2:1 male to female predominance [1–3].
Many anal fistulae are suspected to arise from infected anal glands that open into the anal crypts at the dentate line, a theory known as the cryptoglandular hypothesis [3]. Around 35% of patients develop recurrent disease after initial presentation for cryptoglandular perianal abscess [2, 4]. Risk factors for the development of recurrent abscess and fistulization are related to the underlying nature of the inciting event, i.e. perianal abscess vs. inflammatory bowel disease (IBD). Perianal fistulae are relatively common in Crohn’s disease, occurring in around 20% of patients [5, 6]. Risk factors for the development of fistulizing perianal Crohn’s disease include male gender [6], rectal inflammation [6, 7], and Sephardic Jewish ethnicity [7]. Younger age (less than 40 years) [4] has been reported as a risk factor for recurrence of cryptoglandular disease in patients without IBD. Studies have suggested an increased risk of recurrence in non-diabetic patients [4, 8], although the trend was not found to reach statistical significance . Data for smoking [4, 9] and the use of perioperative antibiotics [4, 8] are mixed.
Imaging was not considered a helpful step in the workup and management of patients with perianal fistula until recently [3]. Despite limited early use, various imaging methods and modalities have been developed, including traditional fistulography, computed tomography, endoanal ultrasound imaging, and magnetic resonance imaging [10]. MRI has become the de facto standard for the imaging evaluation of perianal fistula due to its excellent soft tissue contrast, operator independence, multiplanar capabilities and superior field of view (FOV).
While the spectrum of MRI features of perianal fistulas has recently been well described in the literature, a divide between the radiological and surgical viewpoint still stands as evidenced by the continued use of two different classification schemes. The objective of this article is to review the clinically salient aspects of MRI interpretation of perianal fistulas through discussion of the indications for MRI, radiological vs. surgical classification schemes, and surgical treatment options for perianal fistula.
Magnetic Resonance Imaging
Sequence Selection
Multiple MRI imaging sequences have been described in the literature with varying levels of success for the detection and classification of perianal fistulae [3, 11–14]. For magnetic field strength, both 1.5-and 3-T magnets can be used for this purpose. The increased signal to noise ratio provided by the higher field strength can be used to shorten acquisition time and/or achieve higher spatial resolution [15]. The standard imaging approach at our institution (Table 1) utilizes a T2 weighted (T2W) imaging sequence in conjunction with a T1 weighted (T1W) contrast enhanced gradient echo (GRE) sequence, a combination recently suggested as the most useful for experienced readers [14]. Since the anal canal is tilted forward, the FOV and scan extent are defined using a sagittal T2W single shot image with centerline along the anal canal (Figure 1) and axial and coronal images are obtained relative to the long axis of the anal canal. Levator plate and the entire perineum should be included to evaluate the extent of disease as far inferior as the gluteal folds and as far superior as supralevator region. Frequency selective fat-saturated T2WI provides good spatial resolution and helps increase the conspicuity of T2 hyperintense fistulous tracks and small fluid collections. However, this can be replaced with Single Tau Inversion Recovery (STIR) sequence in the presence of extensive susceptibility artifact as can be seen with metallic hip replacements of extensive post-surgical suture material. Furthermore, 3D T2WI are also obtained providing thin slices, higher signal to noise ratio and above all the ability to reformat images in any plane without increase in scan time. Contrast enhanced T1WI helps assess the degree of inflammation and differentiation of scarring and granulation tissue, especially in patients with pelvic surgery and offers higher diagnostic confidence, especially in cases with equivocal fat saturation [16]. Additionally, seton positioning may be better visualized on the T1W GRE images. High-resolution subtraction MR-fistulography [17] and Diffusion Weighted Imaging [13] may complement MRI findings but are not routinely used at our institution for evaluating anal fistulas. Dynamic contrast-enhanced MR imaging has demonstrated significant correlation between time intensity curves, region of interest volume and Perianal Disease Activity Index and may have a promising role in the assessment of disease activity in perianal Crohn’s disease [18]
Table 1.
Standard anal fistula imaging protocol at our institution at 1.5T.
| Imaging Plane |
Sequence | FOV (cm) |
TR/TE | Slice thickness/ Slice gap (mm) |
Matrix |
|---|---|---|---|---|---|
| Sagittal | T2W Single Shot Turbo Spin Echo | 30 | 4500/130 | 4/5 | 320 × 163 |
| Axial | T2W High Resolution FS* Turbo Spin Echo | 18 | 5424/90 | 3/3.5 | 320 × 315 |
| Coronal Oblique | T2W 3D TSE† with variable flip angle | 25 | 1500/250 | 3/1.5 | 272 × 217 |
| Coronal Oblique | T2W High Resolution FS Turbo Spin Echo | 18 | 4138/90 | 3/3.5 | 288 × 283 |
| Coronal Oblique | T1W Turbo Spin Echo | 20 | 710/15 | 3/3.5 | 448 × 446 |
| Axial | T1W Volume Interpolated 3D GRE‡ Pre contrast | 20 | 5.2/2.5 | 3/3 | 272 × 272 |
| Axial | T1W Volume Interpolated 3D GRE Post contrast | 20 | 5.2/2.5 | 3/3 | 272 × 272 |
| Coronal Oblique | T1W Volume Interpolated 3D GRE Post contrast | 20 | 4.7/2.3 | 3/3 | 192 × 171 |
FS – fat saturated;
TSE – Turbo Spin Echo;
GRE – Gradient Echo
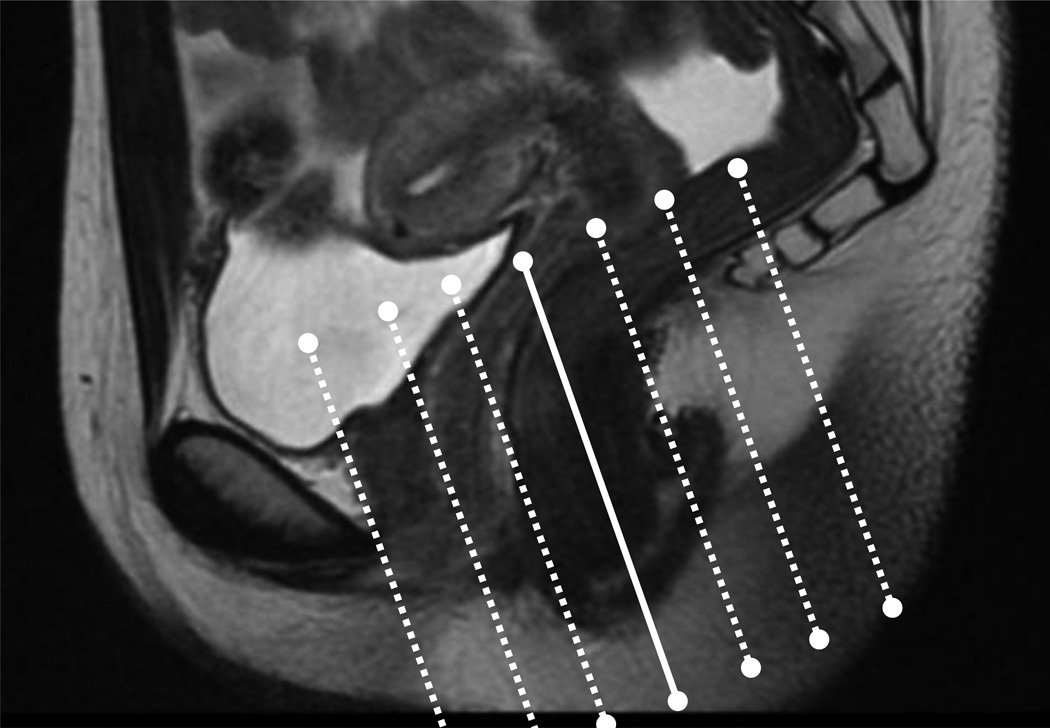
Fig 1.
The standard anal fistula MRI imaging protocol at our institution utilizes a tri-planar scout view and defines the extent of the scan along the sagittal images. The field of view is centered along the anal canal as shown.
Imaging Coils
Endoanal coils have demonstrated mixed results for detecting and classifying fistulae when compared to surface phased array coils, with some patients unable to tolerate coil placement and the limited FOV hampering the evaluation of complex disease [19, 20]. The use of the phased array coil remains standard of care at our institution and many other institutions, although some have advocated the use of both coils to improve diagnosis [19].
Anatomy
The function of the anal canal and associated anatomy is to maintain fecal continence. A basic understanding of the anatomy of the anorectal mechanism is useful to appreciate the common pathways of disease spread in perianal fistula.
The anal canal represents the terminus of the large intestine and measures between 2.5 to 5 centimeters in length [21]. Two muscular complexes, the internal and external sphincters, act in concert to provide the contractile effort needed for fecal continence, with the internal sphincter providing the majority of the resting tone [22]. As shown in Figure 2, the internal sphincter is essentially a continuation of the circular muscular wall of the rectum [21], while the external sphincter is functionally connected via the puborectalis muscle to the levator ani, which forms much of the muscular pelvic floor [21, 22]. The interstitial tissue between the two sphincters provides a pathway for the circumferential and axial spread of disease [23]. Penetration of both sphincters allows disease to enter the fat filled ischioanal fossa, while violation of the levator plate permits access between the superficial ischioanal fossa and pelvic pararectal space [24].
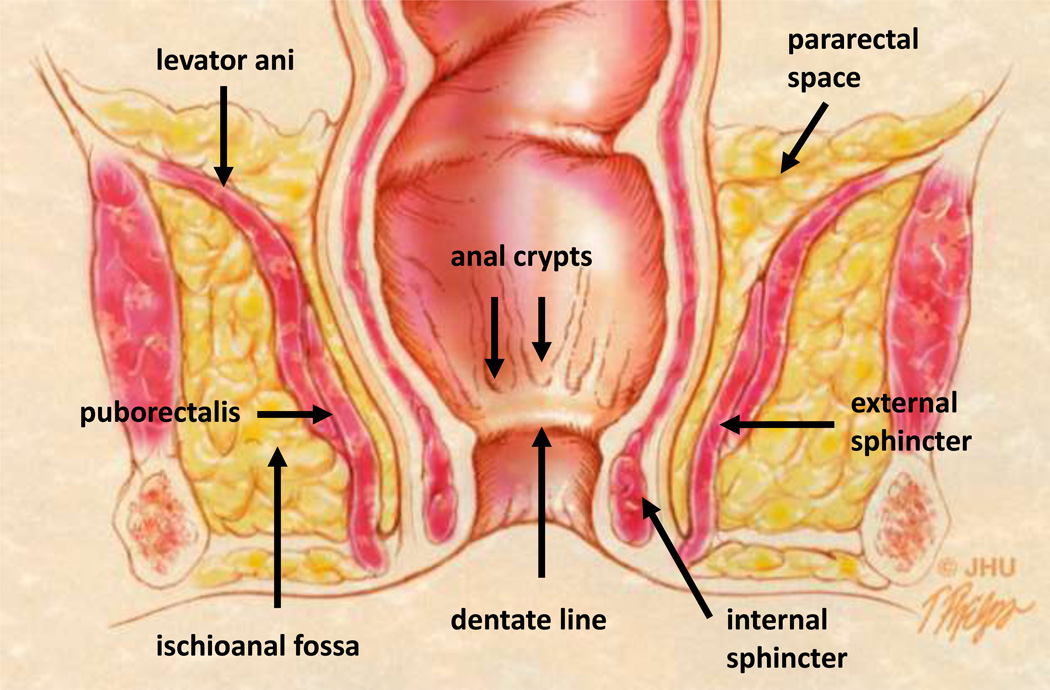
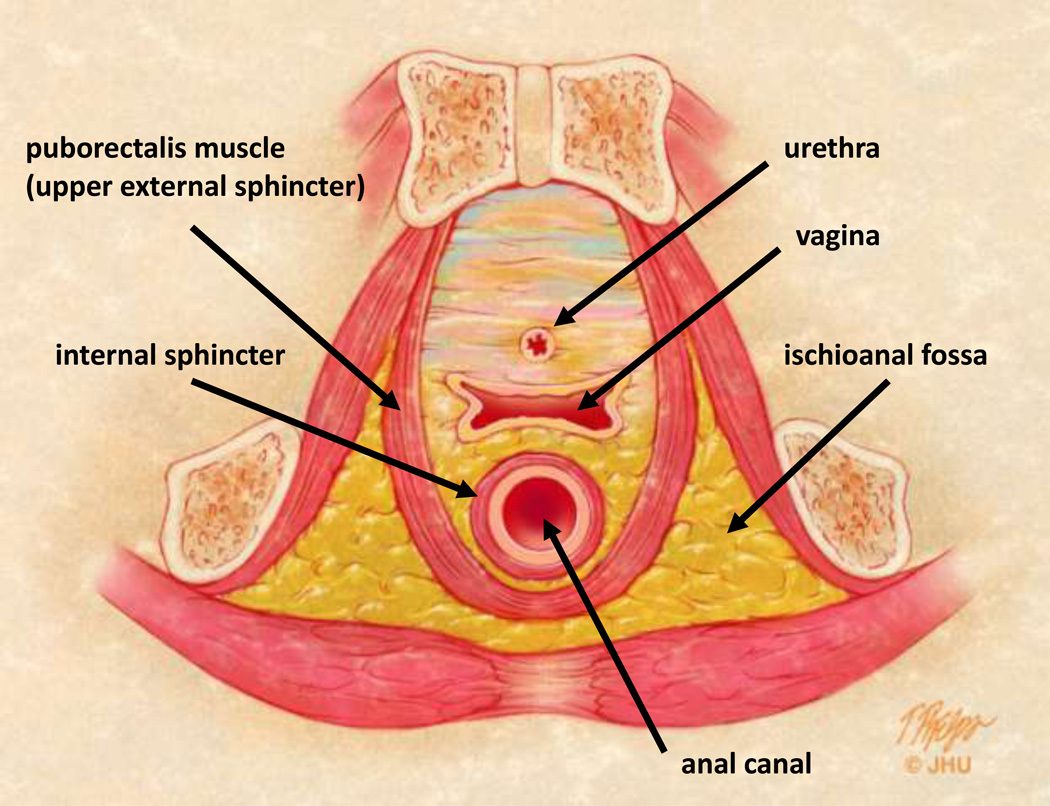
Fig 2.
Pelvic floor and anal canal anatomy.
2A – Coronal anatomic view of the pelvis. The anal glands are the starting location for cryptoglandular disease and emerge in the anal crypts along the dentate line. The interstitial tissue between the internal and external sphincters allows access to the pelvic compartment and pararectal space above the levator ani. The puborectalis muscle forms the superior aspect of the external sphincter and is functionally part of the levator ani muscle complex (17).
2B – Axial anatomic view of the female pelvis and uppermost anal canal mechanism. Cryptoglandular disease can spread circumferentially in the interstitial tissue between the internal and external sphincter. The fat filled ischioanal fossa can also become infected once both sphincters are breached.
MRI Features
For a broad anatomic overview, unenhanced T1 weighted images are ideal for anatomically delineating the sphincter complex, levator plate, and ischiorectal fossa. For evaluation of fistulous tracts, T2 weighted images demonstrate hyperintense fluid within the tract as contrasted to the hypointense fibrous wall of the fistula. T2 weighted images help differentiate the boundaries between internal and external sphincters because sphincters and muscles have low signal intensity while active tracks and extensions have high signal intensity. On gadolinium-enhanced fat suppressed T1 weighted images, fistulous tracts and active granulation tissue demonstrate intense enhancement while any fluid in the track is hypointense.
Chronic fistulous tracts or scars demonstrate low signal intensity on both T1 and T2 weighted images. There is lack of early enhancement of chronic fistulous tracts and scars on gadolinium enhancement images. Abscesses can demonstrate high T2 signal due to the presence of pus in the central cavity. On contrast enhanced fat suppressed T1 weighted images, abscesses demonstrate low signal intensity centrally with ring enhancement. On postoperative MRI, T1 weighted images demonstrate high signal intensity of hemorrhage products and can thereby help differentiate hemorrhage from residual tracks [25].
Classification of Perianal Fistulae
A Comparison of Surgical and Radiological Classification Systems
The archetypal surgical classification system developed by Parks, et al [24] is based on the physical exam of an experienced surgeon and remains a commonly used system for fistula classification. The classification reflects the extent of surgical intervention required for treatment based on the need to violate the sphincter mechanism and the location of the internal opening or source of sepsis. There are five classes listed in order of severity: superficial, intersphincteric, transphincteric, suprasphincteric, and extrasphincteric. Of note, Parks excluded patients with fistulizing Crohn’s disease when generating his original schema [24].
The radiological equivalent of the Parks classification system is known as St James’ University Hospital (SJUH) grading scheme [3, 28] and is based upon the MRI imaging appearance of perianal fistulae in the axial and coronal planes. The added feature of this classification is the identification of secondary extensions as well as complications such as an abscess. This system recognizes six types: Grade 0 refers to a normal appearing anal canal; Grade 1 represents a simple intersphincteric fistula, while Grade 2 represents an intersphincteric fistula with a secondary tract or abscess; Grade 3 fistula refer to simple transphincteric fistulae, while Grade 4 represents a more complicated transphincteric process with a secondary tract or abscess; finally, a Grade 5 fistula represents a complicated abscess with a supra or translevator component.
Superficial fistulae do not penetrate the sphincter mechanism but travel between the mucosal surface and the internal sphincter muscle [26]. This classification accounts for approximately 16% of patients presenting to a major referral center [27]. Interestingly, superficial fistulae were not included in the original classification system [24].
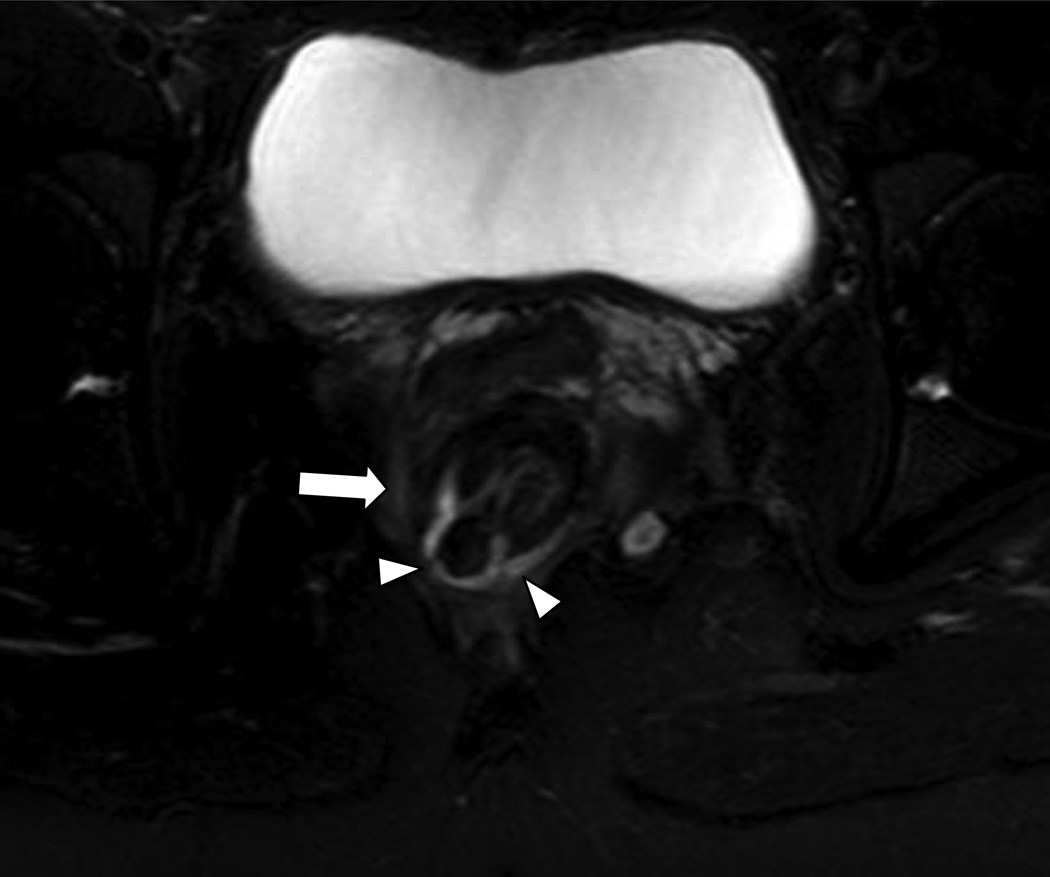
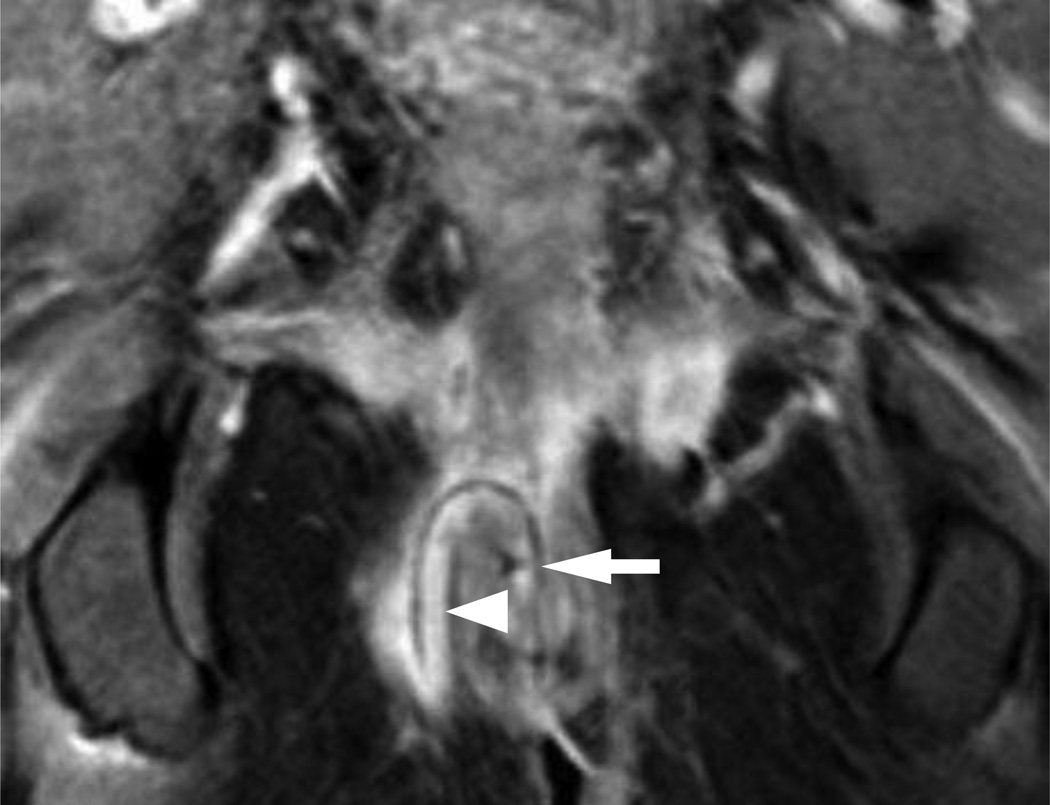
Intersphincteric fistulae are the most common fistulae-in-ano, accounting for 54% of patients present into a major referral center [27]. Intersphincteric fistulae (Figures 4–6) penetrate the internal sphincter mechanism to course between the internal and external sphincter before exiting the perineum [24, 26]. The SJUH further subdivides this category into Grade 1 and 2 based on the absence or presence of a secondary tract or an abscess (Figure 3). In grade 1, the fistulous track is seen between the 2 sphincters as it exits through the perineum (Figure 4). Grade 2 fistula may be associated with a secondary tract without violating the external sphincter (Figure 5) or an associated abscess (Figure 6).
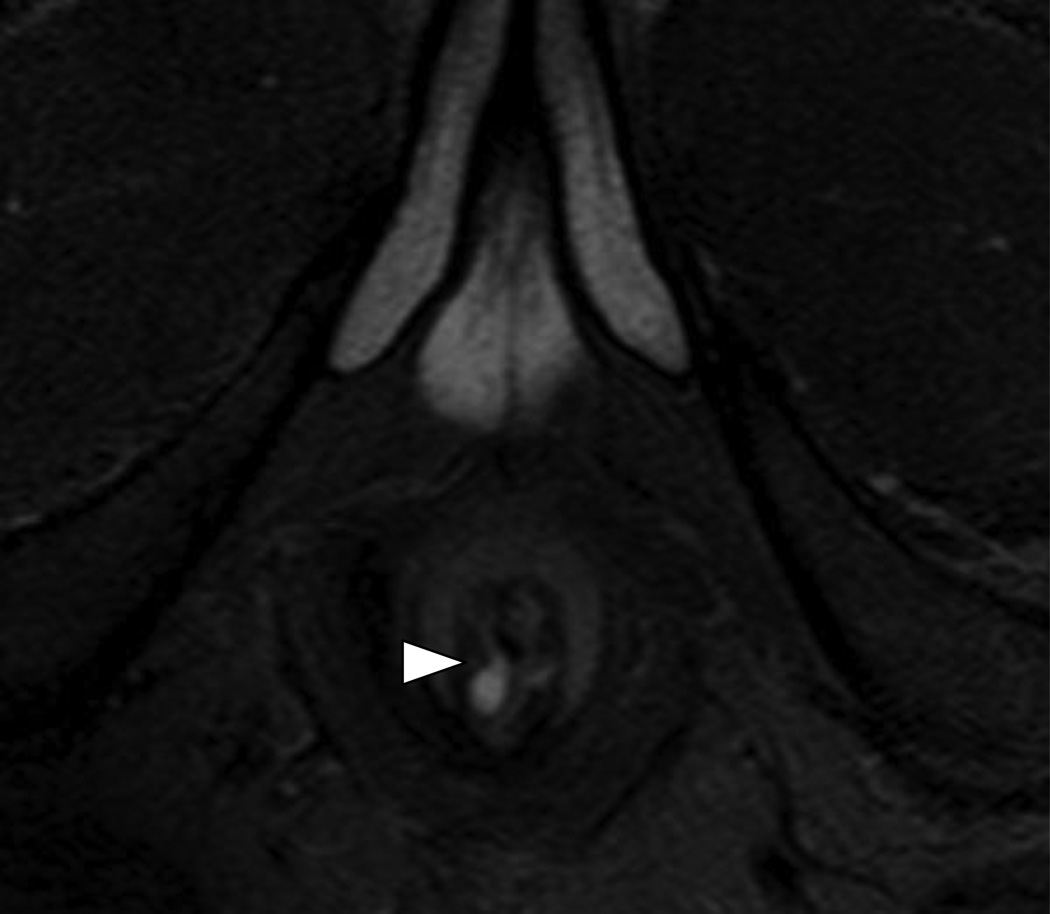
Fig 4.
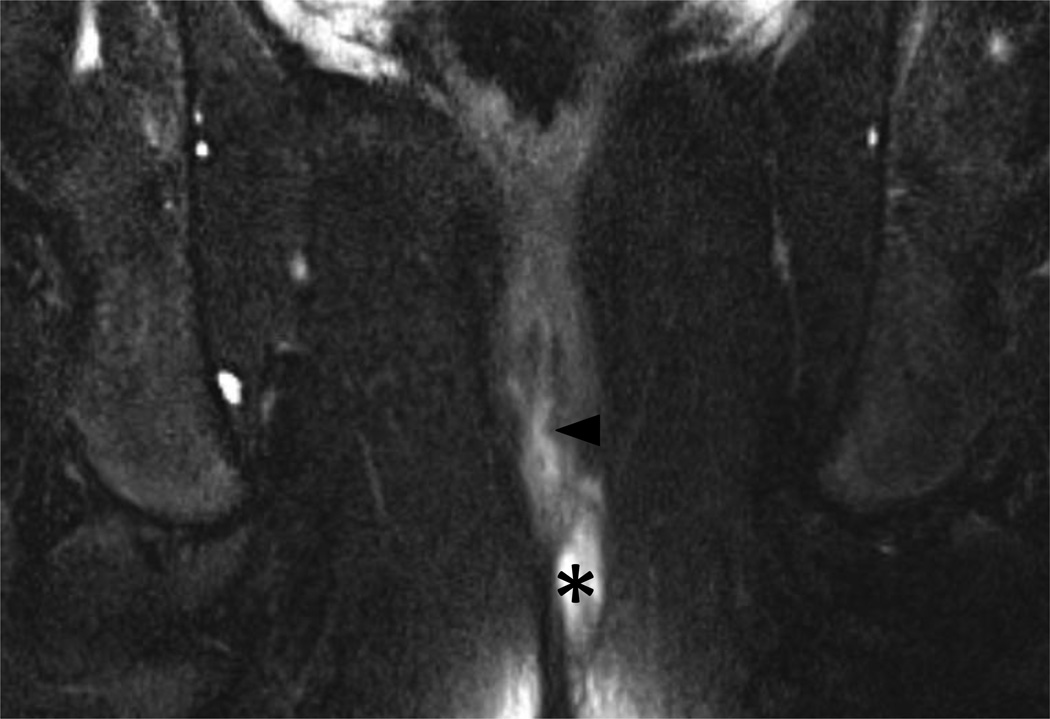
49 year-old male with Grade 1 perianal fistula due to prior perirectal abscesses.
4A – Axial T2W fat suppressed view (TR/TE in milliseconds (ms); 5542/80 ms) of the anal canal at the level of the penis base demonstrating the origin of a Grade 1 (simple intersphincteric) fistula (arrowhead) exiting the internal sphincter.
4B – Coronal T2W fat suppressed view (3347/100 ms) of the anal canal demonstrating the tract of a Grade 1 (simple intersphincteric) fistula (arrowheads) interposed between the internal (single arrow) and external (two arrows) sphincter mechanism.
Fig 6.
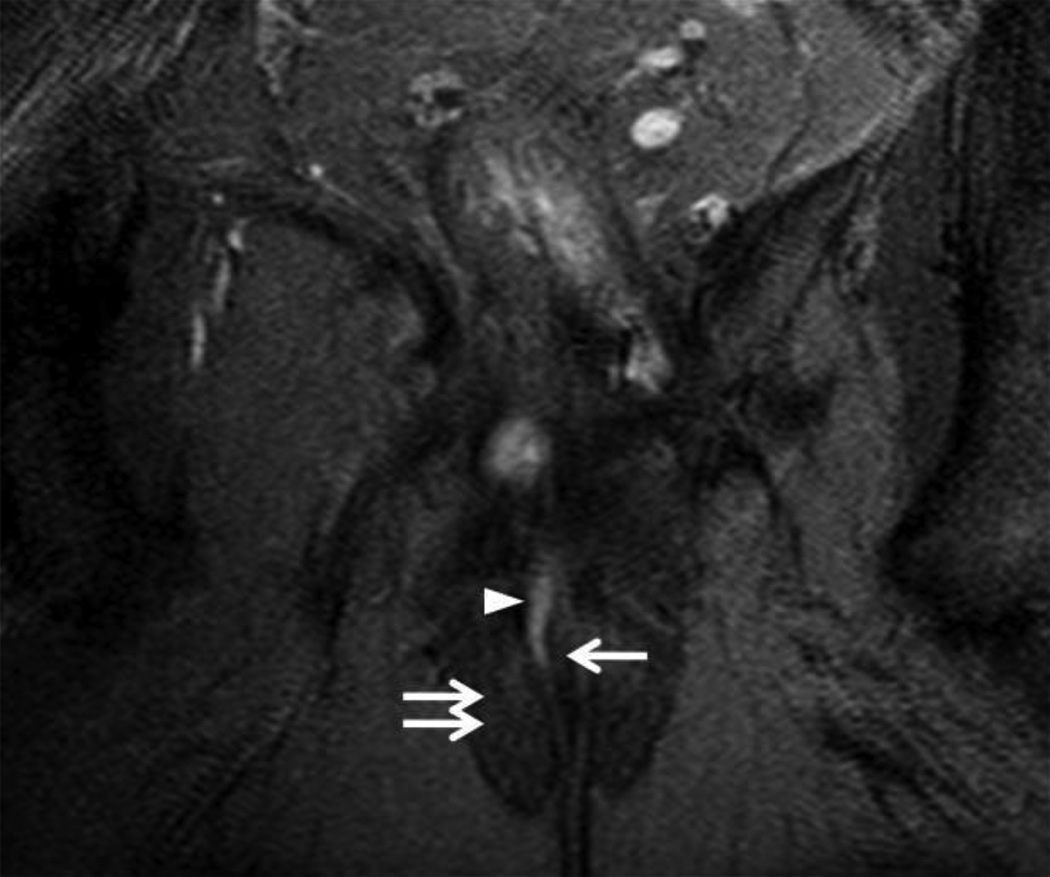
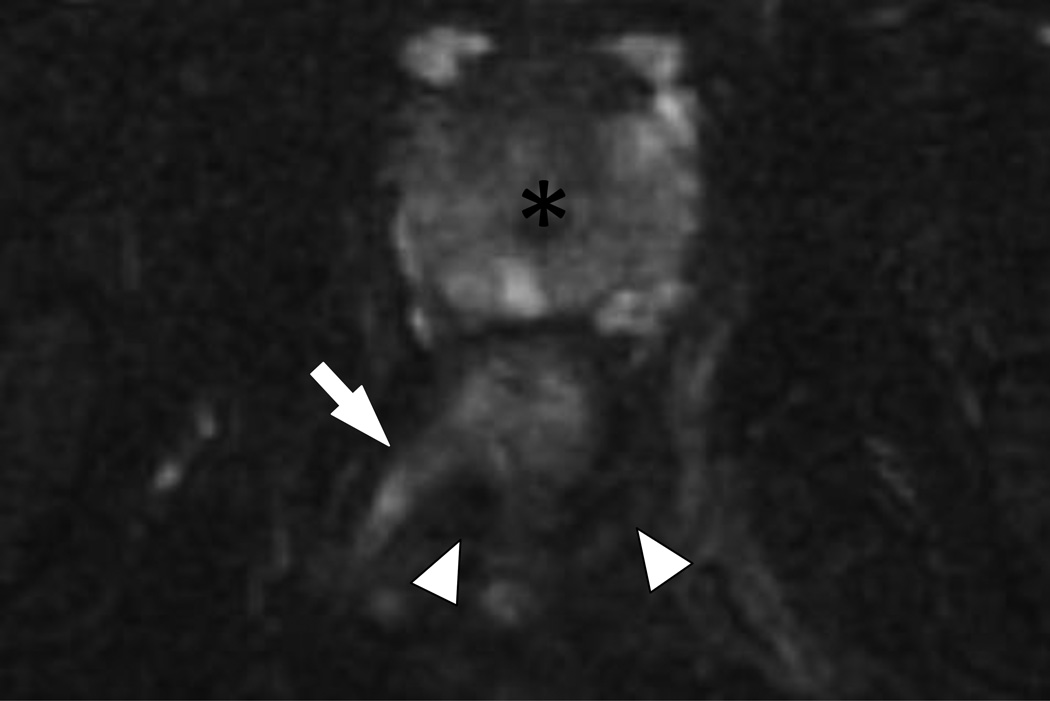
18 year-old male with ileocolonic Crohn’s disease and Grade 2 (complex intersphincteric) perianal fistula.
Coronal fat suppressed T2WI (6750/102 ms) of the anal canal. The inferior extent of a Grade 2 (complex intersphincteric) fistula and abscess is seen (arrowhead) as is the perianal abscess (asterisk).
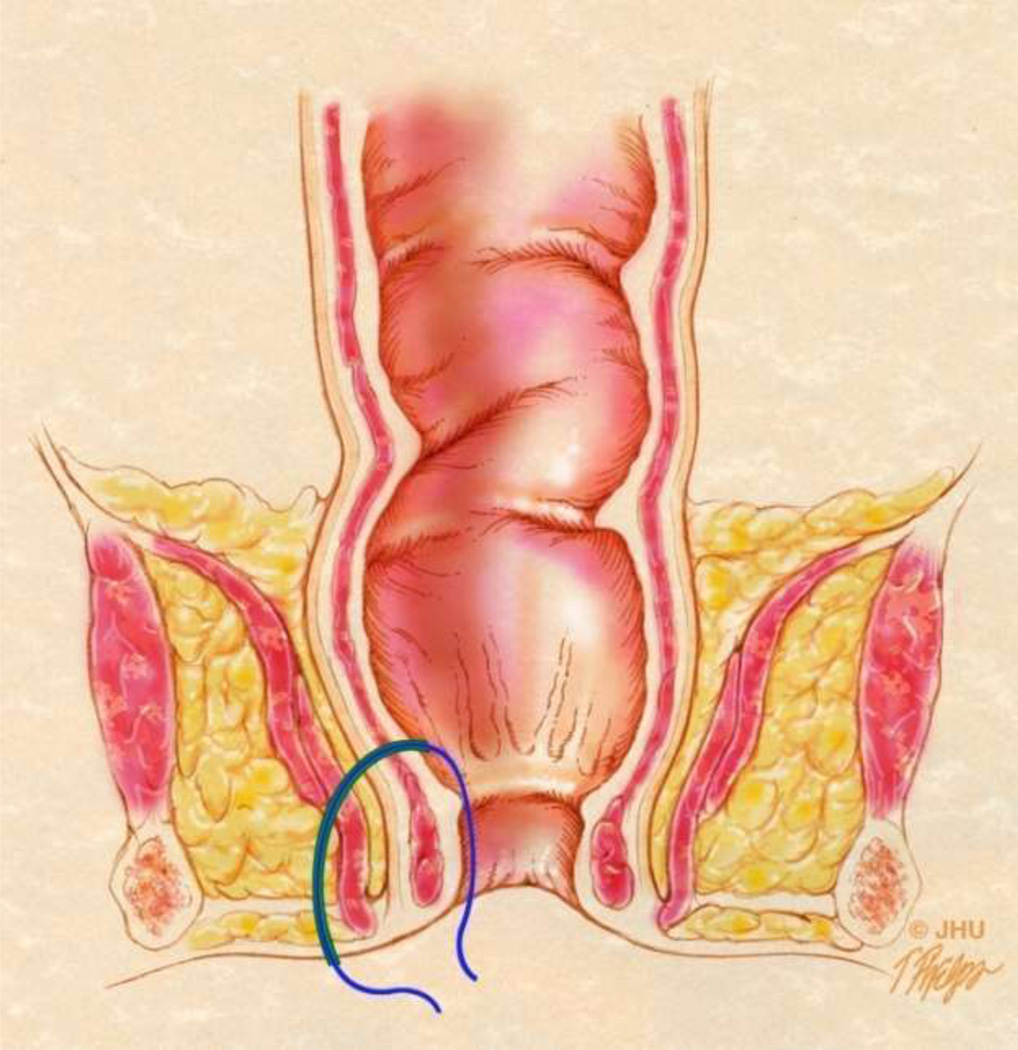
Fig 3.
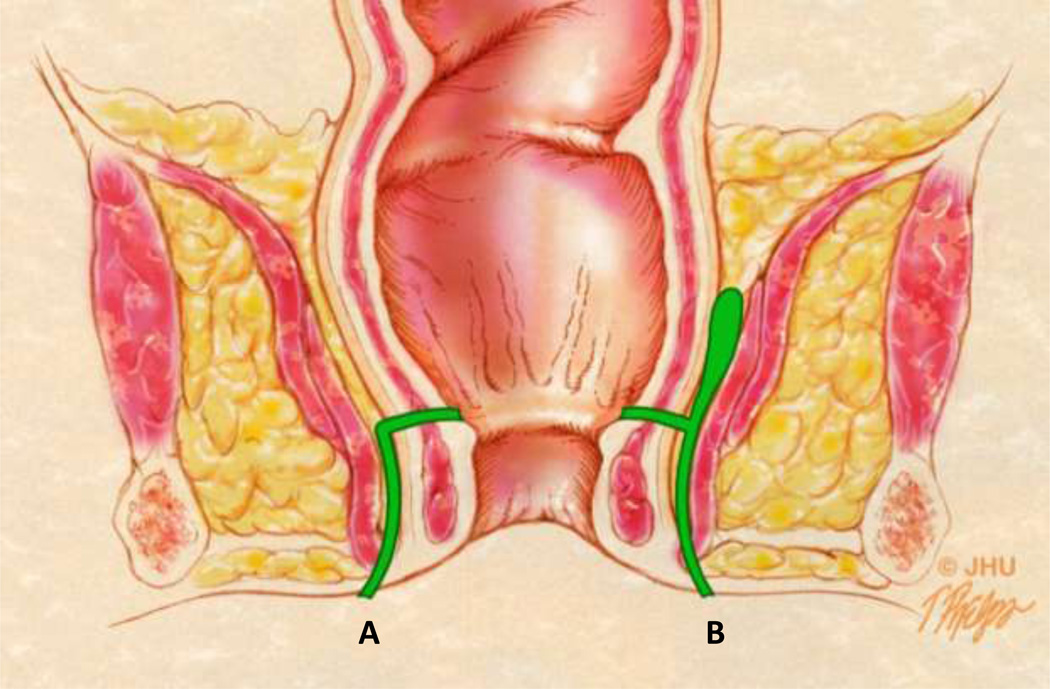
Anatomic drawings of the various types of perianal fistulae using both the St. James and Parks classification systems.
3A – Coronal anatomic view showing different fistulae in green. The tract labeled A represents a Grade 1 simple intersphincteric fistula in the St. James University Hospital (SJUH) classification system. The tract labeled B represents a Grade 2 or complex intersphincteric fistula. Both would be considered intersphincteric fistula in the Parks classification system.
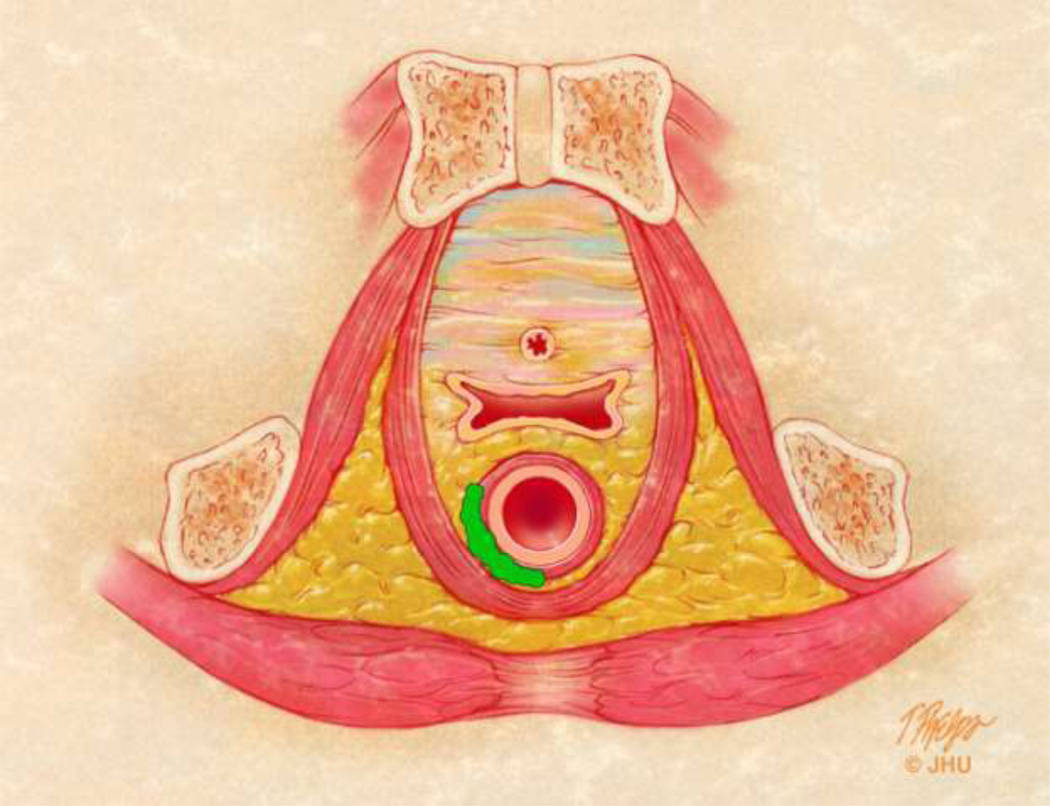
3B – Axial anatomic view of the female pelvis with an example of a horseshoe abscess interposed between the internal and external sphincter mechanisms. Intersphincteric abscesses can be associated with complex intersphincteric fistulae (grade 2). This can also be seen in association with complex transphincteric fistulae (grade 4), and fistulae with supralevator disease (grade 5).
Fig 5.
26 year-old female with Crohn’s disease and horseshoe pattern Grade 2 fistula
Axial T2W high resolution view (5200/80 ms) of the lower pelvis reveals a fistulous track between in the internal and external (arrow) sphincters in a “horseshoe” pattern extending from the 3:30 to 9:30 clock positions (arrowheads).
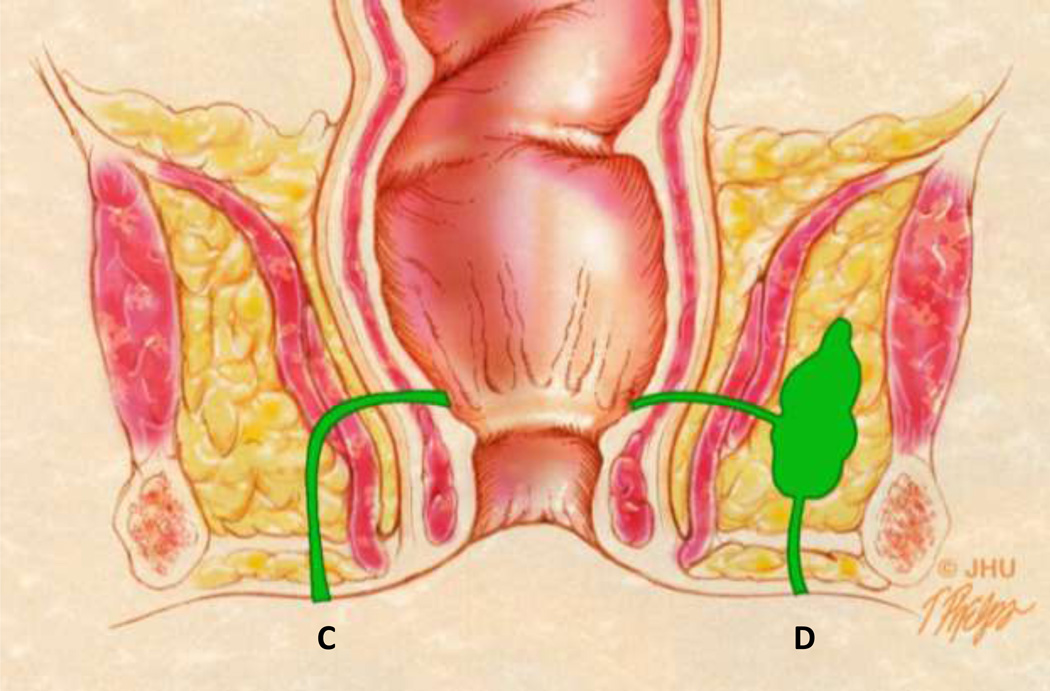
Transphincteric fistulae violate both sphincters before exiting and are less common than the intersphincteric variety, accounting for approximately 21% of patients [27]. Transphincteric fistulae are of 2 variety in the SJUH classified as Grade 3 fistula, a simple transphincteric fistulae, and Grade 4 fistula representing a transphincteric process with a secondary tract or abscess (Figure 7). Grade 3 fistula violates the external sphincter and is not complicated by secondary abscess in the area (Figure 8). Grade 4 fistula is similar to a grade 3 fistula with an associated secondary tract or an abscess (Figure 9).
Fig 7.
Coronal anatomic view showing different fistulae in green. The tract labeled C represents a Grade 3 (SJUH) simple transphincteric fistula. The tract labeled D represents a Grade 4 or complex transphincteric fistula. Both would be considered transphincteric fistula in the Parks classification system.
Fig 8.
49 year-old male with prior perirectal abscess presenting with a Grade 3 (simple transphincteric) perianal fistula. The fistula would also be a transphincteric fistula in the Parks’ classification system.
Axial T2W fat saturated image (4250/107) of the lower pelvis shows a simple transphincteric fistula arising from the right aspect of the superior anal canal (arrow) violating the external sphincter (arrowheads). Prostate (*).
Fig 9.
25 year-old woman with Grade 4 (complex transphincteric) perianal fistula due to prior perirectal abscess.
Post contrast T1W GRE MRI image (6.35/1.53 ms; flip angle 13°) through the lower pelvis and anal canal with rim enhancing abscess (arrow) with a transsphincteric grade 4 fistula penetrating the external sphincter on the left (arrowhead). The vagina (asterisk) is seen anterior to the rectum.
Suprasphincteric fistulae, like intersphincteric fistulae, violate the internal sphincter but course upward along the circumferential layer to enter the pararectal soft tissues before traversing the levator muscle [24, 26] (Figure 10). These rare fistulae account for approximately 3% of cases [7].
Fig 10.
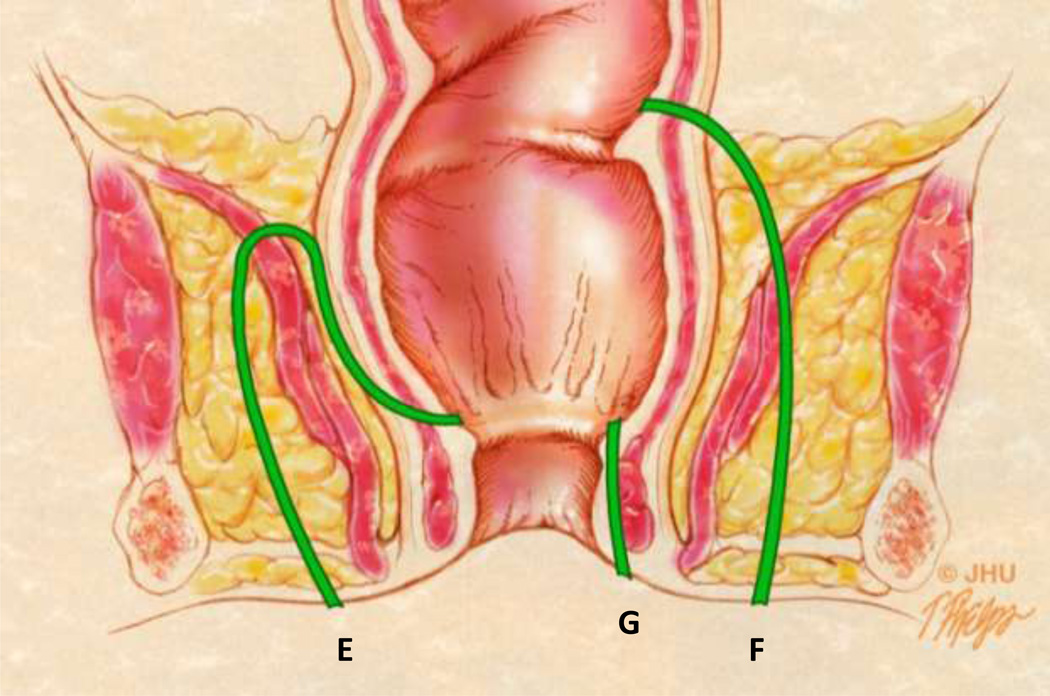
Coronal anatomic view showing different fistulae in green. The tract labeled E represents a suprasphincteric fistula in the Parks classification system. The tract labeled F represents an extrasphincteric fistula. Both would be considered Grade 5 fistula in the SJUH classification system due to the presence of supralevator disease. The tract labeled G is a superficial fistula which does not violate any of the sphincter muscles and is part of a modified Parks classification system.
Extrasphincteric fistulae do not penetrate the sphincter mechanism but arise above the levator from the rectum or other source of sepsis [24, 26] (Figure 10). These rare lesions (accounting for approximately 3% [27]) exit the perineum after penetrating the levator and ischioanal fossa. Both suprasphincteric and extrasphincteric fistulae are classified as grade 5 in the SJUH grading system (Figure 11). Grade 5 fistulae represents a complicated abscess with a supra or translevator component and are mostly due to the presence of pelvic disease with infralevator extension. In practice, most patients with severe disease present with a combination of fistulae of different grades (Figure 12). In such cases a systematic approach in the description of these fistulae with categorization into different grades helps in accurate description at the time of diagnosis and facilitates the evaluation of response to therapy on the follow up examinations.
Fig 11.
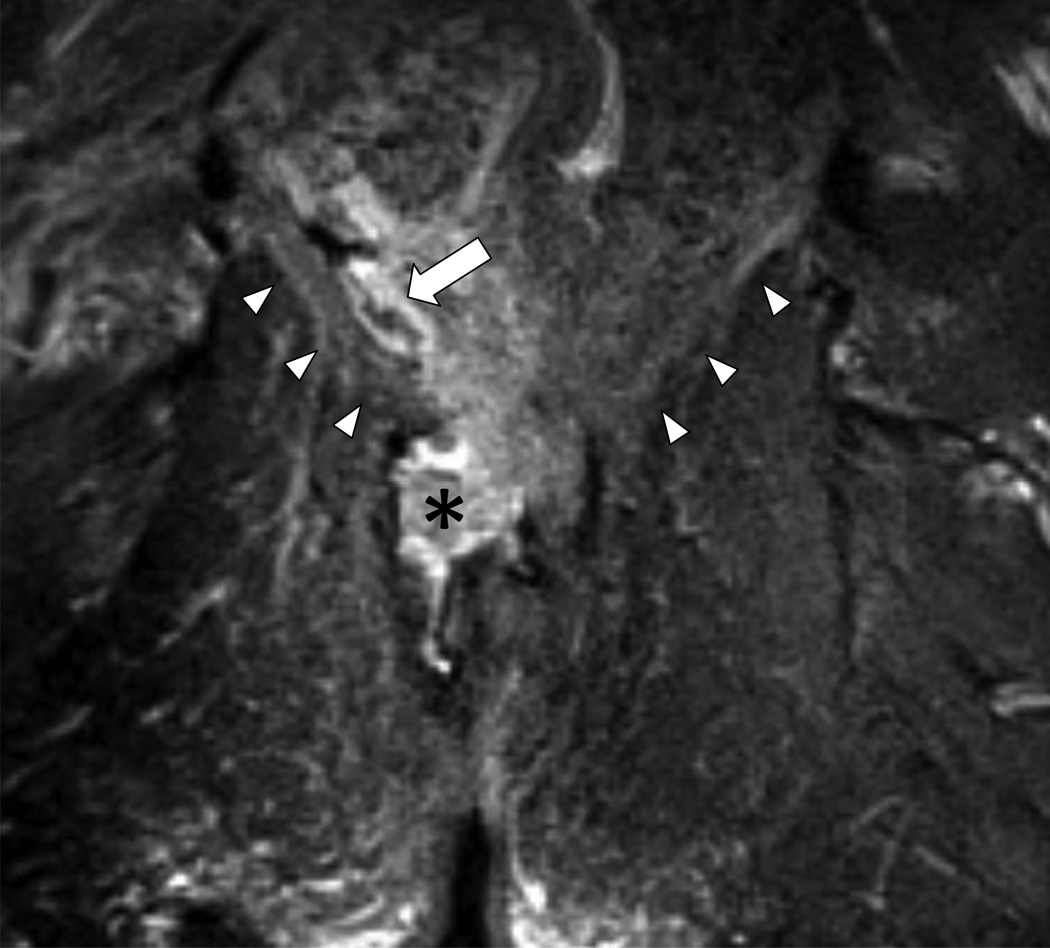
45 year-old man with Grade 5 perianal fistula (suprasphincteric in Parks’ classification system) due to prior perirectal abscess.
T2W fat suppressed coronal MRI (5870/84 ms) through the lower pelvis and anal canal with hyperintense fluid collection and edema above (arrow) and below (asterisk) the levator plate (arrowheads). The supralevator component classifies the fistula as a Grade 5.
Fig 12.
26 year-old female with Crohn’s disease and multiple fistulae, including a Grade 4 (complex transphincteric) and Grade 5 (suprasphincteric) perianal fistula.
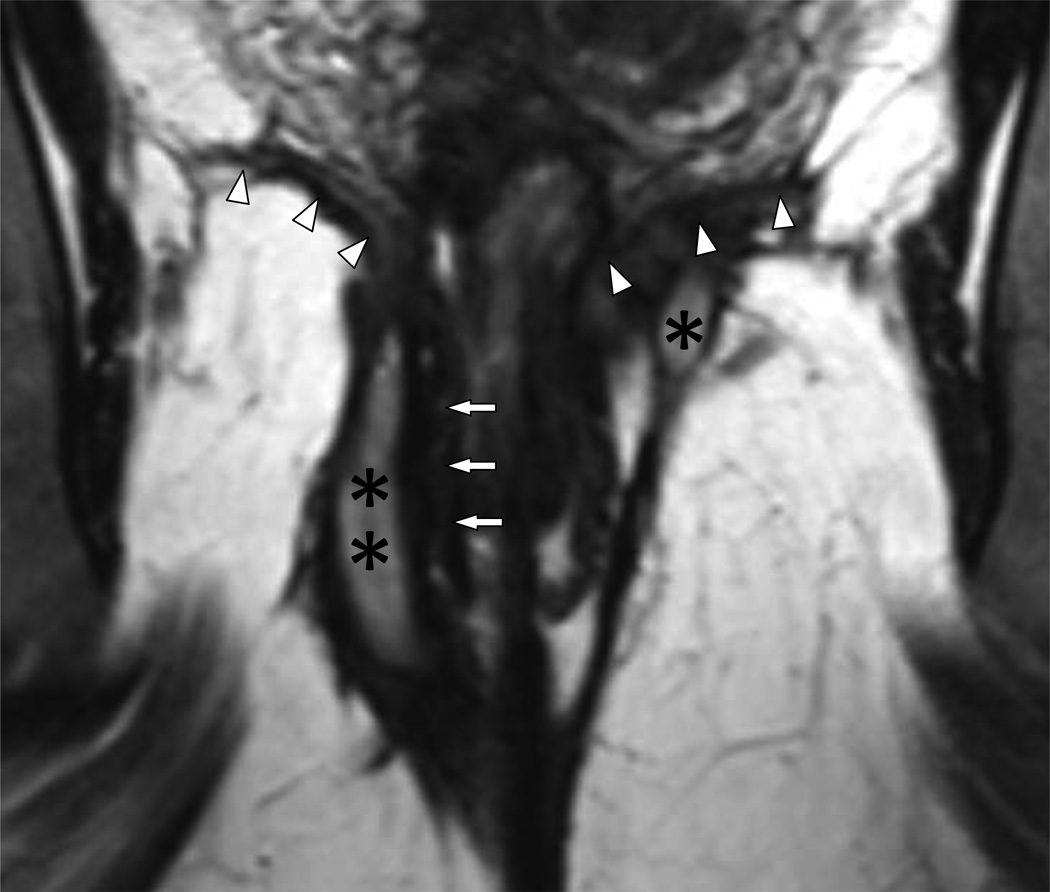
12A – Coronal 3D turbo spin echo view (1874/200 ms) of the lower pelvis with no fat saturation reveals a well-defined fistulous tract and a collection in the ischioanal fossa (double asterisk) outside of the external sphincter (arrows) on the right side and a small abscess (asterisk) beneath the levator plate (arrowheads) on the left qualifying as Grade 4 fistulae.
12B – Coronal fat suppressed T2WI of the lower pelvis (5457/100 ms; 40 mm slab MIP) demonstrating the extent of the complex perianal fistula and abscess. There is extensive disease with supralevator extension (asterisks) above the levator plate (dotted lines) making this Grade 5 disease. Transphincteric setons are seen on both sides (arrows). Also note the improved visualization of the fistulous tracks and the collections on the fat saturated images.
Diagnostic and Clinical Outcome
As multiple medical and surgical treatment options exist, imaging plays a critical role in accurately characterizing perianal fistulas to individualize management strategy. Differences in the classification scheme have been shown to have an impact on prediction of prognosis. Imaging options include fistulography, computed tomography (CT), anal endosonography, and MRI.
MRI classification of perianal fistulae has been significantly associated with clinical outcome, with MRI grades differing significantly between satisfactory and unsatisfactory outcomes [3]. Morris et al reported that in their clinical experience using the St James's University Hospital classification, MR imaging grades 1 and 2 were associated with satisfactory outcome whereas grades 3–5 were associated with unsatisfactory outcome such as need for further surgery (P < 0.001) [3].
MRI evaluation of perianal fistula has also revealed additional diagnostic information in the preoperative setting, especially for complicated disease [29], demonstrated superiority in treatment outcomes over anal endosonography and preoperative digital rectal examination (DRE) [30], and improved outcomes for the surgical treatment of primary [37] and recurrent disease [32]. Beets-Tan et al reported that preoperative MR imaging provided important additional information in 12 of 56 patients with anal fistulas (21%) [29]. This was further subdivided as 4 of 17 patients with recurrent fistulas (benefit in 24%) and 6 of 15 patients with Crohn’s disease (benefit of 40%). In a study of 104 patients with suspected anal fistula by Buchanan et al [30], clinical examination correctly classified the fistula track in 66 patients (61%) compared to 87 patients (81%) by endosongraphy and 97 patients (90%) by MR imaging. In primary fistula in ano, preoperative MRI was shown to have a therapeutic impact in 10% of cases in a prospective study of 30 patients [31]. In recurrent fistula-in-ano, preoperative MRI was shown to have a therapeutic impact with decreased recurrence rates in 75% of cases in a study of 71 patients [32]. Overall, MRI has been identified as the modality of choice for preoperative evaluation of patients with perianal fistula [25].
MRI evaluation and classification of perianal fistulae can be standardized with a high degree of diagnostic accuracy [34], therefore reducing interobserver variability. A prospective study by Beckingham et al comparing digital rectal examination, dynamic contrast enhanced MRI, and surgical exploration in forty-two patients reported a sensitivity of 97% and specificity of 100% for detection of fistulas by dynamic contrast enhanced magnetic resonance imaging [33]. Preoperative evaluation with MRI not only identifies and anatomically delineates the course of perianal fistulas, it also aids in detection of secondary tracts and abscesses [25].
Treatment
Management of perianal fistulae often depends on whether the fistula is due to cryptoglandular disease versus fistulizing Crohn’s disease, the complexity and location of fistula (low versus high) and whether it represents a recurrent process. Crohn’s disease often requires a combined medical and surgical approach, using steroids and immunomodulatory agents in conjunction with palliative surgical procedures such as seton placement [35–37] to permit subsidence of inflammation. Definitive surgical treatment includes fistulotomy or cutting setons for low, simple fistulae versus mucosal advancement flaps and surgical variants for more complex fistulae [35].
While medical management is important in the treatment of perianal fistula, most cases require surgical intervention. Unfortunately, success rates of surgical intervention are low with a high rate of recurrence, especially in Crohn’s disease patients. The risk of post-operative fecal incontinence is also a major consideration. Surgical options include fistulotomy, fistulectomy, noncutting seton, mucosal advancement flap repair, proctectomy, and biomaterial treatment strategies [38].
Fistulotomy
Fistulotomy is the most commonly used surgical procedure for treatment of perianal fistula and is also known as the laying open technique. It involves creating an incision in the fistula tract to open it and merging it with the anal canal in order to allow the fistula to heal. Generally, it is used for intersphincteric fistula and low transsphincteric fistula. In these cases, there is a low risk of fecal incontinence. Employing the laying open technique for complex perianal fistulas, however, results in fecal incontinence in over 50% of cases. Marsupialization of fistulotomy wounds has been reported to improve healing [38].
Fistulectomy
Fistulectomy entails excision of the complete fistula tract. It creates a larger wound than fistulotomy. This technique is commonly used for intersphincteric or low transsphincteric perianal fistulae. A similar rate of recurrence exists with fistulectomy as compared with fistulotomy. Marsupialization of the wound has also been shown to improve healing [38].
Noncutting seton
Noncutting setons are preferred as an alternative to fistulotomy and fistulectomy which can both result in non-healing wounds. Setons can be composed of silk, nylon, polyester, rubber, silicon, plastic, wire, and self-locking cable ties. Setons prevent the closure of the external opening of the fistula which allows drainage and thereby helps to prevent abscess recurrence (Figure 13). Removal of the seton, however, has been shown to result in fistula recurrence in 39% of cases. The rate of fecal incontinence is 5% [38].
Fig 13.
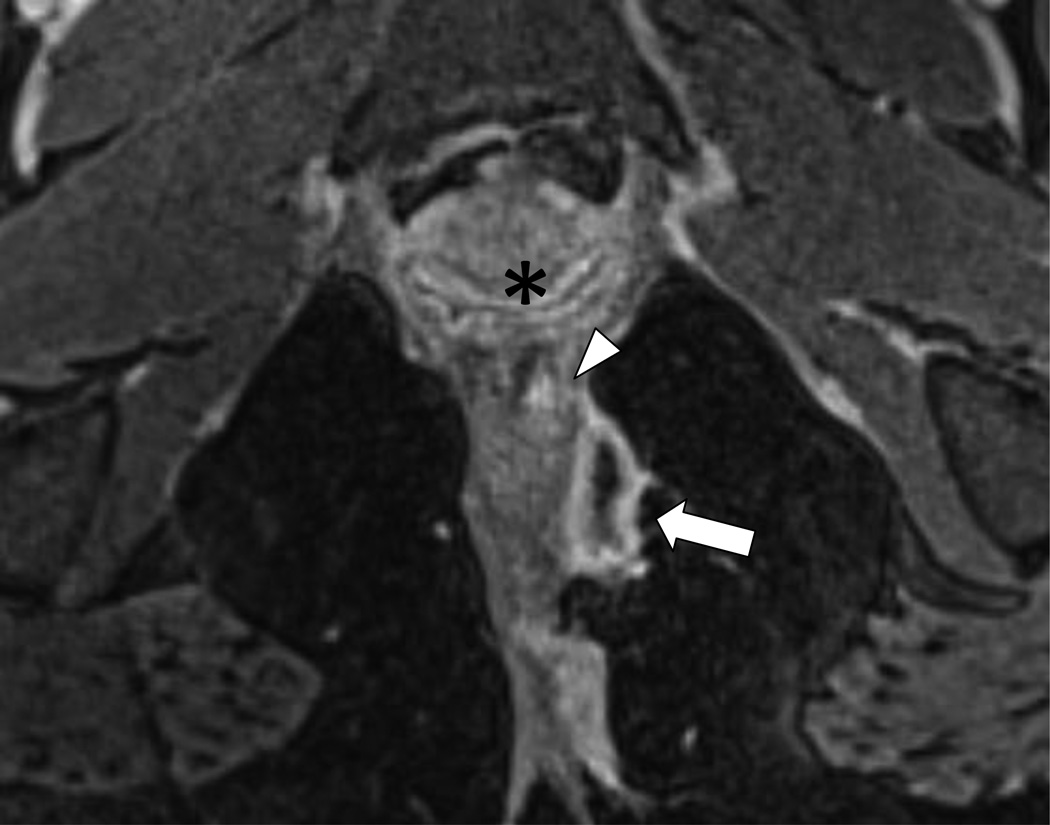
Seton placement through a Grade 3 (SJUH) simple transphincteric fistula.
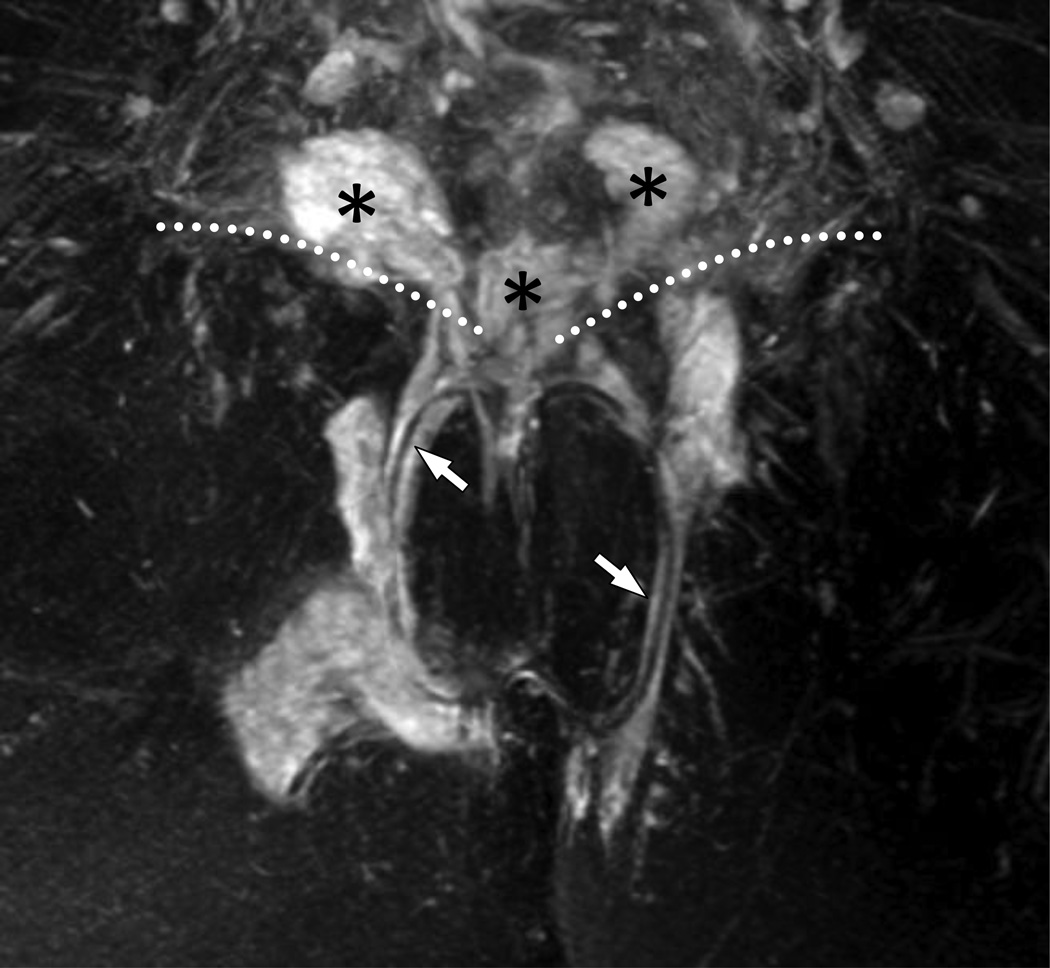
13A – Coronal anatomic drawing demonstrating seton placement through a Grade 3 (SJUH) simple transphincteric fistula
13B – Coronal post contrast T1W GRE MRI (5.76/2.83 ms; flip angle 12°) images demonstrate a seton (white arrow) through a Grade 3 (SJUH) simple transphincteric fistula. External sphincter (arrowhead).
Mucosal advancement flap repair
Mucosal advancement flap repair is the preferred treatment choice for complex perianal fistula. This technique involves fistulectomy with excision of a flap of mucosal tissue around the internal opening of the fistula. The flap is then used to close the fistula tract via suturing (Figure 14). Mucosal advancement flap repair has been shown to be successful in healing perianal fistulae in 55–98%. There is a recurrence rate, however, of 57% in Crohn’s patients and 33% in non-Crohn’s patients at 40.3 month follow-up [38].
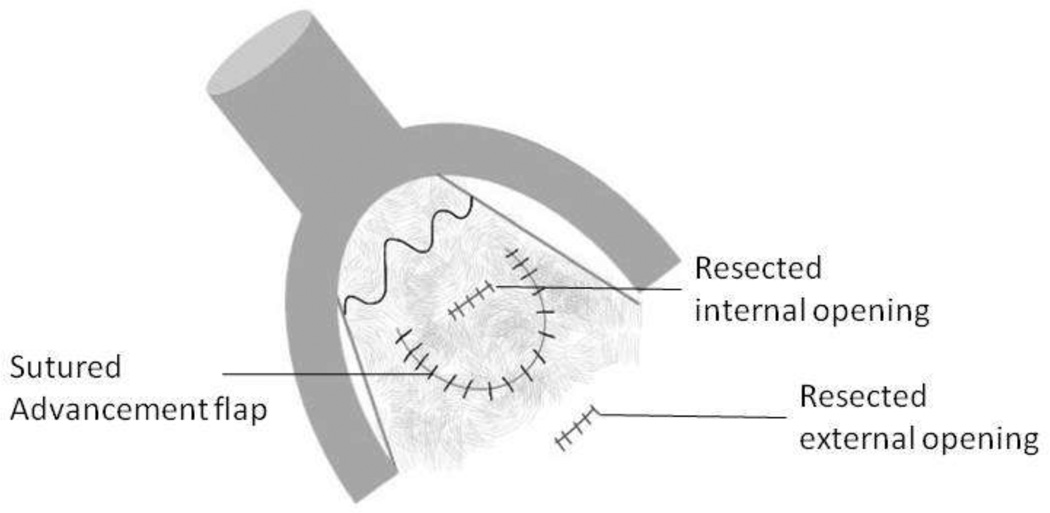
Fig 14.
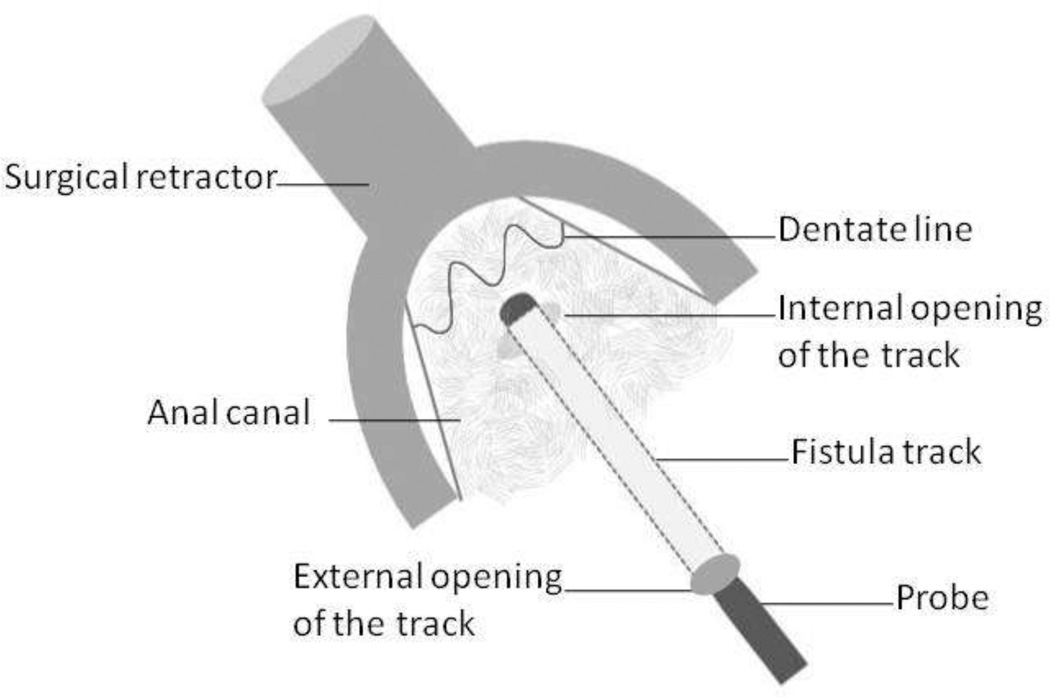
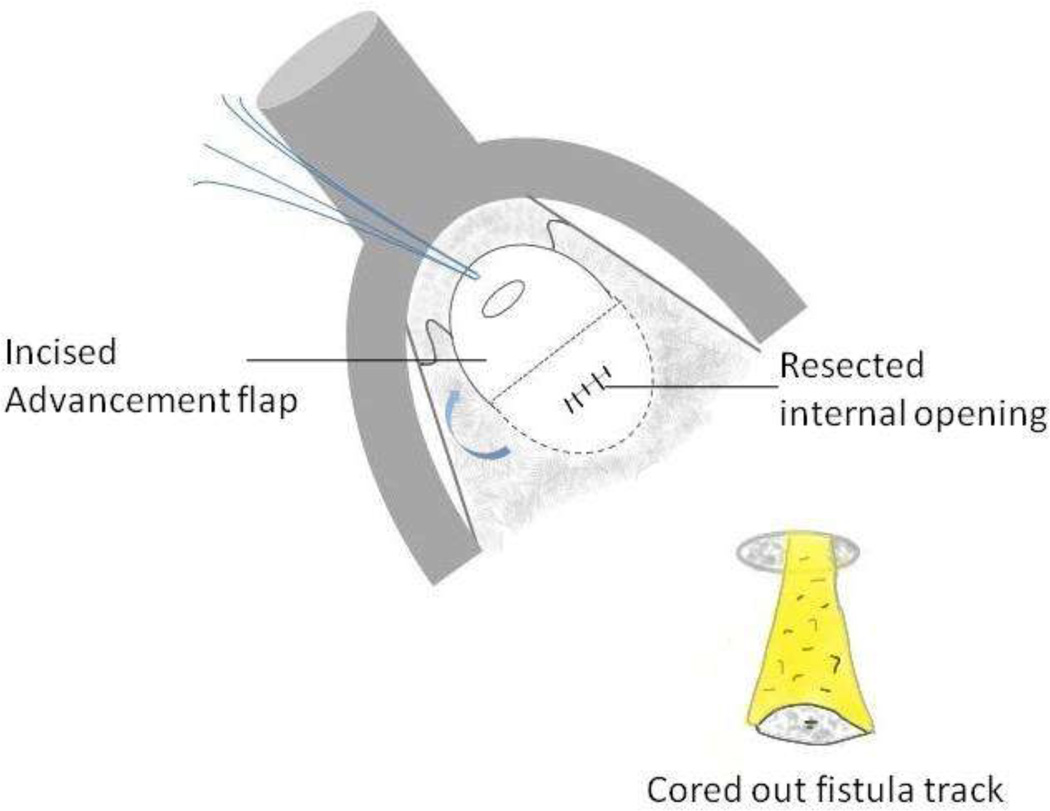
Diagrammatic representation of endorectal advancement flap for repair of an anal fistula.
14A – Internal opening of the fistula is identified using a probe.
14B – The fistula track is cored out. A flap of tissue is then incised around the internal opening of the fistula.
14C – The flap of tissue is then sutured to cover the resected internal opening.
Proctectomy
Proctectomy is often used for treatment of extrasphincteric fistula in Crohn’s patients. It is required in 10–15% of patients and up to 4% of patients may even require proctocolectomy with creation of permanent ileostomy [38].
Biomaterials
More recently, biomaterials have been utilized as potential alternative treatments for perianal fistulae. Biomaterials entail biological glues and collagen-based materials. Fibrin glues, for example, are a tissue adhesive with a fibrinogen component and a thrombin component. The two components are injected into the fistula simultaneously and react to form a fibrin clot. This acts as a sealant for the fistula tract and the fibrin clot has been reported to enhance wound healing. Although the initial success rates were shown to be 60–85%, delayed recurrences were found to occur. Studies in Crohn’s disease patients demonstrated lower success rates. BioGlue, a surgical adhesive made of purified bovine serum albumin and glutaraldehyde, has also been studied with conflicting results. Alternatively, several collagen-based materials have been studied as potential treatment for perianal fistula. One of the most successful collagen-based materials has proven to be small intestine submucosa [SIS]. Promising results led to the development of the Surgisis anal fistula plug (AFP) which is composed of lyophilized porcine submucosa. This cone-shaped plus can be inserted into a fistula tract and sutured in place at both ends. Efficacy rates have been shown to be high with closure of 87% of fistulae, comparable to advanced flap repair. Healing rates, however, have been variable with a reported range of 24–87% [38].
Conclusion
Perianal fistulae is a clinical entity with significant patient morbidity. While multiple surgical options exist, recurrence rates and the risk of fecal incontinence are important considerations in management strategy. MRI provides information about the fistulae with great anatomic detail with respect to secondary tracks and abscesses as well as the surrounding pelvic organs. The use of MRI for the identification and classification of perianal fistulae can provide essential information that has been shown to have both preoperative and prognostic value. Although different surgical and radiological classification schemes for perianal fistula are currently utilized, bridging the divide may contribute to improved patient care. Correct identification of perianal fistulae and an appreciation for common management approaches are useful for effective radiological-surgical communication.
Acknowledgments
Acknowledged support:
NIH NIBIB award T32 EB006351 entitled Training for Clinician Scientists in Imaging Research (KLG)
References
- 1.Zanotti C, Martinez-Puente C, Pascual I, Pascual M, Herreros D, García-Olmo D. An assessment of the incidence of fistula-in-ano in four countries of the European Union. Int J Colorectal Dis. 2007 Dec;22(12):1459–1462. doi: 10.1007/s00384-007-0334-7. [DOI] [PubMed] [Google Scholar]
- 2.Sainio P. Fistula-in-ano in a defined population. Incidence and epidemiological aspects. Ann Chir Gynaecol. 1984;73(4):219–224. [PubMed] [Google Scholar]
- 3.Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000 Jun;20(3):623–635. doi: 10.1148/radiographics.20.3.g00mc15623. [DOI] [PubMed] [Google Scholar]
- 4.Hamadani A, Haigh PI, Liu I-LA, Abbas MA. Who is at risk for developing chronic anal fistula or recurrent anal sepsis after initial perianal abscess? Dis. Colon Rectum. 2009 Feb;52(2):217–221. doi: 10.1007/DCR.0b013e31819a5c52. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz DA, Loftus EV, Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002 Apr;122(4):875–880. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 6.Hellers G, Bergstrand O, Ewerth S, Holmström B. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut. 1980 Jun;21(6):525–527. doi: 10.1136/gut.21.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karban A, Itay M, Davidovich O, Leshinsky-Silver E, Kimmel G, Fidder H, et al. Risk factors for perianal Crohn’s disease: the role of genotype, phenotype, and ethnicity. Am. J. Gastroenterol. 2007 Aug;102(8):1702–1708. doi: 10.1111/j.1572-0241.2007.01277.x. [DOI] [PubMed] [Google Scholar]
- 8.Lohsiriwat V, Yodying H, Lohsiriwat D. Incidence and factors influencing the development of fistula-in-ano after incision and drainage of perianal abscesses. J Med Assoc Thai. 2010 Jan;93(1):61–65. [PubMed] [Google Scholar]
- 9.Devaraj B, Khabassi S, Cosman BC. Recent smoking is a risk factor for anal abscess and fistula. Dis. Colon Rectum. 2011 Jun;54(6):681–685. doi: 10.1007/DCR.0b013e31820e7c7a. [DOI] [PubMed] [Google Scholar]
- 10.Halligan S, Stoker J. Imaging of fistula in ano. Radiology. 2006 Apr;239(1):18–33. doi: 10.1148/radiol.2391041043. [DOI] [PubMed] [Google Scholar]
- 11.Halligan S, Healy JC, Bartram CI. Magnetic resonance imaging of fistula-in-ano: STIR or SPIR? Br J Radiol. 1998 Feb;71(842):141–145. doi: 10.1259/bjr.71.842.9579177. [DOI] [PubMed] [Google Scholar]
- 12.Sabir N, Sungurtekin U, Erdem E, Nessar M. Magnetic resonance imaging with rectal Gd-DTPA: new tool for the diagnosis of perianal fistula. Int J Colorectal Dis. 2000 Nov;15(5–6):317–322. doi: 10.1007/s003840000251. [DOI] [PubMed] [Google Scholar]
- 13.Hori M, Oto A, Orrin S, Suzuki K, Baron RL. Diffusion-weighted MRI: a new tool for the diagnosis of fistula in ano. J Magn Reson Imaging. 2009 Nov;30(5):1021–1026. doi: 10.1002/jmri.21934. [DOI] [PubMed] [Google Scholar]
- 14.Yildirim N, Gökalp G, Öztürk E, Zorluoğlu A, Yilmazlar T, Ercan I, et al. Ideal combination of MRI sequences for perianal fistula classification and the evaluation of additional findings for readers with varying levels of experience. Diagn Interv Radiol. 2012 Feb;18(1):11–19. doi: 10.4261/1305-3825.DIR.4092-10.1. [DOI] [PubMed] [Google Scholar]
- 15.Erturk SM, Alberich-Bayarri A, Herrmann KA, et al. Use of 3.0-T MR imaging for evaluation of the abdomen. Radiographics. 2009 Oct;29(6):1547–1563. doi: 10.1148/rg.296095516. [DOI] [PubMed] [Google Scholar]
- 16.Libicher M, Scharf J, Wunsch A, et al. MRI of pouch-related fistulas in ulcerative colitis after restorative proctocolectomy. J Comput Assist Tomogr. 1998 Jul-Aug;22(4):664–668. doi: 10.1097/00004728-199807000-00029. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer O, Lohrmann C, Langer M. Assessment of anal fistulas with high-resolution subtraction MR-fistulography: comparison with surgical findings. J Mag Reson Imaging. 2004 Jan;19(1):91–98. doi: 10.1002/jmri.10436. [DOI] [PubMed] [Google Scholar]
- 18.Horsthuis K, Lavini C, Bipat S. Perianal Crohn disease: evaluation of dynamic contrast-enhanced MR imaging as an indicator of disease activity. Radiology. 2009 May;251(2):380–387. doi: 10.1148/radiol.2512072128. [DOI] [PubMed] [Google Scholar]
- 19.deSouza NM, Gilderdale DJ, Coutts GA, Puni R, Steiner RE. MRI of fistula-in-ano: a comparison of endoanal coil with external phased array coil techniques. J Comput Assist Tomogr. 1998 Jun;22(3):357–363. doi: 10.1097/00004728-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Halligan S, Bartram CI. MR imaging of fistula in ano: are endoanal coils the gold standard? AJR Am J Roentgenol. 1998 Aug;171(2):407–412. doi: 10.2214/ajr.171.2.9694465. [DOI] [PubMed] [Google Scholar]
- 21.Barleben A, Mills S. Anorectal anatomy and physiology. Surg. Clin. North. Am. 2010 Feb;90(1):1–15. doi: 10.1016/j.suc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 22.de Miguel Criado J, del Salto LG, Rivas PF, del Hoyo LFA, Velasco LG, de las Vacas MIDP, et al. MR imaging evaluation of perianal fistulas: spectrum of imaging features. Radiographics. 2012 Feb;32(1):175–194. doi: 10.1148/rg.321115040. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhammer S. A new approach to the anorectal fistulous abscess based on the high intermuscular lesion. Surg Gynecol Obstet. 1958 May;106(5):595–599. [PubMed] [Google Scholar]
- 24.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976 Jan;63(1):1–12. doi: 10.1002/bjs.1800630102. [DOI] [PubMed] [Google Scholar]
- 25.de Miguel Criado J, Garcia del Salto L, Rivas PF, et al. MR Imaging Evaluation of Perianal Fistulas: Spectrum of Imaging Features. Radiographics. 2012;32:175–194. doi: 10.1148/rg.321115040. [DOI] [PubMed] [Google Scholar]
- 26.Bartram C, Buchanan G. Imaging anal fistula. Radiol. Clin. North. Am. 2003 Mar;41(2):443–457. doi: 10.1016/s0033-8389(02)00122-7. [DOI] [PubMed] [Google Scholar]
- 27.Marks CG, Ritchie JK. Anal fistulas at St Mark’s Hospital. Br J Surg. 1977 Feb;64(2):84–91. doi: 10.1002/bjs.1800640203. [DOI] [PubMed] [Google Scholar]
- 28.Spencer JA, Chapple K, Wilson D, Ward J, Windsor AC, Ambrose NS. Outcome after surgery for perianal fistula: predictive value of MR imaging. AJR Am J Roentgenol. 1998 Aug;171(2):403–406. doi: 10.2214/ajr.171.2.9694464. [DOI] [PubMed] [Google Scholar]
- 29.Beets-Tan RG, Beets GL, van der Hoop AG, Kessels AG, Vliegen RF, Baeten CG, et al. Preoperative MR imaging of anal fistulas: Does it really help the surgeon? Radiology. 2001 Jan;218(1):75–84. doi: 10.1148/radiology.218.1.r01dc0575. [DOI] [PubMed] [Google Scholar]
- 30.Buchanan GN, Halligan S, Bartram CI, Williams AB, Tarroni D, Cohen CRG. Clinical examination, endosonography, and MR imaging in preoperative assessment of fistula in ano: comparison with outcome-based reference standard. Radiology. 2004 Dec;233(3):674–681. doi: 10.1148/radiol.2333031724. [DOI] [PubMed] [Google Scholar]
- 31.Buchanan GN, Halligan S, Williams AB, Cohen CRG, Tarroni D, Phillips RKS, et al. Magnetic resonance imaging for primary fistula in ano. Br J Surg. 2003 Jul;90(7):877–881. doi: 10.1002/bjs.4125. [DOI] [PubMed] [Google Scholar]
- 32.Buchanan G, Halligan S, Williams A, Cohen CRG, Tarroni D, Phillips RKS, et al. Effect of MRI on clinical outcome of recurrent fistula-in-ano. Lancet. 2002 Nov 23;360(9346):1661–1662. doi: 10.1016/S0140-6736(02)11605-9. [DOI] [PubMed] [Google Scholar]
- 33.Beckingham IJ, Spencer JA, Ward J, et al. Prospective evaluation of dynamic contrast enhanced magnetic resonance imaging in the evaluation of fistula in ano. Br J Surg. 1996;83(10):1396–1398. doi: 10.1002/bjs.1800831022. [DOI] [PubMed] [Google Scholar]
- 34.Buchanan GN, Halligan S, Taylor S, Williams A, Cohen R, Bartram C. MRI of fistula in ano: inter- and intraobserver agreement and effects of directed education. AJR Am J Roentgenol. 2004 Jul;183(1):135–140. doi: 10.2214/ajr.183.1.1830135. [DOI] [PubMed] [Google Scholar]
- 35.Williams JG, Farrands PA, Williams AB, Taylor BA, Lunniss PJ, Sagar PM, et al. The treatment of anal fistula: ACPGBI position statement. Colorectal Dis. 2007 Oct;9(Suppl 4):18–50. doi: 10.1111/j.1463-1318.2007.01372.x. [DOI] [PubMed] [Google Scholar]
- 36.Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB. AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003 Nov;125(5):1508–1530. doi: 10.1016/j.gastro.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 37.van der Hagen SJ, Baeten CG, Soeters PB, Russel MGVM, Beets-Tan RG, van Gemert WG. Anti-TNF-alpha (infliximab) used as induction treatment in case of active proctitis in a multistep strategy followed by definitive surgery of complex anal fistulas in Crohn’s disease: a preliminary report. Dis. Colon Rectum. 2005 Apr;48(4):758–767. doi: 10.1007/s10350-004-0828-0. [DOI] [PubMed] [Google Scholar]
- 38.Keshaw H, Foong KS, Forbes A, et al. Perianal fistulae in Crohn's Disease: current and future approaches to treatment. Inflamm Bowel Dis. 2010 May;16(5):870–880. doi: 10.1002/ibd.21137. [DOI] [PubMed] [Google Scholar]