Abstract
Background:
Hodgkin lymphoma (HL) survivors are at increased risk for developing valvular heart disease (VHD). We evaluated the determinants of the risk and the radiation dose-response.
Methods:
A case-control study was nested in a cohort of 1852 five-year HL survivors diagnosed at ages 15 to 41 years and treated between 1965 and 1995. Case patients had VHD of at least moderate severity as their first cardiovascular diagnosis following HL treatment. Control patients were matched to case patients for age, gender, and HL diagnosis date. Treatment and follow-up data were abstracted from medical records. Radiation doses to heart valves were estimated by reconstruction of individual treatments on representative computed tomography datasets. All statistical tests were two-sided.
Results:
Eighty-nine case patients with VHD were identified (66 severe or life-threatening) and 200 control patients. Aortic (n = 63) and mitral valves (n = 42) were most frequently affected. Risks increased more than linearly with radiation dose. For doses to the affected valve(s) of less than or equal to 30, 31–35, 36–40, and more than 40 Gy, VHD rates increased by factors of 1.4, 3.1, 5.4, and 11.8, respectively (P trend < .001). Approximate 30-year cumulative risks were 3.0%, 6.4%, 9.3%, and 12.4% for the same dose categories. VHD rate increased with splenectomy by a factor of 2.3 (P = .02).
Conclusions:
Radiation dose to the heart valves can increase the risk for clinically significant VHD, especially at doses above 30 Gy. However, for patients with mediastinal involvement treated today with 20 or 30 Gy, the 30-year risk will be increased by only about 1.4%. These findings may be useful for patients and doctors both before treatment and during follow-up.
Hodgkin lymphoma (HL) is an archetype of curable malignancy. HL treatment has improved over the last 50 years, leading to a 10-year survival rate of over 80%. However, it is well documented that radiotherapy (RT), as given in the past for HL, is an important cause of increased cardiovascular mortality and morbidity in long-term survivors (1–8). Increased recognition of this risk and combination treatment including both chemotherapy and RT has led to a reduction in doses and volumes of RT used for HL (9). Nevertheless, RT may still result in substantial incidental cardiac exposure if the disease affects the mediastinum (10). Thus, whilst it is thought that the risk of radiation-related heart disease is now lower than for patients treated in the past, the magnitude of the risk for HL patients treated today is unknown.
Recent studies in breast cancer patients have demonstrated a dose-response relationship for radiation-related ischaemic heart disease (11). Valvular heart disease (VHD) is also increased following cardiac irradiation (2,8,12–14). Recent screening studies in HL survivors have reported that 32% of those given mediastinal irradiation developed asymptomatic valvular defects after six years (15), whilst at 20 years 42% had imaging evidence of valvular dysfunction (16). The dose-response relationship for radiation-related VHD is, however, still unknown, and there have been few studies of other risk factors for VHD. The aims of this study were, therefore, to estimate the dose-response relationship for radiation-related VHD and to evaluate other possible determinants of VHD risk in order to assess risk for patients whose treatment involves incidental irradiation of the heart.
Methods
Study Population
A cohort of 1852 five-year survivors of HL treated between 1965 and 1995 was identified through hospital-based cancer registries at The Netherlands Cancer Institute, Amsterdam (NKI-AVL), The Erasmus MC-Daniel den Hoed Cancer Center (DdHK), Rotterdam, and Leiden University Medical Center (LUMC). Details are given elsewhere (1–3,17,18). Patients were included if they: 1) had a histologically confirmed diagnosis of HL when age 15 to 41 years, 2) had no other malignancy (except nonmelanoma skin cancer or carcinoma in situ of breast or cervix) prior to HL, and 3) survived five or more years after HL diagnosis. Follow-up was 98% complete as of January 1, 2002; 54% were followed to 2005 or later.
For the present study, cohort members diagnosed with VHD were identified initially from their hospital records or a postal questionnaire completed by their general practitioner. Case patients were individuals subsequently confirmed by further correspondence with their general practitioner and/or cardiologist to have at least moderately severe VHD (defined by Common Terminology Criteria for Adverse Events [CTCAE] version 4.0, grade ≥2 [19]) as their first diagnosis of clinically significant heart disease after HL diagnosis.
For each case with VHD, three control patients were selected from cohort members, matched on sex, age at HL diagnosis (≤1 year), and date of HL diagnosis (≤3 years). Control patients were free of any cardiac disease of grade 2 or higher on a cutoff date, defined as the date of HL diagnosis plus a time interval equal to the interval from the date of HL diagnosis to the date of VHD diagnosis for the matched case. If fewer than two control patients were found for a case, the matching criteria were relaxed until two were found, up to a maximum difference of four years for age at HL diagnosis and five years for date of diagnosis. Control patients were selected with replacement, so some individuals were a control patient for more than one case patient. The study protocol was approved by the ethics review board of the NKI-AVL.
Data Collection
Details of medical history, HL treatment at diagnosis, and any relapse, cumulative doses of cytotoxic drugs, second malignancies, cardiovascular diseases, and cardiovascular risk factors both at diagnosis of HL and at diagnosis of VHD (index date for control patients) were abstracted from medical records for case patients and two control patients. Where data had already been abstracted for a third matched control patient for use in other studies, this was also included.
Copies of original RT prescription sheets and planning and verification x-rays were obtained. Where original prescriptions were unavailable, information about RT including dates, anatomical areas, dose, fractionation, and treatment energy were abstracted from clinical notes.
Retrospective Radiation Dosimetry Methods
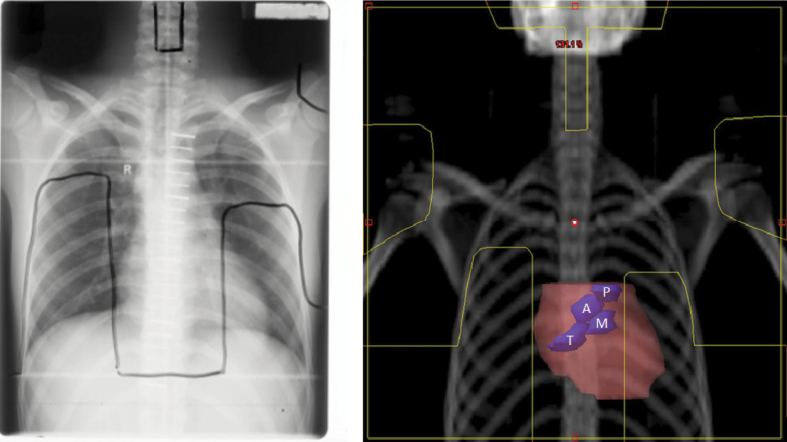
RT regimens were reconstructed using the Eclipse treatment planning system (Version 10.0.28, Varian Medical System, Palo Alto, CA). Two substitute computed tomography (CT) dataset (for men and women, respectively) were chosen from a library of 50 to be representative regarding anatomy and estimated heart dose from a standardized mantle field. Heart and heart valves were outlined as per published guidelines (20). Treatment fields, shielding, prescription points and delivered doses were reproduced from original RT prescriptions and planning x-rays (Figure 1). Dose distributions were calculated using the anisotropic analytic algorithm for megavoltage (MV) treatments and the pencil beam algorithm for cobalt-60 treatments. To simulate the original prescription, dose distributions were calculated initially with the homogeneity correction inactivated to assess delivered dose and then recalculated with the correction reactivated to estimate dose distribution more accurately. Doses were converted to equivalent dose in 2 Gray (Gy) fractions (EQD2) (21) calculated from dose volume histograms, assuming an alpha-beta ratio of two for late cardiac effects (22). When fraction size varied during treatment, EQD2 was calculated separately for each fraction size and then summed. Doses were estimated for the affected valve for case patients and the corresponding valve for control patients. There was only trivial variation in dose across a valve, therefore mean valvular dose was used for analyses.
Figure 1.
An example of an original simulation film used during the 1960s to 1990s to plan radiotherapy for Hodgkin lymphoma, with field borders marked in black (left). The same field has been reconstructed within a modern radiotherapy treatment planning system on a substitute CT dataset with the field overlaid on a digitally reconstructed radiograph, with the field borders marked in yellow (right). The heart (pink) and the heart valves (blue) are outlined to demonstrate their position. A = aortic valve; M = mitral valve; P = pulmonary valve; T = tricuspid valve.
Statistical Analysis
Cumulative risk of VHD in the cohort was calculated treating patients as censored when they developed another heart disease and death as a competing risk. Ninety-five percent confidence intervals (CIs) for cumulative risk were derived using the delta method (23). Incidence rate ratios (RRs) for VHD for different levels of each factor were calculated using logistic regression conditional on sets of individual case patients and their matched control patients. Ninety-five percent confidence intervals for factors with two levels used the Wald method. Ninety-five percent confidence intervals for factors with two or more levels used the amount of information in each category, including the reference category (24). The dose-response was estimated by modelling VHD rate as Κ m(1+βd), where d is dose to the valve of an individual patient, Κ m is a constant specific to each matched set, and β is the proportional increase in VHD rate per unit increase in dose. Nonlinearity was evaluated by including an exponential term: Κ m[1+βd.exp(δd)] and goodness of fit assessed by likelihood ratio test. Multiple regression was used to control for confounding and to assess interactions between radiation dose and other factors. Approximate cumulative risks of VHD for categories of radiation dose were estimated from the VHD rate ratios together with the cumulative risk of VHD for the entire cohort, assuming that the distribution of all individuals in the cohort across the dose categories was equal to that for the control patients. Ninety-five percent confidence intervals were calculated from the profile-likelihood. Statistical tests were two-sided, and P values of less than or equal to .05 were considered stastically significant. Analyses were performed using STATA statistical software version 11.0 (STATAcorp 2009) (25) and Epicure version 1.8 (Hirosoft International) (26).
Results
General
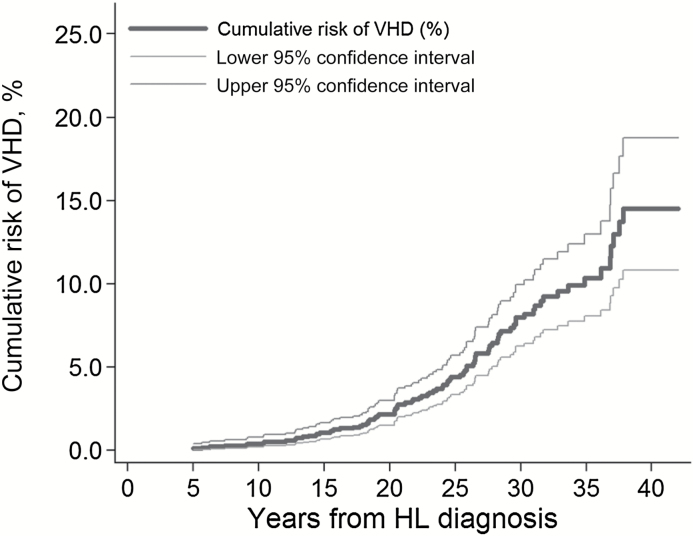
The cohort of 1852 five-year HL survivors was followed for a median of 18.8 years (interquartile range = 13.1–25.6). Eighty-three-point-three percent received mediastinal RT. A total of 89 patients had VHD of at least moderate severity as their first cardiovascular diagnosis, most commonly affecting the aortic (n = 63) and/or mitral (n = 42) valves (Table 1). The median interval between HL diagnosis and VHD diagnosis was 23.3 years, and at 30 years after HL diagnosis the cumulative risk of VHD was 8.0% (95% CI = 6.2% to 10.0%) (Figure 2). The median follow-up after VHD diagnosis was 7.6 years, during which time 56% progressed in grade of severity. Twenty-two of the 89 case patients died from cardiac causes and a further 17 from other causes. Two hundred control patients were included in the case-control study (Table 2), and the length of follow-up was virtually identical for case patients and control patients (Supplementary Table 1, available online).
Table 1.
Characteristics of the cases of valvular heart disease (VHD) recorded as a first cardiac diagnosis, according to the Common Terminology Criteria for Adverse Events (CTAE) version 4 (19), treatments given, and status at the end of follow-up
| Characteristic | Men, No. (%) | Women, No. (%) | Total patients, No. (%) |
|---|---|---|---|
| First valvular defect | |||
| Aortic | 22 (73.3) | 23 (39.0) | 45 (50.6) |
| Mitral | 3 (10.0) | 19 (32.2) | 22 (24.7) |
| Aortic + mitral | 2 (6.7) | 10 (17.0) | 12 (13.5) |
| Aortic + tricuspid | 1 (3.3) | 0 (0.0) | 1 (1.1) |
| Mitral + tricuspid | 0 (0.0) | 3 (5.1) | 3 (3.4) |
| Aortic + mitral + tricuspid | 1 (3.3) | 4 (6.8) | 5 (5.6) |
| Unknown | 1 (3.3) | 0 (0.0) | 1 (1.1) |
| Initial CTCAE grade of VHD | |||
| Grade I | 6 (20.0) | 17 (28.8) | 23 (25.8) |
| Grade II | 16 (53.3) | 25 (42.4) | 41 (46.1) |
| Grade III | 5 (20.0) | 15 (28.8) | 20 (22.5) |
| Grade IV | 1 (1.1) | 2 (2.3) | 3 (3.4) |
| Unknown | 2 (2.3) | - | 2 (2.3) |
| Progression of valve defect | |||
| No | 13 (43.4) | 26 (44.1) | 39 (43.8) |
| Yes | 17 (56.7) | 33 (55.9) | 50 (56.2) |
| Highest CTCAE grade of VHD | |||
| Grade II | 7 (23.3) | 16 (27.1) | 23 (25.8) |
| Grade III | 3 (10.0) | 15 (25.4) | 18 (20.2) |
| Grade IV | 20 (66.7) | 28 (47.5) | 48 (53.9) |
| Basis for Diagnosis of VHD | |||
| Echocardiography | 13 (43.3) | 42 (71.2) | 55 (61.8) |
| Heart catheterisation | 4 (13.3) | 1 (1.7) | 5 (5.6) |
| Both of above | 5 (16.7) | 2 (3.4) | 7 (7.9) |
| Unknown* | 8 (26.7) | 14 (23.7) | 22 (24.7) |
| Treatment for VHD | |||
| Drug therapy | 3 (10.0) | 14 (23.7) | 17 (19.1) |
| Valve replacement or repair | 15 (50.0) | 10 (17.0) | 25 (28.1) |
| Both of above | 3 (10.0) | 2 (3.4) | 5 (5.6) |
| None | 7 (23.3) | 30 (50.9) | 37 (41.6) |
| Unknown | 2 (6.7) | 3 (5.1) | 5 (5.6) |
| Status at end of follow-up | |||
| Alive | 17 (56.7) | 33 (55.9) | 50 (56.2) |
| Dead of cardiac cause† | 8 (26.7) | 14 (23.7) | 22 (24.7) |
| Dead of other cause‡ | 5 (16.7) | 12 (20.3) | 17 (19.1) |
| Totals | 30 (100.0) | 59 (100.0) | 89 (100.0) |
* For these patients 10 had valve replacement or repair, five received drug therapy, five received no treatment, and for two treatments was unknown.
† Congestive heart failure (5), ischaemic heart disease (4), sudden cardiac death (4), valvular heart disease (3), endocarditis (3), other/unspecified cardiac death (3).
‡ Second malignancy (10), other non-neoplastic cause (6), unknown (1).
Figure 2.
Cumulative risk of valvular heart disease as (VHD) a first cardiac diagnosis among five-year survivors of Hodgkin lymphoma (HL) by years since initial HL diagnosis. Cumulative risk was calculated treating patients as censored when they developed another heart disease and death as a competing risk.
Table 2.
Distribution of case patients and control patients who were initially selected for the case-control study by final study status*
| Status within study | Case patients | Control patients |
|---|---|---|
| No. (%) | No. (%) | |
| Initially selected | 143 (100.0) | 429 (100.0) |
| Ineligible | 43 (30.7) | 27 (6.3) |
| Prior cardiovascular diagnosis | 15 (10.5) | 7 (1.6) |
| No VHD | 7 (4.9) | – |
| Grade 1 VHD only | 15 (10.5) | – |
| Medical record lost | 3 (2.1) | 4 (0.9) |
| Lost to follow-up | – | 5 (1.2) |
| Cutoff date changed | – | 7 (1.6) |
| Other reason | 3 (2.1) | 4 (0.9) |
| Selected as control, case excluded | 129 (30.1) | |
| Selected as third control, not used | 55 (12.8) | |
| Administrative reasons for exclusion | 11 (7.7) | 18 (4.2) |
| Case unconfirmed | 8 (5.6) | 15 (3.5) |
| Case without controls | 3 (2.1) | – |
| Control not abstracted | – | 2 (0.5) |
| Control abreacted twice | – | 1 (0.2) |
| Total excluded | 54 (37.8) | 229 (53.4) |
| Total included in study | 89 (62.2) | 200 (46.6) |
| Duplicate controls | 43 (21.5) | |
| Case as control | 13 (6.5) | |
| Once | 12 (6) | |
| Twice | 1 (0.5) | |
| Control as control | 24 (12) | |
| Once | 20 (10) | |
| Twice | 3 (1.5) | |
| Three times | 1 (0.5) | |
| Total number of unique individuals in study | 89 (100.0) | 157 (78.5) |
* VHD = valvular heart disease.
Patient-Related Risk Factors
The total number of cardiovascular risk factors recorded was associated with an increased VHD rate (P trend = .002) (Table 3). When the risk factors were considered individually, obesity at HL diagnosis (RR = 2.1, 95% CI = 1.1 to 3.8, P = .02), hypertension at end of follow-up (RR = 2.0, 95% CI = 1.1 to 3.5, P = .02), and hypercholesterolaemia at end of follow-up (RR = 4.9, 95% CI = 2.6 to 9.1, P < .001) were associated with an increase in VHD. When these factors were considered simultaneously, the associations with obesity at HL diagnosis and hypercholesterolaemia at end of follow-up changed little in magnitude and remained statistically significant, but the association with hypertension at end of follow-up was smaller and no longer statistically significant.
Table 3.
Patient-related cardiovascular risk factors in five-year survivors of Hodgkin lymphoma (HL) who were subsequently diagnosed with valvular heart disease (VHD) as their first cardiac diagnosis (case patients) and in those with no diagnosis of cardiac disease (control patients), and association between these factors and the rate of development of VHD
| Patient-related factor | Case patients/ Control patients | RR* (95% CI) | P | RR with additional adjustment† (95% CI) | P |
|---|---|---|---|---|---|
| Obesity at HL diagnosis | |||||
| No‡ | 60/160 | 1.0 | 1.0 | ||
| Yes | 27/36 | 2.1 (1.1 to 3.8) | .02 | 2.0 (1.0 to 4.0) | .05 |
| Unknown | 2/4 | - | |||
| Obesity at end of FU | |||||
| No‡ | 55/124 | 1.0 | 1.0 | ||
| Yes | 31/58 | 1.2 (0.7 to 2.1) | .60 | 0.8 (0.4 to 1.6) | .62 |
| Unknown | 3/18 | - | - | ||
| Smoker at HL diagnosis | |||||
| No‡ | 44/96 | 1.0 | 1.0 | ||
| Yes | 40/101 | 0.8 (0.5 to 1.4) | .40 | 0.9 (0.5 to 1.7) | .81 |
| Unknown | 5/3 | - | - | ||
| Smoker at end of FU | |||||
| No‡ | 73/146 | 1.0 | 1.0 | ||
| Yes | 12/33 | 0.7 (0.3 to 1.5) | .40 | 0.6 (0.2 to 1.5) | .28 |
| Unknown | 4/21 | - | - | ||
| Diabetes at end of FU | |||||
| No‡ | 78/170 | 1.0 | 1.0 | ||
| Yes | 11/23 | 1.1 (0.5 to 2.5) | .76 | 1.2 (0.5 to 2.9) | .65 |
| Unknown | 0/7 | - | - | ||
| Hypertension at end of FU | |||||
| No‡ | 58/152 | 1.0 | 1.0 | ||
| Yes | 31/43 | 2.0 (1.1 to 3.5) | .02 | 1.3 (0.7 to 2.6) | .39 |
| Unknown | 0/5 | - | - | ||
| Hypercholesterolaemia at end of FU | |||||
| No‡ | 49/168 | 1.0 | 1.0 | ||
| Yes | 40/26 | 4.9 (2.6 to 9.1) | <.001 | 5.0 (2.6 to 9.5) | <.001 |
| Unknown | 0/6 | - | - | ||
| Number of factors | |||||
| 0 | 12/38 | 1.0 (0.5 to 1.9) | - | ||
| 1 | 28/86 | 1.0 (0.7 to 1.6) | - | ||
| 2 | 25/52 | 1.5 (0.9 to 2.4) | - | ||
| 3 | 17/19 | 3.1 (1.5 to 6.3) | - | ||
| 4/5 | 7/5 | 3.7 (1.2 to 11.5) | .002§ | - | - |
* Rate ratios for the rate of development of valvular heart disease calculated conditional on matched sets. Matching variables were gender, age at HL diagnosis and date of HL diagnosis (Supplementary Table 2, available online). CI = confidence interval; FU = follow-up.
† Additional adjustments were: hypercholesterolaemia at end of FU for obesity at HL diagnosis, obesity at HL diagnosis for hypercholesterolaemia at end of FU, and both obesity at HL diagnosis and hypercholesterolaemia at end of FU for other factors.
‡ Reference category.
§ P trend (all other tests are for heterogeneity). All statistical tests were two-sided.
Radiation Dose
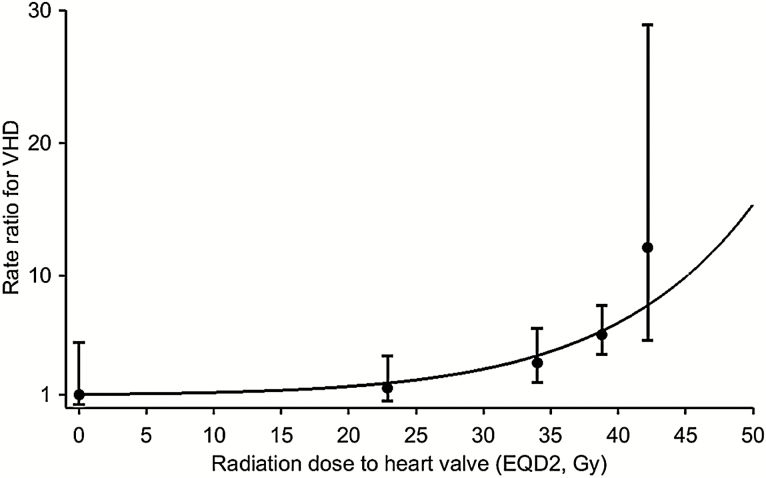
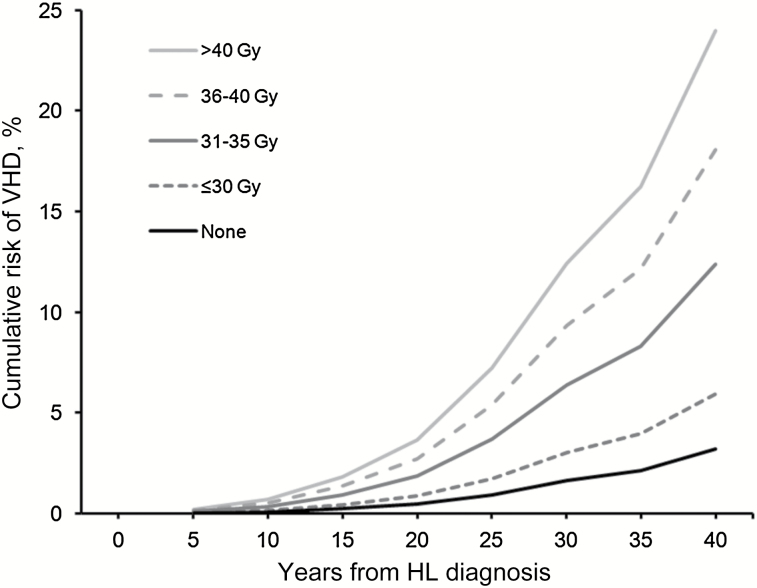
The VHD rate was more closely related to the dose to the affected valve (P trend < .001) than to the prescribed mediastinal dose (P trend = .003) (Table 4). The average of the mean doses to the affected heart valve in case patients was 37.0 Gy (SD = 9.2), compared with only 30.7 Gy (SD = 13.8) for control patients (P difference = .001). For doses to the affected valve(s) of less than or equal to 30, 31–35, 36–40, and more than 40 Gy, VHD rates increased by factors of 1.4, 3.1, 5.4, and 11.8, respectively. There were larger percentages of control patients than case patients in dose categories 0, up to 10, 10–19, 20–30, and 31–35 Gy and larger percentages of case patients than control patients in categories 36–40 and more than 40 Gy (Supplementary Figure 1, available online). The dose-response relationship was nonlinear (P nonlinearity = .03) (Figure 3). For doses to the affected valve of less than or equal to 30 Gy, the VHD rate increased by only 2.5% (95% CI = -2.2% to 29.2%) per Gy, but it increased by 6.5% (95% CI = -0.2% to 42.3%), 11.2% (95% CI = 1.8% to 59.8%), and 24.3% (95% CI = 4.6% to 139.5%) per Gy, respectively, for doses of 31–35 Gy, 36–40 Gy, and more than 40 Gy. Approximate 30-year cumulative risks of VHD were 1.6%, 3.0%, 6.4%, 9.3%, and 12.4% for individuals with radiation doses of 0, less than or equal to 30, 31–35, 36–40, and more than 40 Gy to the affected valve, respectively (Figure 4).
Table 4.
Treatment-related factors in five-year survivors of Hodgkin lymphoma (HL) who were subsequently diagnosed with valvular heart disease (VHD) as a first cardiac diagnosis (case patients) and in those with no diagnosis of cardiac disease (control patients), and association between these factors and the rate of development of VHD
| Treatment-related factor | Case patients/ Control patients | RR* (95% CI) | P | RR with additional adjustment† (95% CI) | P |
|---|---|---|---|---|---|
| Treatment centre | |||||
| NKI-AVL | 35/85 | 1.0 (0.7 to 1.5) | - | ||
| DdHK | 37/81 | 1.2 (0.8 to 1.8) | - | ||
| LUMC | 17/34 | 1.2 (0.7 to 2.2) | .83 | - | - |
| Prescribed mediastinal radiation dose (EQD2) | |||||
| No mediastinal RT | 3/28 | 1.0 (0.3 to 3.3) | 1.0 (0.3 to 3.5) | ||
| ≤30 Gy (median 25.2 Gy) | 2/11 | 0.7 (0.1 to 5.6) | 0.6 (0.1 to 4.7) | ||
| 31–35 Gy (median 35.0 Gy) | 17/41 | 3.4 (2.0 to 6.1) | 3.1 (1.7 to 5.7) | ||
| 36–40 Gy (median 40.0 Gy) | 58/109 | 4.5 (3.2 to 6.2) | 4.1 (2.9 to 5.6) | ||
| >40 Gy (median 42.0 Gy) | 3/8 | 3.9 (1.0 to 14.7) | .003§ | 4.6 (1.2 to 18.5) | .005§ |
| Unknown | 6/3 | - | |||
| Estimated radiation dose to affected valve (EQD2) | |||||
| No mediastinal RT | 2/18 | 1.0 (0.2 to 4.9) | 1.0 (0.2 to 4.8) | ||
| ≤30 Gy (median 22.9 Gy) | 5/33 | 1.5 (0.5 to 3.9) | 1.4 (0.5 to 3.8) | ||
| 31–35 Gy (median 34.0 Gy) | 16/51 | 3.4 (1.9 to 6.0) | 3.1 (1.7 to 5.6) | ||
| 36–40 Gy (median 38.8 Gy) | 45/82 | 5.5 (4.0 to 7.7) | 5.4 (3.9 to 7.7) | ||
| >40 Gy (median 42.2 Gy) | 15/13 | 12.1 (5.1 to 28.9) | <.001§ | 11.8 (4.9 to 28.5) | <.001§ |
| Unknown | 6/3 | - | |||
| Any chemotherapy | |||||
| No‡ | 49/71 | 1.0 | 1.0 | ||
| Yes | 40/129 | 0.7 (0.4 to 1.1) | .12 | 1.3 (0.7 to 2.5) | .38 |
| Anthracycline | |||||
| No‡ | 74/173 | 1.0 | 1.0 | ||
| Yes | 15/27 | 1.4 (0.5 to 3.7) | .55 | 1.2 (0.3 to 4.3) | .79 |
| Vincristine | |||||
| No‡ | 62/105 | 1.0 | 1.0 | ||
| Yes | 27/95 | 0.4 (0.2 to 0.7) | .003 | 0.6 (0.3 to 1.1) | .11 |
| Procarbazine | |||||
| No‡ | 59/104 | 1.0 | 1.0 | ||
| Yes | 30/96 | 0.5 (0.3 to 0.9) | .02 | 0.7 (0.3 to 1.4) | .28 |
| Splenectomy after HL diagnosis | |||||
| No‡ | 45/133 | 1.0 | 1.0 | ||
| Yes | 44/67 | 2.3 (1.3 to 4.3) | .007 | 2.3 (1.1 to 4.5) | .02 |
| Any salvage treatment for HL | |||||
| No‡ | 69/152 | 1.0 | - | ||
| Yes | 20/47 | 0.9 (0.5 to 1.6) | .72 | - | - |
| Unknown | 0/1 | - | |||
| Any invasive second cancer | |||||
| No‡ | 53/132 | 1.0 | - | ||
| Yes | 36/68 | 1.4 (0.8 to 2.4) | .21 | - | - |
* Rate ratios for the rate of development of valvular heart disease calculated conditional on matched sets. Matching variables were sex, age at HL diagnosis, and date of HL diagnosis (Supplementary Table 2, available online). CI = confidence interval; DdHK = The Erasmus MC-Daniel den Hoed Cancer Center; LUMC = Leiden University Medical Center; NKI-AVL = The Netherlands Cancer Institute, Amsterdam; RR = rate ratio; RT = Radiotherapy; EQD2 = Equivalent dose in 2 Gy fractions.
† Additional adjustments were: splenectomy and any chemotherapy for radiation dose, radiation dose to the affected valve and splenectomy for any chemotherapy, radiation dose to affected valve and any chemotherapy for splenectomy, radiation dose to the affected valve, and splenectomy for other variables.
‡ Reference category.
§ P trend (all other tests for heterogeneity). All statistical tests were two-sided.
Figure 3.
Rate ratios (RRs) for valvular heart disease (VHD) by estimated radiation dose (EQD2, Gy) to the affected heart valve compared with no radiation exposure. RRs calculated conditional on matched sets. Matching variables were gender, age at Hodgkin lymphoma (HL) diagnosis and date of HL diagnosis (Supplementary Table 1, available online). Circles are estimates for dose categories: 0 Gy, up to 30 Gy, 31–35 Gy, 36–40 Gy, and >40 Gy and are plotted at the median doses in each category, ie, 0.0, 22.9, 34.0, 38.8, and 42.2 Gy. Vertical lines are 95% confidence intervals. Curved line is the best fitting dose-response relationship (RR = 1+exp[-5.02]dose*exp[0.075*dose]), allowing for curvature (two-sided P nonlinearity = .03, likelihood ratio test). See Supplementary Figure 2 (available online) for additional details.
Figure 4.
Cumulative risks of valvular heart disease (VHD) as their first cardiac diagnosis among five-year survivors of Hodgkin lymphoma (HL) by years since initial HL diagnosis for categories of radiation dose (EQD2) to the affected heart valve. Cumulative risks were calculated treating patients as censored when they developed another heart disease and death as a competing risk.
Other Treatment-Related Risk Factors
Splenectomy after HL diagnosis was associated with an increased VHD rate (RR = 2.3, 95% CI = 1.3 to 4.3, P = .007) (Table 4). This association changed little in magnitude and remained statistically significant after adjusting for radiation dose to the affected valve (RR = 2.3, 95% CI = 1.1 to 4.5, P = .02).
VHD rates were reduced in patients given vincristine (RR = 0.4, 95% CI = 0.2 to 0.7, P = .003) or procarbazine (RR = 0.5, 95% CI = 0.3 to 0.9, P = .02); the reductions were attenuated and no longer statistically significant after adjustment for radiation dose to the affected valve and splenectomy (Table 4). Other factors, including anthracycline chemotherapy (RR = 1.2, 95% CI = 0.3 to 4.3), were not statistically significantly associated with VHD.
Modification of the Effect of Radiation by Other Factors
The possible modifying effect of other factors on the radiation-related VHD rate was evaluated by comparing VHD rate ratios for individuals with radiation dose to the affected valve of greater than or equal to 35 Gy to that for individuals with doses of less than 35 Gy for different levels of each factor (Supplementary Table 2, available online). For individuals treated in calendar periods between 1965 and 1974, 1975 and 1984, and 1985 and 1994, the VHD rate ratios of greater than or equal to 35 Gy vs less than 35 Gy were 1.3, 6.0, and 5.7, respectively (P heterogeneity = .03), but no other factors statistically significantly modified the effect of radiation. Allowing for the effect of splenectomy had little effect on the dose-response relationship in Figure 3 (data not shown).
Discussion
This study provides, for the first time, quantitative estimates of the relationship between radiation dose to the heart valves and subsequent risk of clinically significant VHD. The relationship is nonlinear, with little or no increase following doses below 30 Gy, whilst for doses above 30 Gy the percentage increase in the VHD rate per Gy increases progressively with increasing dose. This study has also provided approximate estimates of 30-year cumulative absolute risks of VHD for categories of radiation dose to the heart valves. The current standard radiation dose for Hodgkin lymphoma is 20 or 30 Gy. Patients with mediastinal involvement whose heart valves are exposed to these doses may expect only around 1.4% higher cumulative risk of clinically significant VHD after 30 years compared with those treated without RT.
Our study considered only VHD occurring before any other diagnosis of heart disease. We did this to avoid confusing the direct effect of treatment for HL on VHD, with that occurring as a secondary consequence of other heart disease, such as cardiac failure and ischaemic heart disease. Our estimates therefore underestimate the total incidence of VHD in the cohort, but correctly estimate that occurring as a direct effect of treatment.
Three recent studies have examined the relationship between VHD and RT for HL. Among 1132 HL survivors treated between 1978 and 1995, VHD was diagnosed in 3% (33 case patients) after a median follow-up of 19.5 years (12). Prescribed mediastinal radiation dose was the only independent risk factor for VHD. No cases of VHD were observed for individuals with doses of 20 Gy, while the 25-year cumulative risks among individuals with prescribed doses of 25, 30, and 36 Gy were 2%, 1%, and 16%, respectively. These findings are consistent with those of our own study.
A second study, including 56 survivors treated between 2002 and 2008 and screened using echocardiography, reported a 32% risk of asymptomatic valvular disorders after median follow-up of 70.5 months (15). The odds ratio for a left-sided valvular defect in patients where more than 25% of the left ventricle received more than 30 Gy vs patients where 25% or less received more than 30 Gy was 4.4 (P = .02), but a dose-response relationship could not be estimated because of the limited number of cases (n = 18). Most VHD cases were mild, and none was severe. After further follow-up, normal tissue complication models were fitted to the data from the cohort (27). The risk of valvular defects increased with maximum heart dose, while the risk of left-sided defects increased with the volume of the left cardiac chambers receiving 30 Gy or more. This is again consistent with our study.
The third study of 143 HL survivors treated between 1978 and 1985 reported that 42% had VHD after a median follow-up of 24 years (16). This high prevalence may be because the mid-mediastinal dose was high (40.3 Gy) and all individuals underwent screening with cardiac magnetic resonance imaging, which detected many asymptomatic defects. No association was observed between doses to specific cardiac structures and cardiac pathologies, perhaps because of the narrow dose range (16). In contrast, our cohort had no systematic screening for cardiac effects, considered only VHD as a first cardiac diagnosis, and had a greater proportion of higher-grade VHD.
Radiation-related VHD has been reported following childhood cancer, with statistically significant increases following mean cardiac doses of 15–34 Gy and 35 Gy (28) or more, and also in breast cancer (29). Taken together, all these studies suggest that cardiac doses of 30 Gy or more increase VHD risk, while at lower doses the increase per Gy is smaller and there may be a threshold dose below which there is no risk.
The use of a substitute CT dataset to derive retrospective dose estimates is a limitation of our study. The substitute CT datasets were chosen to be anatomically and dosimetrically typical, so that our dose estimates are near to the average of the true values. Inter-patient variability in anatomy, particularly cardiac size, shape, and position, could not be accounted for but is likely to be only a minor source of variation, as the heart valves are within the centre of the field and generally not close to lung shielding. Further uncertainties include the lack of original planning and/or verification films for a proportion of patients. Quantitative estimates of the dosimetric uncertainties are not available, rendering it impossible to quantify their effects on our estimates of VHD risk, but it is unlikely to be substantial.
Splenectomy was the only other treatment factor independently associated with an increase in VHD. There may be infectious, immune-related or inflammatory effects of splenectomy that increase the risk of VHD. The aetiology of VHD is incompletely understood, but it is now recognized that, like atherosclerosis, inflammation may play a role (30). Elsewhere, the association between splenectomy and subsequent VHD has been reported to be statistically nonsignificant (12). Splenectomy is no longer routinely used for the staging and treatment of HL. However the effect, if real, could be of relevance to HL survivors given a splenectomy.
Anthracyclines are known to cause cardiomyopathy and congestive cardiac failure (31). An earlier analysis of this cohort reported that anthracyclines increased the risk of VHD from mediastinal radiotherapy (hazard ratio [HR] = 2.1, 95% CI = 1.3 to 3.5) (2). In the present analysis, there was no statistically significant association, although the 95% confidence interval for an effect of anthracycline exposure was wide (0.3 to 4.3) (Table 4). A possible explanation for this apparent inconsistency is that the present analysis considered only case patients whose first clinically significant heart disease was VHD, while the earlier one included all diagnoses of VHD including those following ischaemic heart disease or cardiomyopathy. The effect of anthracyclines with or without RT on VHD has not been extensively investigated in other studies, but an increased risk of hospitalization for cardiac disease following treatment with doxorubicin-containing chemotherapy and RT vs chemotherapy alone has been reported (6).
Neither vincristine nor procarbazine chemotherapy were found to be independent risk factors for VHD. Exposure to vinca alkaloids has previously been associated with an increased risk of death from myocardial infarction following treatment for HL (7), but vincristine has never been associated with VHD or any other cardiac diseases.
Hypertension and hypercholesterolaemia at the end of follow-up were both associated with an increase in VHD, as were an increasing number of cardiac risk factors in total (Table 3). However, it is not possible to conclude that these associations are causal as patients with known VHD (including grade 1 VHD) are likely to be closely monitored for cardiac risk factors and the risk factors for VHD and atherosclerosis are similar (32). Nevertheless, these findings highlight the need to monitor and treat HL patients for conventional cardiovascular risk factors, as they are also at increased risk of coronary artery disease (2).
Obesity at diagnosis, but not at the end of follow-up, was also associated with VHD risk, but again the association may not be causal as mediastinal separation is greater in obese patients, possibly leading to increased inhomogeneity in radiation dose and to areas of increased dose to the heart valves. This dosimetric effect would not have been accounted for by our dose reconstruction methods.
This study confirms that radiation dose to the heart valves is the main risk factor for the development of clinically significant VHD following treatment for HL. It is the first time that a dose-response relationship has been derived in terms that can be easily translated into clinical practice. It will help clinicians assess the risk of VHD for patients treated in the future and will assist in guiding the appropriate follow-up of HL survivors.
Funding
This work was supported by the Dutch Cancer Society (grant NKI 2010–4720) and by core funding to the Oxford University Clinical Trial Services Unit from Cancer Research UK, the British Heart Foundation (BHF), and the UK Medical Research Council. DJC was supported by the BHF Centre for Research Excellence (RE/08/04).
Supplementary Material
The study sponsors had no role in the design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, nor the decision to submit the manuscript for publication.
The authors would like to thank Karen Kooijman, Kim Kersten, Harmke Groot, and Merian van Wouwe for their assistance with data abstraction and Yaochen Wang for her assistance with the processing of radiation dose data.
References
- 1. Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol. 2003;21(18):3431–3439. [DOI] [PubMed] [Google Scholar]
- 2. Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878–1886. [DOI] [PubMed] [Google Scholar]
- 3. De Bruin ML, Dorresteijn LD, van’t Veer MB, et al. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101(13):928–937. [DOI] [PubMed] [Google Scholar]
- 4. Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. J Clin Oncol. 2002;20(8):2101–2108. [DOI] [PubMed] [Google Scholar]
- 5. Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117(2):412–418. [DOI] [PubMed] [Google Scholar]
- 6. Myrehaug S, Pintilie M, Tsang R, et al. Cardiac morbidity following modern treatment for Hodgkin lymphoma: supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk Lymphoma. 2008;49(8):1486–1493. [DOI] [PubMed] [Google Scholar]
- 7. Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst. 2007;99(3):206–214. [DOI] [PubMed] [Google Scholar]
- 8. Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290(21):2831–2837. [DOI] [PubMed] [Google Scholar]
- 9. Specht L, Yahalom J, Illidge T, et al. Modern Radiation Therapy for Hodgkin Lymphoma: Field and Dose Guidelines From the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89(4):854–862. [DOI] [PubMed] [Google Scholar]
- 10. Maraldo MV, Brodin NP, Vogelius IR, et al. Risk of Developing Cardiovascular Disease after Involved Node Radiotherapy versus Mantle Field for Hodgkin Lymphoma. Int J Radiat Oncol Biol Phys. 2012;83(4):1232–1237. [DOI] [PubMed] [Google Scholar]
- 11. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. [DOI] [PubMed] [Google Scholar]
- 12. Schellong G, Riepenhausen M, Bruch C, et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for hodgkin disease in children and adolescents: Report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer. 2010;55(6):1145–1152. [DOI] [PubMed] [Google Scholar]
- 13. Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22(15):3139–3148. [DOI] [PubMed] [Google Scholar]
- 14. Heidenreich PA, Hancock SL, Lee BK, et al. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42(4):743–749. [DOI] [PubMed] [Google Scholar]
- 15. Cella L, Liuzzi R, Conson M, et al. Dosimetric predictors of asymptomatic heart valvular dysfunction following mediastinal irradiation for Hodgkin’s lymphoma. Radiother Oncol. 2011;101(2):316–321. [DOI] [PubMed] [Google Scholar]
- 16. Machann W, Beer M, Breunig M, et al. Cardiac magnetic resonance imaging findings in 20-year survivors of mediastinal radiotherapy for Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 2010;79(4):1117–1123. [DOI] [PubMed] [Google Scholar]
- 17. van Leeuwen FE, Klokman WJ, Hagenbeek A, et al. Second cancer risk following Hodgkin’s disease: a 20-year follow-up study. J Clin Oncol. 1994;12(2):312–325. [DOI] [PubMed] [Google Scholar]
- 18. van Leeuwen FE, Klokman WJ, Veer MB, et al. Long-term risk of second malignancy in survivors of Hodgkin’s disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18(3):487–497. [DOI] [PubMed] [Google Scholar]
- 19. Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0). USA: National Cancer Institute; 2009. [Google Scholar]
- 20. Feng M, Moran JM, Koelling T, et al. Development and Validation of a Heart Atlas To Study Cardiac Exposure to Radiation Following Treatment for Breast Cancer. Int J Radiat Oncol Biol Phys. 2011;79(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joiner MC, Van der Kogel AJ. Basic Clinical Radiobiology. 4th ed: Hodder Arnold; 2009. [Google Scholar]
- 22. Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76(3):656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choudhury JB. Non-parametric confidence interval estimation for competing risks analysis: application to contraceptive data. Stat Med. 2002;21(8):1129–1144. [DOI] [PubMed] [Google Scholar]
- 24. Plummer M. Improved estimates of floating absolute risk. Stat Med. 2004;23(1):93–104. [DOI] [PubMed] [Google Scholar]
- 25.Stata Statistical Software: Version 11. In. College Station, TX: StataCorp; 2009. [Google Scholar]
- 26.Epicure: Version 1.8. In. Seattle, WA: Hirosoft International Corporation. [Google Scholar]
- 27. Cella L, Liuzzi R, Conson M, et al. Multivariate normal tissue complication probability modeling of heart valve dysfunction in hodgkin lymphoma survivors. Int J Radiat Oncol Biol Phys. 2013;87(2):304–310. [DOI] [PubMed] [Google Scholar]
- 28. Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100(2):167–175. [DOI] [PubMed] [Google Scholar]
- 30. Veinot JP. Pathology of inflammatory native valvular heart disease. Cardiovasc Pathol. 2006;15(5):243–251. [DOI] [PubMed] [Google Scholar]
- 31. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. [DOI] [PubMed] [Google Scholar]
- 32. Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29(3):630–634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.