Abstract
Biomedical strategies for tissue engineering and repair utilize specific cells, scaffolds, and growth factors to reconstruct elements of damaged tissue. The cellular element of these strategies is limited, however, by poor efficiency of delivery and retention of therapeutic cells in target sites. We propose that the presence of a cellular anchor that is able to specifically bind a defined element of target tissue will facilitate efficient binding and retention of therapeutic cells, thereby promoting repair of the target site. To do so, we engineered an artificial collagen-specific anchor (ACSA) that is able to specifically bind collagen I. The ACSA was engineered by creating a construct comprising rationally designed consecutive domains. The binding specificity of the ACSA was achieved by employing variable regions of a monoclonal antibody that recognizes a unique epitope present in human collagen I. Meanwhile, cell membrane localization of the ACSA was provided by the presence of a transmembrane domain. We determined that the ACSA was localized within cell membranes and interacted with its intended target, that is, collagen I. We have demonstrated that, in comparison to the control, the cells expressing the ACSA attached better to collagen I and exhibited improved retention in sites of seeding. We have also demonstrated that the presence of the ACSA did not interfere with cell proliferation, the biosynthesis of endogenous collagen I, or the biological functions of native collagen receptors. Since the presented cell delivery system utilizes a common characteristic of major connective tissues, namely the presence of collagen I, the findings described here could have a broad positive impact for improving the repair processes of tendon, ligament, bone, intervertebral disc, skin, and other collagen I-rich connective tissues. If successful, the ACSA approach to deliver cells will serve as an outline for developing cell delivery methods that target other elements of extracellular matrices, including other collagen types, laminins, and fibronectins.
Introduction
Aleading doctrine for tissue engineering and repair approaches involves employing appropriate cells and scaffolds that support them and providing specific growth factors that drive the formation of a specific tissue. Considering the cellular aspect of this principle, one of the limitations of the currently explored methods is the inability to deliver therapeutic cells efficiently and specifically and to retain them in targeted sites.1–3 Although a number of approaches to the targeted delivery of therapeutic cells have been explored, their clinical utility remains quite poor.1
Our study addresses this problem by exploring a new approach to guide, bind, and retain cells within collagen I-rich connective tissues such as the tendon, ligament, bone, and skin. We proposed that the targeted delivery and retention of cells could be achieved by employing an artificial collagen-specific anchor (ACSA) that is able to specifically bind collagen I-rich injury sites. Our main hypothesis is that when the ACSA is expressed on the surface of therapeutic cells delivered to the injury site, these cells will remain in the targeted site instead of dispersing into the surrounding areas and this will enable the efficient restoration of the damaged tissue.
We engineered collagen I specificity of the ACSA by employing critical elements of the monoclonal anti-collagen I antibody that targets the telopeptide region of the α2-chain of human collagen I (α2Ct), as described in Fig. 1A.4–6 This antibody originally produced in mice as an IgA type served as a template for the mouse–human chimeric IgG variant and for the single-chain fragment variable (scFv).5,6 Earlier studies had already described the great utility of all variants to bind fibrillar collagen I molecules and this provided a foundation for the proposed research.4–6
FIG. 1.
A schematic demonstrating critical elements of rational engineering and expression of the artificial collagen-specific anchor (ACSA)-green fluorescent protein (GFP) construct. (A) A schematic representation of procollagen I molecule. Arrows indicate individual C-terminal telopeptide; the star symbol identifies the α2Ct. (B) Critical common steps in transformation of an initial antibody into a chimeric antigen receptor (CAR) or ACSA. (C) Graphic representation of major domains of native leukocyte-associated Ig-like receptor 1 (LAIR-1) and elements of the ACSA-GFP construct. Arrows indicate the region of native LAIR-1 utilized in engineering the ACSA-GFP construct. (D) An illustration depicting the proposed arrangement of the ACSA-GFP in employed NIH/3T3 cells. Symbols: Np and Cp, procollagen N-terminal and C-terminal propeptides; TH, triple helical domain of procollagen I; Nt and Ct, N-terminal and C-terminal telopeptides of procollagen I; α1 and α2, specific chains of procollagen I; CB, extracellular collagen-binding domain of native LAIR-1; T, transmembrane domain; IT, intracellular immune receptor tyrosine-base inhibitory motif critical for LAIR-1-mediated signaling; VH and VL, variable domains of the heavy and the light chain of the archetypical anti-α2Ct antibody. Color images available online at www.liebertpub.com/tea
The antibody-based techniques of targeting therapeutic cells to specific sites have not yet been extensively explored in the area of regeneration and engineering of connective tissues, however, techniques based on a chimeric antigen receptor (CAR) are quite advanced in the area of anticancer therapies (Fig. 1B).7 Specifically, a CAR-based method is employed to direct therapeutic T lymphocytes to target human malignancies (Fig. 1B).8–11 CAR utilizes the antibody–antigen principle to recognize specific epitopes present on the surfaces of cancerous target cells. The CAR is most commonly constructed by employing a specific scFv that is genetically engineered to include the variable regions of a monoclonal antibody raised against a specific marker present on targeted cancerous cells.12 We employed a similar approach to engineer the ACSA construct (Fig. 1).
We also employed fibroblastic cells to find out if they express the ACSA on their surfaces and if the presence of this construct improves binding and retention of engineered cells within collagen I-rich matrices. We wanted to find out how the presence of the ACSA could affect the major biological cellular functions such as cell proliferation and production of extracellular proteins. Furthermore, we wanted to find out if the presence of the ACSA would interfere with the biological functions of native collagen-specific receptors. Moreover, we demonstrated that the presence of the ACSA does not negatively alter the major biological cellular functions such as cell proliferation and production of extracellular proteins. Furthermore, we found that the presence of the ACSA does not interfere with the biological functions of native collagen-specific receptors such as integrins and discoidin domain receptor 1 (DDR1). Thus, these results show not only the great potential of the ACSA to deliver and retain the therapeutic cells to collagen I-rich connective tissues but also indicate its safety.
The presented cell delivery system utilizes a universal characteristic of major connective tissues, namely the presence of fibrillar collagen I. Therefore, its potential applicability is broad. Consequently, the described studies are significant, with a potential wide-ranging positive impact on improving the repair processes of tendon, ligament, bone, intervertebral disc, skin, and other collagen I-rich connective tissues. If successful, the ACSA approach to deliver cells will provide a blueprint for developing cell delivery methods that target other elements of extracellular matrices, including other collagen types, laminins, and fibronectins. Future experiments in preclinical animal-based models will determine the clinical utility of the proposed method to improve the site-specific delivery and retention of therapeutic cells.
Materials and Methods
DNA construct
The DNA construct for the ACSA encoded the following consecutive domains: (1, 2) variable regions of the heavy and light chains of the anti-collagen I antibody (VH, VL), (3) a transmembrane domain, and (4) an intracellular domain (Fig. 1C). The first two domains are identical to those of the scFv variant of the original anti-collagen I antibody described in detail elsewhere.4–6 The third domain included the transmembrane domain and the hinge region of the leukocyte-associated Ig-like receptor 1 (LAIR-1). DNA sequences encoding the extracellular ligand-binding domain and the intracellular regulatory domain of the native LAIR-1 were purposely omitted in the ACSA construct (Fig. 1C). The fourth intracellular domain included the green fluorescent protein (GFP; Fig. 1B). The presence of GFP in the ACSA construct (ACSA-GFP) facilitated the direct monitoring of the expression and localization of the analyzed protein.
The entire DNA construct encoding the ACSA-GFP was synthesized commercially (GenScript USA, Inc.). The construct was cloned into the pLVX-Tight-Puro vector that included a DNA cassette for a puromycin resistance gene (Clontech Laboratories, Inc.). The expression of this construct was regulated by the tetracycline (Tet)-responsive cytomegalic virus promoter. In the experimental approach employed here, the presence of tetracycline or its derivative, doxycycline (Dox), switches the expression of the ACSA-GFP on (Tet-On). In contrast, in the absence of Dox, the expression of the ACSA-GFP ceases.13
Expression of the ACSA-GFP
The DNA construct was employed to transduce NIH/3T3 Tet-On cells that express the tetracycline transactivator (tTA), a protein needed for the Tet-dependent regulation of the Tet-responsive promoter (Clontech Laboratories, Inc.). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 40 μg/mL of l-ascorbic acid phosphate magnesium salt (Wako Pure Chemical Industries, Ltd.). This precursor of vitamin C is routinely added to cultures of collagen-producing cells to facilitate proper synthesis and secretion of endogenous collagens. Following selection with puromycin, stably transduced puromycin-resistant clones cultured in the presence or absence of Dox were selected and then screened for the expression of the ACSA-GFP by Western blot and by fluorescence microscopy, as described.14
Membrane localization of the ACSA-GFP
The selected cells were grown in the presence or absence of 1 μg/mL of Dox, and then the cell membrane and the cytoplasmic fractions were separated (ProteoExtract Transmembrane Protein Extraction Kit; EMD Millipore). Proteins derived from the cell membrane fraction were analyzed by Western blot with the use of the anti-GFP antibody to detect the ACSA-GFP. Cell lysates from nontransduced NIH/3T3 cells were used as a negative control. To further confirm the specificity and membrane localization of the ACSA-GFP construct, a biotinylated form of its target, that is, α2Ct peptide (GenScript, Inc.), was employed in cell-based assays. Specifically, the NIH/3T3 cells were cultured in the presence of Dox to express the ACSA-GFP. Subsequently, the cell layers were washed with FBS-free media, and then the 0.1 μg/mL of biotinylated α2Ct peptide was added to cell culture media. After a 1-h incubation, the cell layers were washed to remove the unbound peptide, and then the cell layers were fixed with methanol. The presence of the biotinylated α2Ct was evaluated with the use of a streptavidin-conjugated fluorophore (AlexaFluor 594; Life Technologies). Microscopic observations were carried out with a fluorescence microscope (Eclipse E600; Nikon) equipped with a digital camera (DS-Qi1Mc; Nikon). In a parallel experiment, a nonspecific biotinylated peptide was employed as a control.
Binding procollagen I-target
The role of the ACSA-GFP construct in binding cells to procollagen I was analyzed, as described.15 In brief, 8-well strips (Nunc-Immobilizer; Thermo Scientific) were coated with 50 μg/mL of purified human procollagen I isolated from cultured fibroblasts, as described.16 Subsequently, the wells were blocked with a 1% solution of bovine serum albumin (BSA; Sigma-Aldrich). A control group of wells coated with 1% BSA was also prepared.
NIH/3T3 cells harboring the ACSA-GFP were labeled with a cell membrane-permeable fluorescent dye according to the manufacturer's protocol (CMFDA; Life Technologies). Subsequently, the cells were added to the wells at 1×105 cells per well. After a 30-min incubation, the cell layers were rinsed and then the attached cells were lysed with 0.5% Triton X-100. The fluorescence of the released dye was measured with the use of a plate reader (Infinite/M-1000; Tecan Group). The differences between the mean values calculated for specific groups were evaluated with the use of the Student's t-test (GraphPad Prism; GraphPad Software, Inc.). In a parallel experiment, the binding of analyzed cells to wells coated with fibronectin (Sigma-Aldrich) was also analyzed.
Binding to the α2Ct target
To further assay the binding specificity of the ACSA-GFP, we studied the binding of cells expressing this construct to a biotinylated form of the α2Ct peptide. In brief, streptavidin (Thermo Scientific) was immobilized covalently to the wells of 8-well strips, and then the biotinylated α2Ct peptide was added to the streptavidin-coated surfaces. Subsequently, cell-binding assays were carried out as described above. Control experiments with surfaces coated with a control biotinylated peptide or with streptavidin only were also carried out.
Cell proliferation assays
The effect of the presence of the ACSA-GFP construct on the proliferation of cells was analyzed, as described.5,6 In brief, we employed the cell counting kit (CCK-8; Sigma-Aldrich) that utilizes the ability of living cells to metabolize [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium] monosodium salt (WST-8) to a water-soluble formazan dye. The amount of formazan produced from WST-8 is measured through absorbance at 450 nm. The values of absorbance are directly proportional to the number of living cells. In our assays, cells harboring the ACSA-GFP were maintained in the presence or absence of Dox. Subsequently, the cells were seeded into 96-well plates at the density of 1×103 cells per well. At designated time points, WST-8 was added to cell cultures for 2 h. After that time, the absorbance was measured according to the manufacturer's protocol. Control groups were also analyzed, including nontransduced NIH/3T3 cells grown in the presence or absence of Dox and corresponding cells cultured in the presence of 0.5 μg/mL of actinomycin D (Sigma-Aldrich), an inhibitor of proliferation.
Cell migration assays
The motility of cells expressing the ACSA-GFP construct was compared to that of cells in which the expression of the analyzed receptor was not activated by Dox. In addition, the motility of nontransduced NIH/3T3 cells was also analyzed. Cell migration assays were done by employing an agarose drop method, as described.17 In brief, 2-μL drops of cell–agarose mixtures containing 1×104 cells were carefully applied onto procollagen I-coated or BSA-coated plastic wells. Next, the agarose drops were allowed to solidify at 4°C, and then 100 μL of DMEM supplemented with 2% BSA was added to each well. At designated time points, the digital images were taken and used to measure the distance of migration of cells by applying a radial mask method, as described.17 Based on these measurements, the mean values and standard errors of the means (SEM) were calculated.
Collagen gel contraction
One of elements of wound healing is the contraction of collagen-rich matrices of connective tissues, a process that largely depends on the cell–matrix interaction. To analyze the ability of cells expressing the ACSA-GFP construct to participate in this process, we employed a collagen gel contraction assay. The cells harboring the ACSA-GFP were detached from cell culture dishes, and then a 100-μL sample containing 3×106 cells was mixed with 400 μL of a neutral collagen solution containing 3 mg/mL of collagen I (BD Biosciences). Individual 500-μL samples were poured into separate wells of a 24-well plate, and then the cell-containing mixtures were allowed to polymerize at 37°C. Subsequently, the polymerized collagen discs were removed from the wells and then transferred to cell culture dishes. Floating cell-containing collagen discs were maintained in DMEM substituted with 10% FBS. At designated time points, the discs were photographed with the use of a stereo microscope (SMZ-1000; Nikon) equipped with a digital camera (DXM-1200F, Nikon).
Biosynthesis of procollagen I and survival of cells expressing the ACSA-GFP
The biosynthesis of procollagen I by cells expressing the ACSA-GFP was analyzed by employing cells cultured in the presence or absence of Dox. Procollagen I secreted by cell culture media was analyzed by Western blot with the use of the anti-collagen I antibody (AFCol-1), as described.6
The survival of cells was analyzed by Western blot assays of cleaved poly(ADP-ribose) polymerase (PARP), a marker of apoptosis, as described.18,19 An intact form of PARP and its processed form were assayed with the anti-PARP antibody that recognizes both variants (Cell Signaling Technology). Cell lysates derived from nontransduced NIH/3T3 cells treated with staurosporine (Sigma-Aldrich) served as a positive control for the presence of cleaved PARP. In all the analyzed groups, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Matrix-dependent cell signaling
Western blot assays were used to analyze the possibility of interference of the ACSA-GFP with the collagen-dependent signaling mediated by integrins and DDR1, as described.20 In brief, NIH/3T3 cells were cultured in 10% FBS. After reaching confluence, the cells were maintained for 24 h in DMEM supplemented with 0.5% FBS and 40 μg/mL of l-ascorbic acid phosphate magnesium salt. Subsequently, the cells were harvested by treatment with an enzyme-free cell dissociation buffer (Life Technologies) and then incubated at 37°C in a gently-mixed suspension for 1 h in the presence of 0.1% heat-inactivated BSA. Next, a fraction of the cells were collected by centrifugation, and then cell pellets were lysed in the presence of inhibitors of phosphatases (Thermo Scientific). The remaining portions of nontransduced cells and the cells harboring the ACSA-GFP construct were seeded onto collagen-coated plates and then cultured in the presence or absence of Dox in DMEM supplemented with 0.1% heat-inactivated BSA. Subsequently, the cells were harvested into the lysis buffer, as described above.
For the integrin-mediated signaling, selected molecules were detected with the following antibodies: (i) anti-focal adhesion kinase (FAK; Cell Signaling Technology #3285) and (ii) anti-extracellular signal-regulated kinase p44/42 (ERK1/2; Cell Signaling Technology #4695). The phosphorylated forms of these proteins were evaluated with the anti-phospho-FAK (Tyr397) antibody (Cell Signaling Technology #3283) and with the anti-phospho-p44/p42 (Erk1/2) (Thr202/Tyr204) antibody (Cell Signaling Technology #9101). For the DDR1-mediated cell signaling activities, the anti-DDR1 antibody was employed to demonstrate the presence of DDR1 (Cell Signaling Technology #5583). The anti-phosphorylated DDR1 (Tyr792) antibody (Cell Signaling Technology #11994) was used to determine its collagen-mediated autophosphorylation, as described.21
Results
Expression of the ACSA-GFP
The DNA construct for the expression of the ACSA-GFP included the sequences encoding the variable regions of the prototypic anti-α2Ct antibody, the transmembrane domain of LAIR-1, and GFP (Fig. 1C). Following the selection of puromycin-resistant clones, microscopic and Western blot assays confirmed the Tet-dependent expression of the analyzed construct (Fig. 2), whose electrophoretic migration distance corresponded to the predicted molecular mass of 67.5 kDa (Fig. 2).
FIG. 2.

Microscopic and Western blot assays of the expression of the ACSA-GFP construct. (A, B) Visualization of cells expressing the ACSA-GFP constructs in cells cultured in the presence of doxycycline (Dox). A nonspecific control peptide was added to cell culture media (A); the absence of peptide-specific fluorescence indicates that the ACSA-GFP construct does not bind to the nonspecific peptide. In contrast, peptide-specific fluorescence is clearly apparent in cells cultured in the presence of the α2Ct peptide, a specific ACSA-GFP target (B). (C) Subcellular localization of the ACSA-GFP construct in transduced cells cultured in the presence (+) or absence (−) of Dox. Symbols: m, cell membrane fraction; c, cytoplasmic fraction; 3T3, nontransduced NIH/3T3 control cells. The molecular masses (kDa) of marker proteins are also indicated. Color images available online at www.liebertpub.com/tea
Membrane localization of the ACSA-GFP
As indicated in Figure 2, the ACSA-GFP-specific band was primarily detected in the cell membrane fraction. The presence of the biotinylated α2Ct peptide bound to the surface of the NIH/3T3 cells expressing the ACSA-GFP, but not to surfaces of cells cultured in the absence of Dox (not shown), further indicates the membrane localization of this construct (Fig. 2). The lack of binding of the control peptide corroborates the specificity of the binding of the ACSA-GFP to the collagen I target.
Binding of cells to procollagen I
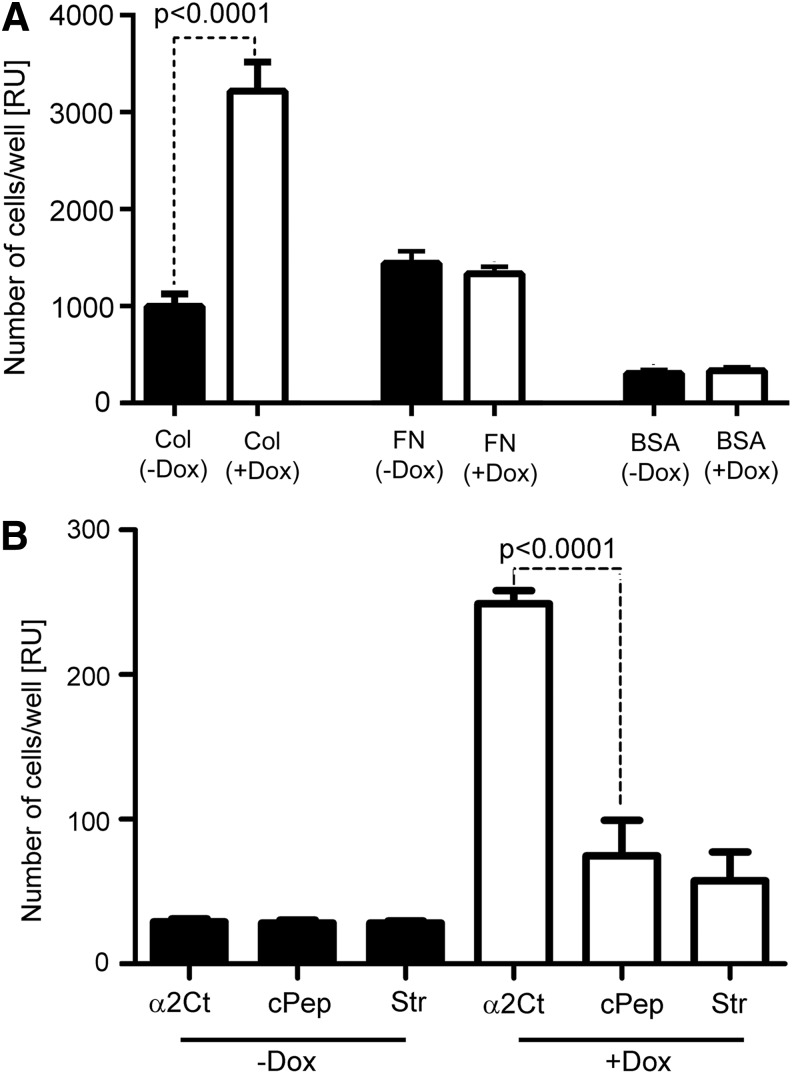
The results of cell attachment assays strongly indicate that the presence of the ACSA-GFP greatly enhances the collagen I-binding characteristics of the analyzed cells (Fig. 3A). Regardless of the presence or absence of the ACSA-GFP construct, no differences in the binding of cells to surfaces coated with fibronectin or with BSA were observed. These results suggest that the ACSA-GFP-mediated interaction was the main mechanism driving the attachment of cells expressing this construct to procollagen I-coated surfaces. Moreover, as a relatively high number of cells expressing the ACSA-GFP construct bound to α2Ct-coated surfaces, but not to surfaces coated with a random peptide, the described studies indicate the high specificity of the ACSA-GFP-procollagen I interaction (Fig. 3B).
FIG. 3.
A graphic representation of results on the attachment of cells. (A) Cells seeded on collagen I-coated (Col), fibronectin-coated (FN), or bovine serum albumin (BSA)-coated surfaces. (B) Cells seeded on control surfaces on surfaces coated with the α2Ct peptide. Symbols: cPep, surfaces coated with nonspecific control peptide; Str, surfaces coated with streptavidin only.
Cell proliferation
As demonstrated by the similar slopes of fitted curves seen in Figure 4, the rate of proliferation of NIH/3T3 cells expressing the ACSA-GFP was comparable to the proliferation rate of cells, in which the expression of this receptor was blocked in the absence of Dox. Similar proliferation kinetics seen in the control group of nontransduced NIH/3T3 cells cultured in the presence or absence of Dox suggests that neither the presence of a transgene encoding the ACSA-GFP construct nor the presence of Dox interfered with the proliferative activities of the analyzed cells (Fig. 4). The validity of the applied test was determined by the observation of inhibition of proliferation of control cells treated with antiproliferative actinomycin D (Fig. 4).
FIG. 4.
Proliferation of cells expressing the ACSA-GFP and nontransduced NIH/3T3 cells. Dotted lines indicate cells cultured in the presence of Dox while the straight lines represent cells maintained in the absence of this antibiotic. Graphic representation of proliferation characteristics of nontransduced NIH/3T3 cells cultured in the presence of actinomycin D is also indicated (AcD-3T3).
Cell migration
As indicated in Figure 5, after 2 days of culturing cells on procollagen I-coated surfaces, the average distance of the migration of cells with no expression of the ACSA-GFP was 0.28 mm (SEM 0.02), while the average migration distance for cells expressing this construct was 0.12 mm (SEM 0.03). The corresponding values after 3 days were 0.5 mm (SEM 0.01) and 0.3 mm (SEM 0.01), respectively. The differences in migration distances were statistically significant for the 2- and 3-day groups (p<0.0001). No difference in migrations was observed between nontransduced NIH/3T3 cells cultured in the presence or absence of Dox (not shown), and the migration behavior of these groups was similar to that seen in transduced cells cultured in the absence of Dox (Fig. 5). No migration of cells seeded on BSA-coated plates was observed during the 3-day culture (Fig. 5).
FIG. 5.

Migration of transduced cells cultured on procollagen I-coated and BSA-coated plates in the absence or presence of Dox. Dotted lines delineate the edges of agarose drops with encapsulated cells and indicate the position of the front of migrating cells present on indicated days. Arrows show the overall migration distances in the depicted experimental groups.
Collagen gel contraction
Both transduced and nontransduced NIH/3T3 cells seeded into collagen gels were able to contract them. The sizes of collagen discs observed on the second and the fifth day of culture were identical, regardless of the cell type encapsulated in them. The lack of collagen gel contraction in the absence of cells confirms this process was indeed cell dependent (Fig. 6).
FIG. 6.

Contraction of collagen gels by nontransduced and transduced cells cultured in the presence or absence of Dox. The top row depicts representative discs at day 0.
Biosynthesis of procollagen I and survival of cells expressing the ACSA-GFP
Analysis of proteins secreted by cells expressing the ACSA-GFP demonstrated that the presence of this construct did not alter the biosynthesis of procollagen I (Fig. 7). The absence of cleaved PARP indicates that the presence of the ACSA-GFP was not associated with increased cell death. The validity of cleaved PARP assays is demonstrated by the presence of cleaved PARP in the staurosporine-treated control group of untransformed NIH/3T3 cells (Fig. 7).
FIG. 7.

Western blot assays of the production of procollagen I (A) and poly(ADP-ribose) polymerase (PARP) in transduced cells (B) harboring the ACSA-GFP construct cultured in the presence (+) or absence of Dox (−). Analysis of control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is also presented (C). In all panels, the last lane represents results from nontransduced cells treated with staurosporine.
Matrix-dependent cell signaling
We tested whether the presence of collagen I-specific ACSA-GFP affects the functions of the native collagen I-specific receptors. Our assays focused on analyzing selected elements of integrin-mediated and DDR1-mediated processes of phosphorylation. Specifically, we analyzed ERK1/2, FAK, and DDR1 in cells cultured in suspension and in collagen-coated plates. As indicated in Figure 8, the phosphorylation of all selected markers in collagen-stimulated cells expressing the ACSA-GFP construct in the presence of Dox was similar to that seen in the control cultured in the absence of Dox, in which the expression of the ACSA-GFP was blocked. Moreover, the phosphorylation patterns seen in transduced NIH/3T3 cells were similar to those patterns seen in nontransduced NIH/3T3 cells (Fig. 8). The lack of apparent anchorage-dependent phosphorylation of analyzed proteins in cells maintained in suspension validates data obtained for the corresponding groups of cells cultured in collagen-coated plates (Fig. 8).
FIG. 8.

Western blot assays of selected proteins participating in integrin and discoidin domain receptor (DDR)-mediated collagen-cell interactions. The total pool of the analyzed proteins and their phosphorylated (P) forms were detected in cell lysates with the use of specific antibodies. Collagen-stimulated and nonstimulated cells harboring the ACSA-GFP construct and not transformed NIH/3T3 control cells cultured in the presence or absence of Dox were analyzed.
Discussion
A number of studies have explored distinct methods to optimize the efficiency of the delivery and retention of therapeutic cells, but the results have not been fully satisfactory.1 Some of these studies explored the systematic delivery of therapeutic cells, while others focused on local delivery approaches. For instance, the systemic delivery of cells was explored for myocardial repair, but this technique raised concerns about the possibility of entrapping the applied cells in nontargeted tissues, thereby raising the possibility of side effects.22–26 Local delivery methods of cells include encapsulating them and placing locally in the area of injury. Employing such an approach demonstrated promising results in bone, cartilage, skin, and pancreas, but the efficacy of local cell delivery was limited, in part, due to poor cell attachment and retention.1,27–32 Consequently, other experimental approaches to improve local retention of therapeutic cells were explored. For instance, some researchers have applied magnetic targeting of cells tagged with iron oxide particles to improve intramyocardial retention of cells in a rat-based model.33 Others functionalized native surface proteins expressed by mesenchymal stem cells by the chemical attachment of antibody-derived fragments to specifically bind sites in the targeted tissues.34,35 Such approaches are limited, however, because cell surface modifications are not stable enough to repair the targeted tissue.1
Our study addressed the need to improve the efficiency of the local cell delivery retention in target sites by employing a concept tested in cancer research. A CAR, is engineered to deliver T cells to specific antigen targets present in cancerous cells. In cancer therapy, the role of CAR is to direct T cells into specific tumor antigens, thereby activating specific cytoplasmic domains that stimulate an anticancer immunological response. Fundamentally similar, our technology differs, however, in one significant area. Specifically, the intention of employing the ACSA is to deliver cells to a specific site and to retain them in a target area rather than, as in a case of CAR, trigger specific intracellular responses upon delivery of cells. Consequently, the ACSA-GFP construct was engineered in a way that excludes the natural extracellular collagen-binding domains and intracellular regulatory domains of native LAIR-1. Only hinge and transmembrane domains of this receptor were employed to generate the ACSA-GFP construct. The fact that LAIR-1 in its native form functions as a collagen-specific receptor was purposely considered by us during the rational engineering of the ACSA-GFP. Specifically, we postulate that choosing LAIR-1-derived hinge and transmembrane domains provide the necessary structural characteristics that enable the ACSA to bind collagen I molecules. This notion is supported by our observations that cells expressing the ACSA-GFP were interacting specifically with intact procollagen I and with its specific α2Ct fragment. Although the key elements of the ACSA-GFP construct include collagen-binding fragments derived from the anti-α2Ct antibody, they do not include the fragment crystallizable (Fc) domain. As the Fc region responsible for generating the immune response of native antibodies is absent, we predict that the immunogenic potential of the ACSA-GFP is minimal. Future experiments at the organismal level done in relevant animal studies will determine the immunogenicity of the ACSA-GFP and potential off-target toxicity of cells expressing collagen-specific anchors. Based on the long-term follow-up data associated with CAR therapy for cancer, however, the overall risk associated with artificial receptor technology is low.36
The ACTS-GFP-collagen I interaction was analyzed in the context of a monomeric collagen I and its biologically relevant fibrillar form present in collagen gels. Although the binding of the ACSA-GFP to collagen fibrils was not demonstrated directly, the ability of the ACSA-GFP to interact with collagen I molecules present in the context of complex fibrils is supported by the observation that the archetypical anti-α2Ct scFv construct recognizes its binding partner in complex collagen fibrils.5
The finding that cells expressing the ACSA-GFP attached to the collagen I target makes them attractive for the delivery of cells into collagen I-rich tissues. This notion was strengthened by the observation that the cells expressing the ACSA-GFP tended to remain in the site of delivery. This behavior was demonstrated by their relatively short migration distances observed on collagen I-coated surfaces. We postulate that improved retention of cells was primarily a result of the anchoring action of the ACSA-GFP rather than its potentially adverse influence on vital cellular processes. This notion was supported by the fact that the proliferation, the viability, secretion of procollagen I, cell-signaling activities mediated by native collagen receptors, and the ability to contract collagen gels were not altered in cells expressing the ACSA-GFP construct.
Since it has been demonstrated that collagen-specific integrins and DDRs are important for the ability of cells to contract gels, our observation that collagen gel contraction by cells expressing the ACSA-GFP is not affected may further indicate that this artificial anchor does not interfere with the binding of cells through the native collagen-specific receptors.37,38 Because the dynamics of contraction observed over a period of 5 days did not differ between the group of cells expressing the ACSA-GFP and that in which the expression was inhibited, it is apparent that the ACSA-GFP does not actively participate in gel contraction. Collagen-specific integrins and DDR1 recognize specific well-defined sites within the collagen I triple helical domain and the ACSA targets the distant α2Ct region. So the collagen gel contraction assays further indicate the high binding specificity of the novel collagen I-binding construct that does not interfere with the binding of native receptors.4,39
The presented motility assays indicate that NIH/3T3 cells expressing the ACSA-GFP retain the ability to migrate, thereby indicating that the artificial anchor does not permanently lock the cells in the place of seeding. We postulate that the ability of engineered cells to move is, in part, the result of a relatively weak binding of the individual ACSA-GFP constructs to collagen I. Although the binding kinetics of the ACSA-GFP were not assayed directly, we determined that the Kd value for the anti-α2Ct scFv-collagen I binding is 70 nM, while the corresponding value for the prototypic IgA-type of the anti-α2Ct antibody, based on which the scFv and the ACSA were created, is 0.2 nM.5,6 Still, in comparison to the binding affinities of native collagen receptors characterized by Kd values of 200 nM for integrins, 25 μM for DDR2, and 18 μM for LAIR-1, the predicted affinity for the ACTS could be relatively high, thereby providing a robust and a specific anchoring element for applied cells.40–43 Moreover, it is predicted that the presence of multiple ACSA-GFP constructs on cell surfaces increases the overall avidity of binding interactions, thereby improving cell retention. Considering the above binding affinities, we propose that while increasing the retention, the interactions mediated by the ACSA constructs do not limit the migration of cells within the targeted injury site.
As the majority of connective tissues such as skin, bone, ligament, and tendon include collagen I-rich extracellular matrices, the potential applicability of the proposed ACSA is broad. Furthermore, the basic concept of employing site-specific receptors to deliver and retain therapeutic cells, tested by us with collagen I as the primary target, can be potentially applied to target other unique macromolecules present in various tissues and organs. With emerging technologies that depend on introducing genetic modifications in therapeutic cells to regenerate damaged tissues, our concept described here represents a valid and timely approach to improve cell delivery and retention.44 Future tests with the use of clinically relevant models will scrutinize the efficacy of rationally engineered anchors for their applications in regenerative medicine.
Acknowledgments
This research was supported, in part, by a grant from American Foundation for Surgery of the Hand awarded to Mark L. Wang. The authors thank Jennifer Fisher Wilson for revising the article.
Disclosure Statement
The authors declare no competing financial interests exist.
References
- 1.Ansboro S., Greiser U., Barry F., and Murphy M.Strategies for improved targeting of therapeutic cells: implications for tissue repair. Eur Cells Mater 23,310, 2012; discussion 318 [DOI] [PubMed] [Google Scholar]
- 2.James R., Kesturu G., Balian G., and Chhabra A.B.Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg 33,102, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Qiu Y., Lim J.J., Scott L., Jr., Adams R.C., Bui H.T., and Temenoff J.S.PEG-based hydrogels with tunable degradation characteristics to control delivery of marrow stromal cells for tendon overuse injuries. Acta Biomater 7,959, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Chung H.J., Steplewski A., Chung K.Y., Uitto J., and Fertala A.Collagen fibril formation. A new target to limit fibrosis. J Biol Chem 283,25879, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fertala J., Kostas J., Hou C., Steplewski A., Beredjiklian P., Abboud J., Arnold W.V., Williams G., and Fertala A.Testing the anti-fibrotic potential of the single-chain Fv antibody against the alpha2 C-terminal telopeptide of collagen I. Connect Tissue Res 55,115, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fertala J., Steplewski A., Kostas J., Beredjiklian P., Williams G., Arnold W., Abboud J., Bhardwaj A., Hou C., and Fertala A.Engineering and characterization of the chimeric antibody that targets the C-terminal telopeptide of the alpha2 chain of human collagen I: a next step in the quest to reduce localized fibrosis. Connect Tissue Res 54,187, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han E.Q., Li X.L., Wang C.R., Li T.F., and Han S.Y.Chimeric antigen receptor-engineered T cells for cancer immunotherapy: progress and challenges. J Hematol Oncol 6,47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Till B.G., Jensen M.C., Wang J., Chen E.Y., Wood B.L., Greisman H.A., Qian X., James S.E., Raubitschek A., Forman S.J., Gopal A.K., Pagel J.M., Lindgren C.G., Greenberg P.D., Riddell S.R., and Press O.W. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 112,2261, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hombach A., Heuser C., Sircar R., Tillmann T., Diehl V., Pohl C., and Abken H.Characterization of a chimeric T-cell receptor with specificity for the Hodgkin's lymphoma-associated CD30 antigen. J Immunother 22,473, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Sadelain M., Brentjens R., and Riviere I.The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol 21,215, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dotti G., Savoldo B., and Brenner M.Fifteen years of gene therapy based on chimeric antigen receptors: “are we nearly there yet?” Hum Gene Ther 20,1229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson I.C., Lenton K.A., Little D.J., Decorso T., Lee F.T., Scott A.M., Zola H., and Hohmann A.W.Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Mol Immunol 34,1157, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Gossen M., Freundlieb S., Bender G., Muller G., Hillen W., and Bujard H.Transcriptional activation by tetracyclines in mammalian cells. Science 268,1766, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Ito H., Rucker E., Steplewski A., McAdams E., Brittingham R.J., Alabyeva T., and Fertala A.Guilty by association: some collagen II mutants alter the formation of ECM as a result of atypical interaction with fibronectin. J Mol Biol 352,382, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Bazan-Socha S., Kisiel D.G., Young B., Theakston R.D., Calvete J.J., Sheppard D., Marcinkiewicz C.Structural requirements of MLD-containing disintegrins for functional interaction with alpha 4 beta 1 and alpha 9 beta1 integrins. Biochemistry 43,1639, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kadler K.E., Hojima Y., and Prockop D.J.Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem 262,15696, 1987 [PubMed] [Google Scholar]
- 17.Ito H., Steplewski A., Alabyeva T., and Fertala A.Testing the utility of rationally engineered recombinant collagen-like proteins for applications in tissue engineering. J Biomed Mater Res A 76,551, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hintze V., Steplewski A., Ito H., Jensen D.A., Rodeck U., and Fertala A.Cells expressing partially unfolded R789C/p.R989C type II procollagen mutant associated with spondyloepiphyseal dysplasia undergo apoptosis. Hum Mutat 29,841, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Jensen D.A., Steplewski A., Gawron K., and Fertala A.Persistence of intracellular and extracellular changes after incompletely suppressing expression of the R789C (p.R989C) and R992C (p.R1192C) collagen II mutants. Hum Mutat 32,794, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlaepfer D.D., Jones K.C., and Hunter T.Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol 18,2571, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leitinger B., Steplewski A., and Fertala A.The D2 period of collagen II contains a specific binding site for the human discoidin domain receptor, DDR2. J Mol Biol 344,993, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Sheng C.C., Zhou L., and Hao J.Current stem cell delivery methods for myocardial repair. Biomed Res Int 2013,547902, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell N.G., and Suzuki K.Cell delivery routes for stem cell therapy to the heart: current and future approaches. J Cardiovasc Transl Res 5,713, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Barbash I.M., Chouraqui P., Baron J., Feinberg M.S., Etzion S., Tessone A., Miller L., Guetta E., Zipori D., Kedes L.H., Kloner R.A., and Leor J.Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108,863, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Ortiz L.A., Gambelli F., McBride C., Gaupp D., Baddoo M., Kaminski N., and Phinney D.G.Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A 100,8407, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmood A., Lu D., Lu M., and Chopp M.Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery 53,697, 2003; discussion 702 [DOI] [PubMed] [Google Scholar]
- 27.Yao J., Korotkova T., and Smith RL.Viability and proliferation of pluripotential cells delivered to tendon repair sites using bioactive sutures—an in vitro study. J Hand Surg 36,252, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann A., and Gross G.Tendon and ligament engineering in the adult organism: mesenchymal stem cells and gene-therapeutic approaches. Int Orthop 31,791, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juncosa-Melvin N., Boivin G.P., Galloway M.T., Gooch C., West J.R, and Butler D.L.Effects of cell-to-collagen ratio in stem cell-seeded constructs for Achilles tendon repair. Tissue Eng 12,681, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Brodke D., Pedrozo H.A., Kapur T.A., Attawia M., Kraus K.H., Holy C.E., Kadiyala S., and Bruder S.P.Bone grafts prepared with selective cell retention technology heal canine segmental defects as effectively as autograft. J Orthop Res 24,857, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Tuli R., Li W.J., and Tuan R.S.Current state of cartilage tissue engineering. Arthritis Res Ther 5,235, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiwari A., Kidane A., Salacinski H., Punshon G., Hamilton G., and Seifalian A.M.Improving endothelial cell retention for single stage seeding of prosthetic grafts: use of polymer sequences of arginine-glycine-aspartate. Eur J Vasc Endovasc Surg 25,325, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Chaudeurge A., Wilhelm C., Chen-Tournoux A., Farahmand P., Bellamy V., Autret G., Menager C., Hagege A., Larghero J., Gazeau F., Clement O., and Menasche P.Can magnetic targeting of magnetically labeled circulating cells optimize intramyocardial cell retention?. Cell Transplant 21,679, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Zanjani E.D., Flake A.W., Almeida-Porada G., Tran N., and Papayannopoulou T.Homing of human cells in the fetal sheep model: modulation by antibodies activating or inhibiting very late activation antigen-4-dependent function. Blood 94,2515, 1999 [PubMed] [Google Scholar]
- 35.Kumar S., and Ponnazhagan S.Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J 21,3917, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Barrett D.M., Singh N., Porter D.L., Grupp S.A., and June C.H.Chimeric antigen receptor therapy for cancer. Annu Rev Med 65,333, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olaso E., Lin H.C., Wang L.H., and Friedman S.L.Impaired dermal wound healing in discoidin domain receptor 2-deficient mice associated with defective extracellular matrix remodeling. Fibrogenesis Tissue Repair 4,5, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooke M.E., Sakai T., and Mosher D.F.Contraction of collagen matrices mediated by alpha2beta1A and alpha(v)beta3 integrins. J Cell Sci 113(Pt 13),2375, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Farndale R.W., Lisman T., Bihan D., Hamaia S., Smerling C.S., Pugh N., Konitsiotis A., Leitinger B., de Groot P.G., Jarvis G.E., and Raynal N.Cell-collagen interactions: the use of peptide Toolkits to investigate collagen-receptor interactions. Biochem Soc Trans 36,241, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Ichikawa O., Osawa M., Nishida N., Goshima N., Nomura N., and Shimada I.Structural basis of the collagen-binding mode of discoidin domain receptor 2. EMBO J 26,4168, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jokinen J., Dadu E., Nykvist P., Kapyla J., White D.J., Ivaska J., Vehvilainen P., Reunanen H., Larjava H., Hakkinen L., and Heino J.Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem 279,31956, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Kapyla J., Ivaska J., Riikonen R., Nykvist P., Pentikainen O., Johnson M., and Heino J.Integrin alpha(2)I domain recognizes type I and type IV collagens by different mechanisms. J Biol Chem 275,3348, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Jiang L., and Barclay A.N.New assay to detect low-affinity interactions and characterization of leukocyte receptors for collagen including leukocyte-associated Ig-like receptor-1 (LAIR-1). Eur J Immunol 39,1167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myers T.J., Granero-Molto F., Longobardi L., Li T., Yan Y., and Spagnoli A.Mesenchymal stem cells at the intersection of cell and gene therapy. Expert Opin Biol Ther 10,1663, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]