Synopsis
This review focuses on the roles of glia and polyamines (PAs) in brain function and dysfunction, highlighting how PAs are one of the principal differences between glia and neurons as they are surprisingly stored, but not synthesized, almost exclusively in glial cells from which they can be released to regulate neuronal synaptic activity. The review includes the novel role of PAs, such as putrescine (PUT), spermidine (SPD) and spermine (SPM) and their precursors and derivatives. However: (i) PAs have not yet been a focus of much glial research; (ii) PAs affect many neuronal and glial receptors, channels and transporters; (iii) PAs are therefore key elements in the development of many diseases and syndromes (iv) thus forming the rationale for PA and glia focused therapy for these conditions.
Keywords: Stress, Brain disorders, Glia, Polyamines
Glia versus neurons
Cajal1 predicted how glia could help in health and disease by saying that glia are “insulating the neurons and switching their signaling”. His work has been analyzed by many scientists.2–9 Cajal knew that glia were more than just connective tissue but could never prove this. He was able to highlight novel features of glial cells. These observations can be considered to be the discovery of the importance of glia as the “second brain.” Cajal who has been considered by many to be the “father of modern neuroscience” actually made a principal glial discovery: he visualized what are now known as radial glial cells (RGCs). Recent studies have shown that these cells are of ectodermic origin which means that RGCs are universal precursors for both neurons and glia. This broke the dogma that glia and neurons have separate origins and lineages.10 Then came the studies that neurogenesis was observed in adult human11 and rat12 brains shattering yet another dogma that neurogenesis was absent in the mature brain.
There is increasing evidence that RGCs build the brain by accommodating in the inner and outer subventricular zone (SVZ) in order to send their processes into the ventricular zone (VZ). These show polarity and are in fact the stem cells of the developing brain.13, 14,15,16. Several morphologically distinct subtypes of RGCs in fetal macaque neocortex produce neurons and are guides for the migration of neural progenitors.17
Therefore, there are many different types of glial cells that are of RGC origin: NG-2, astrocytes, oligodendrocytes, tanycytes (in whole brain), Müller glia (in retina) and Bergmann glia (in cerebellum) as well as ependymal cells (in the ventricular surface). These cells represent the major neuroglial population in the adult central nervous system (CNS). On the other hand, peripheral glial cells such as Schwann cells, satellite glia (in the sympathetic, parasympathetic and sensory ganglia), enteric glia (in the ganglia of the digestive system), pituicytes (astrocytic glia in the posterior pituitary) are also types of neuroglia. Finally, while there are the microglia (mesodermal origin) that are macrophages in the brain, this review will not include the discussion of microglia.One of the major differences between glia and neurons is accumulation of biogenic polyamines; astrocytes expressing arginine decarboxylase can produce agmatine, a principal element in brain PA-exchange, and therefore, glial cells can be agmatine reservoirs.18 In general, the ratio of astrocytes to neurons (A/N) increases in evolution with increasing brain size19 and the highest glial cell (G) to neuron (G/N) ratio is found in brainstem20 where the most important controls of body functions occur, for example control of respiration.21, 22 On the other hand, there is also evidence that the frontal cortex has the highest G/N ratio. RGCs, as well as astrocytes, are filled with the PAs spermine and spermidine.23, 24, 25 There is one surprising function of RGCs that was recently discovered which is that in the adult retina RGCs provide photon signaling and serve as light guiding fibers26, 27.
Interaction of polyamines with receptors and ion channels
Polyamines such as SPD and SPM are involved in glial-neuronal communication, especially during periods of stress such as during ischemia and trauma; the mechanisms of storage and release are not well known. Since neurodegeneration is a major problem during stress, ischemia and CNS diseases, identifying potential neuroprotective mechanisms could provide new targets for therapeutic interventions. In the 1980s, it was discovered that the polyamine SPM was a principal radical group in spider venom28, 29, 30, 31 and the SPM portion of the venom could insert itself into and block glutamate receptors.30 In the late 1990s, PAs were brought to the attention of neuroscientists. However the sources of PAs in the brain were not known.
PAs affect glial inwardly rectifying potassium (Kir)4.1 channels25, 32, 33 and most of the known neuronal receptors and channels.34 In the brain and peripheral nervous system, SPM and SPD are known to have specific intra- and extracellular actions. SPM affects numerous receptors and channels in neurons with differing affinities ranging from ~10 nM – 200 μM.35, 36, 37, 38 Intracellular SPM/SPD induces voltage-dependent block of Kir channels25, 39, 40, as well as neuronal nicotinic acetylcholine receptor channels35, 41, glutamate (Glu)A-2 lacking α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) channels42, N-methyl-D-aspartate receptors (NMDARs)34, 43, olfactory cyclic nucleotide-gated cation channels (CNGC)44, and voltage-gated sodium channels.45 In addition, some NMDAR and AMPAR channels show rectification in the presence of polyamines or their derivatives.31, 36, 42, 46, 47, 48, 49, 50 PAs are the strongest blockers of Kir channels, glutamate receptor-channels (such as AMPAR, NMDAR, KainateR) and acetylcholine receptor (AChR) channels31, 35, 36, 39, 51, 52, 53 and also act as the calcium-sensing receptors agonists54 and antagonists of transient receptor potential cation channel, subfamily M, member 7 (TRPM7) and transient receptor potential cation channel, subfamily V member 1 (TRPV1) channels.38, 55 Also relevant are the extracellular actions of PA on GluA-2-lacking and GluA5/6-enriched AMPA/Kainate receptor channels in interneurons,56, 57, 58 because glial cells may release PAs to control synaptic activity. These receptor channels have an affinity for SPM in the micromolar (μM) range.
Spermine/Spermidine localization in CNS: Bi-directional polyamine signaling between glia and neurons
Surprisingly, the PAs spermidine and spermine are accumulated in glia (Fig. 1) and their distribution is clearly evolutionarily determined; it is found throughout the brain23, retina24, 25, peripheral nervous system59 and in glial-neuronal co-cultures60 of multiple species, including man24. This phenomenon raises key questions: (i) What are the mechanisms that underlie such uneven distribution, accumulation and release from glia?; (ii) What are the consequences of PAs fluxes within the brain on neuronal function?; (iii) What are the roles of PAs in brain disorders and diseases?
Figure 1. Circulation of polyamines (PA) in brain.
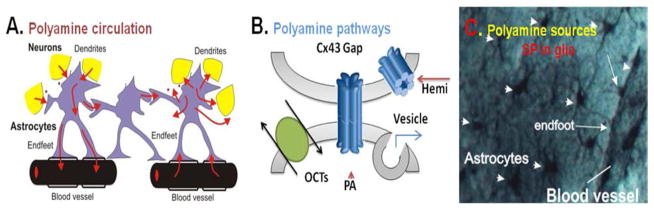
A. Suggested interaction between astrocytes, neuronal dendrites and synapses and blood vessels based on bi-directional polyamine (PA) fluxes (i) between neurons and astrocytes, (ii) between astrocytes in their syncytium and (iii) between astrocytes and blood vessels. Polyamines are taken up and released from glia to neurons as well as propagated distantly through the syncitium (red arrows). B. Suggested PA pathways (uptake and release) in glia via connexin 43 (Cx43) hemichannels, Cx43 gap-junctions, reverse organic cation transporters (OCT) and vesicular release. C. Accumulation of spermine (SP) in astrocytes shown by immunocytochemical method in rat hippocampus. Astrocytes enwrap blood vessels and connect to each other. Note: no spermine and spermidine labels found in neurons in this stratum radiatum area of CA1 rat hippocampus.
Astrocytes enwrap pre- and post-synaptic neuronal terminals, generating a tripartite synapse.61, 62, 63 Failure of synaptic transmission64, 65 and vasodilation66, 67 has been ascribed to the malfunction of perisynaptic and perivascular astrocytes, respectively. Neuronal damage is evident after glial depletion in hepatic encephalopathy68 and neuronal degeneration can occur following apoptosis of glial cells.69 It has long been accepted that glia provide a support function to neurons by buffering extracellular K+ and glutamate.70, 71, 72, 73, 74 However, potential signal functions of glia are much less studied and understood.
Glial cells release signaling molecules such as arachidonate, glutamate, adenosine triphosphate (ATP), D-serine, tumor necrosis factor alpha (TNF-α) and others, which regulate neurons and blood vessels.61, 75–87 Thus, finding the signaling functions of glial cells and potential endogenous glial transmitters are one of the frontiers of glial research. Whilesuch gliotransmitters may be PAs theymostly underestimated and less studied; yet in the context of stress and glial function they might be a key element in helping to modulate neurons.
Polyamines may have harmful or neuroprotective effects via multiple pathways. For example, blocking calcium (Ca)2+-permeable GluR2-lacking AMPA receptor-channels by SPM 42, 56, 88, 89 reduces Ca2+ influx and prevents excitotoxicity.90 Furthermore, potentiation by SPM of GluR-6 kainate receptors on inhibitory neurons58, 91 inhibits the activity of downstream pyramidal cells which can result in a neuroprotective effect. Ischemia, glucose deprivation or mild mechanical trauma all can result in neuronal death due to Ca2+ overload followed by apoptosis92, 93 if not protected by PAs.90 The primary pathway for Ca2+ entry under these conditions is via fast GluR2-lacking AMPA receptors as well as slow NMDA receptors.50, 94, 95, 96 Therefore, the amount of PAs stored and released from glia may underlie the strength of neuroprotection. There might be an aging effect because the amount of PAs stored in the brain declines with age.97, 98, 99, 100, 101, 102 PA sensitive interneurons (lacking GluR2 subunits) within the brain, but not pyramidal neurons (expressing GluR2 subunits), are enveloped by PA-filled astrocytes (ex. in the hippocampus). During ischemic conditions there is a striking disparity in the rate of neuronal death in this region of the brain. Interneurons are spared, whereas pyramidal neurons are very susceptible.90 This observation can be explained by the proximity of astrocytes as they take much better care of their direct neighbors, the interneurons. By contrast, the cortex interneurons and pyramidal cells are much less formally organized. Glial cells are unevenly distributed between the two cell types, making the neuronal sensitivity to ischemia less for interneurons than for pyramidal cells.103. We suggest that the resistance of hippocampal interneurons to ischemia and apoptosis is a result of localized SPM/SPD release from the surrounding glia and the subsequent block of GluR2-lacking AMPA receptors. In support of this, there is compelling evidence for a protective role of exogenous PAs in brain ischemia and neurotransmitter-induced excitotoxicity: for instance the application of naphthylacetyl-spermine (NAS) or SPM/SPD in vitro or in vivo dramatically protects Cornu Ammonis area (CA)1 neurons under these conditions.90, 92, 93, 104 So, we suggest that when experiments are done with brain slices that might have their endogenous PAs washed out of the astrocytes by being disconnected from the blood circulation (a source of PAs) there should be supplementation with external PAs so as to keep PAs buffering the astrocytes which will improve neuronal survival and function.105 This can be directly related to stress and neuroprotection. Another observed phenomenon is that PAs can be oxidized which results in the production of toxic compounds that can be captured by glial cells. Indeed, when cultured in the absence of glia, neurons show a delayed death in response to exogenous PAs.106, 107 Even though extracellular PAs are present in physiological tissues,108 the healthy brain clearly avoids significant PA toxicity, probably because extracellular levels in the cerebrospinal fluid are buffered by the astrocytes. Consistent with this, and in contrast to the findings in pure neuronal cultures, 50 μM SPM applied exogenously in cortical brain slices is well tolerated, and neuronal death is not observed.56 We, therefore, hypothesize that, in the intact brain, glial cells provide protection against trauma and excitotoxicity at least in part by using PAs as a buffer, thereby, preventing oxidation. PAs can then be released at appropriate sites with the potential to block inappropriate calcium permeability through glutamate receptor regulation. Indirect support for this hypothesis is the observation that in perfused brain slices, excitotoxicity induced by anoxia and toxic chemicals (ex. NMDA) in hippocampal slices was prevented if SPM was used.109 If PAs may be considered as neuroprotective agents, then what are the physiological ranges of PA concentrations and what are the free versus bound forms of polyamines in the brain?
The total concentration of intracellular SPM in non-neuronal cells is high (3–10 milli [m]M)97, 110–114, although SPM is greatly buffered in the cells by negatively charged phosphates, ATP, guanosine-5′-triphosphate (GTP), DNA, RNA, membrane proteins and other polypeptides.111,115, 116, 117, 118, 119 Since SPM and SPD are not synthesized in glia120, 121, but instead are accumulated in glia23, 24, 25, 60 (Fig. 1C), the polyamines PUT, SPD and SPM may be exchanged during different conditions such as development, membrane depolarization, substrate co-transport or metabolic downregulation. Our immunolocalization data demonstrate preferential SPM/SPD accumulation in glia that enwrap blood vessels (Fig. 1). SPM and SPD were not found in the majority of neurons of the retina24, 25 and in brain.23 This is consistent with our recent studies suggesting that the free SPM concentration in glia (up to 800 μM33,) may be much higher than estimated in neurons ~10 nano(n)M – 80 μM.35, 36, 37, 38 In the brain, PAs show a strikingly uneven distribution. Using radioactive polyamines59, 116, 117 and polyclonal antibodies specific for PUT60 or SPM/SPD23, 24, 25, we and others have shown that PAs are taken up in brain110, 122, and astrocytes are capable of taking up PAs.60, 123–128 (Fig. 1B).
Therefore, if PAs were to be synthesized in some neurons,129, 130, 131, 132 then they would be released from those neurons, possibly from synaptic neuronal vesicles, to the extracellular space. In fact, a vesicular transport system was found.133, 134 This leads to an immediate accumulation and storage of PAs in glial cells (Fig. 1A). An alternative source of PAs could be from the blood vessels, with which astrocytes maintain intimate contact, wrapping the vascular interface by endfoot processes (Fig. 1A, C). Here, glial cells may use several uptake pathways such as transporters and large pores (Fig. 1B) which will be discussed below.
Brain disorders and glia
Global amnesia, depression, stress, anxiety, autism, glioblastoma multiforme, glaucoma, migraines, neuropathic pain, sleeplessness and drug addiction are among a host of devastating neurological diseases or disorders for which prevention or a cure must be found.135–152 We will show below that these disorders can be tightly linked with the PA machinery.
Since their original discovery by Leeuwenhoek153, the polyamines SPM and SPD have attracted attention of scientists and clinicians.119, 154, 155 In the middle of the 20th century, there was great interest in their role in maintaining DNA structure and the possibility of treating cancer by blocking PA synthesis. Due, in part, to neurological complications in the anti-cancer treatment, this approach failed.156 Later, studies have shown that this could have been predicted because of the existence of multiple effects of PAs on receptors and channels in the brain.
Indeed, multiple biological effects of PAs have been reported including increasing longevity,97, 101, 157, 158 cell proliferation and differentiation,159 receptor and channel regulation,29, 34, 160, 161, 162 modulation of behavior, learning and memory,163–167 as well as antinociceptive,38, 168, 169 neuroprotective,170–172 antidepressant173, 174 and antioxidant effects97, 175
While PAs are still a mystery in the brain18, 60, 110, 114, 122, 132, 157, 176–185, they are known to be very tightly associated with glial cells. Altered PA metabolism may underlie certain brain disorders,186, 187 including depression with suicidal tendency.188 Endogenous depletion of SPD and SPM by dietary means189, 190 or by genetic activation of SPD/SPM-acetyl transferase results in the loss of PAs and a loss of neuroprotection.191, 192 In spite of the fact that SPM/SPD are involved in the pathology of neurodegenerative diseases, they predominantly accumulate in glia and not in neurons.23, 24, 25 However SPD is not synthesized in glial cells120, 121, 179, 193 and thus, SPM cannot be synthesized without SPD, because it is the precursor. A solution to their origins comes from the idea that PAs are probably taken up from external sources by glial cells.114, 126, 194 It has been hypothesized that PAs are taken up by glia from the blood circulation, cerebral spinal fluid (CSF) or from macrophages penetrating the blood-brain barrier. One of the possible pathway is the organic cation transporter (OCT) system which is expressed in glia.195, 196
Several CNS diseases have been shown to be associated with neuroglia such as astrocytes, oligodendrocytes, ependyma and other glial cells. Neurodegenerative diseases where glia play a key role are Alzheimer’s disease143, 197–200, Amyotrophic Lateral Sclerosis 201–203, Alexander disease204, Parkinson’s disease205, Huntington’s disease206, 207, 208, multiple sclerosis 209, 210 and others.
Still other brain disorders and syndromes where glial cells play a pivotal role have been recognized. One of them is directly related to stress and epilepsy where (i) PA-dependent glial Kir4.1 channels are involved74, 211–214 together with (ii) glial connexin gap-junctions215, 216 and (iii) down-regulation of adenosine signaling.151, 217–219 In addition, there are severe disorders such as ischemia and stroke where reactive gliosis and an inability to regulate pH, K+-buffering, glutamate homeostasis and water exchange were found to result in the release of cytotoxic molecules, glial swelling and neuronal death.220–224 The study of both physical221, 225 and chemical brain trauma resulting in the depression of glial metabolism65 or reconstitution following brain edema and inflammation 226, 227 showed that reactive glia no longer were supporting neurons.
Intriguingly, the PA regulated Kir 4.1 channels228, 229 and a PA transporter OCT SLC22A subfamily, OCT3 are mislocalized230 in glial cells involved in brain cancer genesis (gliomas). These tumors produce increased intracranial pressure, metabolic deficiencies, toxicity and cell death.231 Downs Syndrome and Snyder-Robinson Syndrome have malfunction of PA homeostasis.145, 232–234 The neurological manifestations of EAST/SeSAME syndrome with glial Kir4.1 mutations212, 214, 235 is solely of glial origin and blood-brain circulation disorders are seen when astrocytes cannot regulate vasodilation.67, 198, 236 All of these recent findings show the unique role of glial cells and PAs in CNS disorders.
By what mechanisms do glia accumulate and release PAs?
The enzymes ornithine decarboxylase (ODC) and spermidine synthase (SpdS) synthesize PT and SPD, respectively which are the precursors of SPM. In the normal brain, ODC and SpdS expression is typically found only in a few neurons, without any being detectable in glia.120, 121, 179, 237 In addition, we found that SPM-synthase is also absent in glia. This suggests under normal conditions, PAs may be synthesized outside glia in some neurons.120, 130, 131, 179, 238 Ultimately by unknown mechanisms, PAs will accumulate in glia.23–25, 60 Strong evidence for this is the finding that injections of radioactive putrescine into axons result in the transfer of the radioactive label to surrounding glial cells.59 Even in brain areas where many adult neurons lack SpdS activity120 and show low levels of SPM/SPD23 there are still robust and potential sources of PAs in blood capillaries as PAs can permeate the walls of blood vessels18 to which glial cells are attached to by endfeet (Fig. 1 A, C). Therefore, accumulation of PAs in the glial cytoplasm is not primarily due to SPD/SPD synthesis, but instead to either passive or active transport (fluxes) of PAs (Fig. 1).
There are several potential pathways for exchange of SPM/SPD between glia, neurons and blood vessels such as the following:
large pores, including connexin (Cx) and/or pannexin (Panx) hemichannels,
ion channels
exocytotic vesicular release and endocytotic uptake (VRU).
All of these candidate pathways have been identified in glial cells for different molecules, but not yet for SPM/SPD.9, 78, 80, 239–244 It was suggested that PAs could be taken up from external sources by glial cells via hypothetical transporters which would bring PAs into the cells114, 126, 194,195 by transporters localized at the endfeet that are attached to blood vessels and brain ventricles. According to this view, the blood circulation and CSF would be the major sources of PA uptake. Finally, the macrophages that penetrate the blood-brain barrier and enter the brain may be additional sources.
Recent data show that SPD may be taken up by transporters such as polyspecific electrogenic organic cation transporters SLC22A subfamily196 that were suggested as a pathway for monoamines such as dopamine, tetraethylammonium and others,126, 127, 194, 195 that are indeed also well suited for PAs.196 Such transporters may function in a reverse mode releasing PAs by exchanging with other OCT substrates245, or, as it was shown for glutamate reverse transport246 or dopamine reverse transport247, 248 with similar and different mechanisms. Therefore, transporters may fulfill the function of regulating the PA content in the extracellular cleft and thus can regulate neuronal activity.
There may be other minor pathways for PAs; SPM permeates glutamate-receptor channels52, 53, TRPV1 channels38 and glial Kir4.1 channels.249 These are most likely negligible pathways for SPM flux since the channel pores (6 ångström (Å)-9Å) are comparable in size to SPM (4Å diameter and 16Å the length). Additionally, while exocytotic release as described for glutamate78 and ATP250 may be applicable to PAs, there is no still evidence suggesting SPM release via this vesicular pathway.
The most likely major pathways for SPM/SPD exchange are via the large pores such as Cx and Panx hemichannels and transporters such as OCTs. Indeed, SPM/SPD may pass through connexin gap junctions and fill the astrocytic syncitium.105 While several studies suggest that unpaired Cx43 hemichannels are present in the plasma membrane of astrocytes80, 240, 251 and in cell lines252, Cx-hemichannels are normally closed due to the blocking effects of external divalent cations and voltage. When external calcium is decreased80 or internal calcium is increased252 Cx43-hemichannels open, forming large nonselective pores. Glial cells express a number of connexins, the most prevalent is Cx43, which together with Cx26, Cx30 and Cx45, form glial hemichannels and gap junctions with a large pore diameter (10Å-15Å) 253, 254 which SPM can readily pass through. Since Cx38 hemichannels in Xenopus oocytes are permeable to SPD255 and that there are hemichannels in rat cortical astrocytes, most probably Cx43, permeable to PAs105, then this makes the Cx-pathway a likely candidate to be where uptake and release of SPM are regulated. Consistent with this possibility is the finding that gap junctions comprised of Cx43 are not blocked by PAs, while others made from neuronal Cx40 are blocked by SPM.256, 257 An alternative potential pathway for SPM/SPD fluxes are Panx-hemichannels, three of which (Panx1-3) are expressed in glia. These are nonselective pores that can open with normal external calcium levels.243, 251 However, Panx is not blocked by gadolinium258, while SPM flux in astrocytes is blocked by gadolinium making Panxs an unlikely pathway.259 In support of a direct exchange of SPM between the neuronal and glial cytoplasm via Cx hemichannels is the finding that much larger dye molecules can pass between astrocytes, but not between oligodendrocytes and neurons or between neurons.260 We have found that SPM/SPD fluxes are Ca2+- and -gadolinium (Gd3+) sensitive, favoring the possibility that Cx hemichannels rather than Panx hemichannels are the relevant pathway.259–262
As mentioned above, another potential mechanism for SPM/SPD fluxes in glial cells is through polyspecific cation transporters (OCTs).196 These transporters can translocate monovalent, divalent and even polyvalent cations such as SPM and SPD.196, 263 Polyspecific cation transporters (which include OCT1, OCT2, OCT3 and OCTN2, multidrug and toxin extrusion protein or MATE) are present in astrocytes and control signal transmission and energy homeostasis by removing released transmitters and substrates, such as dopamine, norepinephrine, epinephrine, 5-hydroxytryptamine, carnitine and histamine, from the extracellular space.264–267 OCT transporter mRNA has been found in cultured astrocytes195, 268, 269 and is well described.196, 270
Glia-controlled polyamine regulation in the neuronal network
There should be a mechanistic basis to explain diseases, disorders and syndromes that are associated with glia and with PAs. The glial membrane potential (~ −85 mV) is typically 20–30 mV more hyperpolarized than resting neurons, therefore, polyvalent cations, SPM4+ and SPD3+ will be concentrated in glia by electrodiffusion via large pores (like Cx-43) or OCTs (which require electrical transmembrane potential) due to this hyperpolarization. Once inside the cell, PAs will be buffered by polyanions (RNA, ATP, acid proteins, etc.) in the cytosol. When neurons are generating action potentials, there can be large transient falls in extracellular calcium ([Ca2+]o)271, 272 and sodium ([Na+]o), and a rise of potassium ([K+]o)273, all of which will accelerate both glial depolarization and hemichannel opening. Intriguingly, with a delay of only a few minutes during ischemia, there is a dramatic increase of [K+]o (to 55 mM) and a lowering of [Na+]o, [Cl+]o, [H+]o and [Ca2+]o- to 60 mM, 75 mM, pH=6.5 and 0.08 mM from normal levels, respectively.274–277 This provides favorable conditions for Cx43-hemichannel opening that will not block OCTs. Normally, [Ca2+]o can block Cx43 hemichannels.80, 252 During epilepsy, ischemia, spreading depression or trauma, the activity of [Ca2+]o is decreased by an order of magnitude from 1.2 to 0.06 mM.274–277 This is a condition under which Cx43 hemichannels can open,278, 279 potentially allowing PAs to be released outside glia where they may help to remove the external H+ block of Cx43. Also, during ischemia, pH may reach acid levels of ~6.5–6.1274, 276 and OCTs are depressed by acid pH and depolarization.263, 270, 280 Therefore, the Cx43 and OCT pathways function differently: at normal conditions OCTs are a major PA pathway, then with excessive neuronal activation or ischemia there is a stimulation that opens Cx43 hemichannels that will allow the release of PAs, thus, conferring neuroprotection as mentioned above by blocking Ca2+-permeable neuronal channels and preventing apoptosis.
Selective depolarization of astrocytes with aminoadipic acid changes the neuronal firing rate; this is blocked by carbenoxolone, a non-selective blocker of hemichannels.260 This is consistent with the idea that hemichannels are key players in neuronal regulation. SPM/SPD release via hemichannels from astrocytes may be induced by depolarization (Fig. 1B). The consequences of such release will depend critically on which neuronal receptors are exposed to the potentially high localized release.
Polyamines: role in the CNS disorders
While previously unrecognized, recent data highlight dynamic signaling within glia and between glia and neurons via PAs. Many studies have reported neuroprotective effects of PAs.92, 109, 121, 175, 187, 281, 282 Under pathological conditions, PAs may be oxidized and converted to cytotoxic aldehydes and reactive oxygen species, which may be responsible for subsequent neurotoxic damage.197, 281 The down-regulation of the synthesis of SPM due to a mutation in the X-chromosome, causes Snyder-Robinson Syndrome that so severely affects human brain function that the result is mental retardation, hypotonia and cerebellar dysfunction.233, 234 Conversely, unmodified PAs may block Ca2+-permeable receptors and channels which is critical in protecting neurons from apoptosis (see above). Despite these recognized modifications and potential targets, the exact localization and dynamics of PAs in the brain are largely unknown.
What is important to know is that many of the aforementioned disorders show a tight link between PAs and glia. SPM/SPD levels decline with age97, 101, 158 and are involved in Parkinson’s disease.283, 284 These reports clearly demonstrate a vital role for PAs in brain plasticity. For instance, there is evidence that in brain diseases PAs play a principle role in restoring age-related memory impairment101 as there is evidence that PA depletion stops mammalian cell growth and PA supplementation will reverse the effect.285 Treatments with SPD, agmatine, and by genetic modulation that increase endogenous PA levels resulted in not only an increase in life span97, but also in memory restoration.97, 101, 199 PA-rich nutrition increased the life span of aging mice98, 99, 100, 286 while some amines and PAs (such as agmatine, arginine, putrescine, spermidine, spermine) are lost during aging and in the diseased CNS.97, 102, 157, 179–182, 286 Also, PAs inhibit age-associated changes in global DNA methylation as well as dimethylhydrazine-induced tumor genesis.100 It has been hypothesized that information in the brain can be stored (memory) in the chromatin (a complex of DNA-proteins-polyamines) and can be changed during epigenetic chromatin modifications as in Alzheimer’s disease where macrophages penetrate the brain via the “leaky” blood-brain-barrier and probably cleave up “memory-proteins” in chromatin.287 Since, glia (G) outnumber neurons (N) in the human brain (the G/N ratio reaches 11.35 in brainstem and 3.76 in cortex20)) glia can represent a substantial source of memory capacity especially given that PAs are bound to DNA in chromatin, to RNA and to acid proteins.111, 167, 288 There are severe psychiatric disorders directly linked with neuroglia and PA dysfunction such as schizophrenia and mood disorders, where failure of PA exchange is seen179–182, autism with disorders in the PA and Na-H-exchange,149, 289 depression which is associated with decreased glial cell mass, OCTs and PA levels,173, 290 suicide which correlates with PA homeostatic imbalances,291 aging where glial cells lose PAs99, 100, 286 and downregulate Ca2+-signaling236 and fear extinction where PAs reinforce extinction via NMDAR regulation.292 Recently, many human CNS diseases have been linked with PA levels including Alzheimer’s disease102, 293, 294, Parkinson’s disease128, 295, 296, 297, Huntington’s disease167, 298–301 and Amyotrophic lateral sclerosis.186, 187, 302 In spite of a broad phenomenological description in the literature, there still remain functional links between PAs and glia in the brain that have not been mechanistically deciphered. However, what is known is that PAs are ultimately stored in glial cells with the level of storage and PA buffering capacity depending solely on glial cells.
Therefore, research is focusing on glial-based PA-related CNS problems for several reasons. First, PAs are very powerful modulators of the neuronal-glial network.56, 303 Second, PAs are accumulated preferentially in glial cells not in neurons.23, 24, 25 Third, PA homeostasis is critical for life.97, 101,184, 185, 304 Fourth, PA-exchanges play a principal role in brain disorders including inflammation224, 226, 227, depression290, anxiety305, 306 and others (see above). Fifth, PA-sensitive glial Kir4.1, Cx43/Cx30 and OCTs are fundamental for survival of the cells and whole brain.29, 74, 126, 127, 209, 213, 215, 223, 230, 249, 259, 270, 307 Sixth, Cx43 and OCTs are candidates for PA uptake and release.105, 196, 259, 261, 245, 262 Finally, because CNS disorders are closely related to PA exchange in stress and aging102, 170, 157, 179–182 therapeutic supplement by PAs or their precursors have been suggested for neuroprotective treatment.18, 98–102, 110, 286, 308, 309 As for the supplemental therapy, attention should also be focused to PA-carriers such as Cx43 and OCT proteins. While there has been an increase in the research on PAs, the mechanisms of glial dependent PA actions in the CNS is just beginning to be elucidated and it seems that the glial-PA avenue for research has not yet been well blueprinted as more work needs to be done.
Conclusion
As is now evident, PAs are stored in glia23–25 and together with their derivatives and precursors have been suggested as neuroprotective agents.18, 60, 93, 109, 110, 122, 175, 177, 194, 282, 308 However, the oxidation of PAs results in toxic products such as aldehydes that may be harmful for brain cells.107. Lower molecular weight polyamines have less toxicity. One of the PA precursors, agmatine (decarboxylated arginine or 1-(4-aminobutylguanidine) is a natural product discovered over a 100 years ago in herring sperm310 and research indicates its exceptional modulatory action at multiple molecular targets, including neurotransmitter systems, nitric oxide and PA production, therefore providing bases for current therapeutic applications. A triamine (SPD) and a diamine (PUT; 1,4-diaminobutane) were isolated from pro- and eu-karyotic systems; thereby showing that there are many sources of different polyamines in any diet.98–101, 311, 312 Recent preclinical and initial clinical evidence show a very positive effect of agmatine treatment18 making for challenging research opportunities for the use of agmatine in treating diabetes, neurological trauma, neurodegeneration, different types of addictions, mood disorders, cancer, cognitive and memory disorders. It seems that agmatine (amino-guanidine), guanidine based drugs and synthetic amines are examples of the best substrates for organic cation transporters of the SLC22A subfamily196, 313, 314 which have been found and characterized in astrocytes and other glial cells in the brain.263–270, 280 Specifically, recent data show that a polymorphism of OCT1 is directly related to Parkinson’s disease315 and OCT3 is a key modulator of neurodegeneration in the nigrostriatal dopaminergic pathway.316
Therefore, not only should agmatine, SPD and other polyamines be a focus in designing future pharmaceutical tools to treat psychiatric disorders but also the PA transporter systems (for example OCTs315, 316, Cx43105 ) and buffering agents for PAs such as acid proteins, ATP, phosphates, etc.111 should be considered.
Finally, targeting of certain disorders with PAs needs to be taken with great care as for example in the case of gliomas where translocation of glial Kir 4.1 and OCT3 from the glioma cell plasma membrane to the nuclear membrane228–230 makes these very important glial proteins unreachable by drugs from the extracellular space. The future challenge will be to find drugs that are able to reach these crucial areas and modulate the PA system. Only this way will we be able to know if the modulation of the PA system can give us another tool in the fight against brain dysfunction.
Key Points.
Polyamines (Pas) are one of the principal differences between glia and neurons as they are surprisingly stored, but not synthesized, almost exclusively in glial cells from which they can be released to regulate neuronal synaptic activity.
PAs have not yet been a focus of much glial research.
PAs affect many neuronal and glial receptors, channels and transporters.
PAs are key elements in the development of many diseases and syndromes, thus forming the rationale for PA and glia focused therapy for these conditions.
Glossary
- μM
micromolar
- Å
ångström
- A/N
ratio of astrocytes to neurons
- AChR
acetylcholine receptor
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- ATP
adenosine triphosphate
- Ca
calcium
- CA
Cornu Ammonis area
- Ca2+
extracellular calcium
- Cl+
extracellular chloride
- CNGC
cyclic nucleotide-gated cation channels
- CNS
central nervous system
- CSF
cerebral spinal fluid
- Cx
connexin
- G G/N
ratio of glial cells to neurons
- Glu
glutamate
- GTP
guanosine-5′-triphosphate
- H+
extracellular hydrogen
- Ir K
Kir, inwardly rectifying potassium
- K+
extracellular potassium
- m
milli
- MATE
multidrug and toxin extrusion protein
- n
nano
- Na+
extracellular sodium
- NAS
naphthylacetyl-spermine
- NMDAR
N-methyl-D-aspartate receptor
- OCT
organic cation transporter
- ODC
ornithine decarboxylase
- Panx
pannexin
- PAs
polyamines
- PUT
putrescine
- RGCs
radial glial cells
- SPD
spermidine
- SpdS
spermidine synthase
- SPM
spermine
- SVZ
subventricular zone
- TNF-α
tumor necrosis factor alpha
- TRPM7
transient receptor potential cation channel, subfamily M, member 7
- TRPV1
transient receptor potential cation channel, subfamily V, member 1
- VRU
vesicular release and endocytotic uptake
- VZ
ventricular zone
Footnotes
The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramón y Cajal S. Sobre un nuevo proceder de impregnacion de la neuroglia y sus resultados en los centros nerviosos del hombre y animales. Trab Lab Invest Biol Univ Madrid. 1913;11:219–237. [Google Scholar]
- 2.Somjen GG. Nervenkitt: notes on the history of the concept of neuroglia. Glia. 1988;1(1):2–9. doi: 10.1002/glia.440010103. [DOI] [PubMed] [Google Scholar]
- 3.Beatty JT. The Human Brain: Essentials of Behavioral Neuroscience. 1. Sage Publications. Inc; 2001. [Google Scholar]
- 4.Berlucchi G. The origin of the term plasticity in the neurosciences: Ernesto Lugaro and chemical synaptic transmission. J Hist Neurosci. 2002;11(3):305–309. doi: 10.1076/jhin.11.3.305.10396. [DOI] [PubMed] [Google Scholar]
- 5.García-Marín V, García-López P, Freire M. Cajal’s contributions to glia research. Trends Neurosci. 2007a;30(9):479–487. doi: 10.1016/j.tins.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 6.García-Marín V, García-López P, Freire M. Cajal’s contributions to the study of Alzheimer’s disease. J Alzheimers Dis. 2007b;12(2):161–174. doi: 10.3233/jad-2007-12206. [DOI] [PubMed] [Google Scholar]
- 7.DeFelipe J. Cajal and the discovery of a new artistic world: the neuronal forest. Prog Brain Res. 2013;203:201–220. doi: 10.1016/B978-0-444-62730-8.00008-6. [DOI] [PubMed] [Google Scholar]
- 8.Chvátal A, Anderová M, Neprasová H, Prajerová I, Benesová J, Butenko O, Verkhratsky A. Pathological potential of astroglia. Physiol Res. 2008;57(Suppl 3):S101–110. doi: 10.33549/physiolres.931604. [DOI] [PubMed] [Google Scholar]
- 9.Parpura V, Verkhratsky A. Neuroglia at the crossroads of homoeostasis, metabolism and signalling: evolution of the concept. ASN Neuro. 2012;4(4):201–205. doi: 10.1042/AN20120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malatesta P, Hartfuss E, Götz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127(24):5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan MS. Neurogenesis in the 3-month-old rat visual cortex. J Comp Neurol. 1981;195(2):323–338. doi: 10.1002/cne.901950211. [DOI] [PubMed] [Google Scholar]
- 13.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spassky N, Merkle FT, Flames N, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25(1):10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilz GA, Shitamukai A, Reillo I, Pacary E, Schwausch J, Stahl R, Ninkovic J, Snippert HJ, Clevers H, Godinho L, Guillemot F, Borrell V, Matsuzaki F, Götz M. Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat Commun. 2013;4:2125. A. doi: 10.1038/ncomms3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagemann TL, Paylor R, Messing A. Deficits in adult neurogenesis, contextual fear conditioning, and spatial learning in a Gfap mutant mouse model of Alexander disease. J Neurosci. 2013;33(47):18698–18706A. doi: 10.1523/JNEUROSCI.3693-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betizeau M, Cortay V, Patti D, Pfister S, Gautier E, Bellemin-Ménard A, Afanassieff M, Huissoud C, Douglas RJ, Kennedy H, Dehay C. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron. 2013;80(2):442–457. doi: 10.1016/j.neuron.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Piletz JE, Aricioglu F, Cheng JT, Fairbanks CA, Gilad VH, Haenisch B, Halaris A, Hong S, Lee JE, Li J, Liu P, Molderings GJ, Rodrigues AL, Satriano J, Seong GJ, Wilcox G, Wu N, Gilad GM. Agmatine: clinical applications after 100 years in translation. Drug Discov Today. 2013;18(17–18):880–893. doi: 10.1016/j.drudis.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Reichenbach A. Glia: neuron index: review and hypothesis to account for different values in various mammals. Glia. 1989;2:71–77. doi: 10.1002/glia.440020202. [DOI] [PubMed] [Google Scholar]
- 20.Lent R, Azevedo FA, Andrade-Moraes CH, Pinto AV. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur J Neurosci. 2012;35(1):1–9. doi: 10.1111/j.1460-9568.2011.07923.x. [DOI] [PubMed] [Google Scholar]
- 21.Erlichman JS, Leiter JC, Gourine AV. ATP, glia and central respiratory control. Respir Physiol Neurobiol. 2010;173(3):305–311. doi: 10.1016/j.resp.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329(5991):571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laube G, Veh RW. Astrocytes, not neurons, show most prominent staining for spermine/spermidine-like immunoreactivity in adult rat brain. Glia. 1997;19(2):171–179. doi: 10.1002/(sici)1098-1136(199702)19:2<171::aid-glia8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Biedermann B, Skatchkov SN, Bringmann A, Pannicke T, Veh R, Bernstein H-G, Reichenbach A. Spermine/Spermidine is expressed by retinal glial (Müller) cells, and controls distinct K+ channels of their membrane. Glia. 1998;23:209–220. doi: 10.1002/(sici)1098-1136(199807)23:3<209::aid-glia4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Skatchkov SN, Eaton MJ, Krušek J, Veh RW, Biedermann B, Bringmann A, Pannicke T, Orkand RK, Reichenbach A. Spatial distribution of spermine/spermidine content and K+- current rectification in frog retinal glial (Müller) cells. Glia. 2000;31:84–90. doi: 10.1002/(sici)1098-1136(200007)31:1<84::aid-glia80>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Franze K, Grosche J, Skatchkov SN, Schinkinger S, Foja C, Schild D, Uckermann O, Travis K, Reichenbach A, Guck J. Müller cells are living optical fibers in the vertebrate retina. PNAS. 2007 May 15;104:8287–8292. doi: 10.1073/pnas.0611180104. http://www.pnas.org/site/misc/cozzarelliprize.xhtml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reichenbach A, Franze K, Agte S, Junek S, Wurm A, Grosche J, Savvinov A, Guck J, Skatchkov SN. Live Cells as Optical Fibers in the Vertebrate Retina. In: Yasin Moh, Harun Sulaiman W, Arof Hamzah., editors. Selected Topics on Optical Fiber Technology. Chapter 10. InTech; 2012. pp. 247–270. http://www.intechopen.com/books/selected-topics-on-optical-fiber-technology/live-cells-as-optical-fibers-in-the-vertebrate-retina- [Google Scholar]
- 28.Grishin EV, Volkova TM, Arsen’ev AS, Reshetova OS, Onoprienko VV. Structural-functional characteristics of argiopine--the ion channel blockers from the spider Argiope lobata venom. Bioorg Khim. 1986;12(8):1121–1124. [PubMed] [Google Scholar]
- 29.Grishin EV, Volkova TM, Arsen’ev AS. Glutamate receptor antagonists from the spider Argiope lobata venom. Bioorg Khim. 1988;14(7):883–892. [PubMed] [Google Scholar]
- 30.Antonov SM, Grishin EV, Magazanik LG, Shupliakov OV, Vesselkin NP, Volkova TM. Argiopin blocks the glutamate responses and sensorimotor transmission in motoneurones of isolated frog spinal cord. Neurosci Lett. 1987;83(1–2):179–184. doi: 10.1016/0304-3940(87)90237-0. [DOI] [PubMed] [Google Scholar]
- 31.Frølund S, Bella A, Kristensen AS, Ziegler HL, Witt M, Olsen CA, Strømgaard K, Franzyk H, Jaroszewski JW. Assessment of structurally diverse philanthotoxin analogues for inhibitory activity on ionotropic glutamate receptor subtypes: discovery of nanomolar, nonselective, and use-dependent antagonists. J Med Chem. 2010;53(20):7441–7451. doi: 10.1021/jm100886h. [DOI] [PubMed] [Google Scholar]
- 32.Skatchkov SN, Rojas L, Eaton MJ, Orkand RK, Biedermann B, Bringmann A, Pannicke Th, Veh RW, Reichenbach A. Functional expression of Kir 6.1/SUR1-Katp channels in frog retinal Müller glial cells. Glia. 2002;38:256–267. doi: 10.1002/glia.10073. [DOI] [PubMed] [Google Scholar]
- 33.Kucheryavykh YV, Shuba YM, Antonov SM, Inyushin MY, Pearson WL, Kurata H, Cubano L, Reichenbach A, Veh RW, Nichols CG, Eaton MJ, Skatchkov SN. Complex rectification of Müller cell Kir currents. Glia. 2008;56:775–790. doi: 10.1002/glia.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams K. Modulation and block of ion channels: A new biology of polyamines. Cell Signal. 1997;9:1–13. doi: 10.1016/s0898-6568(96)00089-7. [DOI] [PubMed] [Google Scholar]
- 35.Haghighi AP, Cooper E. Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. J Neurosci. 1998;18:4050–4062. doi: 10.1523/JNEUROSCI.18-11-04050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 37.Fakler B, Brandle U, Glowatzki E, Weidemann S, Zenner HP, Ruppersberg JP. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995;13:149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- 38.Ahern GP, Wang X, Miyares RL. Polyamines are potent ligands for the capsaicin receptor TRPV1. JBC. 2006;281:8991–8995. doi: 10.1074/jbc.M513429200. [DOI] [PubMed] [Google Scholar]
- 39.Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- 40.Fakler B, Brandle U, Bond C, Glowatzki E, Konig C, Adelman JP, Zenner H-P, Ruppersberg JP. A structural determinant of differentially sensitivity of cloned inward rectifier K+ channels to intracellular spermine. FEBS Lett. 1994;356:199–203. doi: 10.1016/0014-5793(94)01258-x. [DOI] [PubMed] [Google Scholar]
- 41.Shao Z, Mellor IR, Brierley MJ, Harris J, Usherwood PN. Potentiation and inhibition of nicotinic acetylcholine receptors by spermine in the TE671 human muscle cell line. J Pharmacol Exp Ther. 1998;186:1269–1276. [PubMed] [Google Scholar]
- 42.Koh DS, Burnashev N, Jonas P. Block of native Ca(2+)-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol. 1995;486:305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koenig H, Goldstone AD, Lu CY, Trout JJ. Brain polyamines are controlled by N-methyl-D-aspartate receptors during ischemia and recirculation. Stroke. 1990;21:III98–102. [PubMed] [Google Scholar]
- 44.Lynch JW. Rectification of the olfactory cyclic nucleotide-gated channel by intracellular polyamines. J Membr Biol. 1999;170:213–227. doi: 10.1007/s002329900551. [DOI] [PubMed] [Google Scholar]
- 45.Huang CJ, Moczydlowski E. Cytoplasmic polyamines as permeant blockers and modulators of the voltage-gated sodium channel. Biophys J. 2001;80:1262–1279. doi: 10.1016/S0006-3495(01)76102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antonov SM, Gmiro VE, Johnson JW. Binding sites for permeant ions in the channel of NMDA receptors and their effects on channel block. Nat Neurosci. 1998;1(6):451–461. doi: 10.1038/2167. [DOI] [PubMed] [Google Scholar]
- 47.Davies MS, Baganoff MP, Grishin EV, Lanthorn TH, Volkova TM, Watson GB, Wiegand RC. Polyamine spider toxins are potent un-competitive antagonists of rat cortex excitatory amino acid receptors. Eur J Pharmacol. 1992;227(1):51–56. doi: 10.1016/0922-4106(92)90141-h. [DOI] [PubMed] [Google Scholar]
- 48.Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca(2+)-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci USA. 1995;92:9298–9302. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vataev SI, Oganesian GA, Lukomskaia NIa, Magazanik LG. The action of ionotropic glutamate receptor channel blockers on effects of sleep deprivation in rats. Ross Fiziol Zh Im I M Sechenova. 2013;99(5):575–585. [PubMed] [Google Scholar]
- 50.Abushik PA, Sibarov DA, Eaton MJ, Skatchkov SN, Antonov SM. Kainate-induced calcium overload of cortical neurons in vitro: Dependence on expression of AMPAR GluA2-subunit and down-regulation by subnanomolar ouabain. Cell Calcium. 2013;54(2):95–104. doi: 10.1016/j.ceca.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benveniste M, Mayer ML. Multiple effects of spermine on N-methyl-D-aspartic acid receptor responses of rat cultured hippocampal neurones. J Physiol. 1993;464:131–163. doi: 10.1113/jphysiol.1993.sp019627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bahring R, Bowie D, Benveniste M, Mayer ML. Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J Physiol. 1997;502:575–589. doi: 10.1111/j.1469-7793.1997.575bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Araneda RC, Lan JY, Zheng X, Zukin RS, Bennett MV. Spermine and arcaine block and permeate N-methyl-D-aspartate receptor channels. Biophys J. 1999;76:28999–2911. doi: 10.1016/S0006-3495(99)77445-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quinn SJ, Ye CP, Diaz R, Kifor O, Bai M, Vassilev P, Brown E. The Ca2+-sensing receptor: a target for polyamines. Am J Physiol. 1997;273:C1315–C1323. doi: 10.1152/ajpcell.1997.273.4.C1315. [DOI] [PubMed] [Google Scholar]
- 55.Jiang X, Newell EW, Schlichter LC. Regulation of a TRPM7-like current in rat brain microglia. J Biol Chem. 2003;278:42867–42876. doi: 10.1074/jbc.M304487200. [DOI] [PubMed] [Google Scholar]
- 56.Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature. 1999;401:594–598. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
- 57.Isa T, Iion M, Itazawa S, Ozawa S. Spermine mediates inward rectification of Ca(2+)-permeable AMPA receptor channels. Neuroreport. 1996;6:2045–2048. doi: 10.1097/00001756-199510010-00022. [DOI] [PubMed] [Google Scholar]
- 58.Mott DD, Washburn MS, Zhang S, Dingledine RJ. Subunit-dependent modulation of kainate receptors by extracellular protons and polyamines. J Neurosci. 2003;23:1179–1188. doi: 10.1523/JNEUROSCI.23-04-01179.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindquist TD, Sturman JA, Gould RM, Ingoglia NA. Axonal transport of polyamines in intact and regenerating axons of the rat sciatic nerve. J Neurochem. 1985;44:1913–1919. doi: 10.1111/j.1471-4159.1985.tb07187.x. [DOI] [PubMed] [Google Scholar]
- 60.Gilad GM, Balakrishnan K, Gilad VH. The course of putrescine immunocytochemical appearance in neurons, astroglia and microglia in rat brain cultures. Neurosci Lett. 1999;268:33–36. doi: 10.1016/s0304-3940(99)00375-4. [DOI] [PubMed] [Google Scholar]
- 61.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 62.Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 63.Bacaj T, Tevlin M, Lu Y, Shaham S. Glia are essential for sensory organ function in C. elegans. Science. 2008;322:744–747. doi: 10.1126/science.1163074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keyser DO, Pellmar TC. Synaptic transmission in the hippocampus: critical role of glia. Glia. 1994;10:237–243. doi: 10.1002/glia.440100402. [DOI] [PubMed] [Google Scholar]
- 65.Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia. 1997;21:106–113. [PubMed] [Google Scholar]
- 66.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 67.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468(7321):232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norenberg MD, Neary JT, Bender AS, Dombro RS. Hepatic encephalopathy: a disorder in glial-neuronal communication. Prog Brain Res. 1992;94:261–269. doi: 10.1016/s0079-6123(08)61756-2. [DOI] [PubMed] [Google Scholar]
- 69.Dubois-Dauphin M, Poitry-Yamate C, de Bilbao F, Julliard AK, Jourdan F, Donati G. Early postnatal Müller cell death leads to retinal but not optic nerve degeneration in NSE-Hu-Bcl-2 transgenic mice. Neuroscience. 2000;95:9– 21. doi: 10.1016/s0306-4522(99)00313-9. [DOI] [PubMed] [Google Scholar]
- 70.Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- 71.Newman EA, Frambach DA, Odette LL. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science. 1984;225(4667):1174–1175. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kucheryavykh YV, Kucheryavykh LY, Nichols CG, Maldonado HM, Baksi K, Reichenbach A, Skatchkov SN, Eaton MJ. Downregulation of Kir4.1 Inward Rectifying Potassium Channel Subunits by RNAi Impairs Potassium Transfer and Glutamate Uptake by Cultured Cortical Astrocytes. Glia. 2007;55:274–28. doi: 10.1002/glia.20455. [DOI] [PubMed] [Google Scholar]
- 74.Djukic B, Casper JB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27:11354– 11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci U S A. 2003;100(11):6789–6794. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Newman EA. Glial control of synaptic transmission in the retina. Glia. 2004;47:268–274. doi: 10.1002/glia.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signaling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 79.Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- 80.Ye Z-C, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J Neurosci. 2003;23:3588– 3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 82.Filosa JA, Bonev AD, Straub SV, Meredith AI, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 83.Metea MR, Kofuji P, Newman EA. Neurovascular coupling is not mediated by potassium siphoning from glial cells. J Neurosci. 2007;27:2468–2471. doi: 10.1523/JNEUROSCI.3204-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller RF. D-Serine as a glial modulator of nerve cells. Glia. 2005;47(3):275–283. doi: 10.1002/glia.20073. [DOI] [PubMed] [Google Scholar]
- 85.Sullivan SJ, Miller RF. AMPA receptor-dependent, light-evoked D-serine release acts on retinal ganglion cell NMDA receptors. J Neurophysiol. 2012;108(4):1044–1051. doi: 10.1152/jn.00264.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Viviani B, Corsini E, Galli CL, Marinovich M. Glia increase degeneration of hippocampal neurons through release of tumor necrosis factor-alpha. Toxicol Appl Pharmacol. 1998;150(2):271–276. doi: 10.1006/taap.1998.8406. [DOI] [PubMed] [Google Scholar]
- 87.Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57(4):343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burnashev N. Dynamic modulation of AMPA receptor mediated synaptic transmission by polyamines in principal neurons. Focus on polyamines modulate AMPA receptor-dependent synaptic response in immature layer V pyramidal neurons. J Neurophysiol. 2005;93:2371–2386. doi: 10.1152/jn.01297.2004. [DOI] [PubMed] [Google Scholar]
- 89.Washburn MS, Numberger M, Zhang S, Dingledine R. Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J Neurosci. 1997;17:9393–9406. doi: 10.1523/JNEUROSCI.17-24-09393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noh KM, Yokota H, Mashiko T, Castillo PE, Zukin RS, Bennett MV. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc Natl Acad Sci U S A. 2005;102:12230–12235. doi: 10.1073/pnas.0505408102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mulle C, Sailer A, Swanson GT, Brana C, O’Gorman S, Bettler B, Heinemann SF. Subunit composition of kainate receptors in hippocampal interneurons. Neuron. 2000;28:475–484. doi: 10.1016/s0896-6273(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 92.Liu B, Liao M, Mielke JG, Ning K, Chen Y, Li L, Hayek YH, Gomez E, Zukin RS, Fehlings MG. Ischemic insults direct glutamate receptor subunit 2-lacking AMPA receptors to synaptic sites. J Neurosci. 2006;26:5309–5319. doi: 10.1523/JNEUROSCI.0567-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bell JD, Ai J, Chen Y, Baker A. Mild in vitro trauma induces rapid GluR-2 endocytosis, robustly augments calcium permeability and enhances susceptibility to secondary excitotoxic insult in cultured Purkinje cells. Brain. 2007;130:2528–2542. doi: 10.1093/brain/awm164. [DOI] [PubMed] [Google Scholar]
- 94.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- 95.Bowie D. Redefining the classification of AMPA-selective ionotropic glutamate receptors. J Physiol. 2012;590(Pt 1):49–61. doi: 10.1113/jphysiol.2011.221689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sibarov DA, Bolshakov AE, Abushik PA, Krivoi II, Antonov SM. Na+,K+-ATPase functionally interacts with the plasma membrane Na+,Ca2+ exchanger to prevent Ca2+ overload and neuronal apoptosis in excitotoxic stress. J Pharmacol Exp Ther. 2012;343(3):596–607. doi: 10.1124/jpet.112.198341. [DOI] [PubMed] [Google Scholar]
- 97.Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Fröhlich K-U, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11(11):1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 98.Soda K, Dobashi Y, Kano Y, Tsujinaka S, Konishi F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp Gerontol. 2009;44(11):727–732. doi: 10.1016/j.exger.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 99.Soda K. Polyamine intake, dietary pattern, and cardiovascular disease. Med Hypotheses. 2010;75(3):299–301. doi: 10.1016/j.mehy.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 100.Soda K, Kano Y, Chiba F, Koizumi K, Miyaki Y. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS One. 2013;8(5):e64357. doi: 10.1371/journal.pone.0064357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, Kramer JM, Liu KSY, Schroeder S, Stunnenberg HG, Sinner F, Magnes C, Pieber TR, Dipt S, Fiala A, Schenck A, Schwaerzel M, Madeo F, Sigrist SJ. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci. 2013;16:1453–1460. doi: 10.1038/nn.3512. [DOI] [PubMed] [Google Scholar]
- 102.Liu P, Fleete MS, Jing Y, Collie ND, Curtis MA, Waldvogel HJ, Faull RL, Abraham WC, Zhang H. Altered arginine metabolism in Alzheimer’s disease brains. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.03.013. pii: S0197–4580(14)00267-X. [DOI] [PubMed] [Google Scholar]
- 103.Cervós-Navarro J, Diemer NH. Selective vulnerability in brain hypoxia. Crit Rev Neurobiol. 1991;6:149–182. [PubMed] [Google Scholar]
- 104.Velloso NA, Dalmolin GD, Fonini G, Gindri Sinhorin VD, Ferreira da Silveira A, Rubin MA, Mello CF. Spermine attenuates behavioral and biochemical alterations induced by quinolinic acid in the striatum of rats. Brain Res. 2008;1198:107–114. doi: 10.1016/j.brainres.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 105.Benedikt JM, Inyushin M, Kucheryavykh YV, Rivera Y, Kucheryavykh LY, Nichols CG, Eaton MJ, Skatchkov SN. Intracellular polyamines enhance astrocytic coupling. Neuroreport. 2012;23(17):1021–1025. doi: 10.1097/WNR.0b013e32835aa04b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shalaby IA, Chenard BL, Prochniak MA, Butler TW. Neuroprotective effects of the N-methyl-D-aspartate receptor antagonists ifenprodil and SL-82,0715 on hippocampal cells in culture. J Pharmacol Exp Ther. 1992;260:925–932. [PubMed] [Google Scholar]
- 107.Sparapani M, Dall’Olio R, Gandolfi O, Ciani E, Contestabile A. Neurotoxicity of polyamines and pharmacological neuroprotection in cultures of rat cerebellar granule cells. Exp Neurol. 1997;148:157–166. doi: 10.1006/exnr.1997.6627. [DOI] [PubMed] [Google Scholar]
- 108.Adachi K, Izumi M, Osano Y, Miura N, Takatsu S, Terao S, Mitsuma T. Polyamine concentrations in the brain of vitamin B12-deficient rats. Exp Biol Med. 2003;228:1069–1071. doi: 10.1177/153537020322800913. [DOI] [PubMed] [Google Scholar]
- 109.Ferchmin PA, Pérez D, Biello M. Spermine is neuroprotective against anoxia and N-methyl-D-aspartate in hippocampal slices. Brain Res. 2000;859:273–279. doi: 10.1016/s0006-8993(00)01973-9. [DOI] [PubMed] [Google Scholar]
- 110.Gilad GM, Gilad VH. Polyamines can protect against ischemia-induced nerve cell death in gerbil forebrain. Exp Neurol. 1991;111:349–355. doi: 10.1016/0014-4886(91)90102-i. [DOI] [PubMed] [Google Scholar]
- 111.Watanabe S, Kusama-Eguchi K, Kobayashi H, Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J Biol Chem. 1991;266:20803–20809. [PubMed] [Google Scholar]
- 112.Seiler N. Formation, catabolism and properties of the natural polyamines. In: Carter C, editor. The Neuropharmacology of Polyamines. New York/London: Academic; Harcourt Brace; 1994. pp. 1–36. [Google Scholar]
- 113.Seiler N, Delcros JG, Moulinoux JP. Polyamine transport in mammalian cells. An update. Int J Biochem Cell Biol. 1996;28:843–861. doi: 10.1016/1357-2725(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 114.Masuko T, Kusama-Eguchi K, Sakata K, Kusama T, Chaki S, Okuyama S, Williams K, Kashiwagi K, Igarashi K. Polyamine transport, accumulation and release in brain. J Neurochem. 2003;84:610–617. doi: 10.1046/j.1471-4159.2003.01558.x. [DOI] [PubMed] [Google Scholar]
- 115.Ingoglia NA, Sturman JA, Eisner RA. Axonal transport of putrescine, spermidine and spermine in normal and regenerating goldfish optic nerve. Brain Res 1977. 1977;130:433–445. doi: 10.1016/0006-8993(77)90107-x. [DOI] [PubMed] [Google Scholar]
- 116.Ingoglia NA, Sharma SC, Pilchman J, Baranowski K, Sturman JA. Axonal transport and transcellular transfer of nucleosides and polyamines in intact and regenerating optic nerves of goldfish: speculation on the axonal regulation of periaxonal cell metabolism. J Neurosci. 1982a;2:1412–1423. doi: 10.1523/JNEUROSCI.02-10-01412.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ingoglia NA, Sturman JA, Jaggard P, Perez C. Association of spermine and 4S RNA during axonal transport in regenerating optic nerves of goldfish. Brain Res. 1982b;238:341–351. doi: 10.1016/0006-8993(82)90109-3. [DOI] [PubMed] [Google Scholar]
- 118.Cohen SS. Introduction to the Polyamines. Prentice-Hall, Inc; Englewood Cliffs, New Jersey: 1971. [Google Scholar]
- 119.Wallace HM. The polyamines: past, present and future. Essays Biochem. 2009;46:1–9. doi: 10.1042/bse0460001. [DOI] [PubMed] [Google Scholar]
- 120.Krauss M, Langnaese K, Richter K, Brunk I, Weiske M, Ahnert-Helger G, Veh RW, Laube G. Spermidine synthase is prominently expressed in the striatal patch compartment and in putative interneurons of matrix compartments. J Neurochem. 2006;1:174–189. doi: 10.1111/j.1471-4159.2006.03721.x. [DOI] [PubMed] [Google Scholar]
- 121.Krauss M, Weiss T, Langnaese K, Richter K, Kowski A, Veh RW, Laube G. Cellular and subcellular rat brain spermidine synthase expression patterns suggest region-specific roles for polyamines, including cerebellar pre-synaptic function. J Neurochem. 2007;103:679–693. doi: 10.1111/j.1471-4159.2007.04770.x. [DOI] [PubMed] [Google Scholar]
- 122.Gilad GM, Gilad VH. Polyamine uptake, binding and release in rat brain. Eur J Pharmacol. 1991b;193(1):41–46. doi: 10.1016/0014-2999(91)90198-y. [DOI] [PubMed] [Google Scholar]
- 123.Laschet J, Grisar T, Bureau M, Guillaume D. Characteristics of putrescine uptake and subsequent GABA formation in primary cultured astrocytes from normal C57BL/6J and epileptic DBA/2J mouse brain cortices. Neuroscience. 1992;48:151–157. doi: 10.1016/0306-4522(92)90345-3. [DOI] [PubMed] [Google Scholar]
- 124.Laschet J, Trottier S, Leviel V, Guibert B, Bansard JY, et al. Heterogeneous distribution of polyamines in temporal lobe epilepsy. Epilepsy Res. 1999;35:161–172. doi: 10.1016/s0920-1211(99)00009-1. [DOI] [PubMed] [Google Scholar]
- 125.Seiler N, Delcros JG, Moulinoux JP. Polyamine transport in mammalian cells. An update. Int J Biochem Cell Biol. 1996;28:843–861. doi: 10.1016/1357-2725(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 126.Dot J, Lluch M, Blanco I, Rodriguez-Alvarez J. Polyamine uptake in cultured astrocytes: Characterization and modulation by protein kinases. J Neurochem. 2000;75:1917–1926. doi: 10.1046/j.1471-4159.2000.0751917.x. [DOI] [PubMed] [Google Scholar]
- 127.Dot J, Danchev N, Blanco I, Rodriguez-Alvarez J. Polyamine uptake is necessary for a normal biochemical maturation of astrocytes in culture. NeuroReport. 2002;13:1083–1087. doi: 10.1097/00001756-200206120-00022. [DOI] [PubMed] [Google Scholar]
- 128.De La Hera DP, Corradi GR, Adamo HP, De Tezanos Pinto F. Parkinson’s disease-associated human P5B-ATPase ATP13A2 increases spermidine uptake. Biochem J. 2013;450(1):47–53. doi: 10.1042/BJ20120739. [DOI] [PubMed] [Google Scholar]
- 129.Valentino TL, Lukasiewicz PD, Romano C. Immunocytochemical localization of polyamines in tiger salamander retina. Brain Res. 1996;713:278–285. doi: 10.1016/0006-8993(95)01558-2. [DOI] [PubMed] [Google Scholar]
- 130.Cintra A, Fuxe K, Agnati LF, Persson L, Harfstrand A, Zoli M, Eneroth P, Zini I. Evidence for the existence of ornithine decarboxylase-immunoreactive neurons in the rat brain. Neurosci Lett. 1987;76:269–274. doi: 10.1016/0304-3940(87)90413-7. [DOI] [PubMed] [Google Scholar]
- 131.Dorn A, Müller M, Bernstein HG, Pajunen A, Jarvinen M. Immunohistochemical localization of L-ornithine decarboxylase in developing rat brain. Int J Dev Neurosci. 1987;5:145–150. doi: 10.1016/0736-5748(87)90060-8. [DOI] [PubMed] [Google Scholar]
- 132.Fujiwara K, Bai G, Kitagawa T. Polyamine-like immunoreactivity in rat neurons. Brain Res. 1997;767:166–171. doi: 10.1016/s0006-8993(97)00748-8. [DOI] [PubMed] [Google Scholar]
- 133.Soulet D, Gagnon B, Rivest S, Audette M, Poulin R. A fluorescent probe of polyamine transport accumulates into intracellular acidic vesicles via a two-step mechanism. J Biol Chem. 2004;279(47):49355–49366. doi: 10.1074/jbc.M401287200. [DOI] [PubMed] [Google Scholar]
- 134.Poulin R, Casero RA, Soulet D. Recent advances in the molecular biology of metazoan polyamine transport. Amino Acids. 2012;42(2–3):711–723. doi: 10.1007/s00726-011-0987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. 2008;267(1–2):3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 136.Ricci G, Volpi L, Pasquali L, Petrozzi L, Siciliano G. Astrocyte-neuron interactions in neurological disorders. J Biol Phys. 2009;35(4):317–336. doi: 10.1007/s10867-009-9157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Suzuki M, Van Paesschen W, Stalmans I, Horita S, Yamada H, Bergmans BA, Legius E, Riant F, De Jonghe P, Li Y, Sekine T, Igarashi T, Fujimoto I, Mikoshiba K, Shimadzu M, Shiohara M, Braverman N, Al-Gazali L, Fujita T, Seki G. Defective membrane expression of the Na(+)-HCO(3)(−) cotransporter NBCe1 is associated with familial migraine. Proc Natl Acad Sci U S A. 2010;107(36):15963–15968. doi: 10.1073/pnas.1008705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kettenmann Helmut, Ransom Bruce R. Neuroglia. 3. Oxford University Press; 2012. [Google Scholar]
- 139.Cooper MS. Intercellular signaling in neuronal-glial networks. Biosystems. 1995;34(1–3):65–85. doi: 10.1016/0303-2647(94)01450-l. [DOI] [PubMed] [Google Scholar]
- 140.Cooper ZD, Jones JD, Comer SD. Glial modulators: a novel pharmacological approach to altering the behavioral effects of abused substances. Expert Opin Investig Drugs. 2012;21(2):169–178. doi: 10.1517/13543784.2012.651123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78(1):99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- 142.Verkhratsky A, Noda M, Parpura V, Kirischuk S. Sodium fluxes and astroglial function. Adv Exp Med Biol. 2013a;961:295–305. doi: 10.1007/978-1-4614-4756-6_25. [DOI] [PubMed] [Google Scholar]
- 143.Verkhratsky A, Rodríguez JJ, Parpura V. Astroglia in neurological diseases. Future Neurol. 2013b;8(2):149–158. doi: 10.2217/fnl.12.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Verkhratsky A, Rodríguez JJ, Steardo L. Astrogliopathology: A Central Element of Neuropsychiatric Diseases? Neuroscientist 2013. 2013c Dec 3; doi: 10.1177/1073858413510208. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 145.Seidl R, Beninati S, Cairns N, Singewald N, Risser D, Bavan H, Nemethova M, Lubec G. Polyamines in frontal cortex of patients with Down syndrome and Alzheimer disease. Neurosci Lett. 1996;206(2–3):193–195. doi: 10.1016/s0304-3940(96)12451-4. [DOI] [PubMed] [Google Scholar]
- 146.Seitz R, Ohlmann A, Tamm ER. The role of Müller glia and microglia in glaucoma. Cell Tissue Res. 2013;353(2):339–345. doi: 10.1007/s00441-013-1666-y. [DOI] [PubMed] [Google Scholar]
- 147.Turner JR, Ecke LE, Briand LA, Haydon PG, Blendy JA. Cocaine-related behaviors in mice with deficient gliotransmission. Psychopharmacology (Berl) 2013;226(1):167–176. doi: 10.1007/s00213-012-2897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dalkara T, Kiliç K. How does fasting trigger migraine? A hypothesis. Curr Pain Headache Rep. 2013;17(10):368. doi: 10.1007/s11916-013-0368-1. [DOI] [PubMed] [Google Scholar]
- 149.Zeidán-Chuliá F, Salmina AB, Malinovskaya NA, Noda M, Verkhratsky A, Moreira JC. The glial perspective of autism spectrum disorders. Neurosci Biobehav Rev. 2014;38:160–172. doi: 10.1016/j.neubiorev.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 150.Scofield MD, Kalivas PW. Astrocytic Dysfunction and Addiction: Consequences of Impaired Glutamate Homeostasis. Neuroscientist. 2014 doi: 10.1177/1073858413520347. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Clasadonte J, McIver SR, Schmitt LI, Halassa MM, Haydon PG. Chronic sleep restriction disrupts sleep homeostasis and behavioral sensitivity to alcohol by reducing the extracellular accumulation of adenosine. J Neurosci. 2014;34(5):1879–1891. doi: 10.1523/JNEUROSCI.2870-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jo WK, Law AC, Chung SK. The neglected co-star in the dementia drama: the putative roles of astrocytes in the pathogeneses of major neurocognitive disorders. Mol Psychiatry. 2014;19(2):159–167. doi: 10.1038/mp.2013.171. [DOI] [PubMed] [Google Scholar]
- 153.van Leeuwenhoek A. Observationes D. Anthonii Leeuwenhoek, de natis e semine genitali animalculis. Letter dated November 1677. Philos Trans Roy Soc London. 1678;12:1040–1043. [Google Scholar]
- 154.Dudley HW, Rosenheim O, Starling WW. The chemical constitution of spermine. III. Structure and synthesis. Biochemical Journal. 1926;20:1082–1094. doi: 10.1042/bj0201082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bachrach U. The early history of polyamine research. Plant Physiol Biochem. 2010;48(7):490–495. doi: 10.1016/j.plaphy.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 156.Redgate ES, Boggs S, Grudziak A, Deutsch M. Polyamines in brain tumor therapy. J Neurooncol. 1995;25:167–179. doi: 10.1007/BF01057761. [DOI] [PubMed] [Google Scholar]
- 157.Minois N. Molecular Basis of the ‘Anti-Aging’ Effect of Spermidine and Other Natural Polyamines - A Mini-Review. Gerontology. 2014 doi: 10.1159/000356748. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 158.LaRocca TJ, Gioscia-Ryan RA, Hearon CM, Jr, Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev. 2013;134(7–8):314–20. doi: 10.1016/j.mad.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19:1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 160.Johnson TD. Modulation of channel function by polyamines. Trends Pharmacol Sci. 1996;17:22–27. doi: 10.1016/0165-6147(96)81566-5. [DOI] [PubMed] [Google Scholar]
- 161.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Ann Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 162.Skatchkov SN, Buldakova S, Kucheryavykh YV, Inyushin M, Veh RW, Reichenbach A, Burnashev N, Eaton MJ. Neuronal network regulation in ca1 hippocampus: role of glial polyamines and hemichannels. J Neurochem. 2006c;96(Suppl 1, Symposium 08):138. [Google Scholar]
- 163.Rubin MA, Boemo RL, Jurach A, Rojas DB, Zanolla GR, Obregon AD, Souza DO, Mello CF. Intrahippocampal spermidine administration improves inhibitory avoidance performance in rats. Behav Pharmacol. 2000;11:57–62. doi: 10.1097/00008877-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 164.Rubin MA, Stiegemeier JA, Volkweis MA, Oliveira DM, Fenili AC, Boemo RL, Jurach A, Mello CF. Intra-amygdala spermidine administration improves inhibitory avoidance performance in rats. Eur J Pharmacol. 2001;423:35–39. doi: 10.1016/s0014-2999(01)01061-5. [DOI] [PubMed] [Google Scholar]
- 165.Rubin MA, Berlese DB, Stiegemeier JA, Volkweis MA, Oliveira DM, dos Santos TL, Fenili AC, Mello CF. Intra-amygdala administration of polyamines modulates fear conditioning in rats. J Neurosci. 2004;24:2328–2334. doi: 10.1523/JNEUROSCI.1622-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Velloso NA, Dalmolin GD, Fonini G, Gindri Sinhorin VD, Ferreira da Silveira A, Rubin MA, Mello CF. Spermine attenuates behavioral and biochemical alterations induced by quinolinic acid in the striatum of rats. Brain Res. 2008;1198:107–114. doi: 10.1016/j.brainres.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 167.Velloso NA, Dalmolin GD, Gomes GM, Rubin MA, Canas PM, Cunha RA, Mello CF. Spermine improves recognition memory deficit in a rodent model of Huntington’s disease. Neurobiol Learn Mem. 2009;92(4):574–580. doi: 10.1016/j.nlm.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 168.Genedani S, Piccinini G, Bertolini A. Putrescine has analgesic activity, in rats. Life Sci. 1984;34:2407–2412. doi: 10.1016/0024-3205(84)90429-6. [DOI] [PubMed] [Google Scholar]
- 169.Kolhekar R, Meller ST, Gephart GF. N-methyl-D-aspartate receptor-mediated changes in thermal nociception: allosteric modulation at glycine and polyamine recognition sites. Neurosci. 1994;63:925–936. doi: 10.1016/0306-4522(94)90560-6. [DOI] [PubMed] [Google Scholar]
- 170.Adibhatla RM, Hatcher JF, Sailor K, Dempsey RJ. Polyamines and central nervous system injury: spermine and spermidine decrease following transient focal cerebral ischemia in spontaneously hypertensive rats. Brain Res. 2002;938:81–86. doi: 10.1016/s0006-8993(02)02447-2. [DOI] [PubMed] [Google Scholar]
- 171.Clarkson AN, Liu H, Pearson L, Kapoor M, Harrison JC, Sammut IA, Jackson DM. Neuroprotective effects of spermine following hypoxic-ischemic-induced brain damage: a mechanistic study. FASEB J 2004. 2004;18:1114–1116. doi: 10.1096/fj.03-1203fje. [DOI] [PubMed] [Google Scholar]
- 172.Yin HZ, Tang DT, Weiss JH. Intrathecal infusion of a Ca(2+)-permeable AMPA channel blocker slows loss of both motor neurons and of the astrocyte glutamate transporter, GLT-1 in a mutant SOD1 rat model of ALS. Exp Neurol. 2007;207:177–185. doi: 10.1016/j.expneurol.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zomkowski AD, Santos AR, Rodrigues AL. Putrescine produces antidepressant-like effects in the forced swimming test and in the tail suspension test in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;30:1419–1425. doi: 10.1016/j.pnpbp.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 175.Bellé NA, Dalmolin GD, Fonini G, Rubin MA, Rocha JB. Polyamines reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res. 2004;1008:245–51. doi: 10.1016/j.brainres.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 176.Shaw GG, Pateman AJ. The regional distribution of the polyamines spermidine and spermine in brain. J Neurochem. 1973;20:1225–1230. doi: 10.1111/j.1471-4159.1973.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 177.Halaris A, Piletz JE. Relevance of imidazoline receptors and agmatine to psychiatry: a decade of progress. Ann N Y Acad Sci. 2003;1009:1–20. doi: 10.1196/annals.1304.001. [DOI] [PubMed] [Google Scholar]
- 178.Peters D, Berger J, Langnaese K, Derst C, Madai VI, Krauss M, Fischer KD, Veh RW, Laube G. Arginase and Arginine Decarboxylase - Where Do the Putative Gate Keepers of Polyamine Synthesis Reside in Rat Brain? PLoS One. 2013;8(6):e66735. doi: 10.1371/journal.pone.0066735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bernstein H-G, Müller M. The cellular localization of L-ornithine decarboxylase-polyamine system in the normal and diseased central nervous system. Prog Neurobiol. 1999;57:485–505. doi: 10.1016/s0301-0082(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 180.Bernstein HG, Steiner J, Bogerts B. Glial cells in schizophrenia: pathophysiological significance and possible consequences for therapy. Expert Rev Neurother. 2009;9(7):1059–1071. doi: 10.1586/ern.09.59. [DOI] [PubMed] [Google Scholar]
- 181.Bernstein HG, Derst C, Stich C, Pruss H, Peters D, et al. The agmatine-degrading enzyme agmatinase: a key to agmatine signaling in rat and human brain? Amino Acids. 2011;40:453–465. doi: 10.1007/s00726-010-0657-5. [DOI] [PubMed] [Google Scholar]
- 182.Bernstein HG, Stich C, Jäger K, Dobrowolny H, Wick M, Steiner J, Veh R, Bogerts B, Laube G. Agmatinase, an inactivator of the putative endogenous antidepressant agmatine, is strongly upregulated in hippocampal interneurons of subjects with mood disorders. Neuropharmacology. 2012;62(1):237–246. doi: 10.1016/j.neuropharm.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 183.Abdulhussein AA, Wallace HM. Polyamines and membrane transporters. Amino Acids. 2014;46(3):655–660. doi: 10.1007/s00726-013-1553-6. [DOI] [PubMed] [Google Scholar]
- 184.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pegg AE. The function of spermine. IUBMB Life. 2014;66(1):8–18. doi: 10.1002/iub.1237. [DOI] [PubMed] [Google Scholar]
- 186.Virgili M, Crochemore C, Pena-Altamira E, Contestabile A. Regional and temporal alterations of ODC/polyamine system during ALS-like neurodegenerative motor syndrome in G93A transgenic mice. Neurochem Int. 2006;48:201–207. doi: 10.1016/j.neuint.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 187.Tracey KJ. The inflammatory reflex. Nature. 2002;429:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 188.Sequeira A, Gwadry FG, Ffrench-Mullen JM, Canetti L, Gingras Y, Casero RA, Jr, Rouleau G, Benkelfat G, Turecki G. Implication of SSAT by gene expression and genetic variation in suicide and major depression. Arch Gen Psychiatry. 2006;63:35–48. doi: 10.1001/archpsyc.63.1.35. [DOI] [PubMed] [Google Scholar]
- 189.Adachi K, Izumi M, Osano Y, Miura N, Takatsu S, Terao S, Mitsuma T. Polyamine concentrations in the brain of vitamin B12-deficient rats. Exp Biol Med. 2003;228:1069–1071. doi: 10.1177/153537020322800913. [DOI] [PubMed] [Google Scholar]
- 190.Withrow C, Ashraf S, O’Leary T, Johnson LR, Fitzgerald MEC, Johnson DA. Effect of Polyamine depletion on Cone photoreceptors of the developing rabbit retina. Invest Ophthalmol Vis Sci 2002. 2002;43:3081–3090. [PubMed] [Google Scholar]
- 191.Jänne J, Alhonen L, Keinänen TA, Pietilä M, Uimari A, Pirinen E, Hyvōnen MT, Järvinen A. Animal disease models generated by genetic engineering of polyamine metabolism. J Cell Mol Med. 2005;9:865–882. doi: 10.1111/j.1582-4934.2005.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jänne J, Alhonen L, Pietilä M, Keinänen TA, Uimari A, Hyvōnen MT, Pirinen E, Järvinen A. Genetic manipulation of polyamine catabolism in rodents. J Biochem. 2006;139:155–160. doi: 10.1093/jb/mvj035. [DOI] [PubMed] [Google Scholar]
- 193.Madai VI, Poller WC, Peters D, Berger J, Paliege K, Bernard R, Veh RW, Laube G. Synaptic localisation of agmatinase in rat cerebral cortex revealed by virtual pre-embedding. Amino Acids. 2012;43(3):1399–1403. doi: 10.1007/s00726-011-1195-5. [DOI] [PubMed] [Google Scholar]
- 194.Gilad GM, Gilad VH, Finberg JP, Rabey JM. Neurochemical evidence for agmatine modulation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity. Neurochem Res. 2005;30(6–7):713–719. doi: 10.1007/s11064-005-6865-9. [DOI] [PubMed] [Google Scholar]
- 195.Inazu M, Takeda H, Maehara K, Miyashita K, Tomoda A, Matsumiya T. Functional expression of the organic cation/carnitine transporter 2 in rat astrocytes. J Neurochem. 2006;97:424–434. doi: 10.1111/j.1471-4159.2006.03757.x. [DOI] [PubMed] [Google Scholar]
- 196.Sala-Rabanal M, Li DC, Inyushin M, Skatchkov SN, Nichols CG. Polyamine Transport by the Polyspecific Organic Cation Transporters OCT1, OCT2 and OCT3. Molecular Pharmaceutics. 2013;10(4):1450–1458. doi: 10.1021/mp400024d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Medeiros R, Laferla FM. Astrocytes: Conductors of the Alzheimer disease neuroinflammatory symphony. Exp Neurol. 2012;239C:133–138. doi: 10.1016/j.expneurol.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 198.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sigrist SJ, Carmona-Gutierrez D, Gupta VK, Bhukel A, Mertel S, Eisenberg T, Madeo F. Spermidine-triggered autophagy ameliorates memory during aging. Autophagy. 2014;10(1):178–179. doi: 10.4161/auto.26918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nilsen LH, Witter MP, Sonnewald U. Neuronal and astrocytic metabolism in a transgenic rat model of Alzheimer’s disease. J Cereb Blood Flow Metab. 2014 doi: 10.1038/jcbfm.2014.37. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Johansson A, Engler H, Blomquist G, Scott B, Wall A, Aquilonius SM, Långström B, Askmark H. Evidence for astrocytosis in ALS demonstrated by [11C](L)-deprenyl-D2 PET. J Neurol Sci. 2007;255(1–2):17–22. doi: 10.1016/j.jns.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 202.Martorana F, Brambilla L, Valori CF, Bergamaschi C, Roncoroni C, Aronica E, Volterra A, Bezzi P, Rossi D. The BH4 domain of Bcl-X(L) rescues astrocyte degeneration in amyotrophic lateral sclerosis by modulating intracellular calcium signals. Hum Mol Genet. 2012;21(4):826–840. doi: 10.1093/hmg/ddr513. [DOI] [PubMed] [Google Scholar]
- 203.Sunyach C, Michaud M, Arnoux T, Bernard-Marissal N, Aebischer J, Latyszenok V, Gouarné C, Raoul C, Pruss RM, Bordet T, Pettmann B. Olesoxime delays muscle denervation, astrogliosis, microglial activation and motoneuron death in an ALS mouse model. Neuropharmacology. 2012;62(7):2346–2352. doi: 10.1016/j.neuropharm.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 204.Messing A, Brenner M, Feany MB, Nedergaard M, Goldman JE. Alexander disease. J Neurosci. 2012;32(15):5017–5023. doi: 10.1523/JNEUROSCI.5384-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vila M, Jackson-Lewis V, Guégan C, Wu DC, Teismann P, Choi DK, Tieu K, Przedborski S. The role of glial cells in Parkinson’s disease. Curr Opin Neurol. 2001;14(4):483–489. doi: 10.1097/00019052-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 206.Rempe DA, Nedergaard M. Targeting glia for treatment of neurological disease. Neurotherapeutics. 2010;7(4):335–337. doi: 10.1016/j.nurt.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cisbani G, Freeman TB, Soulet D, Saint-Pierre M, Gagnon D, Parent M, Hauser RA, Barker RA, Cicchetti F. Striatal allografts in patients with Huntington’s disease: impact of diminished astrocytes and vascularization on graft viability. Brain. 2013;136(Pt 2):433–443. doi: 10.1093/brain/aws359. [DOI] [PubMed] [Google Scholar]
- 208.Chakraborty J, Singh R, Dutta D, Naskar A, Rajamma U, Mohanakumar KP. Quercetin improves behavioral deficiencies, restores astrocytes and microglia, and reduces serotonin metabolism in 3-nitropropionic acid-induced rat model of Huntington’s Disease. CNS Neurosci Ther. 2014;20(1):10–19. doi: 10.1111/cns.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Srivastava R, Aslam M, Kalluri SR, Schirmer L, Buck D, Tackenberg B, Rothhammer V, Chan A, Gold R, Berthele A, Bennett JL, Korn T, Hemmer B. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med. 2012;367:115–123. doi: 10.1056/NEJMoa1110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Palumbo S, Bosetti F. Alterations of brain eicosanoid synthetic pathway in multiple sclerosis and in animal models of demyelination: role of cyclooxygenase-2. Prostaglandins Leukot Essent Fatty Acids 2013. 2013 Oct;89(5):273–278. doi: 10.1016/j.plefa.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 211.Hinterkeuser S, Schroder W, Hager JG, Seifert G, Blumcke I, Elger CE, Schramm J, Steinhäuser C. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur J Neurosci. 2000;12:2087–2096. doi: 10.1046/j.1460-9568.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- 212.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van’t Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Inyushin M, Kucheryavykh LY, Kucheryavykh YV, Nichols CG, Buono RJ, Ferraro TN, Skatchkov SN, Eaton MJ. Potassium Channel Activity and Glutamate Uptake are Impaired in Astrocytes of Seizure Susceptible DBA/2 Mice. Epilepsia. 2010;51(9):1707–1713. doi: 10.1111/j.1528-1167.2010.02592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sala-Rabanal M, Kucheryavykh LY, Skatchkov SN, Eaton MJ, Nichols CG. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4.1 (KCNJ10) J Biol Chem. 2010;285:36040–36048. doi: 10.1074/jbc.M110.163170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wallraff A, Köhling R, Heinemann U, Theis M, Willecke K, Steinhauser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 2006;26:5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Steinhäuser C, Seifert G, Bedner P. Astrocyte dysfunction in temporal lobe epilepsy: K+ channels and gap junction coupling. Glia. 2012;60:1192–1202. doi: 10.1002/glia.22313. [DOI] [PubMed] [Google Scholar]
- 217.Steinhäuser C, Boison D. Epilepsy: crucial role for astrocytes. Glia. 2012;60(8):1191. doi: 10.1002/glia.22300. [DOI] [PubMed] [Google Scholar]
- 218.Boison D. Adenosine dysfunction in epilepsy. Glia. 2012;60(8):1234–1243. doi: 10.1002/glia.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hines DJ, Schmitt LI, Hines RM, Moss SJ, Haydon PG. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl Psychiatry. 2013;3:e212. doi: 10.1038/tp.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Takano K, Ogura M, Nakamura Y, Yoneda Y. Neuronal and glial responses to polyamines in the ischemic brain. Curr Neurovasc Res. 2005;2(3):213–223. doi: 10.2174/1567202054368335. [DOI] [PubMed] [Google Scholar]
- 221.D’Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Impaired K(+) homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci. 1999;19(18):8152–8162. doi: 10.1523/JNEUROSCI.19-18-08152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang RL, Zhang ZG, Wang Y, LeTourneau Y, Liu XS, Zhang X, Gregg SR, Wang L, Chopp M. Stroke induces ependymal cell transformation into radial glia in the subventricular zone of the adult rodent brain. J Cereb Blood Flow Metab. 2007;27(6):1201–1212. doi: 10.1038/sj.jcbfm.9600430. [DOI] [PubMed] [Google Scholar]
- 223.Kucheryavykh LY, Kucheryavykh YV, Inyushin M, Shuba YM, Sanabria P, Cubano LA, Skatchkov SN, Eaton MJ. Ischemia increases TREK-2 channel expression in astrocytes: Relevance to glutamate clearance. The Open Neuroscience Journal. 2009;3:40–47. doi: 10.2174/1874082000903010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Quirié A, Demougeot C, Bertrand N, Mossiat C, Garnier P, Marie C, Prigent-Tessier A. Effect of stroke on arginase expression and localization in the rat brain. Eur J Neurosci. 2013;37(7):1193–1202. doi: 10.1111/ejn.12111. [DOI] [PubMed] [Google Scholar]
- 225.Sword J, Masuda T, Croom D, Kirov SA. Evolution of neuronal and astroglial disruption in the peri-contusional cortex of mice revealed by in vivo two-photon imaging. Brain 2013. 2013;136(Pt 5):1446–1461. doi: 10.1093/brain/awt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhang M, Caragine T, Wang H, Cohen PS, Botchkina G, Soda K, Bianchi M, Ulrich P, Cerami A, Sherry B, Tracey KJ. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med. 1997;185(10):1759–68. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhang M, Borovikova LV, Wang H, Metz C, Tracey KJ. Spermine inhibition of monocyte activation and inflammation. Mol Med. 1999;55:595–605. [PMC free article] [PubMed] [Google Scholar]
- 228.Olsen ML, Sontheimer H. Mislocalization of Kir channels in malignant glia. Glia. 2004;46(1):63–73. doi: 10.1002/glia.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem. 2008;107:589–601. doi: 10.1111/j.1471-4159.2008.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kucheryavykh L, Rolón-Reyes K, Kucheryavykh Y, Skatchkov S, Eaton MJ, Sanabria P, Wessinger WD, Inyushin M. Glioblastoma development in mouse brain: general reduction of OCTs and mislocalization of OCT3 transporter and subsequent uptake of ASP+ substrate to the nuclei. J Neurosci Neuroengineer. 2014 doi: 10.1166/jnsne.2014.1091. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012 doi: 10.1016/j.cancergen.2012.10.009. S2210–7762(12)00260-00268. [DOI] [PubMed] [Google Scholar]
- 232.Nelson PG, McCune SK, Ades AM, Nelson KB. Glial-neurotrophic mechanisms in Down syndrome. J Neural Transm Suppl. 2001;1(61):85–94. doi: 10.1007/978-3-7091-6262-0_7. [DOI] [PubMed] [Google Scholar]
- 233.Cason AL, Ikeguchi Y, Skinner C, Wood TC, Holden KR, Lubs HA, Martinez F, Simensen RJ, Stevenson RE, Pegg AE, Schwartz CE. X-linked spermine synthase gene (SMS) defect: the first polyamine deficiency syndrome. Eur J Hum Genet. 2003;11:937–944. doi: 10.1038/sj.ejhg.5201072. [DOI] [PubMed] [Google Scholar]
- 234.Ikeguchi Y, Bewley MC, Pegg AE. Aminopropyltransferases: function, structure and genetics. J Biochem. 2006;139:1–9. doi: 10.1093/jb/mvj019. [DOI] [PubMed] [Google Scholar]
- 235.Scholl UI, Choi M, Liu T, Ramaekers VT, Häusler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A. 2009;106(14):5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Iliff JJ, Nedergaard M. A link between glial Ca2+ signaling and hypoxia in aging? J Cereb Blood Flow Metab. 2013;33(2):170. doi: 10.1038/jcbfm.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kilpeläinen P, Rybnikova E, Hietala O, Pelto-Huikko M. Expression of ODC and its regulatory protein antizyme in the adult rat brain. J Neurosci Res. 2000;62:675–685. doi: 10.1002/1097-4547(20001201)62:5<675::AID-JNR6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 238.Junttila T, Hietanen-Peltola M, Rechardt L, Persson L, Hökfelt R, Pelto-Huikko M. Ornithine decarboxylase-like immunoreactivity in rat spinal motoneurons and motoric nerves. Brain Res. 1993;609:149–153. doi: 10.1016/0006-8993(93)90867-m. [DOI] [PubMed] [Google Scholar]
- 239.Stout CE, Costatin JL, Naus CCG, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 240.Contreras JE, Sáez JC, Bukauskas FF, Bennett MV. Functioning of Cx43 hemichannels demonstrated by single channel properties. Cell Commun Adhes. 2003;10:245–249. doi: 10.1080/cac.10.4-6.245.249. [DOI] [PubMed] [Google Scholar]
- 241.Pannicke T, Faude F, Reichenbach A, Reichelt W. A function of delayed rectifier potassium channels in glial cells: maintenance of an auxiliary membrane potential under pathological conditions. Brain Res. 2000;862:187–193. doi: 10.1016/s0006-8993(00)02144-2. [DOI] [PubMed] [Google Scholar]
- 242.Zhou M, Kimelberg HK. Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression. J Neurosci. 2001;21:7901–7908. doi: 10.1523/JNEUROSCI.21-20-07901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 244.Huang Y, Grinspan JB, Abrams CK, Scherer SS. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia. 2007;55:46–56. doi: 10.1002/glia.20435. [DOI] [PubMed] [Google Scholar]
- 245.Makarov V, Kucheryavykh L, Kucheryavykh Y, Rivera A, Eaton MJ, Skatchkov SN, Inyushin M. Transport Reversal during Heteroexchange: A Kinetic Study. J Biophys. 2013;2013:683256. doi: 10.1155/2013/683256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- 247.Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sulzer D. How Addictive Drugs Disrupt Presynaptic Dopamine Neurotransmission. Neuron. 2011;69(4):628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kucheryavykh YV, Pearson WL, Kurata H, Eaton MJ, Skatchkov SN, Nichols CG. Unique features of Kir4.1 channel rectification. Channels. 2007;1:172–178. doi: 10.4161/chan.4389. [DOI] [PubMed] [Google Scholar]
- 250.Lalo U, Palygin O, Rasooli-Nejad S, Andrew J, Haydon PG, Pankratov Y. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 2014;12(1):e1001747. doi: 10.1371/journal.pbio.1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang F, Smith NA, Xu Q, Goldman S, Peng W, Huang JH, Takano T, Nedergaard M. Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J Neurosci. 2013;33(44):17404–17412. doi: 10.1523/JNEUROSCI.2178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bennett MVL, Contreras JE, Bukauskas FF, Sáez JC. New roles for astrocytes: Gap junstion hemichannels have something to communicate. TINS. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bennett MV, Garré JM, Orellana JA, Bukauskas FF, Nedergaard M, Sáez JC. Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 2012;1487:3–15. doi: 10.1016/j.brainres.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Enkvetchakul D, Ebihara L, Nichols CG. Polyamine flux in Xenopus oocytes through hemi-gap junctional channels. J Physiol. 2003;553(Pt 1):95–100. doi: 10.1113/jphysiol.2003.047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Musa H, Veenstra RD. Voltage-dependent blockade of connexin40 gap junctions by spermine. Biophys J. 2003;84:205–219. doi: 10.1016/S0006-3495(03)74843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Musa H, Fenn E, Crye M, Gemel J, Beyer EC, Veenstra RD. Amino terminal glutamate residues confer spermine sensitivity and affect voltage gating and channel conductance of rat connexin40 gap junctions. J Physiol. 2004;557:863–878. doi: 10.1113/jphysiol.2003.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1b release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Skatchkov SN, Inyushin M, Kucheryavykh YV, Sanabria P, Perez-Melendez V, Laube G, Veh RW, Nichols CG, Sala-Rabanal M, Eaton MJ. Multiple pathways of polyamine accumulation in glia. SFN Abstr. 2009;138(7) [Google Scholar]
- 260.Alvarez-Maubecin V, Garcia-Hernandez F, Williams JT, Van Bockstaele EJ. Functional coupling between neurons and glia. J Neurosci. 2000;20:4091–4098. doi: 10.1523/JNEUROSCI.20-11-04091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Rivera Y, Kucheryavykh YV, Benedikt J, Veh RW, Nichols CG, Rivera A, Eaton MJ, Skatchkov SN. Polyamine fluxes through Cx43-hemichannels in freshly isolated astrocytes and Müller glia. SfN Abstr. 2012;535(16) [Google Scholar]
- 262.Rivera Y, Inyushin M, Kucheryavykh YV, Sala-Rabanal M, Kucheryavykh LY, Benedikt J, Zayas-Santiago A, Veh RV, Nichols CG, Eaton MJ, Skatchkov SN. Pathways for physiological accumulation of polyamines in astrocytes. SFN Abstr. 2013:521.06. [Google Scholar]
- 263.Busch AE, Wuester S, Ulzheimer JC, Waldegger S, Govboulev V, Arndt P, Lang F, Koepsell H. Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. J Biol Chem. 1996;271:32599–32604. doi: 10.1074/jbc.271.51.32599. [DOI] [PubMed] [Google Scholar]
- 264.Gründemann D, Koster S, Kiefer N, Breidert T, Engelhardt M, Spitzenberger F, Obermuller N, Schomig E. Transport of Monoamine Transmitters by the Organic Cation Transporter Type 2, OCT2; J Biol Chem. 1998;273:30915–30920. doi: 10.1074/jbc.273.47.30915. [DOI] [PubMed] [Google Scholar]
- 265.Gründemann D, Schömig E. Gene structures of the human non-neuronal monoamine transporters EMT and OCT2. Hum Genet. 2000;106:627–35. doi: 10.1007/s004390000309. [DOI] [PubMed] [Google Scholar]
- 266.Schömig E, Spitzenberger F, Engelhardt M, Martel F, Ording N, Gründemann D. Molecular cloning and characterization of two novel transport proteins from rat kidney. FEBS Lett. 1998;425:79–86. doi: 10.1016/s0014-5793(98)00203-8. [DOI] [PubMed] [Google Scholar]
- 267.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227– 1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 268.Takeda H, Inazu M, Matsumiya T. Astroglial dopamine transport is mediated by norepinephrine transporter. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:620–623. doi: 10.1007/s00210-002-0640-0. [DOI] [PubMed] [Google Scholar]
- 269.Inazu M, Takeda H, Matsumiya T. The role of glial monoamine transporters in the central nervous system. Nihon Shinkei Seishin Yakurigaku Zasshi. 2003;23:171–178. [PubMed] [Google Scholar]
- 270.Inyushin M, Kucheryaykh Y, Kucheryavykh L, Sanabria P, Jiménez-Rivera C, Struganova I, Eaton M, Skatchkov S. Membrane potential and pH-dependent accumulation of decynium-22 (1,1′-diethyl-2,2′-cyanine iodide) flourencence through OCT transporters in astrocytes. Bol Asoc Med P R. 2010;102(3):5–12. [PMC free article] [PubMed] [Google Scholar]
- 271.Autere A-M, Lamsa K, Kaila K, Taira T. Synaptic activation of GABAA receptors induces neuronal uptake of Ca2+ in adult rat hippocampal slices. J Neurophysiol. 1999;81:811–816. doi: 10.1152/jn.1999.81.2.811. [DOI] [PubMed] [Google Scholar]
- 272.Fedirko N, Avshalumov M, Rice ME, Chesler M. Regulation of postsynaptic Ca2+influx in hippocampal CA1 pyramidal neurons via extracellular carbonic anhydrase. J Neurosci. 2007;27:1167–1175. doi: 10.1523/JNEUROSCI.3535-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ballanyi K, Grafe P, Ten Bruggencate G. Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J Physiol. 1987;382:159–174. doi: 10.1113/jphysiol.1987.sp016361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Siemkowicz E, Hansen AJ. Brain extracellular ion composition and EEG activity following 10 minutes ischemia in normo- and hyperglycemic rats. Stroke. 1981;12(2):236–240. doi: 10.1161/01.str.12.2.236. [DOI] [PubMed] [Google Scholar]
- 275.Nilsson P, Laursen H, Hillered L, Hansen AJ. Calcium movements in traumatic brain injury: the role of glutamate receptor-operated ion channels. J Cereb Blood Flow Metab. 1996;16(2):262–270. doi: 10.1097/00004647-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 276.Hansen AJ, Nedergaard M. Brain ion homeostasis in cerebral ischemia. Neurochem Pathol. 1988;9:195–209. doi: 10.1007/BF03160362. [DOI] [PubMed] [Google Scholar]
- 277.Hansen AJ, Zeuthen T. Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta Physiol Scand. 1981;113(4):437–445. doi: 10.1111/j.1748-1716.1981.tb06920.x. [DOI] [PubMed] [Google Scholar]
- 278.Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochem Int. 2004;45:259–264. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 279.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54(7):758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 280.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165(5):1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ivanova S, Botchkina GI, Al-Abed Y, Meistrell M, III, Batliwalla F, Dubinsky JM, Iadecola C, Wang H, Gregersen PK, Eaton JW, Tracey KJ. Identification of Enzymatically Formed 3-Aminopropanal as an Endogenous Mediator of Neuronal and Glial Cell Death. J Exp Med. 1998;188:327–340. doi: 10.1084/jem.188.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Bell MR, Belarde JA, Johnson HF, Aizenman CD. A neuroprotective role for polyamines in a Xenopus tadpole model of epilepsy. Nat Neurosci. 2011;14:505–512. doi: 10.1038/nn.2777. [DOI] [PubMed] [Google Scholar]
- 283.Antony T, Hoyer W, Cherny D, Heim G, Jovin TM, Subramaniam V. Cellular polyamines promote the aggregation of alpha-synuclein. J Biol Chem. 2003;278:3235–3240. doi: 10.1074/jbc.M208249200. [DOI] [PubMed] [Google Scholar]
- 284.Goers J, Uversky VN, Fink AL. Polycation-induced oligomerization and accelerated fibrillation of human alpha-synuclein in vitro. Protein Sci. 2003;12:702–707. doi: 10.1110/ps.0230903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Mandal S, Mandal A, Johansson HE, Orjalo AV, Park MH. Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells. Proc Natl Acad Sci U S A. 2013;110(6):2169–2174. doi: 10.1073/pnas.1219002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Nishimura K, Shiina R, Kashiwagi K, Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem. 2006;139:81–90. doi: 10.1093/jb/mvj003. [DOI] [PubMed] [Google Scholar]
- 287.Arshavsky YI. Alzheimer disease and cellular mechanisms of memory storage. J Neuropathol Exp Neurol. 2014;73(3):192–205. doi: 10.1097/NEN.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 288.Matthews HR. Polyamines, chromatin structure and transcription. Bioessays. 1993;15(8):561–566. doi: 10.1002/bies.950150811. [DOI] [PubMed] [Google Scholar]
- 289.Kondapalli KC, Hack A, Schushan M, Landau M, Ben-Tal N, Rao R. Functional evaluation of autism-associated mutations in NHE9. Nat Commun. 2013;4:2510. doi: 10.1038/ncomms3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhu HJ, Appel DI, Gründemann D, Richelson E, Markowitz JS. Evaluation of organic cation transporter 3 (SLC22A3) inhibition as a potential mechanism of antidepressant action. Pharmacol Res. 2012;65(4):491–496. doi: 10.1016/j.phrs.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 291.Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonté B, Turecki G, Mechawar N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36(13):2650–2658. doi: 10.1038/npp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Gomes GM, Mello CF, da Rosa MM, Bochi GV, Ferreira J, Barron S, Rubin MA. Polyaminergic agents modulate contextual fear extinction in rats. Neurobiol Learn Mem. 2010;93(4):589–595. doi: 10.1016/j.nlm.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 293.Morrison LD, Kish SJ. Brain polyamine levels are altered in Alzheimer’s disease. Neurosci Lett. 1995;197(1):5–8. doi: 10.1016/0304-3940(95)11881-v. [DOI] [PubMed] [Google Scholar]
- 294.Inoue K, Tsutsui H, Akatsu H, Hashizume Y, Matsukawa N, Yamamoto T, Toyo’oka T. Metabolic profiling of Alzheimer’s disease brains. Sci Rep. 2013;3:2364. doi: 10.1038/srep02364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Grabenauer M, Bernstein SL, Lee JC, Wyttenbach T, Dupuis NF, Gray HB, Winkler JR, Bowers MT. Spermine binding to Parkinson’s protein alpha-synuclein and its disease-related A30P and A53T mutants. J Phys Chem B. 2008;112(35):11147–11154. doi: 10.1021/jp801175w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lewandowski NM, Ju S, Verbitsky M, Ross B, Geddie ML, Rockenstein E, Adame A, Muhammad A, Vonsattel JP, Ringe D, Cote L, Lindquist S, Masliah E, Petsko GA, Marder K, Clark LN, Small SA. Polyamine pathway contributes to the pathogenesis of Parkinson disease. Proc Natl Acad Sci U S A. 2010;107(39):16970–16975. doi: 10.1073/pnas.1011751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Paik MJ, Ahn YH, Lee PH, Kang H, Park CB, Choi S, Lee G. Polyamine patterns in the cerebrospinal fluid of patients with Parkinson’s disease and multiple system atrophy. Clin Chim Acta. 2010;411(19–20):1532–1535. doi: 10.1016/j.cca.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 298.Lesort M, Chun W, Tucholski J, Johnson GV. Does tissue transglutaminase play a role in Huntington’s disease? Neurochem Int. 2002;40(1):37–52. doi: 10.1016/s0197-0186(01)00059-6. [DOI] [PubMed] [Google Scholar]
- 299.Colton CA, Xu Q, Burke JR, Bae SY, Wakefield JK, Nair A, Strittmatter WJ, Vitek MP. Disrupted spermine homeostasis: a novel mechanism in polyglutamine-mediated aggregation and cell death. J Neurosci. 2004;24:7118–7127. doi: 10.1523/JNEUROSCI.1233-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Acevedo-Torres K, Berríos L, Rosario N, Dufault V, Skatchkov S, Eaton MJ, Torres-Ramos CA, Ayala-Torres S. Mitochondrial DNA damage is a hallmark of chemically induced and the R6/2 transgenic model of Huntington’s disease. DNA Repair (Amst) 2009;8:126–36. doi: 10.1016/j.dnarep.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, Anderson MA, Mody I, Olsen ML, Sofroniew MV, Khakh BS. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat Neurosci. 2014 doi: 10.1038/nn.3691. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gomes-Trolin C, Nygren I, Aquilonius SM, Askmark H. Increased red blood cell polyamines in ALS and Parkinson’s disease. Exp Neurol. 2002;177(2):515–520. doi: 10.1006/exnr.2002.7952. [DOI] [PubMed] [Google Scholar]
- 303.Ferchmin PA, Eterović VA, Rivera EM, Teyler TJ. Spermine increases paired-pulse facilitation in area CA1 of hippocampus in a calcium-dependent manner. Brain Res. 1995;689(2):189–196. doi: 10.1016/0006-8993(95)00568-b. [DOI] [PubMed] [Google Scholar]
- 304.Casero RA, Pegg AE. Polyamine catabolism and disease. Biochem J 2009. 2009;421:323–38. doi: 10.1042/BJ20090598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Fiori LM, Wanner B, Jomphe V, Croteau J, Vitaro F, Tremblay RE, Bureau A, Turecki G. Association of polyaminergic loci with anxiety, mood disorders, and attempted suicide. PLoS One. 2010;5(11):e15146. doi: 10.1371/journal.pone.0015146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Le Roy C, Laboureyras E, Laulin JP, Simonnet G. A polyamine-deficient diet opposes hyperalgesia, tolerance and the increased anxiety-like behaviour associated with heroin withdrawal in rats. Pharmacol Biochem Behav. 2013;103(3):510–519. doi: 10.1016/j.pbb.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 307.Kucheryavykh YV, Kucheryavykh LY, Nichols CG, Maldonado HM, Baksi K, Reichenbach A, Skatchkov SN, Eaton MJ. Downregulation of Kir4.1 Inward Rectifying Potassium Channel Subunits by RNAi Impairs Potassium Transfer and Glutamate Uptake by Cultured Cortical Astrocytes. Glia. 2007;55:274–228. doi: 10.1002/glia.20455. [DOI] [PubMed] [Google Scholar]
- 308.Gilad GM, Gilad VH. Overview of the brain polyamine-stress-response: regulation, development, and modulation by lithium and role in cell survival. Cell Mol Neurobiol. 2003;23(4–5):637–649. doi: 10.1023/A:1025036532672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Rubin MA, Berlese DB, Stiegemeier JA, Volkweis MA, Oliveira DM, dos Santos TL, Fenili AC, Mello CF. Intra-amygdala administration of polyamines modulates fear conditioning in rats. J Neurosci. 2004;24:2328–2334. doi: 10.1523/JNEUROSCI.1622-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Kossel A. Über das Agmatin. Zeitschrift für Physiologische Chemie. 1910;66:257–261. [Google Scholar]
- 311.Bardócz S, Duguid TJ, Brown DS, Grant G, Pusztai A, White A, Ralph A. The importance of dietary polyamines in cell regeneration and growth. Br J Nutr. 1995;73(6):819–828. doi: 10.1079/bjn19950087. [DOI] [PubMed] [Google Scholar]
- 312.Kalač P. Health effects and occurrence of dietary polyamines: A review for the period 2005-mid 2013. Food Chem. 2014;161C:27–39. doi: 10.1016/j.foodchem.2014.03.102. [DOI] [PubMed] [Google Scholar]
- 313.Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16(7):871–81. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- 314.Kimura N, Masuda S, Katsura T, Inui K. Transport of guanidine compounds by human organic cation transporters, hOCT1 and hOCT2. Biochem Pharmacol. 2009;77(8):1429–1436. doi: 10.1016/j.bcp.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 315.Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. OCT1 polymorphism is associated with response and survival time in anti-Parkinsonian drug users. Neurogenetics. 2011;12(1):79–82. doi: 10.1007/s10048-010-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, Tieu K. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci USA. 2009;106(19):8043–8048. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]


