Abstract
Genetically modified Schwann cells (SCs) that overexpress neurotrophic factors (NFs), especially those that overexpress multiple NFs, hold great potential for promoting nerve regeneration. Currently, only one NF can be upregulated in most genetically modified SCs, and simultaneously upregulating multiple NFs in SCs remains challenging. In this study, we found that the overexpression of c-Jun, a component of the AP-1 transcription factor, effectively upregulated the expression and secretion of multiple NFs, including glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, artemin, leukemia inhibitory factor, and nerve growth factor. The c-Jun gene-modified SCs showed a normal morphology in scanning electron microscopy and fluorescent staining analysis. In addition, the c-Jun-modified SCs showed enhanced proliferation and migration abilities compared with vector control cells. We used transwell chambers to establish coculture systems imitating the in vivo conditions in which transplanted SCs might influence native SCs and neurons. We found that the c-Jun-modified SCs enhanced native SC migration and promoted the proliferation of native SCs in the presence of axons. Further analysis revealed that in the c-Jun group, the average length and the total area of neurites divided by the total area of the explant body were μm 1180±25 and 6.4±0.4, respectively, which were significantly greater compared with the other groups. These findings raise the possibility of constructing an optimal therapeutic alternative for nerve repair using c-Jun-modified SCs, which have the potential to promote axonal regeneration and functional recovery by upregulating multiple NFs. In addition, these cells exhibit enhanced migration and proliferation abilities, enhance the biological functions of native SCs, and promote neurite outgrowth.
Introduction
In contrast to the central nervous system (CNS), axons in the periphery display a remarkable potential to regenerate after injury.1 Nevertheless, axon regrowth in the peripheral nervous system (PNS) is hampered by nerve gaps created by injury, especially in lengthy defects.2 Currently, autologous transplantation is still the gold standard and the most common treatment for this condition.3,4 However, this treatment has several drawbacks, including donor site morbidity, the scarcity of available donor tissue, and the frequent incidence of the formation of donor-site neuroma.5,6 Nerve conduits (NCs) have been developed as an attractive alternative because they can restore the nerve pathway (which is a prerequisite for successful regeneration) while avoiding the disadvantages of autologous transplants.7–9 NCs have been applied in a clinical setting and have been reported to be beneficial for patients. However, the limitations of those conduits soon became evident, including suboptimal functional recovery and a limited transplant distance.10,11
Schwann cells (SCs), which play a pivotal role during nerve regeneration in vivo, are an attractive type of transplant cell with potentiated functionality due to the expression of multiple neurotrophic factors (NFs), the organization of Büngner bands, and the ability to form myelin.12–14 Recent studies have revealed that delivery of SCs to NCs is advantageous for nerve repair, particularly when using SCs that overexpress an NF, such as basic fibroblast growth factor (FGF-2),15,16 glial cell line-derived neurotrophic factor (GDNF),17,18 nerve growth factor (NGF),19 brain-derived neurotrophic factor (BDNF),20 or neurotrophin-3 (NT-3).21 However, an increasing number of studies have indicated that the application of multiple NFs, rather than a single type, holds greater therapeutic promise.4,22,23 If we can modify a cell to upregulate multiple NFs, nerve regeneration and functional recovery might be greatly enhanced. Currently, simultaneous upregulation of multiple NFs in genetically modified SCs remains challenging.
c-Jun, a component of the AP-1 transcription factor, is expressed in immature SCs and repressed in adult myelinating SCs.24 c-Jun is strongly reactivated and functions as a master regulator of the axonal response to nerve injury.25 Inactivation of c-Jun in SCs results in the downregulation of various NFs, a striking failure of functional recovery and neuronal death. Additionally, it has been noted that GDNF and artemin (Artn), which both encode ligands for the Ret receptor tyrosine kinase, are novel direct c-Jun target genes.26 The leukemia inhibitory factor (LIF) gene has also been demonstrated to be transcriptionally regulated by c-Jun.27 Furthermore, a variety of nerve regeneration-related genes are indirectly regulated by c-Jun, including BDNF and NGF.25,26
Thus, we hypothesized that the overexpression of c-Jun might upregulate multiple NFs in SCs, which would in turn further promote neuronal survival, axonal outgrowth, and functional recovery. In addition, the grafted SCs would not only affect neuronal survival and axon regeneration, but also interact with native SCs. However, limited information is available from previous studies regarding the influence of transplanted SCs on the biological activities of native SCs.
In the present study, a lentiviral vector encoding c-Jun-IRES-eGFP (LV/c-Jun) or eGFP (LV/GFP) was used to transduce purified SCs in vitro. We examined the expression and secretion of multiple NFs, including GDNF, BDNF, Artn, LIF, NT-3, and NGF in LV/c-Jun-transduced SCs. We next investigated the biological characteristics of the transduced SCs and the influence of c-Jun-modified SCs on native SC proliferation and migration. Furthermore, we explored the effect of the c-Jun-modified SCs on the neurite outgrowth of dorsal root ganglion (DRG) explants.
Materials and Methods
Production of lentiviral vectors
Lentiviral vector stocks of LV/c-Jun and LV/GFP were generated based on previous description.28,29 Briefly, an LV/c-Jun stock encoding c-Jun and GFP and an LV/GFP stocks encoding GFP were produced through cotransfection of the vector, packaging, and envelope plasmids into 293T cells. After 2 days, the medium containing the viral particles was harvested and, if needed, concentrated through ultracentrifugation. The number of transducing particles was determined by infecting 293T cells and counting the number of GFP-expressing cells after 48 h. Titers were expressed as transducing units (TU) per milliliter, and the concentrated stocks ranged from 108 to 109 TU/mL.
Isolation and purification of SCs
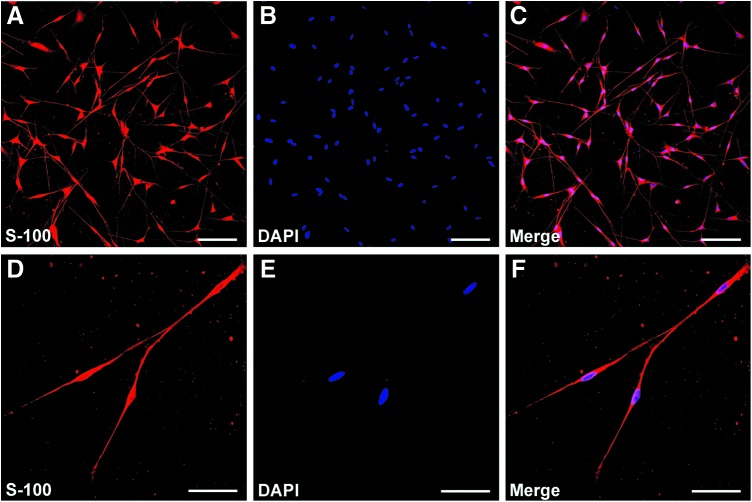
SCs were prepared and purified following the protocol described previously.30,31 All the experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85–23, revised 1985). Briefly, SCs were isolated from the sciatic nerves of 1–2 days newborn Sprague Dawley rats (provided by the Experimental Animal Center of the Forth Military Medical University) and further selected from fibroblasts using anti Thy1.1 (1:5 hybridoma cell supernatant T11D7) and rabbit complement (1:5; Serotec). The final preparations consisted of 98% SCs, as determined by immunofluorescence for S100 (Fig. 1), which is a specific SC marker. SCs were maintained in Dulbecco's modified Eagle's medium: Nutrient Mixture F-12 (DMEM/F12; Gibico) medium with 10% fetal calf serum (FCS; Gibico), antibiotics (penicillin and streptomycin solution), 2 μM/mL forskolin (Sigma-Aldrich), and 20 μg/mL bovine pituitary extract (Biomedical Technologies Stoughton) at 37°C under humidified 5% CO2. SC cultures were passaged no more than five times before conducting experiments.
FIG. 1.
Immunohistochemistry of Schwann cells (SCs) isolated from the sciatic nerve of Sprague Dawley rats. Immunofluorescence staining showing the expression of S100 (red; A, D) with DAPI nuclear counterstaining (blue; B, E). Merge images reveal an SC purity of more than 98% (C, F). Scale bars: (A–C)=100 μm, (D–F)=40 μm. Color images available online at www.liebertpub.com/tea
Transduction of SCs with lentiviral vectors
When SCs reached 30–40% confluence, they were pretreated with 5 μg/mL polybrene (Sigma-Aldrich) for 30–60 min. Then, LV/GFP or LV/c-Jun vectors were added to the dish at a multiplicity of infection of 1:50 for 24 h. The transduced SCs were visible directly by the expression of the reporter gene GFP under a fluorescence microscope.
Scanning electron microscopy
The morphology of SCs in each group was detected with scanning electron microscopy (SEM). Briefly, 72 h after LV transfection, SCs were digested by trypsin, resuspended in fresh medium, and plated on coverslips. Forty-eight hours after plating, SCs were fixed with 4% paraformaldehyde at room temperature for 30 min, washed three times with distilled water, and dehydrated with serial ethanol solutions. The specimens were then dried under vacuum at room temperature, sputter-coated with gold, and subjected to SEM at an accelerating voltage of 5 kV (SEM; Hitachi S-3400N) to examine the surface morphology of the cells.
Cell proliferation assay
SCs were resuspended in fresh prewarmed complete medium, then counted and plated at a density of 3×105 cells/mL on coverslips. Next, 5-ethynyl-2-deoxyuridine (EdU) was applied 4 h before fixation of cultures, which was followed by the EdU immunostaining using the Cell-Light™ EdU DNA Cell Proliferation Kit (Ribobio) according to the manufacturer's protocol. To determine the SCs proliferation level, the EdU labeling index was calculated by determining the number of EdU-labeled nuclei divided by the number of DAPI-labeled nuclei from five random fields. The assays were conducted in triplicate.
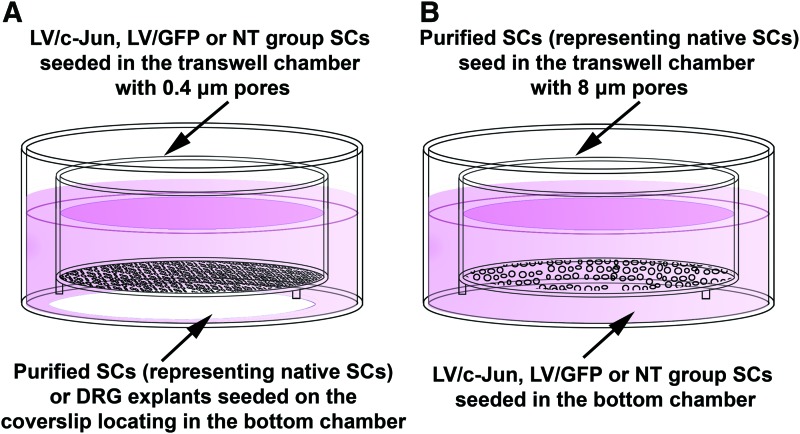
To determine whether c-Jun-modified SCs influenced the proliferation of native SCs, purified SCs representing native SCs or DRG explants were seeded in the bottom chamber and c-Jun-modified SCs, LV/GFP-transduced SCs or nontransduced (NT) SCs were seeded in the top chamber (Fig. 2A). Three days after seeding, the proliferation of SCs in the bottom chambers was measured as described above. To exclude non-SCs from the analysis of DRG explants culture, the SCs were also immunostained with S100 (green) to confirm that the EdU-positive cells were indeed SCs. The EdU labeling index was determined from the number of EdU-labeled S100-positive cells divided by the number of S100-positive cell.
FIG. 2.
Schematic diagram of the coculture systems employed in this study. (A) The coculture system used to investigate the influence of c-Jun-modified SCs on native SC proliferation and dorsal root ganglion (DRG) explant outgrowth. (B) The coculture system used to investigate the influence of c-Jun-modified SCs on native SC migration. Color images available online at www.liebertpub.com/tea
Cell migration assay
SC migration was assessed using transwell chambers with 8 μm pores (Nunc). The bottom surface of each membrane was coated with 10 μg/mL fibronectin (Sigma), and the bottom chamber contained 10% FCS. Cells were transferred to the top of each transwell chamber and allowed to migrate at 37°C in 5% CO2. After 48 h, the upper surface of each membrane was cleaned with a cotton swab, and cells that migrated to the lower surface were fixed in 4% paraformaldehyde followed by Hematoxylin and Eosin staining. The cells on the bottom surface of each membrane were counted under a light microscope (AH3; Olympus). Each condition was analyzed in triplicate. Four fields were examined from each filter. The values for migration were assessed based on the mean number of SCs per field, which was calculated from five random fields. The assays were performed using triplicate wells.
To study whether the c-Jun-modified SCs influenced the migration of native SCs, purified SCs representing native SCs were seeded in the top chamber and c-Jun-modified SCs, LV/GFP-transduced SCs or NT SCs were seeded in the bottom chamber (Fig. 2B). After 48 h, the migration of SCs in the top chamber was determined as described above.
DRG explant cultures
To evaluate nerve regeneration, DRG explant cultures were prepared as described previously.32 Briefly, DRGs from neonatal (postnatal day 1) rats were dissected and digested with 0.125% Trypsin (Invitrogen) for 5 min, then placed on coverslips coated with rat tail collagen, and 2 h after attachment, the coverslips were transferred to the bottom chamber. Then, c-Jun-modified SCs, LV/GFP-transduced SCs, or NT SCs were transferred to the top of each transwell chamber (Fig. 2A) and maintained in DMEM/F12 medium for 48 h in vitro before fixation and immunostaining, as described below. After immunostaining, the axonal length from the edge of DRG explants to the tip of axons as well as the total area of neurites and the explant body in all groups were measured using the ImageJ software version 1.46 m (http://rsb.info.nih.gov/ij/). Neurite outgrowth was evaluated based on the average length of the five longest axons and the total area of neurites divided by the total area of the explant body, as described previously.32,33
Quantitative real-time polymerase chain reaction
For the quantitative real-time polymerase chain reaction (qRT-PCR) analysis, total RNA isolation was performed using the RNeasy Mini Prep kit (Qiagen). cDNA was synthesized using Superscript III reagents according to the manufacturer's instructions (Invitrogen). qRT-PCR was performed in an Eppendorf Mastercycler ep realplex thermal cycler using the iQ SYBR Green Supermix (Bio-Rad) according to the manufacturer's instructions. The sequences of the primers for BDNF, NGF, GDNF, Artn, LIF, NT-3, and β-actin (internal control) are shown in Table 1. The qRT-PCR conditions were as follows: denaturation at 95°C, 30 s; primer annealing at 59°C, 30 s; and elongation at 72°C, 40 s. The quantification of PCR products was performed using the 2−ΔΔCt method, and the quantity of mRNA was normalized to the housekeeping gene β-actin. The assays were performed in triplicate.
Table 1.
Primer Sequences Used for Quantitative Real-Time Polymerase Chain Reaction
| Gene | GenBank accession No. | Direction | Sequence | Length (bp) |
|---|---|---|---|---|
| c-Jun | NM_021835.3 | Upper | 5′ GCCTGCCTCTCTCAACTATGTA 3′ | 126 |
| Lower | 5′ TAGGACACCCAAACAAACAAAC 3′ | |||
| Gdnf | NM_019139.1 | Upper | 5′ AGAGGGAAAGGTCGCAGAG 3′ | 142 |
| Lower | 5′ CTTCACAGGAACCGCTACAA 3′ | |||
| Bdnf | NM_001270630 | Upper | 5′ GCCCAACGAAGAAAACCATA 3′ | 98 |
| Lower | 5′ CCAGCAGAAAGAGCAGAGGA 3′ | |||
| Artn | NM_053397.1 | Upper | 5′ ATGTCGCCCTACCTGGAAC 3′ | 114 |
| Lower | 5′ GGCTCTGTCTGTCCCTCATC 3′ | |||
| Lif | NM_022196.2 | Upper | 5′ TCAAACTCAATGCGACTACAGA 3′ | 103 |
| Lower | 5′ ACACAGGGCACATCCACAT 3′ | |||
| Ngf | NM_001277055 | Upper | 5′ CAGGCAGAACCGTACACAGA 3′ | 183 |
| Lower | 5′ AAACAGTTTGGGGTCCACAG 3′ | |||
| Nt-3 | NM_001270868 | Upper | 5′ CCAGGCGGATATCTTGAAAAA 3′ | 179 |
| Lower | 5′ AGCGTCTCTGTTGCCGTAGT 3′ | |||
| Actb | NM_031144.3 | Upper | 5′ CCCATCTATGAGGGTTACGC 3′ | 150 |
| Lower | 5′ TTTAATGTCACGCACGATTTC 3′ |
Enzyme-linked immunosorbent assay
After LV transduction (72 h), the SCs was counted, and the culture medium was collected to determine the amount of BDNF, NGF, GDNF, LIF, Artn, and NT-3 secreted by the cultured SCs. Cell culture supernatants were centrifuged and assayed using the enzyme-linked immunosorbent assay (ELISA) kits following the manufacturer's instructions (Antibodies Online). The plates were read at 450 nm and analyzed using a microELISA reader (Multiscan MK3; Thermo Labsystems). Then, the amount of BDNF, NGF, GDNF, LIF, Artn, and NT-3 secreted by the SCs was normalized to the cell number in each group. The assays were performed in triplicate.
Immunocytochemistry
SCs or DRG explants cultures were fixed with 4% paraformaldehyde for 15 min at room temperature, treated with 0.2% Triton X-100 for 10 min, and then incubated for 30 min with a blocking solution (10% normal goat serum). Primary antibodies were then applied to the cultures overnight at 4°C. The following day, the cultures were incubated with either an indocarbocyanine-conjugated goat anti-rabbit (1:1000; Abcam, Inc.) or fluorescein-conjugated goat anti-mouse (1:1000; Abcam, Inc.) antibody, and DAPI (10 μg/mL; Sigma), a fluorescent nuclear dye. The following primary antibodies were used: monoclonal mouse anti-growth associated protein-43 (GAP-43, 1:500; Sigma) (to identify neurite outgrowth) and polyclonal rabbit anti-S100 protein (1:100; Abcam, Inc.) (to identify SCs).
Statistical analysis
All data were expressed as the means±standard deviation. One-way ANOVA was used for statistical comparison of the means. Significant results were analyzed with the Tukey's post hoc test (GraphPad Prism 5.0). A p-value of<0.05 was considered statistically significant.
Results
Successful and efficient transduction of SCs
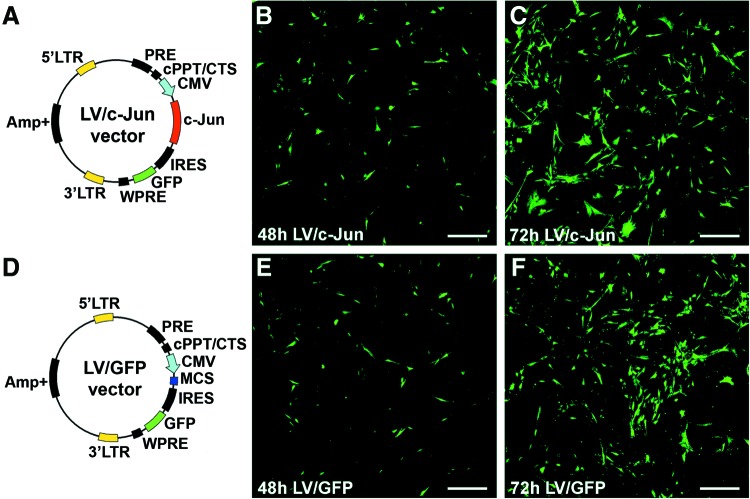
The LV vector used in this study efficiently transduced SCs. GFP expression in both LV/c-Jun- and LV/GFP-transduced SCs was visualized directly under a fluorescence microscope 48 h after transduction (Fig. 3B, E). Seventy-two hours posttransfection, more than 90% of the SCs in both groups expressed GFP (Fig. 3C, F). The genetic maps of the LV vector plasmids are also shown in Figure 3A and D.
FIG. 3.
The lentiviral plasmid maps and direct fluorescence images of reporter gene GFP expression. (A) Plasmid map of the c-Jun-IRES-eGFP vector (LV/c-Jun); (D) plasmid map of the eGFP vector (LV/GFP). Direct fluorescence images of reporter gene GFP expression in SCs at 48 h (B, E) and 72 h (C, F) after LV/c-Jun (B, C) or LV/GFP (E, F) transduction. In both groups, over 90% of SCs expressed GFP 72 h after transduction. Scale bars: (B, C, E, F)=150 μm. Color images available online at www.liebertpub.com/tea
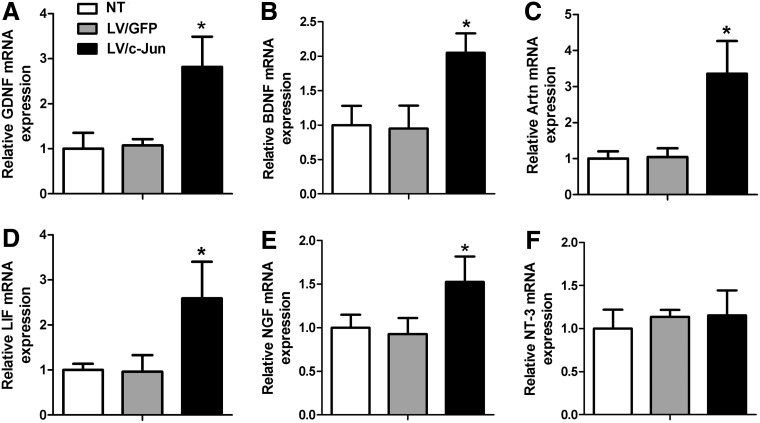
c-Jun-modified SCs increased the mRNA expression of multiple NFs
The mRNA levels of GDNF, BDNF, Artn, LIF, and NGF in the LV/c-Jun group were 2.6, 2.2, 3.2, 2.7, and 1.6 times higher, respectively, than in the LV/GFP group (*p<0.05; Fig. 4A–E). In contrast, no statistically significant difference in the expression of NT-3 genes was observed between the three groups (p>0.05; Fig. 4F). For comparison, the levels of GDNF, BDNF, Artn, LIF, NGF, and NT-3 mRNA in SCs in the LV/GFP group were similar to those in the NT group.
FIG. 4.
mRNA levels of multiple neurotrophic factors (NFs) 72 h after LV/c-Jun transduction. The mRNA levels of glial cell line-derived neurotrophic factor (GDNF) (A), brain-derived neurotrophic factor (BDNF) (B), artemin (Artn) (C), leukemia inhibitory factor (LIF) (D), nerve growth factor (NGF) (E), and neurotrophin-3 (NT-3) (F) were determined in the LV/c-Jun, LV/GFP and nontransduced (NT) groups through quantitative real-time polymerase chain reaction. The ratios of mRNA levels in SCs in LV/c-Jun and LV/GFP groups to those in the NT group are shown. Graph bars: mean±standard deviation (SD); *p<0.05.
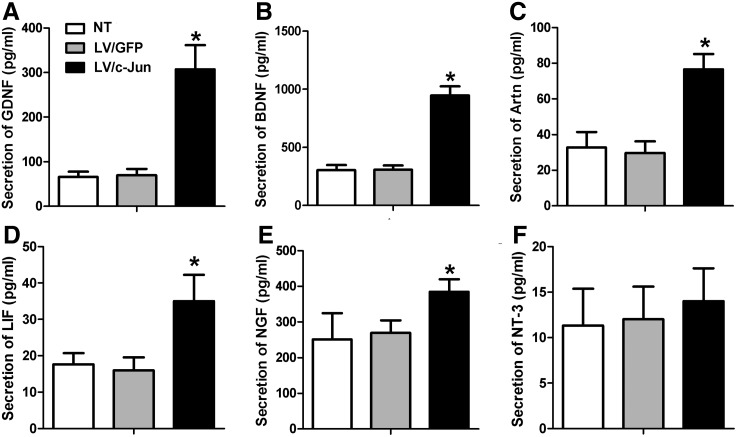
c-Jun-modified SCs increased the secretion of multiple NFs
The secretion levels of GDNF, BDNF, Artn, LIF, and NGF were 308±54, 947±79, 77±8, 35±7, and 384±35 pg/mL, respectively, in the LV/c-Jun group, which were significantly higher than in the LV/GFP group (*p<0.05; Fig. 5A–E). However, the secretion of NT-3 exhibited no significant difference between the three groups (p>0.05; Fig. 5F). As shown in Figure 5, the secretion levels of GDNF, BDNF, Artn, LIF, and NGF in the LV/GFP group were comparable to those in the NT group.
FIG. 5.
The secretion of multiple NFs 72 h after LV/c-Jun transduction. The secretion of GDNF (A), BDNF (B), Artn (C), LIF (D), NGF (E), and NT-3 (F) was determined in the LV/c-Jun, LV/GFP and NT groups through enzyme-linked immunosorbent assay. Graph bars: mean±SD; *p<0.05.
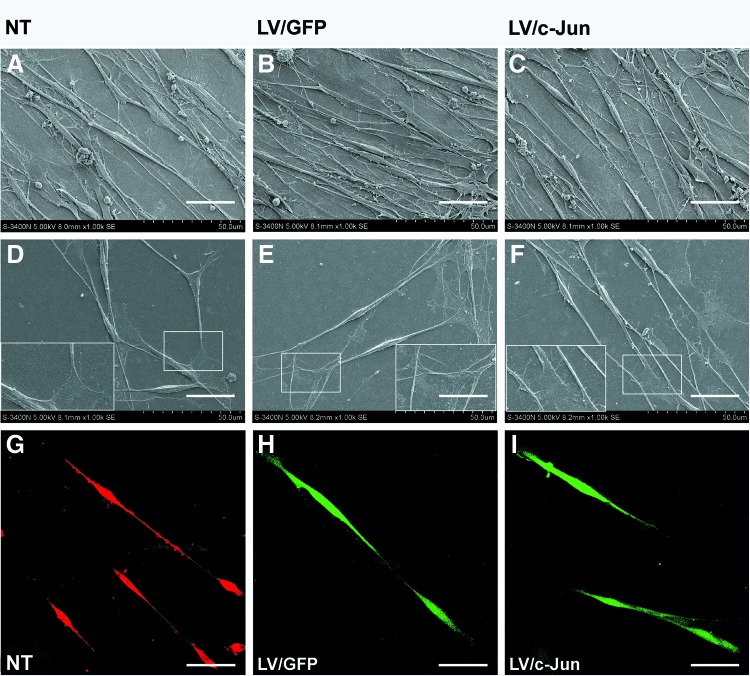
c-Jun-transduced SCs showed normal morphological characteristics
The normal SC morphology contributes to the construction of the regeneration track.25 Thus, we investigated whether the overexpression of c-Jun had an effect on SC morphology. SEM analysis showed that c-Jun-modified SCs exhibited a typical bi- or tripolar morphology (Fig. 6C, F), similar to the LV/GFP-transduced SCs (Fig. 6B, E) and NT SCs (Fig. 6A, D). The fluorescence results also confirmed the normal morphological appearance of c-Jun-modified SCs as observed during SEM analysis (Fig. 6I).
FIG. 6.
Immunohistochemistry and scanning electron microscopy (SEM) analysis of the morphological characteristics of c-Jun-transduced SCs. The morphology of LV/c-Jun- (I) and LV/GFP (H)-transduced SCs shown directly based on the expression of GFP (green). The morphology of NT SCs (G) shown by labeling with S100 (red) antibodies. SEM analysis confirmed that c-Jun-transduced SCs (C, F) exhibit a typical bi- or tripolar morphology, similar to LV/GFP-transduced SCs (B, E) and NT SCs (A, D). Scale bars: (A–F)=25 μm, (G–I)=100 μm. Color images available online at www.liebertpub.com/tea
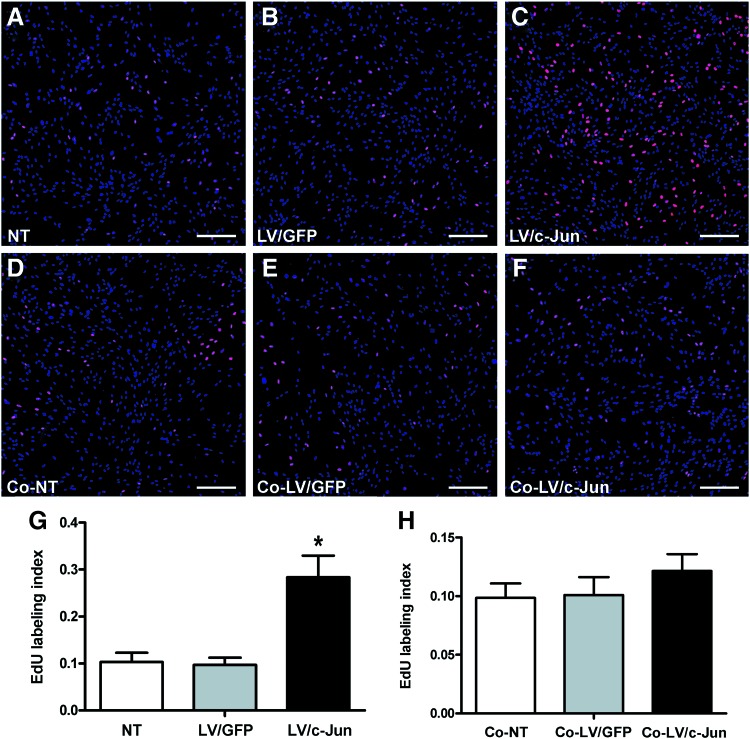
c-Jun-transduced SCs showed an increased proliferation potential, but had no effect on the proliferation of native SCs in the absence of neurons
We next investigated the proliferation of c-Jun-modified SCs through EdU staining. The number of proliferating cells in the c-Jun-modified group was 2.9- and 2.7-fold higher than in the LV/GFP and NT groups, respectively (*p<0.05; Fig. 7A–C, G), suggesting that c-Jun overexpression promotes SC proliferation.
FIG. 7.
The proliferation ability of c-Jun-transduced SCs and the influence of c-Jun-transduced SCs on native SC proliferation in the absence of axons. (A–F) The total number of the cells shown by DAPI (blue) staining; the number of proliferating SCs was determined through 5-ethynyl-2-deoxyuridine (EdU, red) staining. (G) EdU labeling index of c-Jun-transduced SCs, LV/GFP-transduced SCs and NT SCs. (H) In the coculture system, the EdU labeling index of SCs in the bottom chambers represented the influence of c-Jun-transduced SCs, LV/GFP-transduced SCs and NT SCs on native SC proliferation in the absence of axons. Scale bar: (A–F)=150 μm. Graph bars: mean±SD; *p<0.05. Color images available online at www.liebertpub.com/tea
We used a coculture system to mimic the in vivo conditions in which transplanted SCs may influence native SCs (Fig. 2A). Our results showed no significant difference in the percentage of EdU-labeled SCs between the three groups (p>0.05; Fig. 7D–F, H). These findings suggested that the c-Jun-modified SCs increased the proliferation potential, but had no effect on native SC proliferation in the absence of neurons.
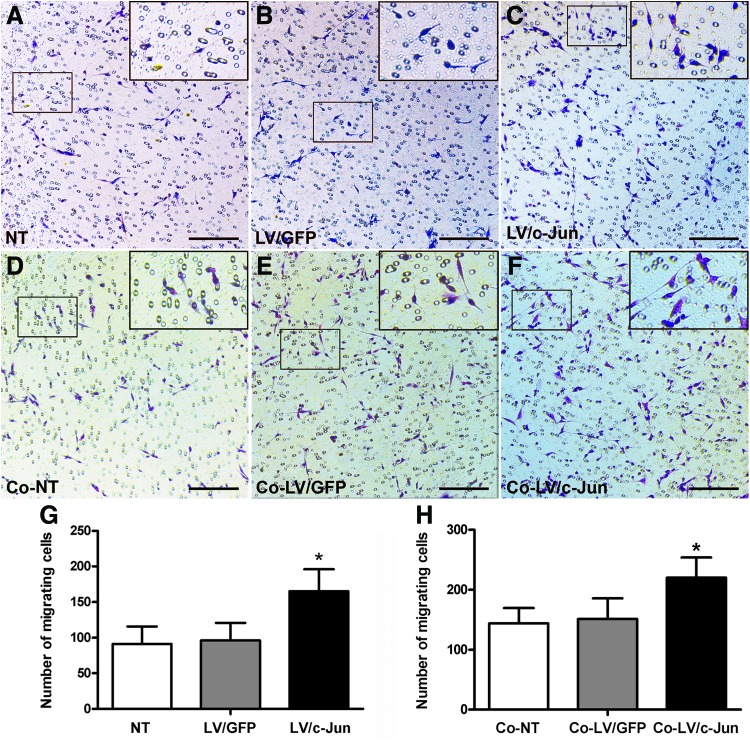
c-Jun-modified SCs showed active migration and facilitated the migration of native SCs
As shown in Figure 6, the number of migrating SCs in the c-Jun-modified group was 1.7- and 1.8-fold higher than in the LV/GFP and NT groups, respectively (*p<0.05; Fig. 8A–C, G). We further investigated the effect of the c-Jun-modified SCs on the migration of native SCs using the coculture system shown in Figure 2B. The c-Jun-modified SCs significantly enhanced native SC migration compared with the other two groups (*p<0.05; Fig. 8D–F, H).
FIG. 8.
Migration ability of c-Jun-transduced SCs and the influence of c-Ju-transduced SCs on native SC migration. (A–F) The cells (purple) that migrated to the lower surface of a transwell chamber with 8 μm pores were detected through Hematoxylin and Eosin staining. (A–C, G) The migration ability of c-Jun-transduced SCs, LV/GFP-transduced SCs and NT SCs. (D–F, H) The influence of the LV/c-Jun, LV/GFP and NT group SCs on native SC migration in the coculture system. Insets represent the magnification of the representative area. Scale bar: (A–F)=150 μm. Graph bars: mean±SD; *p<0.05. Color images available online at www.liebertpub.com/tea
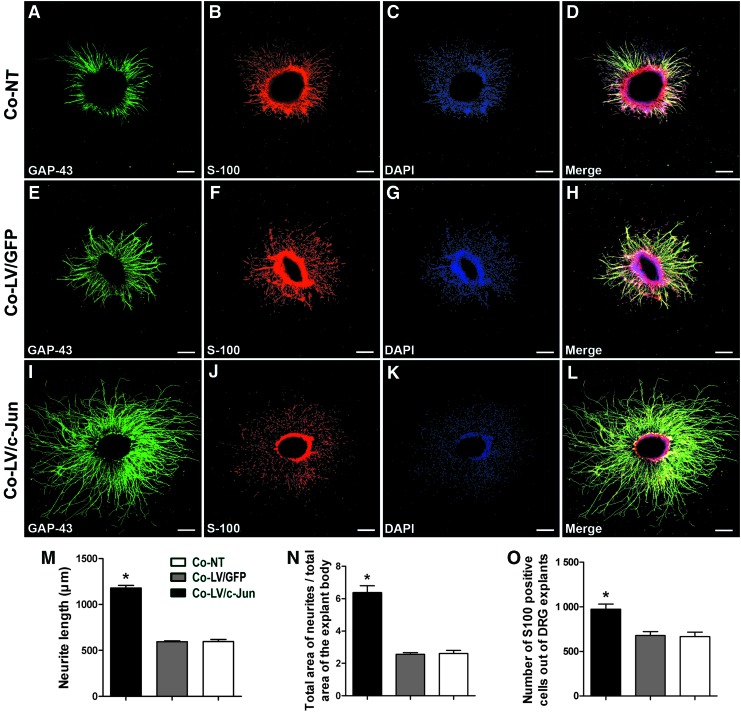
c-Jun-modified SCs promoted neurite outgrowth from DRG explants
To directly determine the effect of the c-Jun-modified SCs on neurite outgrowth, we used a coculture system (Fig. 2A). The average length of the five longest axons reached 1180±25 μm in the c-Jun-modified group, which was significantly higher than in the LV/GFP and NT groups (595±9 and 597±21; *p<0.05; Fig. 9). Larger explants tend to show larger outgrowths simply because they contain more neurons. To exclude this influence, the total area of neurites divided by the total area of the explant body was used. This measure of outgrowth includes only variations in outgrowth that are independent of explant size. The total area of neurites divided by the total area of the explant body was 6.4±0.4 in the c-Jun-modified group, which was significantly larger than in the LV/GFP and NT groups (2.5±0.1 and 2.6±0.2; *p<0.05; Fig. 9N). Strikingly, the number of S100-positive cells per DRG explants was 974±57 in the LV/c-Jun group, compared with 678±45 in the GFP group and 665±52 in the NT group (*p<0.05; Fig. 9O). These results, together with the observed influence of the c-Jun-modified SCs on the native SC migration data, collectively supported the interpretation that c-Jun-modified SCs can promote native SC migration. However, these experiments could not rule out the possibility that the c-Jun-modified SCs can enhance the proliferation of SCs already in contact with axons.
FIG. 9.
Effect of c-Jun-transduced SCs on neurite outgrowth. (A–L) Representative immunostaining images of DRG explants in each group. Axons were labeled with growth associated protein-43 (GAP-43; green); SCs from DRG explants were labeled with S100 (red); cell nuclei were labeled with DAPI (blue). A comparison of the average length of the five longest axons (M), the total area of neurites divided by the total area of the explant body (N) and the total number of SCs (S100-positive cells) from DRG explants (O) in the three groups is shown. Scale bar=250 μm. Graph bars: mean±SD; *p<0.05. Color images available online at www.liebertpub.com/tea
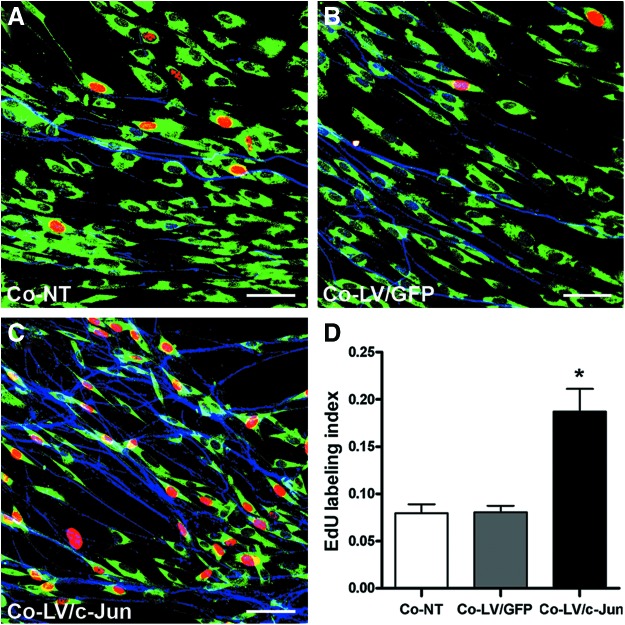
c-Jun-modified SCs promoted the proliferation of native SCs in the presence of neurons
We further investigated the influence of the c-Jun-modified SCs on the proliferation of native SC in the presence of neurons. We used EdU to label the proliferating cells 3 days after DRG explants were cocultured with c-Jun-modified SCs. The SCs were also immunostained with S100 (green) to confirm that the EdU-positive cells were indeed SCs. The EdU labeling index reached 18.69%±2.42% in the LV/c-Jun group as compared with 8.04%±0.69% in the GFP group and 7.74%±0.94% in the NT group (*p<0.05; Fig. 10A–D). These results indicated that c-Jun-modified SCs were capable of promoting proliferation of native SCs in the presence of neurons.
FIG. 10.
Effect of c-Jun-transduced SCs on native SC proliferation in the presence of axons. (A–C) SCs were labeled with S100 (green), proliferating cells were labeled with EdU (red), and axons were labeled with GAP-43 (blue). (D) Comparison of the EdU labeling index of SCs in the presence of axons in the three groups. Scale bar=50 μm. Graph bars: mean±SD; *p<0.05. Color images available online at www.liebertpub.com/tea
Discussion
It is well established that viable SCs are essential for neuronal survival and axonal regrowth in nerve guidance conduits due to the growth-supportive milieu created by the SCs.3 In addition, genetically modified SCs that overexpress a single type of NF, including BDNF, NGF, GDNF, or NT-3, are even more effective than nonmodified SCs for promoting neuronal survival and axonal regeneration.29,32,34,35 Recently, an increasing number of studies have shown that co-delivery of multiple NFs holds greater therapeutic promise than the delivery of a single factor.4,22 However, the simultaneous upregulation of multiple NFs remains a great challenge. In the present study, we used a gene therapy approach to overexpress the transcription factor c-Jun in SCs and examined whether the genetically modified SCs could effectively upregulate multiple NFs. We next investigated the biological characteristics of the c-Jun-transduced SCs and their influence on native SC proliferation and migration. Furthermore, we explored the effect of c-Jun-modified SCs on neurite outgrowth in DRG explants.
We found that the overexpression of c-Jun in SCs significantly upregulated the mRNA levels of GDNF, LIF, Artn, BDNF, and NGF. More importantly, the secretion of BDNF, GDNF, LIF, Artn, and NGF was also significantly enhanced in LV/c-Jun-transduced SCs. These results are consistent with the previous findings that LIF, GDNF, and Artn are transcriptionally regulated by c-Jun.26,27 Currently, we have no clear explanation for the observation that BDNF and NGF, which are not direct target genes of c-Jun, were also significantly upregulated in LV/c-Jun-transduced SCs. One possible explanation is that c-Jun is a major component of the AP-1 transcription factor complex that is not only able to homodimerize and heterodimerize with other Jun or Fos members to form transcriptionally active complexes, but can also heterodimerize efficiently with other transcription factors, such as members of the ATF/CREB family,36 which might contribute to the increased expression of BDNF and NGF.
Previous studies have found that normal SC morphology is essential and contributes to the construction of regeneration tracks.25 The absence of c-Jun in SCs results in flattening and a paucity of processes, leading to disruption of the structure of the essential regeneration tracks and failure of targeted regeneration. In the present study, we confirmed that the overexpression of c-Jun in SCs produced no change in the morphological characteristics of SCs. The c-Jun-modified SCs showed typical bi- or tripolar morphology in fluorescence images and SEM analysis. Efficient SC proliferation contributes to the construction of Büngner bands and the secretion of a variety of adhesion molecules and NFs that promote neuron survival and axonal regeneration. Our data confirmed that c-Jun-transduced SCs showed an increased proliferation ability. However, the c-Jun-modified SCs exerted no effect on cell proliferation in neuron-free cultures of purified SCs. Interestingly, in DRG explant cultures, there was a significant increase in the percentage of EdU-labeled SCs in the LV/c-Jun group, indicating that c-Jun-modified SCs can promote the proliferation of native SCs that are in contact with axons. To the best of our knowledge, there are two possible explanations that may account for this finding. First, c-Jun-modified SCs can upregulate the expression of GDNF, which has been found to promote the proliferation of SCs in the presence of axons.18,25 Second, increased expression of BDNF in c-Jun-modified SCs might result in the increased release of neuregulins, which are mitogenic for SCs, from DRG neurons.37 When lengthy nerve defects are repaired by NCs, SCs in the proximal and distal segments migrate into the conduits, forming regeneration tracks and producing the extracellular matrix, which are prerequisites for effective nerve regeneration and functional recovery. Considering SCs as a candidate transplant cell, the migration ability and the potential to promote native SC migration are of great importance. Our study suggested that c-Jun-modified SCs exhibit a greater migration ability than LV/GFP SCs and NT SCs. In addition, the c-Jun-modified SCs also significantly enhanced the migration of purified SCs, indicating their potential to promote the migration of native SCs when transplanted in vivo. Previous studies have shown that genetically overexpressing or directly administrating GDNF can promote neuron survival, nerve regeneration, and myelin formation.17,18,34 Similarly, overexpression of NGF and BDNF in SCs also has beneficial effects for peripheral nerve repair.19,20 Artn exerts multiple effects on sensory neuron generation, survival, regeneration, and functional recovery.38–41 LIF has also been shown to play a positive role in nerve regeneration.42 These findings highlight the importance of combining a cellular substrate with NFs to promote nerve regeneration. However, an increasing number of studies have shown that co-delivery of multiple factors, rather than a single factor, holds greater therapeutic promise for promoting nerve regeneration because peripheral nerves contained different neuronal and glial subpopulations.4,20,22,23 In the present study, overexpression of the c-Jun gene in SCs significantly increased the expression and secretion of multiple NFs, including GDNF, BDNF, Artn, LIF, and NGF. In addition, the c-Jun-modified SCs significantly increased the average length of axons and the average area occupied by DRG axons in the coculture system, highlighting their potential to promote neuronal survival, axonal regeneration, and functional recovery in response to PNS injury. However, the application of c-Jun-modified SCs is not restricted to PNS repair. c-Jun-transduced SCs can enhance the secretion of GDNF, BDNF, and NGF, which have been widely reported to promote neuronal survival and nerve regeneration in CNS.43–46 In addition, the migration potential of transplanted cells plays a pivotal role in successful regeneration due to their ability to penetrate the glial scar, which is believed to be a leading factor in the generation of a stop mechanism for axon elongation.47–49 The c-Jun-modified SCs showed an enhanced migration ability, which further increases their potential applications in the CNS.
Conclusion
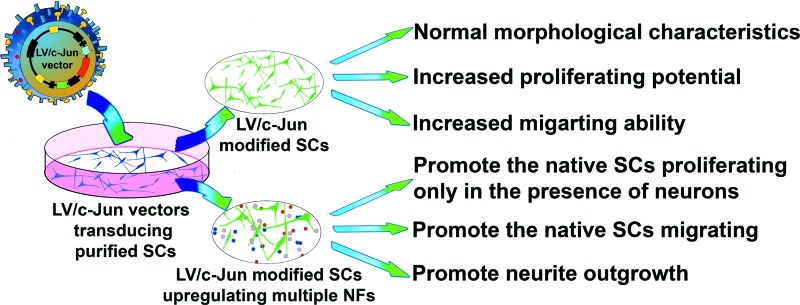
In the present study, we found that c-Jun gene-modified SCs can enhance the expression and secretion of multiple NFs, including GDNF, BDNF, Artn, LIF, and NGF. These genetically modified SCs significantly enhanced neurite outgrowth from DRG explants. The c-Jun-modified SCs not only showed normal morphological characteristics and increased proliferation and migration abilities, but also promoted the migration of native SCs. Interestingly, c-Jun-modified SCs could only promote the proliferation of native SCs in the presence of neurons. These findings thus provide a basis for the development of an optimal therapeutic alternative for nerve repair using c-Jun-modified SCs, which have the potential to promote neuronal survival, axonal regeneration, and functional recovery by upregulating multiple NFs and exhibiting enhanced migration and proliferation abilities, as well as enhancing the biological functions of native SCs (Fig. 11).
FIG. 11.
Schematic diagram of the key findings of the present study. Color images available online at www.liebertpub.com/tea
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (973 Program No. 2014CB542206), the National Natural Science Foundation of China (No. 81201389 and No. 30973052), and the Program for ChangJiang Scholars and Innovative Research Teams in Universities (IRT13051). The authors thank their technicians, Ms. Lifeng Lan, Ms. Jing Fan, Mr. Haifeng Zhang, and Mr. Yongqiang Li, for their excellent technical assistance.
Disclosure Statement
No competing financial interests exist.
References
- 1.Sulaiman O.A., and Gordon T.Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia 32,234, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Huang J., Lu L., Hu X., Ye Z., Peng Y., Yan X., Geng D., and Luo Z.Electrical stimulation accelerates motor functional recovery in the rat model of 15-mm sciatic nerve gap bridged by scaffolds with longitudinally oriented microchannels. Neurorehabil Neural Repair 24,736, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Daly W., Yao L., Zeugolis D., Windebank A., and Pandit A.A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface 9,202, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madduri S., di Summa P., Papaloizos M., Kalbermatten D., and Gander B.Effect of controlled co-delivery of synergistic neurotrophic factors on early nerve regeneration in rats. Biomaterials 31,8402, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Jaquet J.B., Luijsterburg A.J., Kalmijn S., Kuypers P.D., Hofman A., and Hovius S.E.Median, ulnar, and combined median-ulnar nerve injuries: functional outcome and return to productivity. J Trauma 51,687, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Pabari A., Yang S.Y., Mosahebi A., and Seifalian A.M.Recent advances in artificial nerve conduit design: strategies for the delivery of luminal fillers. J Control Release 156,2, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro-Resende V.T., Koenig B., Nichterwitz S., Oberhoffner S., and Schlosshauer B.Strategies for inducing the formation of bands of Bungner in peripheral nerve regeneration. Biomaterials 30,5251, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Tohill M.P., Mann D.J., Mantovani C.M., Wiberg M., and Terenghi G.Green fluorescent protein is a stable morphological marker for schwann cell transplants in bioengineered nerve conduits. Tissue Eng 10,1359, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y., Wang A., Patel S., Kurpinski K., Diao E., Bao X., Kwong G., Young W.L., and Li S.Engineering bi-layer nanofibrous conduits for peripheral nerve regeneration. Tissue Eng Part C Methods 17,705, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meek M.F., and Coert J.H.Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery 61,E1340, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Doolabh V.B., Hertl M.C., and Mackinnon S.E.The role of conduits in nerve repair: a review. Rev Neurosci 7,47, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Krick K., Tammia M., Martin R., Hoke A., and Mao H.Q.Signaling cue presentation and cell delivery to promote nerve regeneration. Curr Opin Biotechnol 22,741, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Lago N., Casas C., Muir E.M., Rogers J., and Navarro X.Effects of Schwann cell transplants in an experimental nerve amputee model. Restor Neurol Neurosci 27,67, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez F.J., Verdu E., Ceballos D., and Navarro X.Nerve guides seeded with autologous schwann cells improve nerve regeneration. Exp Neurol 161,571, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Timmer M., Robben S., Muller-Ostermeyer F., Nikkhah G., and Grothe C.Axonal regeneration across long gaps in silicone chambers filled with Schwann cells overexpressing high molecular weight FGF-2. Cell Transplant 12,265, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Jungnickel J., Haase K., Konitzer J., Timmer M., and Grothe C.Faster nerve regeneration after sciatic nerve injury in mice over-expressing basic fibroblast growth factor. J Neurobiol 66,940, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Shakhbazau A., Mohanty C., Shcharbin D., Bryszewska M., Caminade A.M., Majoral J.P., Alant J., and Midha R.Doxycycline-regulated GDNF expression promotes axonal regeneration and functional recovery in transected peripheral nerve. J Control Release 172,841, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Eggers R., Hendriks W.T., Tannemaat M.R., van Heerikhuize J.J., Pool C.W., Carlstedt T.P., Zaldumbide A., Hoeben R.C., Boer G.J., and Verhaagen J.Neuroregenerative effects of lentiviral vector-mediated GDNF expression in reimplanted ventral roots. Mol Cell Neurosci 39,105, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Shakhbazau A., Kawasoe J., Hoyng S.A., Kumar R., van Minnen J., Verhaagen J., and Midha R.Early regenerative effects of NGF-transduced Schwann cells in peripheral nerve repair. Mol Cell Neurosci 50,103, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Godinho M.J., Teh L., Pollett M.A., Goodman D., Hodgetts S.I., Sweetman I., Walters M., Verhaagen J., Plant G.W., and Harvey A.R.Immunohistochemical, ultrastructural and functional analysis of axonal regeneration through peripheral nerve grafts containing Schwann cells expressing BDNF, CNTF or NT3. PLoS One 8,e69987, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shakhbazau A., Shcharbin D., Bryszewska M., Kumar R., Wobma H.M., Kallos M.S., Goncharova N., Seviaryn I., Kosmacheva S., Potapnev M., and Midha R.Non-viral engineering of skin precursor-derived Schwann cells for enhanced NT-3 production in adherent and microcarrier culture. Curr Med Chem 19,5572, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Madduri S., Feldman K., Tervoort T., Papaloizos M., and Gander B.Collagen nerve conduits releasing the neurotrophic factors GDNF and NGF. J Control Release 143,168, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Boyd J.G., and Gordon T.Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp Neurol 183,610, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Parkinson D.B., Bhaskaran A., Arthur-Farraj P., Noon L.A., Woodhoo A., Lloyd A.C., Feltri M.L., Wrabetz L., Behrens A., Mirsky R., and Jessen K.R.c-Jun is a negative regulator of myelination. J Cell Biol 181,625, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arthur-Farraj P.J., Latouche M., Wilton D.K., Quintes S., Chabrol E., Banerjee A., Woodhoo A., Jenkins B., Rahman M., Turmaine M., Wicher G.K., Mitter R., Greensmith L., Behrens A., Raivich G., Mirsky R., and Jessen K.R.c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75,633, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontana X., Hristova M., Da C.C., Patodia S., Thei L., Makwana M., Spencer-Dene B., Latouche M., Mirsky R., Jessen K.R., Klein R., Raivich G., and Behrens A.c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J Cell Biol 198,127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozec A., Bakiri L., Hoebertz A., Eferl R., Schilling A.F., Komnenovic V., Scheuch H., Priemel M., Stewart C.L., Amling M., and Wagner E.F.Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature 454,221, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Ciucurel E.C., and Sefton M.V.Del-1 overexpression in endothelial cells increases vascular density in tissue-engineered implants containing endothelial cells and adipose-derived mesenchymal stromal cells. Tissue Eng Part A 20,1235, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y., Leaver S.G., Plant G.W., Hendriks W.T., Niclou S.P., Verhaagen J., Harvey A.R., and Cui Q.Lentiviral-mediated transfer of CNTF to schwann cells within reconstructed peripheral nerve grafts enhances adult retinal ganglion cell survival and axonal regeneration. Mol Ther 11,906, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Birge R.B., Wadsworth S., Akakura R., Abeysinghe H., Kanojia R., MacIelag M., Desbarats J., Escalante M., Singh K., Sundarababu S., Parris K., Childs G., August A., Siekierka J., and Weinstein D.E.A role for schwann cells in the neuroregenerative effects of a non-immunosuppressive fk506 derivative, jnj460. Neuroscience 124,351, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Hadlock T., Sundback C., Hunter D., Cheney M., and Vacanti J.P.A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng 6,119, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Zhang L., Ma Z., Smith G.M., Wen X., Pressman Y., Wood P.M., and Xu X.M.GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia 57,1178, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortimer D., Feldner J., Vaughan T., Vetter I., Pujic Z., Rosoff W.J., Burrage K., Dayan P., Richards L.J., and Goodhill G.J.Bayesian model predicts the response of axons to molecular gradients. Proc Natl Acad Sci U S A 106,10296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng L.X., Deng P., Ruan Y., Xu Z.C., Liu N.K., Wen X., Smith G.M., and Xu X.M.A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. J Neurosci 33,5655, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavdas A.A., Chen J., Papastefanaki F., Chen S., Schachner M., Matsas R., and Thomaidou D.Schwann cells engineered to express the cell adhesion molecule L1 accelerate myelination and motor recovery after spinal cord injury. Exp Neurol 221,206, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Raivich G., Bohatschek M., Da C.C., Iwata O., Galiano M., Hristova M., Nateri A.S., Makwana M., Riera-Sans L., Wolfer D.P., Lipp H.P., Aguzzi A., Wagner E.F., and Behrens A.The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron 43,57, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Ma Z., Wang J., Song F., and Loeb J.A.Critical period of axoglial signaling between neuregulin-1 and brain-derived neurotrophic factor required for early Schwann cell survival and differentiation. J Neurosci 31,9630, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong D.G., Park W.K., and Park S.Artemin activates axonal growth via SFK and ERK-dependent signalling pathways in mature dorsal root ganglia neurons. Cell Biochem Funct 26,210, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Wang R., Rossomando A., Sah D.W., Ossipov M.H., King T., and Porreca F.Artemin induced functional recovery and reinnervation after partial nerve injury. Pain 155,476, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jankowski M.P., Rau K.K., Soneji D.J., Anderson C.E., and Koerber H.R.Enhanced artemin/GFRalpha3 levels regulate mechanically insensitive, heat-sensitive C-fiber recruitment after axotomy and regeneration. J Neurosci 30,16272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andres R., Forgie A., Wyatt S., Chen Q., de Sauvage F.J., and Davies A.M.Multiple effects of artemin on sympathetic neurone generation, survival and growth. Development 128,3685, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Tham S., Dowsing B., Finkelstein D., Donato R., Cheema S.S., Bartlett P.F., and Morrison W.A.Leukemia inhibitory factor enhances the regeneration of transected rat sciatic nerve and the function of reinnervated muscle. J Neurosci Res 47,208, 1997 [PubMed] [Google Scholar]
- 43.Namiki J., Kojima A., and Tator C.H.Effect of brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. J Neurotrauma 17,1219, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Chu T.H., Wang L., Guo A., Chan V.W., Wong C.W., and Wu W.GDNF-treated acellular nerve graft promotes motoneuron axon regeneration after implantation into cervical root avulsed spinal cord. Neuropathol Appl Neurobiol 38,681, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Tuszynski M.H., Weidner N., McCormack M., Miller I., Powell H., and Conner J.Grafts of genetically modified Schwann cells to the spinal cord: survival, axon growth, and myelination. Cell Transplant 7,187, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Grill R.J., Blesch A., and Tuszynski M.H.Robust growth of chronically injured spinal cord axons induced by grafts of genetically modified NGF-secreting cells. Exp Neurol 148,444, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Zujovic V., Thibaud J., Bachelin C., Vidal M., Coulpier F., Charnay P., Topilko P., and Baron-Van E.A.Boundary cap cells are highly competitive for CNS remyelination: fast migration and efficient differentiation in PNS and CNS myelin-forming cells. Stem Cells 28,470, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Cao L., Zhu Y.L., Su Z., Lv B., Huang Z., Mu L., and He C.Olfactory ensheathing cells promote migration of Schwann cells by secreted nerve growth factor. Glia 55,897, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Grimpe B., Pressman Y., Lupa M.D., Horn K.P., Bunge M.B., and Silver J.The role of proteoglycans in Schwann cell/astrocyte interactions and in regeneration failure at PNS/CNS interfaces. Mol Cell Neurosci 28,18, 2005 [DOI] [PubMed] [Google Scholar]