Abstract
MicroRNAs are small endogenous noncoding RNAs that regulate protein expression by hybridization to imprecise complementary sequences of target mRNAs. Changes in abundance of muscle-specific microRNA, miR-1, have been implicated in cardiac disease, including arrhythmia and heart failure. However, the specific molecular targets and cellular mechanisms involved in the action of miR-1 in the heart are only beginning to emerge. In this study we investigated the effects of increased expression of miR-1 on excitation-contraction coupling and Ca2+ cycling in rat ventricular myocytes using methods of electrophysiology, Ca2+ imaging and quantitative immunoblotting. Adenoviral-mediated overexpression of miR-1 in myocytes resulted in a marked increase in the amplitude of the inward Ca2+ current, flattening of Ca2+ transients voltage dependency and enhanced frequency of spontaneous Ca2+ sparks while reducing the sarcoplasmic reticulum Ca2+ content as compared with control. In the presence of isoproterenol, rhythmically paced, miR-1-overexpressing myocytes exhibited spontaneous arrhythmogenic oscillations of intracellular Ca2+, events that occurred rarely in control myocytes under the same conditions. The effects of miR-1 were completely reversed by the CaMKII inhibitor KN93. Although phosphorylation of phospholamban was not altered, miR-1 overexpression increased phosphorylation of the ryanodine receptor (RyR2) at S2814 (CaMKII) but not at S2808 (PKA). Overexpression of miR-1 was accompanied by a selective decrease in expression of the protein phosphatase PP2A regulatory subunit B56α involved in PP2A targeting to specialized subcellular domains. We conclude that miR-1 enhances cardiac excitation-contraction coupling by selectively increasing phosphorylation of the L-type and RyR2 channels via disrupting localization of PP2A activity to these channels.
Keywords: Ryanodine receptor, miR-1, CaMKII, PP2A, arrhythmia
INTRODUCTION
Cardiac contractility relies on release of Ca2+ from the sarcoplasmic reticulum (SR) and alterations in intracellular Ca2+ cycling have been implicated in different cardiac diseases, including arrhythmia and heart failure (HF). Normally, SR Ca2+ release is activated by Ca2+ that enters the cell through voltage-dependent Ca2+ channels of the sarcolemma during the plateau phase of the cardiac action potential (AP). This process, known as Ca2+-induced Ca2+ release (CICR)1, involves the ryanodine receptor (RyR2) channels on the SR and is essential for activation of contractile filaments during myocardial contraction2. Relaxation occurs when Ca2+ released to the cytosol is re-sequestered to the SR by the phospholamban-controlled SR Ca2+ ATPase (SERCA). Cardiac contractility is modulated by reversible phosphorylation of the components of SR Ca2+ release machinery, including the L-type Ca2+ channel (dihydropyridine receptor, DHPR), RyR2 and phospholamban (PLB), by protein kinase A (PKA)2, 3, Ca2+/calmodulin-dependent protein kinase (CaMKII)4, 5 and phosphatases PP1 and PP2A6–8. While in general both PKA and CaMKII potentiate SR Ca2+ release and enhance contractility2, the underlying mechanisms of these effects, and in particular the role of RyR2 phosphorylation/dephosphorylation, remain highly controversial3, 9–14.
MicroRNAs are recently discovered molecules consisting of ~22 nucleotides that regulate gene expression by annealing to target messenger RNAs inhibiting translation or promoting mRNA degradation15. Of approximately 600 microRNAs identified in vertebrates, several, including miR-1, are muscle-specific16–18. MiR-1 has been shown to be involved in cardiac development and apoptosis17, 18 and is reportedly upregulated in hearts from individuals with coronary artery disease19 and HF20. Several targets for miR-1 have been identified in the heart, including connexin 43 and Kir2.1 and miR-1-mediated reductions in expression of these proteins has been implicated in arrhythmogenesis19. However, considering the inherent capacity of miRNAs to target a broad range of proteins, the link between miR-1 and HF and sudden cardiac death is far from being clear and more miR-1 targets involved in these disease states are likely remain to be identified. In the present study we investigated the effects of increased expression of miR-1 on excitation-contraction coupling and Ca2+ cycling in rat ventricular myocytes by using methods of cellular electrophysiology and Ca2+ imaging. Our results identified a new potentially important target for miR-1 in the heart, namely the PP2A regulatory subunit B56α. Through translational inhibition of this mRNA target, miR-1 causes CaMKII-dependent hyperphosphorylation of RyR2, enhances RyR2 activity, and promotes arrhythmogenic SR Ca2+ release.
MATERIALS AND METHODS
The cellular and subcellular effects of adenovirally mediated miR-1 overexpression were studied in isolated adult rat ventricular myocytes maintained in culture for 36–48 hrs. Cytosolic Ca2+ changes were monitored using confocal microscopy, and whole cell currents and membrane potential were recorded with the patch-clamp technique. Changes in levels of RNA, protein expression and protein phosphorylation were studied using standard approaches.
An expanded Materials and Methods section can be found in the online data supplement available at http://circres.ahajournals.org.
RESULTS
MiR-1 Stimulates ICa and SR Ca2+ Release in Cardiac Myocytes
Myocytes were infected with either an adenoviral construct for expression of miR-1 (Ad-miR-1)21 or a construct containing a nontranslatable DNA segment that served as control (Ad-control). As determined by RT PCR, miR-1 abundance was increased ~2 fold in myocytes infected with Ad-miR-1 compared with control cells (Online Figure I). First, we investigated the effects of overexpression of miR-1 on EC coupling in voltage clamped cardiac myocytes. Inward Ca2+ currents (ICa) and intracellular Ca2+ transients were simultaneously measured in cardiac myocytes depolarized to membrane potentials in the range between –40 and 60 mV (Fig. 1). Overexpression of miR-1 resulted in a significant increase in the amplitude of ICa at membrane potentials of -40 to 20 mV (Fig. 1B). Additionally, the voltage-dependence of ICa (I–V curve) was shifted to the left 4 mV in miR-1 myocytes (Online Figure II). Despite increased ICa, the trigger for SR Ca2+ release, maximum Ca2+ transient amplitude was not changed by miR-1 overexpression (Fig. 1A,B). The failure of enhanced ICa to increase maximum SR Ca2+ release is attributable to reduced SR Ca2+ content limiting Ca2+ release in miR-1 cells (see below). At the same time, the voltage-dependence of Ca2+ transients was markedly broadened and flattened compared with control due to increased Ca2+ transient amplitude at small and large depolarizations (Fig. 1B). While the increase of Ca2+ transient amplitude at small depolarizing pulses can be accounted for by increased ICa, the increase of Ca2+ transients at large depolarizing steps occurred without an increase in ICa and is indicative of enhanced responsiveness of Ca2+ release channels to ICa (i.e. enhanced EC coupling gain).
Fig. 1. MiR-1 Overexpression Stimulates ICa and SR Ca2+ Release in Cardiac Myocytes.
(A) Representative recordings of Ca2+ transients and ICa evoked by depolarizing steps from a holding potential of −50 mV to −30, 0 and +30 mV in a control myocyte and a myocyte overexpressing miR-1. The voltage steps were applied at intervals of 5 s. (B, C) Voltage dependencies of ICa (bottom) and Ca2+ transient amplitude (top) in control (blue) and miR-1-overexpressing (red) myocytes at baseline conditions (B) and upon application of 100 nmol/L ISO (C). *Significantly different vs. control at P<0.05, One Way ANOVA (N=5–7).
The effects of miR-1 overexpression on the SR Ca2+ release were further studied by measuring spontaneous local Ca2+ release events, Ca2+ sparks, in intact quiescent myocytes loaded with Fluo-3 AM (Fig. 2). Spark frequency in miR-1 overexpressing cells was significantly higher than in control cells (4.39±0.54 and 2.88±0.41 100 µm−1 s−1 respectively, Fig. 2A,C). We assessed the Ca2+ loading state of the SR in miR-1 vs. control myocytes by application of caffeine. Judging from the amplitude of caffeine-induced Ca2+ transients, SR Ca2+ content was reduced to 75% of control in miR-1 myocytes (Fig. 2B,D). The lowered SR Ca2+ content is a likely result of increased spark-mediated SR Ca2+ leak and it provides an explanation for the lack of potentiation of maximum Ca2+ release by increased ICa in miR-1 overexressing myocytes. Therefore, miR-1 caused profound changes in EC coupling, including increased ICa, enhanced RyR2 channel functional activity and a reduction in the SR Ca2+ content.
Fig. 2. MiR-1 Overexpression Increases Ca2+ Spark Frequency and Decreases SR Ca2+ Content in Intact Cardiac Myocytes.
(A, B) Representative line-scan images of Ca2+ sparks (A) and time-dependent profiles of global Ca2+ releases induced by application of 10 mmol/L caffeine (B) recorded in a control myocyte and a myocyte overexpressing miR-1 at reference conditions and after treatment with the CaMKII inhibitor KN93 (1 µmol/L). (C) Averaged spark frequency for the three myocyte groups; n was 33, 53 and 45 for control, miR-1 and miR-1 treated with KN93, respectively. (D) Averaged amplitude of caffeine-induced Ca2+ transients; n was 14, 14 and 8 for control, miR-1 and miR-1 treated with KN93, respectively. *Significantly different vs. control at P<0.05, One Way ANOVA.
The Stimulatory Effects of MiR-1 in ICa and SR Ca2+ Release are Caused by Phosphorylation of DHPRs and RyR2s
The effects of miR-1 overexpression upon ICa in cardiomyocytes, namely the increased ICa amplitude and left-ward shift of ICa voltage dependence (Fig. 1B,C, Online Figure II), are similar to the effects of β-adrenergic stimulation. To examine whether the action of miR-1 involves the same mechanisms that mediate the response to β-adrenegic stimulation, we investigated the effects of the β-adrenergic agonist isoproterenol (ISO) on ICa and Ca2+ transients in miR-1 vs. control myocytes. As shown in Fig. 1B,C, ISO increased ICa amplitude ~2-fold in control but was virtually ineffective in miR-1 myocytes. Similarly, while ISO resulted in a ~2-fold increase in Ca2+ transient amplitude in control myocytes, ISO failed to cause significant changes in the amplitude and voltage-dependence of Ca2+ transients in miR-1 myocytes. These results could be explained by miR-1 and ISO acting through the same intracellular signaling mechanisms resulting in phosphorylation of target proteins including DHPR and RyR2.
RyR2 is phosphorylated at least at three different sites: at S2808 by PKA22 and possibly CaMKII23; at S2814 by CaMKII22, and at S2030 by PKA24. To test directly the hypothesis that miR-1 overexpression results in increased phopshorylation of RyR2 at these sites, we quantified RyR2 phosphorylation using phosphospecific antibodies. MiR-1 overexpression caused a marked increase in phosphorylation at the CaMKII site S2814 while leaving phosphorylation of the PKA site S2808 unaltered. The involvement of CaMKII in RyR2 phosphorylation at S2814 was further confirmed by the ability of KN93, a CaMKII inhibitor, to prevent phosphorylation at this site in miR-1 myocytes (Fig. 3A,B,C). Interestingly, RyR2s were not detectibly phosphorylated at S2030 either in the control or miR-1 groups even when the myocytes were exposed to 100 nmol/L ISO (Online Figure III). MiR-1 did not change phopshorylation of PLB at its PKA or CaMKII sites, S16 and T17, respectively (Fig. 3A,D) but increased phosphorylation of DHPRs (Fig. 3A,E).
Fig. 3. MiR-1 Overexpression Increases RyR2 and DHPR Phosphorylation and Does not Affect Phosphorylation of PLB.
(A) Representative Western blots. RyR2 phosphorylation at sites S2814 and S2808 and PLB phosphorylation at S16 and T17 was measured with phospho-specific antibodies. DHPR phosphorylation level was assessed using the Pro-Q Diamond phosphoprotein gel stain technology (Invitrogen). Total protein content (i.e. RyR2, PLB, or DHPR) was measured in the same samples on a different blot and used as a control for loading. Ventricular cells isolated from one heart were split into 5 groups, infected with the control adenovirus or the adenovirus encoding miR-1 and kept in culture for 48 hrs. Subsequently, myocytes were exposed to 100 nmol/L ISO (3 min) and 1 µmol/L KN93 (30 min), paced at 1 Hz for 1 min and flash frozen. (B) Data pooled from 6 experiments for S2808. (C) Data pooled from 7 experiments for S2814. (D) Data pooled from 7 independent experiments for T17. (E) Data pooled from 5 independent experiments for phospho-DHPR. *,†Significantly different vs. control (*) or vs. control+ISO (†)at P<0.05, paired Student’s t test.
To test the involvement of CaMKII in the functional effects of miR-1 on SR Ca2+ release in myocytes, we examined the impact of KN93 on miR-1-dependent changes in Ca2+ cycling. KN93 reversed the effects of miR-1 on frequency of Ca2+ sparks and on the SR Ca2+ content of myocytes (Fig. 2), as well as on Ca2+ currents and Ca2+ transients in voltage-clamped cells (Online Figure IV). Collectively, these results suggest that miR-1 overexpression results in augmented phosphorylation of DHPR and RyR2 by CaMKII, while the phosphorylation of PLB is unaltered.
MiR-1 Inhibits Expression of the PP2A Regulatory Subunit B56α
The phoshorylation-dependent effects of miR-1 on Ca2+ handling point to the possibility that miR-1 targets components of the phosphorylation-dephosphorylation system in myocytes. Bioinformatic sequence analysis revealed that the PP2A regulatory subunit B56α is a potential target of miR-1 since it harbors 7-basepair long complimentary seed sequence in its 3′-UTR region (Fig. 4A). Binding of miR-1 to B56α encoding RNA was confirmed by a luciferase reporter assay (Fig. 4B). Moreover, the miR-1 targeting of B56α in cardiac myocytes was confirmed by quantitative immunoblot analysis using an anti-B56α antibody (Fig. 5B,C). Importantly, miR-1 overexpression did not appreciably change expression levels of a number of relevant Ca2+ and phosphorylation handling proteins, including DHPR, RyR2, SR Ca ATPase (SERCA2a), PLB, sodium/calcium exchanger (NCX1), calsequestrin (CASQ2), catalytic subunits of PP1, PP2A and CaMKII, as well as phosphodiesterases 3A and 4D. As shown in Fig. 5A expression of all of these proteins was similar in miR-1 vs. control myocytes. Of note, miR-1 overexpression resulted in a decrease in expression of PKA β catalytic subunit, which is also a predicted target of miR-1. However, down-regulation of this kinase cannot account for the increase in phosphorylation of target proteins by miR-1 in our experiments.
Fig. 4. PP2A Regulatory Subunit B56α is a Target for Silencing by MiR-1.
(A) Complementarity between miR-1 and the putative B56α 3′-UTR site targeted (985–991 bp downstream from the human B56α stop codon, www.Targetscan.org). The putative miR-1 binding site harbored in the B56α 3′-UTR is conserved across species (i.e. human, mouse, rat, dog and chicken). The binding of miR-1 to the B56α 3′-UTR target site fulfills the requirement of a 7-bp seed sequence of complementarity at the miRNA end. (B) CHO cells were transfected with psiCHECK or the psiCHECK-B56α luciferase reporter construct and either miR-1 or scrambled miRNA at the concentrations indicated. Twenty-four hours following transfection, luciferase activities were measured. Renilla luciferase activity was normalized to firefly luciferase activity and mean activities ± S.E. from five independent experiments are shown (P<0.01 vs. CHO cells transfected with psiCHECK-B56α at each concentration shown).
Fig. 5. MiR-1 Overexpression Decreases Amount of PP2A Regulatory Subunit B56α in Myocytes.
(A) Representative immunoblots of lysates from ventricular myocytes infected with control adenoviruses or adenoviruses encoding miR-1 prepared from 2 hearts. PP2AC indicates protein phosphatase 2A catalytic subunit; PP1C, protein phosphatase 1 catalytic subunit; PKAβC, protein kinase A catalytic subunit β isoform; CaMKIIδC, Ca2+-calmodulin dependent protein kinase catalytic subunit δ isoform; CaMKII T287, CaMKII phosphorylated at threonine 287 probed with phospho-specific antibody; PDE3A and PDE4D, phosphodiesterases types 3A and 4D respectively; Cav1.2, α1C subunit of L-type Ca2+ channel; CASQ2, cardiac isoform of calsequestrin; SERCA2a, cardiac isoform of SR Ca2+-ATPase; NCX1, Na+/Ca2+ exchanger type 1. 40 µg of protein per lane was used for analysis of all proteins. There is no significant difference in the density of these proteins between the 2 groups except PKAβC. (B, C) Representative immunoblot (B) and pooled data for PP2A regulatory subunit B56α in control myocytes and myocytes overexpressing miR-1 (C). Data were obtained from 10 independent experiments. *Significantly different vs. control, P<0.05, paired Student’s t test. (D) Control (top) and miR-1-overexpressing (bottom) cardiomyocytes fixed after 48 hours in culture coimmunolabeled with B56α- (left, green) and RyR2-specific antibodies (middle, red).
An important condition for B56α to be able to specifically influence phosphorylation of the DHPR and RYR2 channels is that it must be in close proximity to these channels. To examine the cellular distribution of B56α with respect to RyR2 in cardiac myocytes, we performed immunostaining experiments. As demonstrated in Fig. 5D, B56α shows a substantial degree of co-localization with RyR2 at the Z-lines. Thus, B56α and RyR2 (and hence DHPR) appear to coexist in specific subcellular compartments of ventricular myocytes. MiR-1 overexpression reduced B56α abundance uniformly without a change in the overall distribution pattern across the cells. In accordance with the targeting role of B56α, the distribution of PP2A catalytic subunit changed from localized preferentially at the Z-lines to more diffused following overexpression of miR-1 (Online Figure V).
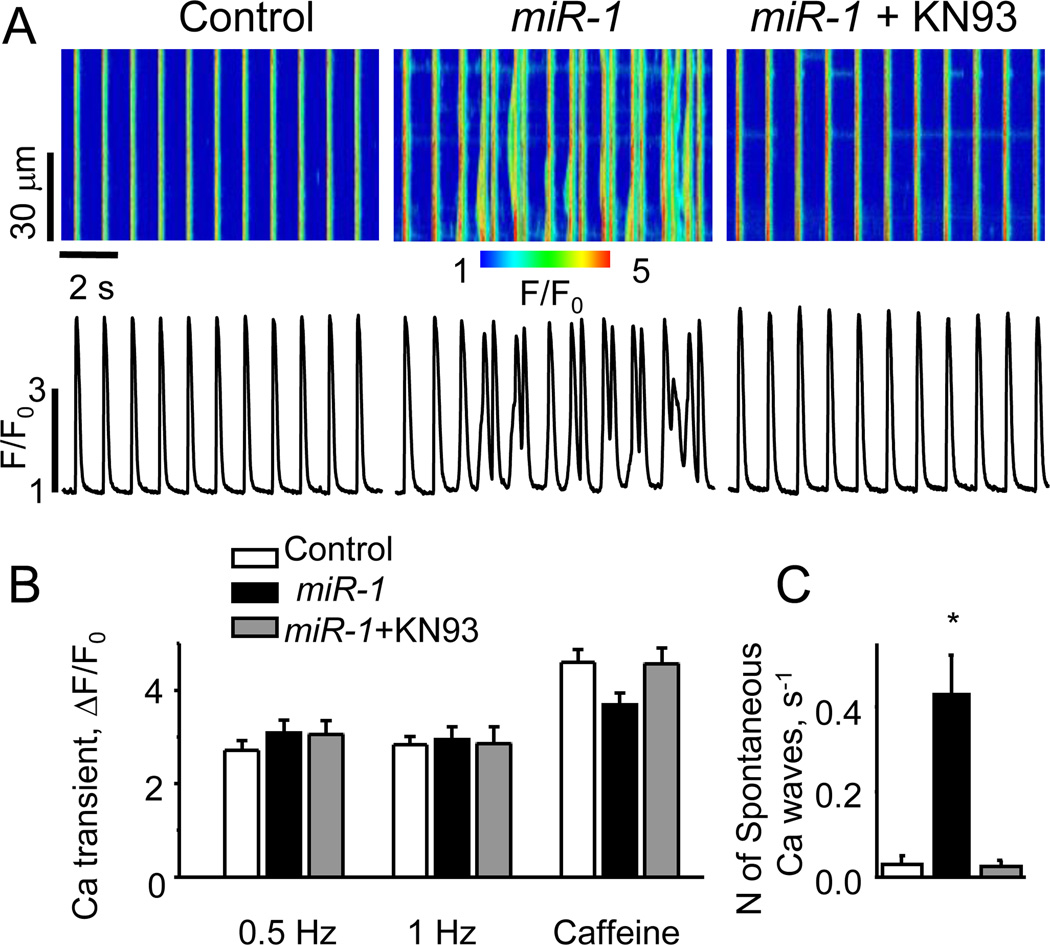
β-Adrenergic Stimulation Promotes Arrhythmia in MiR-1 Overexpressing Myocytes
RyR2 hyperphosphorylation by either PKA or CaMKII has been suggested to present a possible mechanism for Ca2+-dependent arrhythmia, including catecholaminergic polymorphic ventricular tachycardia (CPVT)25, 26. To determine whether miR-1 overexpression renders myocytes prone to arrhythmogenic alterations in Ca2+ cycling, we examined miR-1 and control myocytes as to the occurrence of spontaneous, extrasystolic Ca2+ transients and associated delayed afterdepolarizations (DADs)27, 28 (Fig. 6, Online Figure VI). Control and miR-1 myocytes were paced at 1 Hz in the presence or absence of ISO (100 nmol/L). In the presence of ISO, the two groups showed no significant differences in the amplitude of Ca2+ transients and SR Ca2+ load when paced at 1 Hz (Fig. 6A,B). Control myocytes exposed to ISO exhibited relatively few Ca2+ waves and DADs. However, the number of spontaneous extrasystolic Ca2+ waves per second increased more than 10-fold in miR-1 overexpressing cells compared with controls (Fig. 6C). Simultaneously with Ca2+ waves, these cells exhibited DADs (Online Figure VI). Remarkably, incubation of miR-1 overexpressing myocytes with 1 µmol/L KN93 completely abolished the miR-1-mediated boost in arrhythmogenic activity (Fig. 6A,C). In the absence of ISO, spontaneous Ca2+ waves were only rarely observed in paced miR-1 myocytes. However, the properties of SR Ca2+ release in these cells were strikingly altered compared to control. The amplitude of Ca2+ transients in miR-1 myocytes was ~2-fold higher than in control (Online Figure VII). Additionally, miR-1 myocytes exhibited substantial spontaneous diastolic SR Ca2+ release, seen as Ca2+ sparks, much less pronounced in control cells (see insets in Online Figure VII). Collectively, these results suggest that miR-1 overexpression renders RyR2s hyperactive even in the absence of PKA phosphorylation and that arrhythmogenic spontaneous Ca2+ release is linked to RyR2 phosphorylation by CaMKII rather by PKA.
Fig. 6. MiR-1 Overexpression Increases Arrhythmogenic Potential of Myocytes Undergoing Repetitive Stimulation in the Presence of Isoproterenol.
(A) Representative line-scan images and corresponding time-dependent profiles of Fluo-3 fluorescence in rat ventricular myocytes infected with Ad-Control and Ad-miR-1 adenoviruses. Ca transients were evoked by electrical field stimulation at 1 Hz in the presence of 100 nmol/L ISO. MiR-1-overexpressing myocytes displayed increased incidence of arrhythmogenic spontaneous diastolic Ca2+ waves. Incubation of miR-1-overexpressing myocytes with KN93 (1 µmol/L for 30 min) restored normal rhythmic activity. (B) Summarized data for Ca2+ transient amplitude and SR Ca2+ content for control, miR-1-overexpressing myocytes and miR-1-myocytes treated with KN93 in the presence of 100 nmol/L ISO. The myocytes were field-stimulated at 0.5 and 1 Hz for 1 min each then exposed 10 mmo/L caffeine. (C) Averaged number of spontaneous Ca2+ waves per second in field-stimulated myocytes at 1 Hz in the presence of 100 nmol/L ISO. *Significantly different at P<0.05, One Way ANOVA. Number of cells studied (n=8–16).
DISCUSSION
In the present study, we investigated the effects of the muscle-specific microRNA, miR-1, on excitation-contraction coupling and regulation of SR Ca2+ release in cardiac myocytes. Our major findings are that miR-1 enhanced the functional activity of RyR2 channels and thus resulted in increased EC coupling gain, elevated diastolic SR Ca2+ leak, and reduced SR Ca2+ content, and promoted arrhythmogenic disturbances in myocyte Ca2+ cycling. These effects were attributable to translational inhibition by miR-1 of the B56α regulatory subunit of protein phosphatase PP2A in turn causing hyperphosphorylation of RyR2 by CaMKII. These findings have important implications for the functional role of microRNAs and mechanisms of arrhythmogenesis in cardiac muscle.
Our conclusion that miR-1 altered myocyte Ca2+ signaling through disruption of site-specific PP2A phosphatase activity was supported by the following results. 1) miR-1 expression in myocytes resulted in reduced expression of B56α involved in subcellular localization of PP2A activity; 2) miR-1 increased phosphorylation of the DHPR and RyR2 without affecting phosphorylation of PLB localized in a separate cellular domain; 3) B56α co-localized with RyR2 in myocytes; and 4) The functional effects of miR-1 on EC coupling and intracellular Ca2+ cycling were reversed by the inhibitor of CaMKII KN93. The PP2A catalytic subunit has been shown to complex with DHPR7 and RyR28 and is critical to dephosphorylation of these proteins following their phosphorylation by PKA and/or CaMKII7, 8. Consistent with the role of B56α in conveying PP2A phosphatase activity to DHPR, B56α has been shown to co-immunoprecipitate with the α subunit of the cardiac L-type Ca2+ channel (Cav1.2α)29. As to RyR2, it has been previously reported that the PP2A catalytic subunit associates with the RyR2 complex through the regulatory subunit, PR130, which binds directly to RyR2 by a leucine zipper motif30. However, since PR130 does not present a target for miR-1, it is unlikely to play a role in the effects of miR-1 in our experiments. In support of the involvement of B56α, it has been shown to interact with the anchoring protein, ankyrin-B31, which in turn binds to RyR232 thereby linking PP2A activity to the RyR2. Of note, myocytes from genetically altered mice lacking ankyrin-B exhibit increased propensity for spontaneous Ca2+ release and DADs when challenged with ISO similar to the effects of miR-1 in our study (see for discussion below). Moreover, human mutations in ankyrin-B have been linked to CPVT an arrhythmic disorder associated with abnormal Ca2+ handling and DADs33, 34. Based on our findings, disruption of PP2A activity may contribute to, or account for, those abnormalities.
The RyR2 is phosphorylated by PKA and CaMKII at a number of different sites22–24. In our study the alterations of RyR2 function and SR Ca release in miR-1 overexpressing myocytes appear to be caused by CaMKII rather than PKA for the following reasons. First, the potentiation of RyR2 function was associated with increased phosphorylation of RyR2 at the CaMKII site S2814 but not at the PKA site S2808, as determined by phosphospecific antibodies directed to these sites. Furthermore, the effects of miR-1 could be prevented by the CaMKII inhibitor KN93. The observed changes in myocyte Ca2+ handling, including increased EC coupling gain, reduced SR Ca2+ content and increased SR RyR2-mediated Ca2+ leak are similar to those observed by others in cells overexpressing CaMKII26 and are consistent with miR-1 effects being mediated by CaMKII. Thus, collectively, these results suggest that miR-1 influences myocyte Ca2+ signaling by regulating the spatial distribution of phosphatase PP2A activity and hence promoting RyR2 phosphorylation by CaMKII.
Additionally, phosphorylation of the L-type Ca2+ channel (α1C and/or β subunits of DHPR) by PKA, PKC and CaMKII is known to occur at a number of sites and cause potentiation of the L-type Ca2+ current4, 7, 29, 35. At the present time, we do not know which of the specific phosphorylation pathways and sites are involved in modulation of ICa by miR-1 in our study. In principle, by targeting PP2A activity in the vicinity of the L-type Ca2+ channels, miR-1 would be expected to enhance phosphorylation at all sites. The failure of ISO to further stimulate the enlarged ICa in miR-1 cells (Fig. 1) supports the possibility that PKA plays a role in mediating the effects of miR-1 on ICa. At the same time, the fact that CaMKII inhibition attenuates the miR-1-induced increase in ICa (Online Figure IV) points to the possibility that CaMKII might be also involved. Future studies using genetic mouse models with targeted mutations of the phosphorylation sites on the L-type Ca2+ channel may help to resolve this issue.
An important finding of this work is that miR-1 resulted in profound disturbances in myocyte Ca2+ cycling manifested as extrasystolic spontaneous Ca2+ releases. Spontaneous Ca2+ releases are arrhythmogenic as they results in DADs and EADs27, 28. DADs and EADs in turn present triggering events for arrhythmia. Interestingly, these abnormal Ca2+ releases typically occurred after stimulation of the miR-1 overexpressing cells with ISO and were only rarely observed in ISO-treated control myocytes or untreated miR-1 overexpressing cells. Correlating these functional effects with the state of RyR2 phosphorylation at S2808 and S2814 revealed that disrupted Ca2+ cycling was associated with maximum phosphorylation at S2814 but not necessarily at S2808, and again this abnormal Ca2+ cycling was completely preventable by inhibition of CaMKII. The increased occurrence of global spontaneous Ca2+ waves and DADs in miR-1 cells in the presence of ISO is likely to be due to stimulation of SERCA-mediated SR Ca2+ uptake rather than caused by additional effects upon CAMKII-phosphorylated RyR2s. The stimulatory effects of CaMKII on SR Ca2+ release and the involvement of CaMKII in the pro-arrhythmic effects of miR-1 are consistent with previous studies which showed that CaMKII activation is involved in arrhythmia induction in various pathologic settings including cardiac hypertrophy and heart failure26, 36.
Previously, we reported that exogenous phosphatases, including PP2A, elevate cardiac SR Ca2+ leak by stimulation of RyR2s10. This result is in apparent contradiction with the stimulatory effects of increased RyR2 phosphorylation described in the present study. Recently, we found that both phosphorylation and dephosphorylation can stimulate RyR2 activity resulting in increased SR Ca2+ leak in cardiomyocytes37. Although we do not have a definitive explanation for these apparently contradicting results, they can be rationalized by considering that RyR2 is a multimer with multiple sets of phosphorylation sites. It is possible that RyR2 activity is lowest at intermediate phosphorylation of a certain set of sites (i.e. S2814) whereas both hypo- or hyper-phosphorylation of the sites leads to increased activity. It is also possible that the observed stimulatory effects of kinases and phosphatases are mediated by different sets of sites. Elucidation of the precise mechanisms of action of phosphorylation and dephosphorylation on RyR2 should await further studies.
MiR-1 levels have been shown to increase in human cardiac diseases, such as infarction and heart failure, and elevated miR-1 levels have been implemented in the development of arrhythmia in these disease settings19, 20. Yang et al.19 attributed the pro-arrhythmic effects of miR-1 to down regulation of connexin 43 and the inward rectifier K channel (Kir2.1), which they identified as targets for miR-1. Our study demonstrated that altered Ca2+ signaling presents another potential arrhythmogenic mechanism for miR-1. In vivo, electrical remodeling and alterations of Ca2+ signaling are likely to act together to induce arrhythmia. For example, the probability of DADs reaching the threshold for AP potential for any given magnitude of spontaneous Ca2+ release would be expected to increase on inhibition of Kir2.1 due to increased membrane resistance38.
A potential limitation of our study is that the experiments were performed using primary cultures of adult rat ventricular myocytes, which are known to change their characteristics over time in culture. We did not find a significant difference in ICa density either with or without ISO between cells cultured for 48 hrs and freshly isolated myocytes (Online Fig VIII). Therefore, the EC coupling machinery of rat cardiomyocytes during the first 48 hrs seems to be largely preserved. This result is consistent with the recent study by Banyasz et al39, which showed that the most profound changes in morphometric and functional parameters, including T-tubular density and ICa, in rat ventricular myocytes take place after three days in culture.
In conclusion, our results identified a new important target for miR-1 in the heart, namely the PP2A regulatory subunit B56α. Through translational inhibition of this mRNA target, miR-1 causes CaMKII-dependent hyperphosphorylation of RyR2, enhances RyR2 activity, and promotes arrhythmogenic SR Ca2+ release. This mechanism could contribute to induction of arrhythmia in disease states accompanied by elevated miR-1.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This study was supported by American Heart Association Scientist Development Grants (DT, AEB), and NIH grants (SG, TSE, DSF).
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985;85:247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 3.Wehrens XH, Marks AR. Novel therapeutic approaches for heart failure by normalizing calcium cycling. Nat Rev Drug Discov. 2004;3:565–573. doi: 10.1038/nrd1440. [DOI] [PubMed] [Google Scholar]
- 4.Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation–contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Grueter CE, Colbran RJ, Anderson ME. CaMKII, an emerging molecular driver for calcium homeostasis, arrhythmias, and cardiac dysfunction. J Mol Med. 2007;85:5–14. doi: 10.1007/s00109-006-0125-6. [DOI] [PubMed] [Google Scholar]
- 6.Steenaart NAE, Ganim JR, DiSalvo J, Kranias EG. The phospholamban phosphatase associated with cardiac sarcoplasmic reticulum is a type 1 enzyme. Arch Biochem Biophys. 1992;293:17–24. doi: 10.1016/0003-9861(92)90359-5. [DOI] [PubMed] [Google Scholar]
- 7.Davare MA, Horne MC, Hell JW. Protein phosphatase 2A is associated with class C L-type calcium channels (Cav1.2) and antagonizes channel phosphorylation by cAMP-dependent protein kinase. J Biol Chem. 2000;275:39710–39717. doi: 10.1074/jbc.M005462200. [DOI] [PubMed] [Google Scholar]
- 8.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 9.Bers DM, Eisner DA, Valdivia HH. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res. 2003;93:487–490. doi: 10.1161/01.RES.0000091871.54907.6B. [DOI] [PubMed] [Google Scholar]
- 10.Terentyev D, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, Gyorke S. Protein phosphatases decrease sarcoplasmic reticulum calcium content by stimulating calcium release in cardiac myocytes. J Physiol. 2003;552:109–118. doi: 10.1113/jphysiol.2003.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, Walsh MP, Warltier DC, Cheng H, Chen SR. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 12.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101:746–749. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 13.Yang D, Zhu WZ, Xiao B, Brochet DX, Chen SR, Lakatta EG, Xiao RP, Cheng H. Ca2+/Calmodulin Kinase II-Dependent Phosphorylation of Ryanodine Receptors Suppresses Ca2+ Sparks and Ca2+ Waves in Cardiac Myocytes. Circ Res. 2007;100:399–407. doi: 10.1161/01.RES.0000258022.13090.55. [DOI] [PubMed] [Google Scholar]
- 14.Bridge JHB, Savio-Galimberti E. What Are the Consequences of Phosphorylation and Hyperphosphorylation of Ryanodine Receptors in Normal and Failing Heart? Circ Res. 2008;102:995–997. doi: 10.1161/CIRCRESAHA.108.176172. [DOI] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 17.Latronico MV, Catalucci D, Condorelli G. Emerging role of microRNAs in cardiovascular biology. Circ Res. 2007;101:1225–1236. doi: 10.1161/CIRCRESAHA.107.163147. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Luo X, Lu Y, Yang B. miRNAs at the heart of the matter. J Mol Med. 2008 doi: 10.1007/s00109-008-0341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 20.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 21.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs Play an Essential Role in the Development of Cardiac Hypertrophy. Circ Res. 2007;100:416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 22.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez P, Bhogal MS, Colyer J. Stoichiometric phosphorylation of cardiac ryanodine receptor on serine 2809 by calmodulin-dependent kinase II and protein kinase A. J Biol Chem. 2003;278:38593–38600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- 24.Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, Walsh MP, Warltier DC, Cheng H, Chen SR. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 25.Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, Landry DW, Kontula K, Swan H, Marks AR. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 26.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1422. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 27.Kass RS, Tsien RW. Fluctuations in membrane current driven by intracellular calcium in cardiac Purkinje fibers. Biophys J. 1982;38:259–269. doi: 10.1016/S0006-3495(82)84557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res. 2007;100:105–111. doi: 10.1161/01.RES.0000252828.17939.00. [DOI] [PubMed] [Google Scholar]
- 29.Hall DD, Feekes JA, Arachchige Don AS, Shi M, Hamid J, Chen L, Strack S, Zamponi GW, Horne MC, Hell JW. Binding of protein phosphatase 2A to the L-type calcium channel Cav1.2 next to Ser1928, its main PKA site, is critical for Ser1928 dephosphorylation. Biochemistry. 2006;45:3448–3459. doi: 10.1021/bi051593z. [DOI] [PubMed] [Google Scholar]
- 30.Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, Rosemblit N, Marks AR. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol. 2001;153:699–708. doi: 10.1083/jcb.153.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhasin N, Cunha SR, Mudannayake M, Gigena MS, Rogers TB, Mohler PJ. Molecular basis for PP2A regulatory subunit B56alpha targeting in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293:H109–H119. doi: 10.1152/ajpheart.00059.2007. [DOI] [PubMed] [Google Scholar]
- 32.Bourguignon LY, Chu A, Jin H, Brandt NR. Ryanodine receptor-ankyrin interaction regulates internal Ca2+ release in mouse T-lymphoma cells. J Biol Chem. 1995;270:17917–17922. doi: 10.1074/jbc.270.30.17917. [DOI] [PubMed] [Google Scholar]
- 33.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogné K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 34.Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT, Bennett V. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci U S A. 2004;101:9137–9142. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Liu G, Zakharov SI, Morrow JP, Rybin VO, Steinberg SF, Marx SO. Ser1928 is a common site for Cav1.2 phosphorylation by protein kinase C isoforms. J Biol Chem. 2005;280:207–214. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 37.Terentyev D, Terentyeva R, Gyorke S. Both Phosphorylation and Dephosphorylation of Ryanodine Receptor Enhance Sarcoplasmic Reticulum Calcium Leak in Canine Ventricular Myocytes. Circulation. 2006;114:II_92. [Google Scholar]
- 38.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 39.Banyasz T, Lozinskiy I, Payne CE, Edelmann S, Norton B, Chen B, Chen-Izu Y, Izu LT, Balke CW. Transformation of adult rat cardiac myocytes in primary culture. Exp Physiol. 2008;93:370–382. doi: 10.1113/expphysiol.2007.040659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.