Abstract
Importance
The Cornea Donor Study (CDS) showed that donor age is not a factor in survival of most penetrating keratoplasties for endothelial disease. Secondary analyses confirm the importance of surgical indication and presence of glaucoma in outcomes at 10 years.
Objective
To assess the relationship between donor and recipient factors and corneal graft survival in the CDS.
Design
Multi-center prospective, double-masked, controlled clinical trial
Setting
105 surgeons from eighty clinical sites enrolled participants and forty-three eye banks provided corneas.
Participants
1090 subjects undergoing corneal transplantation for a moderate risk condition, principally Fuchs’ dystrophy or pseudophakic/aphakic corneal edema (PACE)
Intervention(s) for Clinical Trials or Exposure(s) for observational studies
Corneas from donors <66 years or ≥ 66 years were assigned, double-masked to donor age. Surgery and post-operative care were performed according to surgeons’ usual routines. Subjects were followed for up to twelve years.
Main Outcome Measure(s)
Graft failure defined as a regraft or a cloudy cornea for 3 consecutive months.
Results
The 10-year graft failure rate was higher in cases with PACE than with Fuchs’ dystrophy (37% versus 20%, p < 0.001) and in cases with a history of glaucoma prior to penetrating keratoplasty, particularly with prior glaucoma surgery (58% with prior glaucoma surgery and medications at time of surgery versus 22% with no history of glaucoma, p<0.001). There were trends towards increased graft failure in recipients who were older (p=0.04), African-American (p=0.11), or had a smoking history (p=0.02). Lower endothelial cell density (ECD) and higher corneal thickness (CT) at 6 months, 1 year, and 5 years were associated with subsequent graft failure (p=0.04 to <0.001).
Conclusions and Relevance
Most penetrating corneal grafts for Fuchs’ dystrophy or PACE remain clear at 10 years. The risk of failure is greater for those with PACE and when there is a history of glaucoma. ECD and CT measurements during the course of post-keratoplasty follow up are associated with risk of failure. However, even with very low ECD and high CT at 5 years, most corneas remain clear at 10 years.
Introduction
The Cornea Donor Study (CDS) was designed primarily to evaluate the effect of donor age on graft survival and endothelial cell loss in penetrating keratoplasty for endothelial disease. At 5 years there was no difference in graft survival (86%) between participants who received corneas from donors 12-65 and 66-75 years old.1 By 10-12 years there was a small, but non-significant, difference (77% survival for the younger group and 71% for the older group).2 However, there was a suggestion of an age effect at the extremes of the donor age range: 96% survival for 80 donors 12 to 33 and 62% survival for 130 donors 72 to 75 years old.
The effects of recipient, donor, and surgical factors other than donor age on graft survival at 5 years have been reported in prior publications.3-8 The most prominent finding was that eyes with Fuchs’ dystrophy had a substantially lower failure rate (7%) than eyes with pseudophakic or aphakic corneal edema (PACE) (27%).7 Donor endothelial cell density (ECD) had no effect on outcomes, but 6-month post op ECD < 1700 cells/mm2 and corneal thickness (CT) > 600 µm at one year were associated with an increased risk of failure at 5 years.8 Most other factors studied had marginal or no effect on outcomes.
The extension of the CDS to 10-12 years of follow-up provides an opportunity to examine the longer term effects of donor and recipient factors on graft survival and in particular to assess the relationship of ECD and CT at 5 years to the subsequent course of the grafts.
Methods
Study Protocol
Complete details of the CDS protocol have been previously reported.1, 9, 10 The study protocol was approved by the institutional review board at each investigational site. Between January 2000 and August 2002, 1090 eligible patients (median age 72 years, interquartile range 65 to 76 years) at 80 sites had a penetrating keratoplasty for Fuchs’ dystrophy (62%), PACE (34%; 93% pseudophakic and 7% aphakic) or another corneal endothelial disorder (4%). Written informed consent was obtained from each subject for the first five years of follow up, and 663 participants without a regraft by 5 years were re-consented for follow up through 2012.
Eligible donor corneas met Eye Bank Association of America standards for human corneal transplantation. Additional donor eligibility criteria included age between 10 and 75 years and an eye bank-measured ECD of 2300 to 3300 cells/mm2. Median donor age at the time of death was 61 years (interquartile range 52 to 69 years). Clinical investigators and subjects were masked to certain characteristics of the donor tissue, including age and ECD. Donor tissue was assigned without regard to recipient age or other subject characteristics. Preoperative management, penetrating keratoplasty surgical technique, and postoperative care were provided according to each investigator’s directive. In the first six months of the study, follow up visit frequency was left to each investigator’s routine. Then the minimum follow-up schedule included a visit between months 6 and 12 and then annual visits through 2012. CT, measured using an ultrasonic pachymeter by the investigator’s usual routine, was optional at post-keratoplasty follow-up visits. Measurements were recorded to the nearest micrometer (µm).
Graft clarity was assessed at each visit. The definition of graft failure, based on the definition used in the Collaborative Corneal Transplantation Studies (CCTS),11, 12 was a regraft or, in the absence of regraft, a cloudy cornea in which there was loss of central graft clarity sufficient to compromise vision for a minimum of three consecutive months. Details regarding graft failure classification have been published.1
A subset of the CDS participants also consented to participate in the Specular Microscopy Ancillary Study. Preoperative specular microscopic images of the central donor corneal endothelium were provided by participating eye banks. Postoperative specular microscopic images of the central corneal endothelium of the graft were obtained at the 6-month and annual follow-up visits. The preoperative donor images and postoperative recipient images were evaluated for quality and ECD by a central reading center, the Cornea Image Analysis Reading Center (formerly the Specular Microscopy Reading Center) at Case Western Reserve University and University Hospitals Eye Institute, using a previously described variable frame analysis method.13
Statistical Methods
Cumulative probabilities of graft failure (subsequently referred to as “graft failure rates”) along with 99% confidence intervals were calculated using the Kaplan-Meier method at 10 years. Proportional hazards regression was used to assess the association of baseline recipient factors with graft failure in univariate and multivariate analyses. Covariates with P<0.10 were included in a multivariate model to control for potential confounding factors; however, due to multiple comparisons only covariates with P<0.01 were considered statistically significant. The proportional hazards assumption was violated for diagnosis and donor age in the final multivariate baseline recipient factors model. The baseline hazard function was stratified by donor age, but hazard ratios were modeled for diagnosis so that the values could be displayed. Results were similar for the other recipient factors when the baseline hazard functions were also stratified by corneal diagnosis (data not shown). The association of lens status with graft failure was assessed in separate proportional hazard regressions for Fuchs and PACE patients adjusting for participant age and smoking status, and stratifying the baseline hazard function by donor age. Additional analyses were performed on the subset of patients who had available ECD and/or CT measurements. Multivariate proportional hazards models were fit conditional on graft survival at 5.5 years which was the upper limit for the 5 year visit window. Similar models were run at 6 months and 1 year. No significant deviations from the proportional hazards assumptions were detected for follow-up ECD or CT values.
In all multivariate models, missing data were treated as a separate category for discrete covariates and a missing value indicator was added for continuous covariates. Similar methods were used to assess the association of donor factors with graft failure. All reported p-values are two-sided. Statistical analyses were conducted using SAS version 9.3 software (SAS Institute Inc., Cary, NC).
Results
Graft failure occurred in 224 (21%) of the 1090 participants. In univariate and multivariate analyses, the 10-year graft failure rate was higher in cases with PACE than in cases with Fuchs’ dystrophy (37% versus 20%, p < 0.001) and in cases with a history of glaucoma (either or both prior glaucoma surgery prior to penetrating keratoplasty and use of intraocular-pressure lowering medications at time of penetrating keratoplasty), particularly when there had been prior glaucoma surgery (58% in cases with prior glaucoma surgery and on intraocular-pressure lowering medications at time of surgery versus 22% with no history of glaucoma, p<0.001) (Table 1). There were trends towards increased graft failure in recipients who were older (p=0.04), or had a history of smoking (p=0.02) that did not meet our threshold for statistical significance accounting for multiple comparisons (Table 1). African-American recipients were associated with increased graft failure in univariate analysis (p=0.002) and this trend was also observed in multivariate analysis, but did not reach statistical significance (hazard ratio (HR) 1.5; p=0.11).
Table 1.
Association of Baseline Recipient Factors and Graft Failure
| Recipient Baseline Factors* | N | 10-yr Graft Failure ± 99% CIa |
Univariate Models | Multivariate Modelb | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazard Ratio (99% CI) |
P-value | Hazard Ratio (99% CI) |
P-value | |||
| Age at Penetrating Keratoplasty | <0.001 | 0.04 | ||||
| <60 years | 162 | 19% ± 7% | 1.0 | 1.0 | ||
| 60 – <70 years | 284 | 21% ± 6% | 1.0 (0.6 – 1.8) | 0.9 (0.5 – 1.6) | ||
| ≥70 years | 644 | 29% ± 5% | 1.5 (0.9 – 2.5) | 1.2 (0.7 – 2.1) | ||
|
| ||||||
| Gender | 0.61 | |||||
| Male | 393 | 24% ± 6% | 1.0 | |||
| Female | 697 | 26% ± 5% | 1.1 (0.7-1.5) | |||
| Race/Ethnicity | 0.002 | |||||
|
| ||||||
| White (Non-Hispanic) | 1011 | 24% ± 4% | 1.0 | |||
| African-American | 50 | 38% ± 17% | 2.1 (1.1 – 4.1) | |||
| Other** | 29 | 53% ± 28% | 2.1 (0.9 – 5.1) | |||
|
| ||||||
| Diagnosis | <0.001 | <0.001 | ||||
| Fuchs’ Dystrophy | 676 | 20% ± 4% | 1.0 | 1.0 | ||
| Pseudophakic/Aphakic Corneal Edema | 369 | 37% ± 7% | 2.5 (1.8 – 3.6) | 2.1 (1.4 – 3.0) | ||
| Other*** | 45 | 23% ± 13% | 1.7 (0.7 – 3.9) | 1.2 (0.5 – 2.8) | ||
|
Glaucoma History at Time of
Penetrating Keratoplasty |
<0.001 | <0.001 | ||||
| No Medications and No Surgery | 920 | 22% ± 4% | 1.0 | 1.0 | ||
| IOP-lowering medication; No Surgery |
99 | 32% ± 12% | 1.6 (0.9 – 2.8) | 1.2 (0.7 – 2.2) | ||
| Prior glaucoma surgery; No IOP- lowering medications |
26 | 50% ± 24% | 2.9 (1.4 – 6.3) | 2.6 (1.2 – 5.6) | ||
| Prior glaucoma surgery and lOP- lowering medications |
45 | 58% ± 20% | 4.5 (2.5 – 8.1) | 4.1 (2.2 – 7.5) | ||
|
| ||||||
| Smoker Penetrating Keratoplasty | 0.06 | 0.02 | ||||
| No | 988 | 24% ± 4% | 1.0 | 1.0 | ||
| Yes | 102 | 35% ± 13% | 1.5 (0.9 – 2.5) | 1.6 (0.9 – 2.8) | ||
|
| ||||||
| History of Diabetesd | 0.90 | |||||
| No | 899 | 24% ± 4% | 1.0 | |||
| Yes | 141 | 23% ± 9% | 1.0 (0.6 – 1.7) | |||
CI = Confidence interval
The 10-year Kaplan-Meier estimates are provided for illustration. The proportional hazards models include all follow up data from surgery to end of study.
The multivariate model was generated through stepwise selection of variables with criterion P<0.10. The baseline hazard function was stratified by donor age because it violated the proportional hazard assumption. The proportional hazards assumption was also violated for diagnosis . Results were similar when the baseline hazard function was also stratified by diagnosis (data not shown).
c P values are from models with continuous (both linear and quadratic) recipient age.
Unknown for 50 subjects.
Recipient bed size, vitrectomy in addition to penetrating keratoplasty, and post-operative intraocular pressure were all associated with graft failure in univariate analyses but were not associated in multivariate analysis due to confounding with corneal diagnosis or glaucoma history.
Includes 8 Asians, 13 Hispanics, and 8 others.
Includes 12 with interstitial keratitis, 7 with posterior polymorphous dystrophy, 6 with perforating corneal injury, and 20 with other cause of endothelial failure.
Further exploration showed that the effects of both recipient corneal diagnosis and glaucoma history were primarily limited to the first five years following surgery (data not shown). During the first five years, the hazard ratio for graft failure for PACE compared with Fuchs’ dystrophy was 4.3 (99% confidence interval (CI) 2.6 to 7.1; p<0.001) while among grafts still functioning at 5 years, the corresponding hazard ratio for subsequent failure was 1.1 (99% CI: 0.6 to 2.1; p=0.65). Similarly for glaucoma history, during the first five years the hazard ratio for cases with prior history of glaucoma surgery and on medical treatment at the time of penetrating keratoplasty was 7.2 (99% CI: 3.8 to 13.5; p<0.001) compared with no history of glaucoma, and the corresponding hazard ratio afterwards was 0.5 (99% CI: <0.1 to 7.3; p=0.55).
Among cases with Fuchs’ dystrophy, the 10-year graft failure rate was similar in postoperative phakic and pseudophakic eyes (16% versus 20%, p=0.34), with almost all of the pseudophakic eyes having posterior chamber intraocular lenses (96% of 501). Among cases with PACE, graft failure by 10 years was more common when an anterior chamber lens was present postoperatively than with a posterior chamber lens (57% vs. 30%, multivariate HR=1.9, 99% CI: 1.1-3.4; p= 0.02) (Table 2). This hazard ratio did not vary meaningfully over the 10 years of follow-up (data not shown). Notably, eyes with an anterior chamber lens preoperatively that was retained postoperatively (N=81) had a 59% graft failure rate by 10 years, while those who had an anterior chamber lens exchanged for a posterior chamber lens (N=28) had a 23% failure rate (multivariate HR=0.4, 99% CI: 0.1-1.2; p=0.04) (eTable 1). Other than lens status, the effect of the baseline recipient factors on graft failure was similar in Fuchs’ dystrophy and PACE cases (Table 3).
Table 2.
Association of Lens Status and Graft Failure According to Corneal Diagnosis
| N | 10-yr Graft Failure ± 99% CI* |
Multivariate Models** | ||
|---|---|---|---|---|
| Hazard Ratio (99% CI) |
P-value | |||
| Fuchs dystrophy | ||||
| Preoperative phakic, postoperative phakic | 153 | 16% ± 7% | 1.0 | 0.62 |
| Preoperative phakic, postoperative pseudophakic1 | 299 | 18% ± 6% | 0.9 (0.4 – 1.8) | |
| Preoperative pseudophakic or aphakic, postoperative pseudophakic2 | 202 | 23% ± 8% | 1.0 (0.5 – 2.3) | |
| Postoperative aphakic | 22 | 31% ± 19% | 1.5 (0.5 – 4.9) | |
| Pseudophakic or aphakic corneal edema | ||||
| Postoperative pseudophakic (PC IOL) | 218 | 30% ± 9% | 1.0 | 0.02 |
| Postoperative pseudophakic (sutured PCL) | 54 | 30% ± 14% | 1.1 (0.5 – 2.5) | |
| Postoperative pseudophakic (AC IOL) | 89 | 57% ± 17% | 1.9 (1.1 – 3.4) | |
| Postoperative aphakic | 8 | NA | NA | |
AC = anterior chamber; IOL = intraocular lens; PC = posterior chamber; PCL = posterior chamber lens
NA = Graft failure rates/hazard ratios are not reported for groups with fewer than 15
Kaplan-Meier estimates
Models adjusted for participant age (linear and quadratic terms) and smoking status, and baseline hazard function stratified by donor age.
Intraocular lenses: 288 posterior chamber, 8 sutured posterior chamber, and 3 anterior chamber.
Intraocular lenses: 195 posterior chamber, 3 sutured posterior chamber, and 4 anterior chamber.
Table 3.
Association of Baseline Recipient Factors and Graft Failure According to Corneal Diagnosis
| Preoperative Diagnosisa | ||||
|---|---|---|---|---|
|
| ||||
| Fuchs’ Dystrophy | Pseudophakic/Aphakic corneal edema | |||
|
| ||||
| Baseline Factors | N | 10-yr Graft Failureb
± 99% CI |
N | 10-yr Graft Failureb
± 99% CI |
| Overall | 676 | 20% ± 4% | 369 | 37% ± 7% |
|
| ||||
| Age at Surgery | ||||
| <60 years | 126 | 13% ± 6% | 29 | 54% ± 23% |
| 60 – <70 years | 201 | 19% ± 7% | 70 | 25% ± 12% |
| ≥70 years | 349 | 23% ± 6% | 270 | 38% ± 9% |
|
| ||||
| Gender | ||||
| Male | 210 | 16% ± 6% | 158 | 36% ± 11% |
| Female | 466 | 21% ± 5% | 211 | 37% ± 10% |
|
| ||||
| Race/Ethnicity | ||||
| White (Non-Hispanic) | 651 | 19% ± 4% | 322 | 35% ± 8% |
| Non-White (including Hispanic) | 25 | 43% ± 24% | 47 | 45% ± 20% |
| Black | 19 | 32% ± 21% | 27 | 42% ± 22% |
| Hispanic | 2 | NA | 9 | NA |
| Other | 4 | NA | 11 | NA |
|
| ||||
|
Glaucoma History at Time of Penetrating
Keratoplasty |
||||
| No Medications and No Surgery | 627 | 19% ± 4% | 259 | 31% ± 8% |
| IOP-lowering medication; No Surgery | 34 | 24% ± 14% | 61 | 36% ± 17% |
| Prior glaucoma surgery; No IOP- lowering medications |
8 | NA | 15 | 57% ± 33% |
| Prior glaucoma surgery and IOP- lowering medications |
7 | NA | 34 | 68% ± 23% |
|
| ||||
| Smoker (at time of surgery) | ||||
| No | 628 | 19% ± 4% | 325 | 36% ± 8% |
| Yes | 48 | 26% ± 14% | 44 | 44% ± 20% |
|
| ||||
| History of Diabetesc | ||||
| No | 587 | 19% ± 4% | 276 | 36% ± 8% |
| Yes | 67 | 17% ± 10% | 69 | 31% ± 16% |
CI = Confidence interval
* Graft failure rates not reported for groups with N < 15.
45 subjects with “Other” diagnosis not included.
Kaplan-Meier estimates.
Excludes 46 additional subjects with unknown history of diabetes.
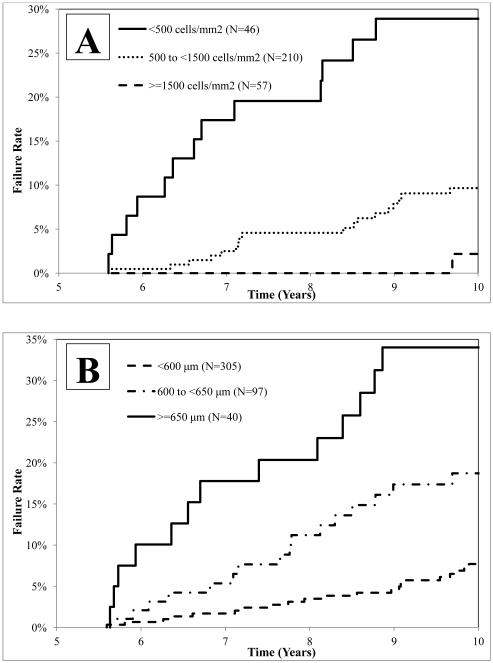
Measurements of ECD and CT at 6 months, 1 year, and 5 years were strongly associated with an increased probability of subsequent graft failure (Table 4). Among cases with a surviving graft at 5 years, the conditional probability of failure by 10 years was 29% among 46 cases with 5-year ECD <500 cells/mm2 compared with 10% for the 210 cases with 5-year ECD 500-1499 cells/mm2, and 2% for the 57 cases with ECD ≥1,500 cells/mm2 (p<0.001) (Table 4 and Figure 1). With respect to CT, the conditional probability of failure by 10 years was 34% among the 40 cases with 5-year CT ≥650 μm compared with 19% among the 97 cases with 5-year CT 600-649 μm, and 8% among the 305 cases with 5-year CT <600 μm (p<0.001) (Table 4 and Figure 1). The correlation between 5-year ECD and CT measurements was −0.31 (N=273, 95% CI: −0.41 to −0.20, p-value<0.001). Graft failure rates combining the 5-year ECD and CT data are shown in eTable 2. The addition of preoperative recipient diagnosis, glaucoma history, and donor age to the model did not appreciably increase the ability to predict the probability of subsequent graft failure (data not shown). As at 5 years, no other donor factors, including eye-banking parameters and ABO matching, and no operative factors correlated with failure at 10 years.
Table 4.
Association of Endothelial Cell Density and Corneal Thickness Measurements During Follow Up with Graft Failure
| N | Conditional 10- yr Graft Failure ± 99% CI |
Multivariate Modela | ||
|---|---|---|---|---|
| Follow-up Factors | Hazard Ratio (99% CI) |
P-value | ||
| Model 1 (conditional on 6 months survival) | ||||
| ECD at 6 months (N=295)b | <0.001 | |||
| ≥2700 cells/mm2 | 93 | 6% ± 4% | 1.0 | |
| 2200 to <2700 cells/mm2 | 102 | 20% ± 9% | 3.6 (1.0 – 13.4) | |
| 1700 to <2200 cells/mm2 | 58 | 25% ± 13% | 5.0 (1.3 – 19.5) | |
| <1700 cells/mm2 | 42 | 41% ± 18% | 10.5 (2.7 – 40.4) | |
| CT at 6 months (N=641)b | 0.001 | |||
| <500 μm | 120 | 14% ± 7% | 1.0 | |
| 500 to <550 μm | 280 | 19% ±6% | 1.5 (0.7 – 3.1) | |
| 550 to <600 μm | 178 | 28% ± 9% | 2.0 (0.9 – 4.3) | |
| ≥600 μm | 63 | 36% ±15% | 2.8 (1.1 – 6.8) | |
| Model 2 (conditional on 1 year survival) | ||||
| ECD at 1 year (N=368)c | <0.001 | |||
| ≥2700 cells/mm2 | 83 | 4% ± 3% | 1.0 | |
| 2200 to <2700 cells/mm2 | 105 | 13% ±7% | 2.7 (0.6 – 12.0) | |
| 1700 to <2200 cells/mm2 | 92 | 17% ± 9% | 3.2 (0.7 – 14.2) | |
| <1700 cells/mm2 | 88 | 39% ±13% | 10.0 (2.5 – 39.3) | |
| CT at l year (N=633)c | 0.04 | |||
| <500 μm | 96 | 18% ± 9% | 1.0 | |
| 500 to <550 μm | 266 | 18% ± 6% | 1.0 (0.5 – 2.4) | |
| 550 to <600 μm | 201 | 23% ± 8% | 1.3 (0.6 – 2.9) | |
| ≥600 μm | 70 | 28% ± 12% | 2.2 (0.8 – 5.5) | |
| Model 3 (conditional on 5 years survival) | ||||
| ECD at 5 years (N=313)d | <0.001 | |||
| ≥1500 cells/mm2 | 57 | 2% ± 2% | 1.0 | |
| 1250 to <1500 cells/mm2 | 25 | 9% ± 7% | 6.5 (0.3 – 127.8) | |
| 1000 to <1250 cells/mm2 | 30 | 7% ± 6% | 4.0 (0.2 – 94.5) | |
| 750 to <1000 cells/mm2 | 49 | 7% ± 5% | 3.5 (0.2 – 69.7) | |
| 500 to <750 cells/mm2 | 106 | 12% ± 6% | 5.5 (0.4 – 80.3) | |
| <500 cells/mm2 | 46 | 29% ± 14% | 16.6 (1.1 – 241.7) | |
| CT at 5 years (N=442)d | <0.001 | |||
| <550 μm | 148 | 7% ± 4% | 1.0 | |
| 550 to 600 μm | 157 | 8% ± 4% | 1.2 (0.4 – 3.2) | |
| 600 to 650 μm | 97 | 19% ± 8% | 2.0 (0.8 – 5.3) | |
| ≥650 μm | 40 | 34% ± 16% | 3.7 (1.3 – 10.7) | |
CT = Corneal Thickness; ECD = Endothelial Cell Density
Models are conditional on graft survival by the specified time and include subjects with CT or ECD values at the specified time. P values in table are from models with continuous ECD and CT values.
At 6 months, 1035 subjects had a surviving graft, ECD measurements were missing for 740 subjects, and CT measurements were missing for 394 subjects .
At 1 year, 985 subjects had a surviving graft, ECD measurements were missing for 617 subjects, and CT measurements were missing for 352 subjects.
At 5 years, 651 subjects had a surviving graft, ECD measurements were missing for 338 subjects, and CT measurements were missing for 209 subjects.
Figure 1. Among those with a Surviving Graft at 5 Years, Graft Failure Rates over Time Stratified by 5 Year ECD and CT Values.
In panel A, conditional on graft survival at 5.5 years (upper limit for the 5 year visit window), Kaplan-Meier cumulative probabilities of graft failure are shown for the <500, 500 to <1500, and ≥1,500 cells/mm2 5 year ECD groups. In panel B, conditional on graft survival at 5.5 years, Kaplan-Meier cumulative probabilities of graft failure are shown for the <600, 600 to <650, and ≥650 μm 5 year CT groups.
Discussion
Analysis of the CDS data after 10-12 years of follow up largely showed similar associations of baseline recipient factors with graft failure as were seen after 5 years in eyes undergoing penetrating keratoplasty for corneal endothelial disease. Graft failure was again shown to be more likely in cases of PACE than Fuchs’ dystrophy and in cases with a prior history of glaucoma, particularly when prior glaucoma surgery had been performed. In addition, there were trends suggesting higher failure rates in recipients who were 70 years or older, African-American, or had a history of smoking. No other donor factors were significantly associated with graft failure by 10-12 years other than the previously reported suggestion of an association between the extremes of donor age and graft outcome.2 As at 5 years, there was no indication that ABO blood type incompatibility between donor and recipient was important; however, this must be viewed in the context that the study eyes were not considered to be at high risk for rejection failure.6
Both ECD and CT during the course of the study were strongly associated with subsequent graft failure. However, despite these significant associations, neither factor was strongly predictive that graft failure would occur. Even with ECD < 500 cells/mm2 at 5 years, the probability of graft survival at 10 years was 71% and likewise, even when CT was ≥ 650 µm at 5 years, the probability of graft survival at 10 years was 66%. Combining ECD and CT data with donor age, preoperative recipient diagnosis and glaucoma history did not improve the prediction of success. These data may be useful for clinicians in counseling patients and to provide reassurance that the majority of grafts will remain clear for a number of years even when the ECD is <500 cells/mm2.
By 10 years, recipient diagnosis remained the most important predictor of outcome, with PACE grafts having failed at almost twice the rate of grafts for Fuchs’ dystrophy. PACE increased the rate of early failures but grafts in eyes with PACE that survived the first five years had a failure rate from 5 to 12 years similar to Fuchs’ dystrophy cases. This is consistent with the hypothesis that many eyes developing PACE have a pathologic response to intraocular lens (IOL) presence that persists after keratoplasty.7 That is, many PACE eyes are a subset of all pseudophakic eyes, those with poor tolerance of IOLs. Those eyes manifesting this IOL effect may be dropping out of the surviving graft group early, leaving those with PACE not attributable to continued IOL effects after the first 5 years. The lack of effect of lens status in Fuchs’ eyes and the detrimental effect of anterior chamber vs. posterior chamber IOLs in PACE eyes throughout the 10 year follow-up bolster this notion. The post-keratoplasty presence of an anterior chamber IOL was associated with at 1.9 fold increased risk of failure over posterior chamber IOLs in PACE eyes. Unlike the overall IOL effect, the detrimental effect of anterior chamber IOLs persisted from 5 to 10 years. This adverse effect of anterior chamber IOLs on graft survival has been noted in the past.14, 15 Those PACE eyes in which the anterior chamber IOL was replaced with a posterior chamber IOL at keratoplasty had an approximate 60% reduction in the risk of failure, confirming this effect.
Preoperative glaucoma, particularly prior surgical glaucoma treatment in PACE eyes, also was associated with early failures. There were insufficient numbers of glaucoma cases to determine whether this was true for Fuchs’ eyes and data were not collected to evaluate the effect of intraocular pressure control after the first postoperative month. Other studies have associated pre- and post-operative glaucoma with corneal graft failure in both PACE and Fuchs’ eyes.16, 17 Glaucoma surgery, particularly with tube drainage devices, has been strongly associated with graft failure.18 These failures are likely related to endothelial cell decline, but the mechanism is unknown.
Recipient diabetes did not contribute to graft failure but there was a trend for a higher failure rate among smokers than nonsmokers. Smoking has been associated with the severity of corneal edema in Fuchs’ dystrophy.19 Association of smoking and other risk factors with Fuchs’ endothelial corneal dystrophy is possibly mediated through oxidative endothelial damage.19, 20 There also were trends towards a higher failure rate in recipients older than 70 years and in African-Americans. An association between non-white race and corneal graft failure has been noted previously.21
In summary, analysis of the CDS data after 10-12 years of follow up extends our understanding of the association of donor and recipient factors with graft failure in eyes undergoing penetrating keratoplasty for corneal endothelial disease. The sample size and the duration and completeness of follow up exceed those of the few other prospective trials of penetrating keratoplasty in the literature. Most grafts following penetrating keratoplasty for Fuchs’ dystrophy or PACE will remain clear at 10 years. Of the preoperative risk factors studied, the risk of failure is greater for cases of PACE and when there is a history of glaucoma. ECD and CT measurements during the course of post-keratoplasty follow up are associated with risk of failure. However, even with very low ECD and high CT at 5 years, most corneas will remain clear at 10 years. The applicability of the CDS data to endothelial keratoplasty, which has replaced penetrating keratoplasty as the procedure of choice for the corneal endothelial diseases22 studied in CDS, cannot be predicted. Penetrating keratoplasty may still have advantages in some complex cases requiring intraocular lens exchange or anterior segment reconstruction. It is likely that the principles examined here are broadly applicable to endothelial keratoplasty. Further trials to examine donor and eye banking parameters for endothelial keratoplasty such as the Cornea Preservation Time Study are warranted.
Supplementary Material
Acknowledgements
Roy W. Beck, MD, PhD had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Craig Kollman, PhD and Dan Raghinaru, MS conducted the data analysis. The manuscript was drafted by Alan Sugar, MD, Robin Gal, MSPH, Dan Raghinaru, MS, Craig Kollman, PhD, and Roy Beck, PhD. Mariya Dontchev, MPH, Christopher Croasdale, MD, Robert Feder, MD, Edward Holland, MD, Jonathan Lass, MD, Jonathan Macy, MD, Mark Mannis, MD, Patricia Smith, MD, and Sarkis Soukiasian, MD provided critical review for intellectual content. All authors approved the submitted version of the manuscript and are accountable for all aspects of the work. All authors made substantial contributions to conception or design of the work, or the acquisition, analysis or interpretation of the work.
Financial Support: Supported by cooperative agreements with the National Eye Institute, National Institutes of Health, Department of Health and Human Services EY12728 and EY12358. Additional support provided by: Eye Bank Association of America, Bausch & Lomb, Inc., Tissue Banks International, Vision Share, Inc., San Diego Eye Bank, The Cornea Society, Katena Products, Inc., ViroMed Laboratories, Inc., Midwest Eye-Banks (Michigan Eye-Bank, Illinois Eye-Bank, Cleveland Eye Bank and Lions Eye Bank of New Jersey), Konan Medical Corp., Eye Bank for Sight Restoration, SightLife, Sight Society of Northeastern New York (Lions Eye Bank of Albany), Lions Eye Bank of Oregon
The sponsors were not involved in the design and conduct of the study, collection, management, analysis and interpretation of the data, preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest: None to report
Conflict of Interest: The members of the writing committee have no conflics of interest to report.
Trial Registration: clinicaltrials.gov NCT00006411
References
- 1.Cornea Donor Study Investigator Group The effect of donor age on corneal transplantation outcome: results of the cornea donor study. Ophthalmology. 2008;115:620–6. doi: 10.1016/j.ophtha.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornea Donor Study Group The effect of donor age on penetrating keratoplasty survival after 10 years in the Cornea Donor Study. Ophthalmology. 2013;120:2419–27. doi: 10.1016/j.ophtha.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornea Donor Study Investigator Group Corneal thickness as a predictor of corneal transplant outcome. Cornea. 2013;32:729–36. doi: 10.1097/ICO.0b013e31827b14c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornea Donor Study Investigator Group Donor age and corneal endothelial cell loss five years after successful cornea transplantation: specular microscopy ancillary study results. Ophthalmology. 2008;115:627–32. doi: 10.1016/j.ophtha.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornea Donor Study Investigator Group Donor risk factors for graft failure in the cornea donor study. Cornea. 2009;28:981–5. doi: 10.1097/ICO.0b013e3181a0a3e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornea Donor Study Investigator Group The effect of ABO blood incompatibility on corneal transplant failure in conditions with low-risk of graft rejection. Am J Ophthalmol. 2009;147:432–8. doi: 10.1016/j.ajo.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornea Donor Study Investigator Group Recipient risk factors for graft failure in the cornea donor study. Ophthalmology. 2009;116:1023–8. doi: 10.1016/j.ophtha.2008.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornea Donor Study Investigator Group Endothelial cell density to predict endothelial graft failure after penetrating keratoplasty. Arch Ophthalmol. 2010;128:63–9. doi: 10.1001/archophthalmol.2010.128.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornea Donor Study Group Baseline donor characteristics in the Cornea Donor Study. Cornea. 2005;24:389–96. doi: 10.1097/01.ico.0000151503.26695.f0. [DOI] [PubMed] [Google Scholar]
- 10.Cornea Donor Study Group Clinical profile and early surgical complications in the Cornea Donor Study. Cornea. 2006;25:164–70. doi: 10.1097/01.ico.0000164832.69668.4b. [DOI] [PubMed] [Google Scholar]
- 11.Collaborative Corneal Transplantation Studies Research Group The Collaborative Corneal Transplantation Studies (CCTS): effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110:1392–403. [PubMed] [Google Scholar]
- 12.Collaborative Corneal Transplantation Studies Research Group Design and methods of the Collaborative Corneal Transplantation Studies. Cornea. 1993;12:93–103. doi: 10.1097/00003226-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Cornea Donor Study Group Specular Microscopy Ancillary Study methods for donor endothelial cell density determination of Cornea Donor Study images. Curr Eye Res. 2006;31:319–27. doi: 10.1080/02713680500536738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunette I, Stulting RD, Rinne JR, et al. Penetrating keratoplasty with anterior or posterior chamber intraocular lens implantation. Arch Ophthalmol. 1994;112:1311–19. doi: 10.1001/archopht.1994.01090220061024. [DOI] [PubMed] [Google Scholar]
- 15.Schein OD, Kenyon KR, Steinert RF, et al. A randomized trial of intraocular lens fixation techniques with penetrating keratoplasty. Ophthalmology. 1993;100:1437–43. doi: 10.1016/s0161-6420(93)31458-2. [DOI] [PubMed] [Google Scholar]
- 16.Stewart RM, Jones MN, Batterbury M, et al. Effect of glaucoma on corneal graft survival according to indication for penetrating keratoplasty. Am J Ophthalmol. 2011;151:257–62. doi: 10.1016/j.ajo.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Anshu A, Lim LS, Htoon HM, Tan DTH. Postoperative risk factors influencing corneal graft survival in the Singapore Corneal Transplant Study. Am J Ophthalmol. 2011;151:442–8. doi: 10.1016/j.ajo.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Banitt M, Lee RK. Management of patients with combined glaucoma and corneal transplant surgery. Eye. 2009;23:1972–79. doi: 10.1038/eye.2008.377. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Igo RP, Fondran J, et al. Association of smoking and other risk factors with Fuchs' endothelial corneal dystrophy severity and corneal thickness. Invest Ophthalmol Vis Sci. 2013;54:5829–35. doi: 10.1167/iovs.13-11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurkunas UV, Bitar MS, Funaki T, Azizi B. Evidence of oxidative stress in the pathogenesis of Fuchs endothelial corneal dystrophy. Am J Pathol. 2010;177:2278–89. doi: 10.2353/ajpath.2010.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price MO, Thompson RW, Jr., Price FW., Jr. Risk factors for various causes of failure in initial corneal grafts. Arch Ophthalmol. 2003;121(8):1087–92. doi: 10.1001/archopht.121.8.1087. [DOI] [PubMed] [Google Scholar]
- 22.Eye Bank Association of America 2012 Eye Banking Statistical Report. 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.