Abstract
Decellularized tissues have proven to be versatile matrices for the engineering of tissues and organs. These matrices usually consist of collagens, matrix-specific proteins, and a set of largely undefined growth factors and signaling molecules. Although several decellularized tissues have found their way to clinical applications, their use in the engineering of cartilage tissue has only been explored to a limited extent. We set out to generate hydrogels from several tissue-derived matrices, as hydrogels are the current preferred cell carriers for cartilage repair. Equine cartilage, meniscus, and tendon tissue was harvested, decellularized, enzymatically digested, and functionalized with methacrylamide groups. After photo-cross-linking, these tissue digests were mechanically characterized. Next, gelatin methacrylamide (GelMA) hydrogel was functionalized with these methacrylated tissue digests. Equine chondrocytes and mesenchymal stromal cells (MSCs) (both from three donors) were encapsulated and cultured in vitro up to 6 weeks. Gene expression (COL1A1, COL2A1, ACAN, MMP-3, MMP-13, and MMP-14), cartilage-specific matrix formation, and hydrogel stiffness were analyzed after culture. The cartilage, meniscus, and tendon digests were successfully photo-cross-linked into hydrogels. The addition of the tissue-derived matrices to GelMA affected chondrogenic differentiation of MSCs, although no consequent improvement was demonstrated. For chondrocytes, the tissue-derived matrix gels performed worse compared to GelMA alone. This work demonstrates for the first time that native tissues can be processed into crosslinkable hydrogels for the engineering of tissues. Moreover, the differentiation of encapsulated cells can be influenced in these stable, decellularized matrix hydrogels.
Introduction
In tissue engineering, there is a rationale for designing biomimetic materials that recreate the native cell niche.1,2 Tissue-derived matrices, for example, can provide structural and biological support for matrix formation by embedded or invading cells.3 Native tissues that have been decellularized while maintaining the natural growth factors and signaling molecules may thus provide the ultimate biomimetic environment.4 Despite the clinical translation of decellularized materials for the repair of skin, bone, heart valves, and so on, the application in the field of cartilage regeneration has only been explored to a limited extent.5,6
There is a strong need for novel biomaterials for cartilage tissue engineering.7,8 Hydrogels are good potential candidates, since they can support the chondrogenic morphology and simultaneously serve as a temporary scaffold for matrix formation. Moreover, hydrogels are easily delivered to a cartilage defect and allow for engineering of advanced cartilage constructs.9
Currently, fibrin glue is the clinical standard for cell delivery to cartilage defects.10 However, this gel is relatively unstable with a high degradation rate and is thus a delivery vehicle rather than a support structure for cartilage matrix formation.11,12 Therefore, several new hydrogels are being designed, in which ideally the degradation rate is balanced with active cartilage matrix formation, and biochemical cues are incorporated to direct the behavior of encapsulated cells.13,14 Hydrogels derived from natural tissues are interesting candidates to meet these requirements, as they may form a potential scaffold for cells and include the appropriate biochemical cues. To this end, native matrix gels have been acquired from several tissues through digestion with pepsin enzymes.15–18 These hydrogels allowed the invasion of cells and subsequent matrix deposition. However, tissue-derived matrix gels so far have only been physically cross-linked resulting in low shape stability, which is a serious drawback.
In this study, decellularized matrices were derived from cartilage, meniscus, and tendon tissues. Cartilage has a high level of collagen II and glycosaminoglycans (GAGs); meniscus has an intermediate level of GAGs, and a mixture of collagen types I and II, whereas tendon is predominantly composed of collagen type I and a small amount of GAGs.19 Decellularized cartilage tissue may contain the biochemical cues that are present in the native chondrocyte niche. Intuitively, a collagen type II scaffold would be the scaffold of choice for cartilage engineering, whereas there are indications that this type of collagen induces catabolic pathways in cultured chondrocytes.20–22 On the other hand, collagen type I scaffolds have proven efficacy in the regeneration of cartilage tissue.23,24 Therefore, both types of collagen and a mixed composition (meniscus tissue) were evaluated here. The tissue-derived matrices were used for the functionalization of gelatin methacrylamide (GelMA) hydrogels. GelMA has been identified as a stable, versatile hydrogel for tissue regeneration.25–27 It can be synthesized at low cost and gelatin is widely available as a substrate; cell adhesion sites are abundant and cells are allowed to migrate through the hydrogel and remodel the newly formed tissue. We previously showed that functionalizing GelMA with GAGs can substantially improve cartilage matrix formation in in vitro models.28 In this study, cartilage, meniscus, and tendon tissues were processed into hydrogels that were covalently cross-linked to GelMA hydrogel. The potential of these matrices to enhance cartilage tissue formation by chondrocytes and mesenchymal stromal cells (MSCs) in vitro was evaluated.
Materials and Methods
Development of crosslinkable tissue-derived matrix hydrogels
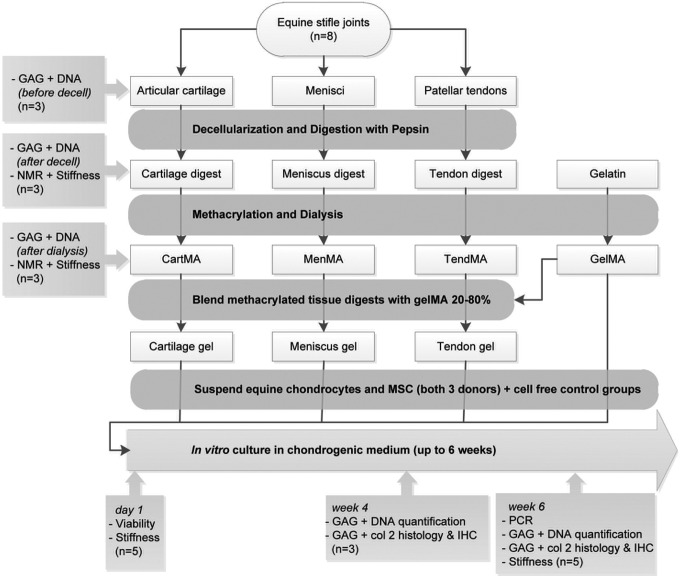
Stifle joints (n=8; age 3–10 years) were obtained from horses that had died or were euthanized due to nonorthopedic ailments. Consent was obtained from the owner before tissue harvest and tissue was only obtained from macroscopically healthy joints. The stifle joints were dissected and the full-thickness cartilage was harvested from the entire femoral condyles. Also, both complete menisci and the patellar tendons were harvested, 0.5 cm from their anchoring site in the bone. The tissues were dissected into small pieces (∼5×5×5 mm for meniscus and tendon, and ∼5×5×2 mm for cartilage tissue) and all donors were pooled. Three random samples were taken from each tissue type to measure the GAG and DNA content. The obtained values will thus reflect the variability between donors and sample location (e.g., cartilage from the medial or lateral condyle). Then the tissues were separately milled in liquid nitrogen (A11 basic analytical mill; IKA). The cartilage fragments were subsequently sieved through pores of 710 μm. Cells were removed from the tissues by treatment with 10 mM Tris/1% triton on a roller bench for 24 h; sonication for 2 h at 55±10 kHz and a nuclease solution consisting of 1 U/mL desoxyribonuclease and 1 U/mL ribonuclease in phosphate-buffered saline (PBS) on a roller bench for 72 h at 37°C. The resulting matrices (from cartilage, meniscus, and tendon tissue) were freeze-dried and digested with pepsin (Sigma-Aldrich) in a 0.01 M hydrochloric acid solution at 37°C on a roller bench, until clear suspensions were obtained. The pH of the solutions was raised to 9.0 for 1 h (using 1 M NaOH), to irreversibly inactivate the remaining pepsin enzymes,29 before adjustment to 7.5 (using 1 M HCl). Next, methacrylic anhydride (Sigma) was added dropwise (2.5 mL/g matrix) and was allowed to react with the matrices under constant stirring for 1 h. The methacrylated tissue-derived matrices (CartMA, MenMA and TendMA) were dialyzed against distilled water for 7 days at 40°C to remove unreacted methacrylic acid and anhydride. After freeze-drying, the tissue digests were dissolved in PBS 10% (w/v), containing photoinitiator Irgacure 2959 (0.1% [w/v], Ciba; BASF). A schematic overview of the experimental set-up is presented in Figure 1.
FIG. 1.
Experimental setup of the study. Col, collagen; GAG, glycosaminoglycans; IHC, immunohistochemistry; MES, mesenchymal stromal cells; n, the number of analyzed samples per donor; NMR, nuclear magnetic resonance; PCR, polymerase chain reaction.
Characterization of tissue-derived matrices
Collagen type I (rat tail; BD Biosciences), collagen type II (from chicken sternal cartilage; Sigma), meniscus, tendon, and cartilage digests (not methacrylated) were electrophoresed on a Bolt 4–12% Bis-Tris Plus gel (Novex; Life Technologies) under reducing conditions (1.25% 2-mercaptoethanol). The proteins were visualized with Page Blue (Thermo Scientific) and compared to a multicolor High Range protein ladder (Thermo Scientific). Images were recorded using a Epson perfection 4490 Photo scanner.
The success of the methacrylation procedure was evaluated using proton nuclear magnetic resonance (1H NMR). 1H NMR spectra of 10 mg/mL solutions of decellularized cartilage, meniscus, and tendon tissue digests, and their methacrylated equivalents (respectively cartMA, menMA, and tendMA), were recorded on a Mercury 300 MHz instrument (Varian Associates, Inc.; NMR Instruments). Chemical shifts were recorded in ppm with reference to the solvent peak (δ=4.8 ppm for D2O).
Blending of tissue digests with GelMA
For cellular differentiation experiments, the methacrylated tissue digests (cartMA, menMA, and tendMA), were separately blended with GelMA at 37°C, and will be referred to as the cartilage, meniscus, and tendon group, respectively. GelMA was synthesized by reaction of type A gelatin (Sigma) with methacrylic anhydride as described previously.30,31 The final composition of the hydrogels was 8% GelMA and 2% tissue digest in 1×PBS with 0.1% photoinitiator (all w/v). GelMA (10% w/v) was used as a control.
Photo-cross-linking of hydrogels
Photo-cross-linking of all hydrogels was performed for 15 min in a custom-made Teflon mold (width×height=4×2 mm) using 365 nm UV light in a UVP CL-1000L cross-linker (UVP). The cross-linked samples were stored overnight at 37°C in PBS before analyzing the compressive modulus.
Compressive mechanical testing
The compressive modulus of all three uncross-linked and cross-linked (-MA) tissue digests was measured, and compared to cross-linked GelMA gels (all n=5). The compressive modulus was also measured at day 1 and week 6 of in vitro culture for all experimental groups (cross-linked cartilage, meniscus, tendon, and GelMA gels, all n=5). Measurements were performed by uniaxial unconfined compression in air at room temperature. Hydrogels and hydrogel-cell constructs were compressed to ∼20% strain in 2 min using a Dynamic Mechanical Analyzer (DMA 2980; TA Instruments). The compressive modulus was calculated from the linear derivative of the stress/strain curve at 10–15% strain.
Isolation of equine chondrocytes and mesenchymal stromal cells (MSCs)
Full-thickness cartilage was harvested under sterile conditions from the stifle joint of fresh equine cadavers (n=3, age 3–10 years) with macroscopically healthy cartilage and with consent of the owners. After overnight digestion in 0.15% type II collagenase (Worthington Biochemical Corp) at 37°C, the suspension was filtered, stored at −196°C, and encapsulated in the hydrogels at passage 1, according to a previously described protocol.25
With approval of the Institutional Animal Ethical Committee, bone marrow aspirates were obtained from the iliac crest of healthy horses under general anesthesia (n=3). The mononuclear fraction was isolated according to a previously described protocol.32 The cells were stored at −196°C and encapsulated in the hydrogels at passage 3–4. The multilineage potential of cells cultured from the bone marrow aspirates was confirmed by a three-way differentiation assay as previously described.32,33
Viability assay
To evaluate the effect of the addition of the methacrylated tissue digests to GelMA on embedded cells, the viability of chondrocytes and MSCs (both three donors) was analyzed on day 1. The cells had been encapsulated in the cartilage, meniscus, tendon, and GelMA hydrogels at a concentration of 5×106 cells/mL. A LIVE/DEAD Viability Assay (Molecular Probes MP03224) was performed according to the manufacturer's instructions. Live and dead cells were counted for three samples per time point, at four locations within each construct. Viability was calculated as follows: (live cells/total cells)×100.
Cellular differentiation experiments
Equine chondrocytes and MSCs (both three donors, 15×106 cells/mL) were embedded in the cartilage, meniscus, tendon, and GelMA groups. The gels were cultured in vitro for up to 6 weeks in chondrogenic differentiation medium. For chondrocyte-laden hydrogels this consisted of Dulbecco's modified Eagle's medium (DMEM) (41965; Invitrogen) supplemented with 0.2 mM l-ascorbic acid 2-phosphate, 0.5% human serum albumin (SeraCare Life Sciences), 1×ITS-X (Invitrogen), 100 U/mL penicillin, 100 μg/mL streptomycin, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Invitrogen), and 5 ng/mL transforming growth factor β-2 (TGFβ-2). MSC-laden samples were cultured in DMEM (31966; Invitrogen) supplemented with 0.2 mM l-ascorbic acid 2-phosphate, 1×ITS+ premix (BD Biosciences), 0.1 μM dexamethasone, 100 U/mL penicillin and 100 μg/mL streptomycin, and 10 ng/mL TGFβ-2 (R&D Systems). Cell-free hydrogels cultured for 6 weeks served as a negative control group.
Histology, immunohistochemistry, and biochemistry
After 4 and 6 weeks of culture (three and five replicates respectively), the DNA and GAG content was quantified for all donors and groups. To this end, the samples were digested overnight in papain solution (200 μL per sample [0.01 M cysteine, 250 μg/mL papain, 0.2 M NaH2PO4, and 0.01 M ethylenediaminetetraacetic acid] at 60°C). Total DNA was quantified using the Picogreen DNA assay (Invitrogen) according to the manufacturer's instructions. Total GAG content was determined by photospectrometry at 525 and 595 nm, after reaction with dimethyl-methylene blue using a microplate reader (Biorad). The ratio of both absorbances was calculated and the GAG content was quantified using a chondroitin sulfate (Sigma) standard. The concentrations of GAG and DNA in each papain digest were normalized per donor to the 4-week GelMA control group. GAG/DNA was calculated to display the single cell synthetic activity for the production of cartilage-specific matrix.
After 6 weeks of culture, three samples from each donor were taken for histology and immunohistochemistry. Samples were dehydrated through a graded ethanol series, cleared in xylene, and embedded in paraffin. The samples were sectioned into 5 μm slices and a triple stain of hematoxylin (Klinipath BV) fast green and Safranin-O (Merck) was applied to identify GAG deposition. The stained sections were examined using a light microscope (Olympus BX51).
Collagen type II was stained by immunohistochemistry after deparaffinization and rehydration of the sections, according to a previously described protocol25 (primary antibody: 1:100, monoclonal mouse, II-II6B3; Developmental Studies Hybridoma Bank (DSHB); secondary antibody: 1:200, P0447; Dako). Isotype controls were performed by using mouse isotype IgG1 monoclonal antibody at concentrations similar to those used for the stainings.
Gene expression
After 6 weeks of culture, the samples were homogenized in TRIzol reagent (Invitrogen). The best-performing chondrocyte and MSC donors were selected for polymerase chain reaction (PCR)-analysis, based on a safranin-O staining of the GelMA control groups at week 6. The RNA was isolated according to the manufacturer's guidelines (chloroform was substituted by 3-bromo-chloropropane34). RNA was cleared of any DNA contamination by DNase digestion. Total RNA yield was determined spectrophotometrically (NanoDrop ND1000; Isogen Life Science) and 500 ng total RNA per sample was reverse transcribed into complementary DNA (cDNA) using Superscript III (Invitrogen) and random primers. SybrGreen™ quantitative real-time PCR was subsequently performed on an iQ-5 real time PCR detection system (Bio-Rad Laboratories). Each sample was run in duplicate and a three-fold serial dilution of pooled cDNA was used as a standard curve.
Primers were designed using computer software (primer BLAST, www.ncbi.nlm.nih.gov/tools/primer-blast) and obtained from Eurogentec. The specific genes of interest were collagen type IA1 (COL1A1), collagen type IIA1 (COL2A1), aggrecan (ACAN), matrix metalloproteinases-3 (MMP-3), MMP-13, and MMP-14. Hypoxanthine-guanine-phosphoribosyltransferase 1 (HPRT-1) and signal recognition particle 14 kDa (SRP-14) were selected as reference genes. Following fold-change calculation using the standard curve method, the geometric mean of these two reference genes was used to calculate the normalized mRNA expression of each target gene. Primer sequences of selected genes are provided in Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/tea.
Statistical analysis
An independent samples T-test, assuming unequal variances, was performed to compare the DNA and GAG content of the tissues before and after the decellularization protocol and also to compare the stiffness of cross-linked and uncross-linked cell-free hydrogels. A Univariate Analysis of Variance with a Tukey HSD post hoc test was performed to compare GAG, DNA, GAG/DNA, and the compressive modulus between the groups of the cell culture experiments. Because the level of matrix formation differed significantly between the cell donors, a randomized block design was used, correcting for donor effects. GAG and DNA were normalized to the GelMA control group at week 4 for each separate cell donor; the compressive modulus was normalized to GelMA day 1. The statistical analyses were done using SPSS statistics (IBM, version 20). For the PCR data, Kruskal Wallis and Dunn's multiple comparison post hoc tests were used to test differences between experimental conditions for each primer pair using Graphpad software (Graphpad Prism version 5.2 for Windows). Differences were considered significant when p<0.05 for all tests.
Results
Decellularization procedure
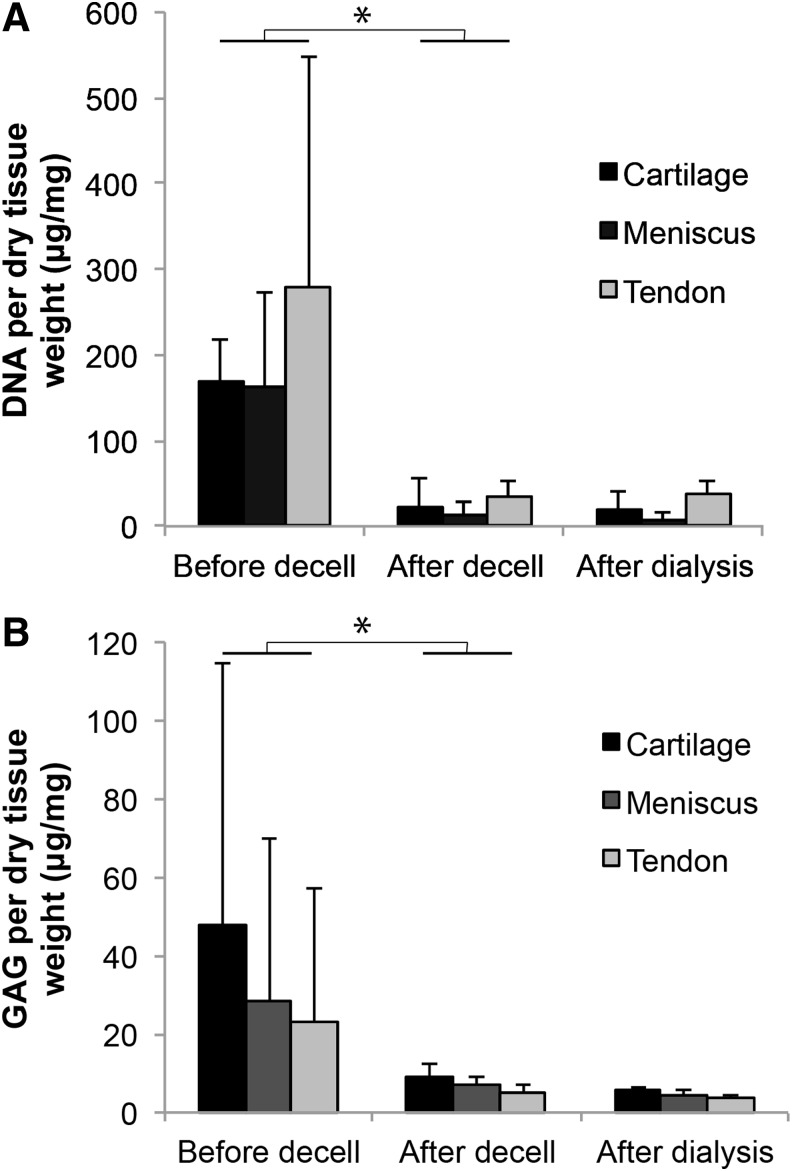
The DNA content of the cartilage, meniscus, and tendon tissues significantly decreased after decellularization, to values below 50 μg/mg dry weight (Fig. 2A). The DNA content was unaltered by the subsequent dialysis. The GAG content of all three tissues showed large variations between donors and/or sample location (Fig. 2B). Yet, the variation and the absolute GAG content decreased considerably after the decellularization procedure and remained unaltered after dialysis. About 58±10% of methacrylated and decellularized material could be obtained from the original tissues (expressed in dry weight).
FIG. 2.
The effect of the decellularization protocol on DNA and GAG content of equine cartilage, meniscus, and tendon tissues (*p<0.05). (A) The DNA content of all tissues was significantly reduced by the decellularization protocol. (B) The GAG content of all tissues directly after harvesting from the knee joint (before decell) shows large standard deviations, caused by donor and location variations. The subsequent decellularization procedure significantly reduced and standardized the GAG contents.
Characterization of tissue-derived matrices
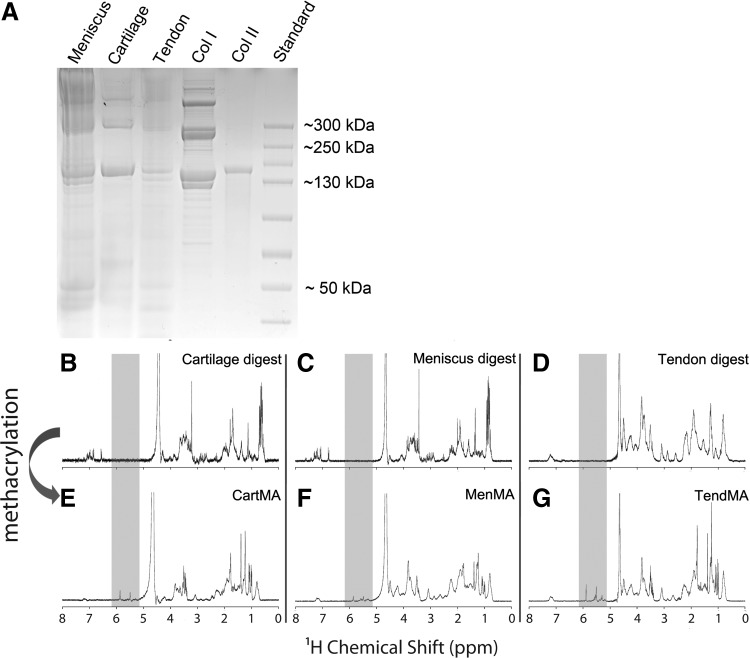
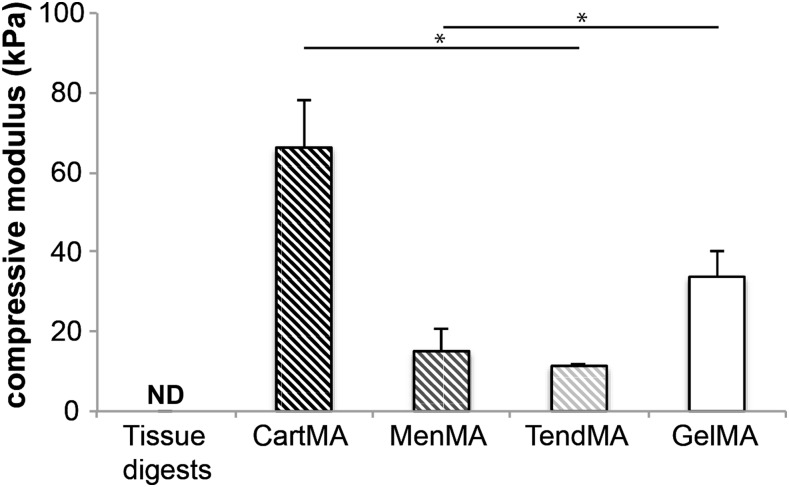
The decellularized cartilage, meniscus, and tendon matrices were successfully digested with pepsin enzymes until clear or close to clear solutions were obtained. Short treatment of the tissue particles (cartilage) or fibers (meniscus and tendon) with pepsin resulted in viscous substances, whereas overnight treatment resulted in low-viscosity solutions. Overnight digestion was used to facilitate the methacrylation procedure. Gel electrophoresis showed that all digested tissues consisted of polymers with a molecular weight predominantly in the range of 130–300 kDa (Fig. 3A). The profile of the collagen I solution reflects the collagen monomers (two subunits), dimers (two subunits), and trimers. This typical profile can also be observed in the tendon and meniscus digests. The collagen type II control only displays monomers with one subunit. This single unit monomer can also be found in the cartilage digest. Successful methacrylation of the solutions was confirmed with NMR by the double peak that emerged between 5 and 6 ppm after methacrylation of all three tissue digests (Fig. 3B–G). Comparing the stiffness of methacrylated tissue digests (cartMA, menMA, and tendMA) with GelMA and nonmethacrylated tissue digests revealed significant variations (Fig. 4). CartMA was stiffer than all other groups (66.2±11.9 kPa, p<0.05) and GelMA was stiffer (33.8±6.5 kPa, p<0.05) compared to menMA (15.1±5.4 kPa) and tendMA (11.4±0.6 kPa). The nonmethacrylated tissue digests remained low-viscosity solutions after treatment with UV light and, therefore, their stiffness could not be determined. Considering the variable degree of stiffness and to functionalize the existing GelMA hydrogel platform, the methacrylated tissue digests were blended with GelMA for the cellular differentiation experiments.
FIG. 3.
Characterization of tissue-derived matrices. (A) Protein gel of tissue digests, collagen type I and II. (B–D) NMR of the tissue digests shows an inhomogeneous tissue profile after the decellularization procedure, reflecting the natural polymers. (E–G) The two peaks that appeared after methacrylation (shaded area, between 5 and 6 ppm) show the presence of vinyl protons, and confirm the success of the methacrylation procedure.
FIG. 4.
Compressive modulus of photo-cross-linked 10% hydrogels (*p<0.05). Nonmethacrylated tissue digests were not crosslinkable and hence their compressive modulus could not be determined (ND). The compressive modulus of cross-linked tissue digests (cartMA, menMA, and tendMA) and GelMA varied significantly. GelMA, gelatin methacrylamide.
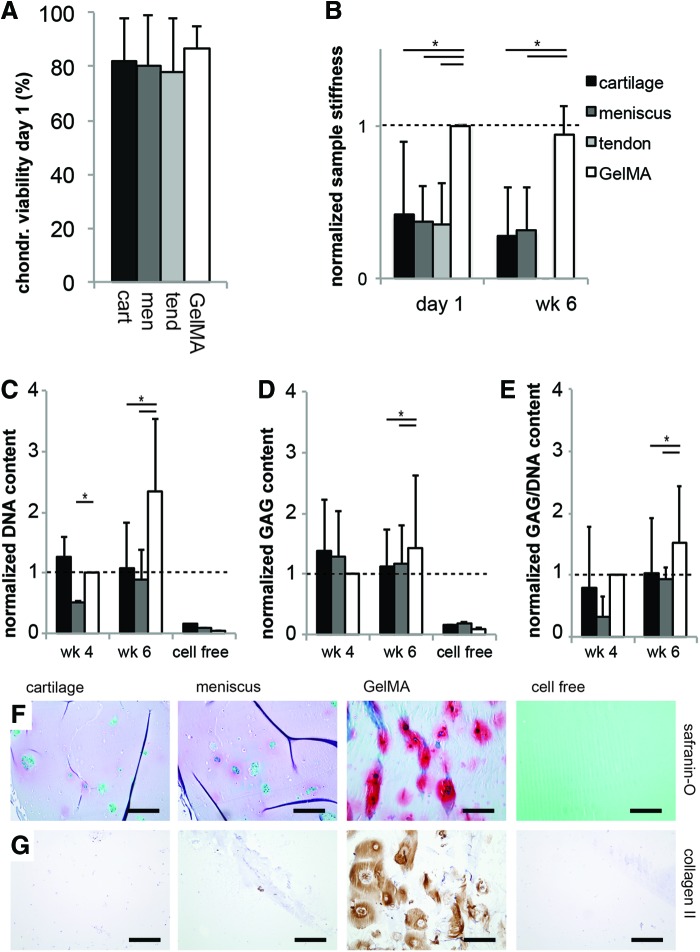
Viability and cartilage matrix formation by embedded chondrocytes
Encapsulation of chondrocytes resulted in high viability at day 1 in all tissue-derived matrix hydrogels (Fig. 5A). During in vitro culture, the tendon samples rapidly disintegrated for all chondrocyte donors and could, therefore, not be included in the differentiation analysis. GelMA gels had a higher stiffness 1 day after cell encapsulation compared to the cartilage, meniscus, and tendon groups (Fig. 5B). A comparable pattern was observed after 6 weeks of culture. The DNA and GAG content and GAG/DNA of hydrogels was comparable for all groups after 4 weeks, but it was significantly higher in the GelMA group compared with cartilage and meniscus after 6 weeks (Fig. 5C–E). The absolute GAG content in the GelMA control samples was 1.17±0.28 μg/mg wet weight after 4 weeks and increased to 2.73±2.13 μg/mg after 6 weeks of culture. GAG and DNA in all experimental groups were significantly higher than the cell free control samples. The superior cartilage-specific matrix formation after 6 weeks in the GelMA group was confirmed by histology for GAGs (Fig. 5F) and immunohistochemistry for collagen type II (Fig. 5G). The differences between the experimental groups at week 4 were comparable to week 6. Therefore, only week 6 data were presented for both cell types. The cell free cartilage gels contained no significant traces of GAGs and collagen type II after 6 weeks.
FIG. 5.
Survival and chondrogenic differentiation of chondrocytes embedded in the tissue-derived matrix gels (*p<0.05). (A) The cartilage, meniscus, and tendon gels were not cytotoxic for embedded chondrocytes; (B) the stiffness of the tissue-derived hydrogels was lower than GelMA directly after encapsulation (day 1) and after 6 weeks of culture; (C–E) the DNA, GAG, and GAG/DNA values after 6 weeks of culture were lower in both experimental groups compared with the GelMA group; (F) GAG formation (red) and (G) collagen type II content (brown) were superior in the GelMA group after 6 weeks. The tendon group disintegrated during culture of chondrocytes and is therefore not displayed for GAG and DNA content. The DNA, GAG, and GAG/DNA content were normalized per donor to GelMA week 4; sample stiffness was normalized to GelMA day 1. All scale bars are 200 μm; “cell free” sample is cartilage gel. Color images available online at www.liebertpub.com/tea
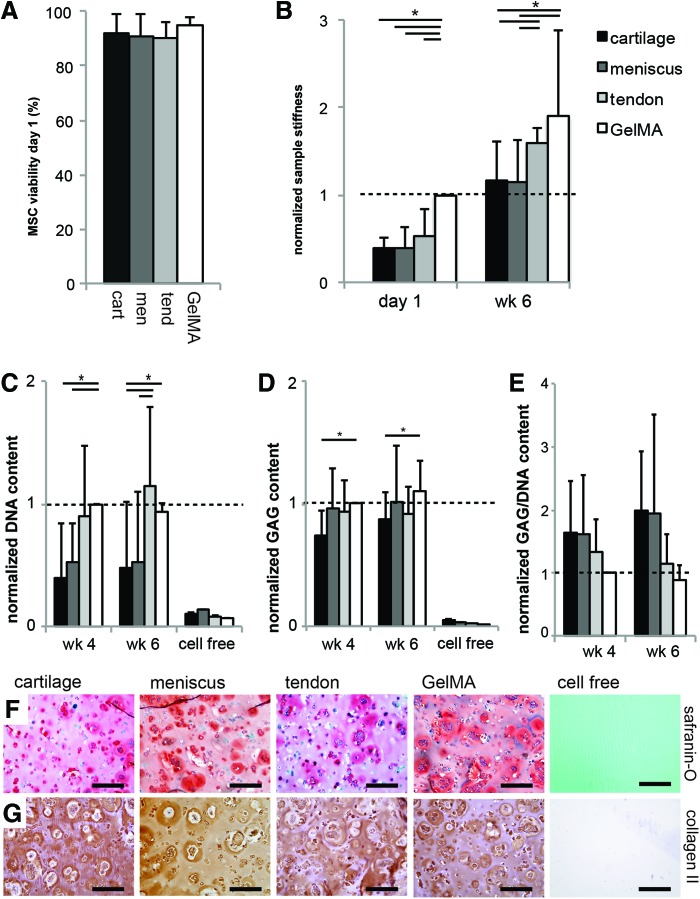
Viability and cartilage matrix formation by embedded MSCs
MSCs in all experimental groups retained a high viability 1 day after encapsulation in the hydrogels (Fig. 6A). The stiffness of GelMA gels was highest 1 day after encapsulation of the MSCs (Fig. 6B). GelMA and tendon gels showed superior stiffness after 6 weeks of cell culture. The DNA content was higher in the tendon and GelMA gels compared to the cartilage and meniscus gels, both after 4 and 6 weeks of culture (Fig. 6C). A comparable amount of GAG was measured for all groups; only the cartilage gels had a statistically lower amount than the GelMA group (Fig. 6D). The absolute GAG content in the GelMA control samples was 3.48±3.62 μg/mg wet weight after 4 weeks and 3.82±4.38 μg/mg after 6 weeks of culture. The GAG/DNA values for the cartilage and meniscus gels were larger than found in the GelMA group (due to a lower DNA content in cartilage and meniscus gels), although these differences were not statistically significant. Staining of GAGs and collagen type II showed abundant cartilage-specific matrix formation by MSCs in all groups (Fig. 6F, G).
FIG. 6.
Survival and chondrogenic differentiation of MSCs embedded in the tissue-derived matrix gels (*p<0.05). (A) The cartilage, meniscus, and tendon gels were not cytotoxic for embedded MSCs; (B) directly after encapsulation of the MSCs (day 1), the stiffness of GelMA samples was highest; after 6 weeks of culture, GelMA and the tendon group were stiffer; (C, D) differences were observed in the DNA and GAG content of the experimental groups; (E) GAG/DNA content was higher in the cartilage and meniscus gels, although this difference was not statistically significant; (F) GAGs (red) and (G) collagen type II (brown) localization in all groups after 6 weeks. The DNA and GAG content were normalized per donor to GelMA week 4; sample stiffness was normalized to GelMA day 1. All scale bars are 200 μm; “cell free” sample is cartilage gel. Color images available online at www.liebertpub.com/tea
Gene expression levels
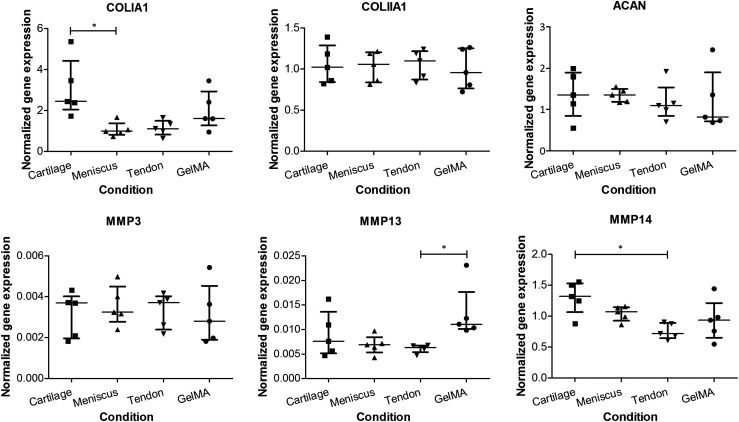
The poor interaction of the chondrocytes with the various tissue cultures (Fig. 5) led to insufficient RNA yields in the cartilage and meniscus groups (the tendon group having disintegrated and not being available for that reason), so that the chondrocytes were excluded from the gene expression analysis. In contrast, the RNA yields of MSCs were sufficient for all samples. ACAN, and COL2 expression was similar for all experimental groups (Fig. 7). The expression of COL1A1 was significantly lower in meniscus than in cartilage gels. Catabolic enzyme MMP-13 and MMP-14 expression was significantly lower in the tendon group compared with GelMA and the cartilage group respectively. These were the only target genes for which a significant difference existed between the experimental conditions.
FIG. 7.
Gene expression by MSCs in tissue-derived matrix gels (*p<0.05). Gene expressions for aggrecan and collagen type II were similar in all hydrogels cultured with MSCs. Collagen I expression was higher in the cartilage hydrogel compared with meniscus. MMP-3 expression was similar in all cultures, whereas expression of MMP-13 and MMP-14 was lower in tendon gels compared with GelMA and cartilage respectively. Separate data points were presented (n=5) including the median and interquartile range.
Discussion
In this study, crosslinkable hydrogels were created, based on matrix derived from cartilage, meniscus, and tendon tissues. These substrates allowed the biological functionalization of the existing GelMA hydrogel system, in which they influenced the quantity and quality of matrix produced by embedded cells. All tissue types negatively influenced outcomes for chondrocytes, whereas encapsulated MSCs exhibited variable differentiation patterns, depending of the type of tissue the matrix gel was derived from.
To our knowledge, this is the first report in which decellularized tissues are covalently cross-linked into stable hydrogels. Previously, the Badylak group has derived hydrogels from the extracellular matrix of the dermis, urinary bladder, and the central nervous system.15–17 These hydrogels allowed the infiltration and adhesion of cells and influenced their differentiation. In addition, hydrogels derived from heart, cartilage, and adipose were recently shown capable of supporting tissue formation by embedded cells.18 Analogous to our work, the hydrogels were obtained by pepsin digestion of the respective tissues. However, only physical cross-linking of the natural polymers was accomplished, resulting in considerable contraction of the hydrogel constructs during culture. This behavior is comparable to other natural polymer networks like collagen gels and fibrin glue.12,35,36 The resulting shape instability, however, is unfavorable for the sustainable filling and regeneration of a tissue defect, especially for load-bearing tissues such as cartilage. We have now successfully overcome this issue by covalent cross-linking of the matrix-derived hydrogels through the addition of methacrylamide groups. These substrates were subsequently incorporated into GelMA hydrogel, because we aimed to further develop this versatile hydrogel, which was proven efficient for cartilage matrix formation by embedded cells.25,28,31 The methacrylation procedure resulted in a very substantial increase in the compressive modulus, which was largest in the cartilage-derived matrix and of the same order of magnitude as occurring in the GelMA reference matrix. The methacrylation procedure can hence be considered as an important asset in the production of extracellular matrices for tissues that have to withstand biomechanical forces.
The chondrogenic potential of MSCs in extracellular matrix gels was greater than that of chondrocytes. MSCs have previously shown capable of forming a cartilage specific matrix in 3D-hydrogel systems.37 The quality and quantity of cartilaginous tissue formed by MSCs was found comparable to that of chondrocytes, although dependent on the type of hydrogel.38 From our work we can conclude that equine MSCs thrive relatively well in GelMA gels. Nevertheless, chondrocytes from the different donors did show less donor variation than the MSCs, which is a known phenomenon for the latter cell type.39 Still, MSCs are of great interest as an allogeneic cell source for the single-stage repair of cartilage tissue.40 A limitation for the application of MSCs in vivo is that they require the addition of growth factors to be directed toward the chondrogenic lineage.33 Recently, chondrocytes have shown they are capable of guiding MSC differentiation, and therefore, a coculture of MSCs and chondrocytes holds promise for the repair of cartilage tissue.40
The absolute GAG production by chondrocytes embedded in the GelMA hydrogels is similar to the amounts reported in previous in vitro studies.28,31 However, a consistent negative effect of the incorporation of all three tissue-derived components was observed on the differentiation of chondrocytes. This is in line with previous reports on the culture of chondrocytes on collagen matrices.20–22 Collagen type II matrix induces catabolic pathways in cultured articular chondrocytes, including the upregulation of MMP-3, MMP-13, and MMP-14.21,22 In addition, earlier work from our group showed poor GAG production of chondrocytes cultured on decellularized cartilage matrix scaffolds (collagen type II) and even accelerated degradation of the scaffolds.20 In the current work, scaffold (i.e., hydrogel) disintegration was observed only for the tendon hydrogels (collagen type I), which may be a result of the catabolic activities of the chondrocytes, analogous to those observed in osteoarthritis.41,42 Unfortunately, we were unable to analyze MMP expression by chondrocytes, since the tendon gels had disintegrated and RNA yield was too low in the cartilage and meniscus groups. In contradiction to these findings, successful cultures of chondrocytes were shown on synthetic collagen type I and II matrices.43–46 The literature is thus inconclusive about the effect of collagen matrices on chondrocyte behavior, although the upregulation of inflammatory pathways has been clearly indicated.
In interpreting cell–matrix interactions, the difference between pure collagen scaffolds and tissue-derived matrices should be taken into account. In the latter, other matrix components, growth factors and/or signaling molecules may contribute to effects on cell behavior.2,4 For example, it was shown that several growth factors were retained in decellularized bladder submucosa matrix47 and small intestinal submucosa matrix.48 The decellularization protocol in this study was relatively mild compared to previous studies.6 Therefore, a panel of growth factors is likely to be retained within the matrix, although this was not specifically studied here. The main components of the tissue-derived matrices were shown to be collagens type I and II, the proportions depending on the original tissue. However, the exact biochemical composition of the polymers, for example, the amount of available reactive amines to be methacrylated, remains unknown. As a result, the degree of methacrylation of the tissue-derived matrices was not controlled, and will hence vary between tissue types and tissue donors. This limitation should be taken into account during the interpretation of the responses of embedded cells.
The absence of trypsin as a protein-cleaving enzyme in the current protocol lead to the preservation of some of the GAGs (∼10%, compared to 0% when treated with trypsin20) while the DNA content could be decreased to acceptable standards, that is, below 50 μg/mg dry tissue.6 Chondrocytes probably benefit more from GAG than collagen components in hydrogel cultures.28,31 Therefore, the current outcomes could be improved by a modified decellularization protocol, preserving still more GAGs.
For the chondrogenic differentiation of MSCs, our results show a slight preference for a collagen type I rich matrix (tendon gels) over collagen type II (cartilage gels). For example, MSCs proliferated more in tendon and GelMA gels and the sample stiffness was higher after culture, compared to meniscus and cartilage gels. In addition, MSCs in the tendon gels showed reduced expression of MMP-13 and MMP-14, although gene expression of cartilage-specific matrix genes was similar. Recently, Yang et al. also analyzed the effects of tendon-derived matrix on MSCs and found a reduced expression of collagen type I, which is considered a marker for inferior fibrocartilage formation.36 The lowest GAG production by MSCs was observed in the cartilage matrix gels, along with a higher gene expression of collagen type I. Previous research, however, revealed a stimulatory effect of cartilage matrix on the chondrogenic differentiation of MSCs in vivo.49,50 In addition, other work showed that chondrogenesis of bovine MSCs was slightly enhanced in collagen type II compared with collagen type I gels.51 It appears that certainly collagens influence the differentiation of MSCs, yet the optimal collagen composition still needs to be unraveled and will require specific tailoring for the target tissue to be engineered.
Further research should identify the specific biological cues in native tissues that are favorable to cells in tissue engineering. Unfortunately, the specific factors responsible for the cell behavior in decellularized tissues are still largely unknown. A better understanding of the native cell niche can guide the process of producing biomimetic materials.
Conclusion
Crosslinkable hydrogels were created from cartilage, meniscus, and tendon tissue through a process that included enzymatic digestion and methacrylation. The methacrylation procedure successfully increased the stiffness of these matrices, which is a critical factor in the manufacturing of scaffolds for use in musculoskeletal tissue repair. Moreover, a stable hydrogel platform was created by covalent incorporation of these tissue-derived matrices in versatile GelMA hydrogels. The response of embedded cells to these matrices depended on the cell type and the matrix components. Chondrocytes performed relatively poor in the tissue-derived matrix gels compared with the GelMA control group. The chondrogenic differentiation capacity of MSCs was comparable across all hydrogels.
Supplementary Material
Acknowledgments
The authors would like to thank Karin van Leersum and Trudy van Ruiten for their assistance in the experimental work. The antibody against collagen type II (II-II6B3), developed by T.F Linsenmayer, was obtained from the DSHB developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. J.V. was supported by a grant from the Dutch government to the Netherlands Institute for Regenerative Medicine (NIRM, Grant No. FES0908) and J.M. was supported by the Dutch Arthritis Foundation.
Disclosure Statement
No competing financial interests exist.
References
- 1.DeForest C.A., and Anseth K.S.Advances in bioactive hydrogels to probe and direct cell fate. Annu Rev Chem Biomol Eng 3,421, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Badylak S.F., Weiss D.J., Caplan A., and Macchiarini P.Engineered whole organs and complex tissues. Lancet 379,943, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Badylak S.F., Freytes D.O., and Gilbert T.W.Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater 5,1, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Badylak S.F., Brown B.N., Gilbert T.W., Daly K.A., Huber A., and Turner N.J.Biologic scaffolds for constructive tissue remodeling. Biomaterials 32,316, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Benders K.E., van Weeren P.R., Badylak S.F., Saris D.B., Dhert W.J., and Malda J.Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol 31,169, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Crapo P.M., Gilbert T.W., and Badylak S.F.An overview of tissue and whole organ decellularization processes. Biomaterials 32,3233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiller K.L., Maher S.A., and Lowman A.M.Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev 17,281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huey D.J., Hu J.C., and Athanasiou K.A.Unlike bone, cartilage regeneration remains elusive. Science 338,917, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malda J., Visser J., Melchels F.P., Jungst T., Hennink W.E., Dhert W.J., Groll J., and Hutmacher D.W.25th anniversary article: engineering hydrogels for biofabrication. Adv Mater 25,5011, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Mastbergen S.C., Saris D.B., and Lafeber F.P.Functional articular cartilage repair: here, near, or is the best approach not yet clear? Nat Rev Rheumatol 9,277, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Deponti D., Di Giancamillo A., Mangiavini L., Pozzi A., Fraschini G., Sosio C., Domeneghini C., and Peretti G.M.Fibrin-based model for cartilage regeneration: tissue maturation from in vitro to in vivo. Tissue Eng Part A 18,1109, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Homminga G.N., Buma P., Koot H.W., van der Kraan P.M., and van den Berg W.B.Chondrocyte behavior in fibrin glue in vitro. Acta Orthop Scand 64,441, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Moreira Teixeira L.S., Leijten J.C., Wennink J.W., Chatterjea A.G., Feijen J., van Blitterswijk C.A., Dijkstra P.J., and Karperien M.The effect of platelet lysate supplementation of a dextran-based hydrogel on cartilage formation. Biomaterials 33,3651, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen L.H., Kudva A.K., Guckert N.L., Linse K.D., and Roy K.Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage. Biomaterials 32,1327, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Freytes D.O., Martin J., Velankar S.S., Lee A.S., and Badylak S.F.Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 29,1630, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Wolf M.T., Daly K.A., Brennan-Pierce E.P., Johnson S.A., Carruthers C.A., D'Amore A., Nagarkar S.P., Velankar S.S., and Badylak S.F.A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 33,7028, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medberry C.J., Crapo P.M., Siu B.F., Carruthers C.A., Wolf M.T., Nagarkar S.P., Agrawal V., Jones K.E., Kelly J., Johnson S.A., Velankar S.S., Watkins S.C., Modo M., and Badylak S.F.Hydrogels derived from central nervous system extracellular matrix. Biomaterials 34,1033, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pati F., Jang J., Ha D.H., Won Kim S., Rhie J.W., Shim J.H., Kim D.H., and Cho D.W.Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 5,3935, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mescher A.L.Junqueira's Basic Histology: Text and Atlas, 12th edition. New York, NY: The McGraw-Hill Companies, 2010 [Google Scholar]
- 20.Benders K.E.M., Boot W., van Weeren P.R., Gawlitta D., Bergman H.J., Saris D.B.F., Dhert W.J.A., and Malda J.Multipotent stromal cells outperform chondrocytes on cartilage-derived matrix scaffolds. Cartilage 5,221, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klatt A.R., Paul-Klausch B., Klinger G., Kuhn G., Renno J.H., Banerjee M., Malchau G., and Wielckens K.A critical role for collagen II in cartilage matrix degradation: collagen II induces pro-inflammatory cytokines and MMPs in primary human chondrocytes. J Orthop Res 27,65, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Fichter M., Korner U., Schomburg J., Jennings L., Cole A.A., and Mollenhauer J.Collagen degradation products modulate matrix metalloproteinase expression in cultured articular chondrocytes. J Orthop Res 24,63, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Delcogliano M., de Caro F., Scaravella E., Ziveri G., De Biase C.F., Marotta D., Marenghi P., and Delcogliano A.Use of innovative biomimetic scaffold in the treatment for large osteochondral lesions of the knee. Knee Surg Sports Traumatol Arthrosc 22,1260, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Zheng M.H., Willers C., Kirilak L., Yates P., Xu J., Wood D., and Shimmin A.Matrix-induced autologous chondrocyte implantation (MACI): biological and histological assessment. Tissue Eng 13,737, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Boere K.W., Visser J., Seyednejad H., Rahimian S., Gawlitta D., van Steenbergen M.J., Dhert W.J., Hennink W.E., Vermonden T., and Malda J.Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomater 10,2602, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Nikkhah M., Eshak N., Zorlutuna P., Annabi N., Castello M., Kim K., Dolatshahi-Pirouz A., Edalat F., Bae H., Yang Y., and Khademhosseini A.Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials 33,9009, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nichol J.W., Koshy S.T., Bae H., Hwang C.M., Yamanlar S., and Khademhosseini A.Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31,5536, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levett P.A., Melchels F.P., Schrobback K., Hutmacher D.W., Malda J., and Klein T.J.A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater 10,214, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Piper D.W., and Fenton B.H.Ph stability and activity curves of pepsin with special reference to their clinical importance. Gut 6,506, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Den Bulcke A.I., Bogdanov B., De Rooze N., Schacht E.H., Cornelissen M., and Berghmans H.Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 1,31, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Schuurman W., Levett P.A., Pot M.W., van Weeren P.R., Dhert W.J., Hutmacher D.W., Melchels F.P., Klein T.J., and Malda J.Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol Biosci 13,551, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Gawlitta D., Farrell E., Malda J., Creemers L.B., Alblas J., and Dhert W.J.Modulating endochondral ossification of multipotent stromal cells for bone regeneration. Tissue Eng Part B Rev 16,385, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., and Marshak D.R.Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Chomczynski P., and Mackey K.Substitution of chloroform by bromochloropropane in the single-step method of Rna isolation. Anal Biochem 225,163, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Fujiwara T., Kubo T., Kanazawa S., Shingaki K., Taniguchi M., Matsuzaki S., Gurtner G.C., Tohyama M., and Hosokawa K.Direct contact of fibroblasts with neuronal processes promotes differentiation to myofibroblasts and induces contraction of collagen matrix in vitro. Wound Repair Regen 21,588, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Yang G., Rothrauff B.B., Lin H., Gottardi R., Alexander P.G., and Tuan R.S.Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials 34,9295, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams C.G., Kim T.K., Taboas A., Malik A., Manson P., and Elisseeff J.In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng 9,679, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Erickson I.E., Huang A.H., Chung C., Li R.T., Burdick J.A., and Mauck R.L.Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A 15,1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter-Arnold J.L., Neilsen N.L., Amelse L.L., Odoi A., and Dhar M.S.In vitro analysis of equine, bone marrow-derived mesenchymal stem cells demonstrates differences within age- and gender-matched horses. Equine Vet J 46,589, 2014 [DOI] [PubMed] [Google Scholar]
- 40.de Windt T.S., Hendriks J.A., Zhao X., Vonk L.A., Creemers L.B., Dhert W.J., Randolph M.A., and Saris D.B.Concise review: unraveling stem cell cocultures in regenerative medicine: which cell interactions steer cartilage regeneration and how? Stem Cells Transl Med 3,723, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu L., Peng H., Glasson S., Lee P.L., Hu K., Ijiri K., Olsen B.R., Goldring M.B., and Li Y.Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum 56,2663, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y., Su J., Yu J., Bu X., Ren T., Liu X., and Yao L.An essential role of discoidin domain receptor 2 (DDR2) in osteoblast differentiation and chondrocyte maturation via modulation of Runx2 activation. J Bone Miner Res 26,604, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Rutgers M., Saris D.B., Vonk L.A., van Rijen M.H., Akrum V., Langeveld D., van Boxtel A., Dhert W.J., and Creemers L.B.Effect of collagen type I or type II on chondrogenesis by cultured human articular chondrocytes. Tissue Eng Part A 19,59, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Schwarz S., Elsaesser A.F., Koerber L., Goldberg-Bockhorn E., Seitz A.M., Bermueller C., Durselen L., Ignatius A., Breiter R., and Rotter N. Processed xenogenic cartilage as innovative biomatrix for cartilage tissue engineering: effects on chondrocyte differentiation and function. J Tissue Eng Regen Med, 2012. doi: 10.1002/term.1650 [DOI] [PubMed] [Google Scholar]
- 45.Nehrer S., Breinan H.A., Ramappa A., Shortkroff S., Young G., Minas T., Sledge C.B., Yannas I.V., and Spector M.Canine chondrocytes seeded in type I and type II collagen implants investigated in vitro. J Biomed Mater Res 38,95, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Nehrer S., Breinan H.A., Ramappa A., Young G., Shortkroff S., Louie L.K., Sledge C.B., Yannas I.V., and Spector M.Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials 18,769, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Chun S.Y., Lim G.J., Kwon T.G., Kwak E.K., Kim B.W., Atala A., and Yoo J.J.Identification and characterization of bioactive factors in bladder submucosa matrix. Biomaterials 28,4251, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Voytik-Harbin S.L., Brightman A.O., Kraine M.R., Waisner B., and Badylak S.F.Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem 67,478, 1997 [PubMed] [Google Scholar]
- 49.Chen C.C., Liao C.H., Wang Y.H., Hsu Y.M., Huang S.H., Chang C.H., and Fang H.W.Cartilage fragments from osteoarthritic knee promote chondrogenesis of mesenchymal stem cells without exogenous growth factor induction. J Orthop Res 30,393, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Xue J.X., Gong Y.Y., Zhou G.D., Liu W., Cao Y., and Zhang W.J.Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells induced by acellular cartilage sheets. Biomaterials 33,5832, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Bosnakovski D., Mizuno M., Kim G., Takagi S., Okumura M., and Fujinaga T.Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng 93,1152, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.