Abstract
Objective
Corin is a natriuretic peptide converting enzyme that cleaves precursor pro-B-type natriuretic peptide (proBNP) to active BNP (diuretic, natriuretic, and vasodilatory properties). Increased plasma BNP is a known diagnostic and prognostic heart failure (HF) biomarker in ambulatory and surgical patients. Recent studies indicate that plasma corin is significantly decreased in chronic HF patients, yet perioperative plasma corin concentrations have not been assessed in cardiac surgical patients. The objectives of this study were to determine the effect of coronary artery bypass graft (CABG) surgery with cardiopulmonary bypass (CPB) on plasma corin concentrations and to assess the association between change in perioperative plasma corin concentration and long-term postoperative HF hospitalization or death. We hypothesized that plasma corin concentrations decrease significantly from preoperative baseline during postoperative days 1-4, and that hospitalization or death from HF during the 5 years after surgery is significantly associated with higher relative difference (preoperative baseline to postoperative nadir) in plasma corin concentrations.
Design
Prospective observational pilot study.
Setting
Two institutions: Brigham and Women’s Hospital, Boston, Massachusetts and the Texas Heart Institute, St. Luke’s Hospital, Houston, Texas.
Patients
99 European ancestry subjects who underwent isolated primary CABG surgery with CPB.
Intervention
Non-emergency isolated primary CABG surgery with CPB.
Measurements
Plasma corin concentration was assessed preoperatively and at four postoperative time points (postoperative days 1-4). HF hospitalization or HF death events during the five years following surgery were identified by review of hospital and death records.
Main Results
Postoperative plasma corin concentrations were significantly lower than preoperative baseline concentrations (P<0.0001). Perioperative corin concentrations were significantly higher in males than in females (P<0.0001). 15 subjects experienced long-term postoperative HF events. Subjects who experienced HF hospitalization or HF death during study follow-up had significantly higher relative difference in plasma corin concentration (preoperative baseline to postoperative nadir) than subjects who did not experience HF events during study follow-up (P=0.03).
Conclusions
Plasma corin concentrations decrease significantly from preoperative concentrations following CABG surgery. HF hospitalization or HF death during the 5 years following CABG surgery with CPB is associated with larger relative decrease in plasma corin concentration from preoperative baseline. Further investigation is warranted to determine the role of corin in postoperative HF biology.
Keywords: CORIN protein, human; Natriuretic Peptide; Heart Failure; Coronary Artery Bypass Grafting; Biological Markers; Cardiac Surgery
Introduction
Heart failure (HF) affects approximately 5.1 million Americans1 and is associated with increased hospitalization, mortality, and health care expenditures1, 2. While coronary artery bypass graft (CABG) surgery is performed to improve quality of life and survival3, HF severe enough to result in hospitalization or death occurs in greater than 10% of patients during the first 5 years following isolated primary CABG surgery4, and remains the leading cause of death following this procedure5. Despite advances in HF prevention and treatment6, the biology of HF is still incompletely understood.
The natriuretic peptide system has counter-regulatory natriuretic, diuretic, and vasodilatory properties7. The convertase protein corin is a key component of natriuretic peptide processing that cleaves the less biologically active precursor pro-B type natriuretic peptide (proBNP) into biologically active BNP as well as an inactive NT-proBNP fragment8, 9 (Figure 1). Corin protein is thought to be produced primarily in the cardiac myocyte but has been demonstrated to be expressed in both the human heart and the kidney10 as well as circulating in the bloodstream11. Circulating corin levels may be partially regulated by degree of shedding from cardiac myocytes and protein autocleavage12. Presently available commercial BNP assays capture within their BNP concentration measurements both the precursor proBNP as well as the processed and more biologically active BNP8, 13, 14. The less biologically active proBNP has been shown to be preferentially elevated in ambulatory HF patients15.
Figure 1.
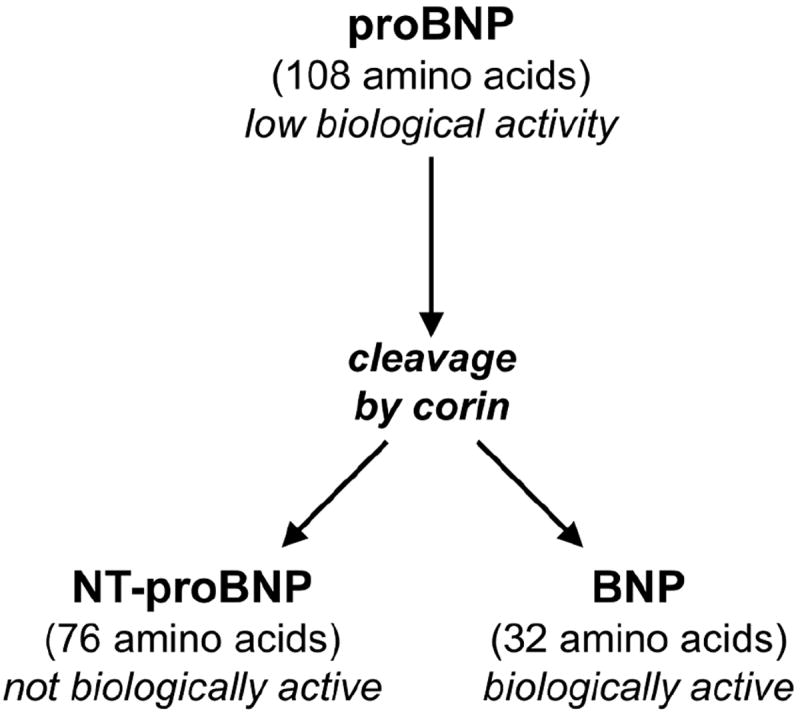
Illustration of precursor proBNP processing by corin to NT-proBNP (inactive fagment) and BNP (biologically active fragment).
Corin may play a role in HF biology. In addition to ambulatory HF patients having increased plasma concentrations of proBNP in relation to BNP15, plasma corin levels are lower in ambulatory HF patients than in controls11, 16. Furthermore, degree of reduced plasma corin concentration in ambulatory patients is correlated with HF severity11. Dong and colleagues demonstrated that patients with NYHA class II, III, and IV heart failure had progressively lower levels of circulating plasma corin (450, 377, 282 pg/mL respectively)11. Corin over-expression in mice with dilated cardiomyopathy has been associated with enhanced myocardial contractile function, reduced HF signs, and decreased mortality17. It has been hypothesized that active corin deficiency, which could manifest as decreased circulating plasma corin, leads to decreased natriuretic peptide processing with a resulting relative overabundance of less biologically active proBNP and a deficiency of the processed active BNP fragment18.
Natriuretic peptides have been studied in cardiac surgical populations. Plasma BNP concentration is known to rise significantly after CABG surgery when compared to preoperative baseline19, 20. We have previously shown that both increased preoperative and peak postoperative20 BNP concentrations are associated with increased short and long-term postoperative morbidity and mortality, including increased long-term HF related hospitalization or death during the 5 years following on-pump CABG surgery4, 19-21.
While corin has been studied both in animal models and in ambulatory HF cohorts, the perioperative response pattern of plasma corin concentration to surgical stress has yet to be defined in CABG surgical patients. Furthermore, no study has assessed for an association between decline in perioperative corin concentration and increased postoperative HF. We therefore performed an exploratory pilot study of 99 patients of European ancestry who underwent isolated primary CABG surgery with cardiopulmonary bypass (CPB) to: (1) characterize the perioperative pattern of plasma corin concentrations; and (2) assess for an association between decreased postoperative plasma corin concentration and the occurrence of HF hospitalization or HF death up to 5 years following surgery. We hypothesized that plasma corin concentrations decrease significantly from preoperative baseline during postoperative days 1-4, and that hospitalization or death from HF during the 5 years after surgery is significantly associated with higher relative difference (preoperative baseline to postoperative nadir) in plasma corin concentrations.
Methods
Study Population
1922 patients who underwent cardiac surgery with CPB at Brigham and Women’s Hospital or the Texas Heart Institute were prospectively enrolled between August 2001 and February 2009 in an ongoing observational parent cohort study known as the CABG Genomics Program, which has the overall aim of identifying genetic and biomarker predictors of adverse events after cardiac surgery (http://clinicaltrials.gov/show/NCT00281164). Institutional Review Board approvals were obtained and each study subject provided written informed consent prior to study initiation. Patients from the overall CABG Genomics Program cohort were not considered for inclusion in the current study if any of the following clinical criteria were present: emergency surgery, concurrent valve surgery, prior cardiac surgery, preoperative inotropic support or intra-aortic balloon pump, surgery without CPB or aortic cross-clamp, preoperative hemodialysis, or a preoperative creatinine greater than 3 mg/dL.
The 99 subjects included in the present study’s analysis were initially selected from 100 subjects who were included in a pilot study assessment of a genetic hypothesis, and therefore subjects who declared themselves not to be of Northern or Southern European ancestry were excluded in order to avoid potential influence of population stratification. Those without genotyping data were additionally excluded in order to allow comparison of perioperative corin concentrations in minor allele carriers of a CORIN gene intronic single nucleotide polymorphism (SNP) rs12645164 versus non-carriers of this SNP. Of the 1238 CABG Genomics subjects (after exclusions for clinical factors, non-European ancestry and available genotyping), 14 subjects were homozygous for the minor allele of rs12645164. 100 total patients were therefore selected for the pilot study by matching each of the 14 minor allele homozygotes 3:1 with both heterozygotes and major allele homozygotes, with one subject of older age matched 4:1 (matching was performed according to age, gender, study institution, and left ventricular ejection fraction). This genetic pilot study did not identify a significant difference in corin concentrations between minor allele carriers and non-carriers of rs12645164.
For the present study analysis, we assessed 99 of these pilot study subjects (1 subject excluded for missing preoperative corin concentration) in order describe overall pattern of perioperative plasma corin release (preoperative and postoperative days 1-4), the influence of gender on pattern of perioperative plasma corin release, and the association between decline in perioperative plasma corin concentration and the occurrence of HF hospitalization or HF death up to 5 years after on-pump CABG surgery. A schematic outlining patient selection for this study’s analysis is shown in Figure 2.
Figure 2.

Schematic illustrating the steps taken to select the study’s 99 subjects from the overall CABG Genomics study cohort. *One older subject in the minor allele homozygous group was matched 1:4 for age, gender, study site and left ventricular ejection fraction, with matching to 4 subjects in the heterozygote group and 4 subjects in the major allele homozygous group according to age >65 years instead of age by decile.
Blood Collection
Preoperative and postoperative day 1-4 plasma EDTA samples were collected and assessed, with plasma corin concentrations measured using ELISA (R&D systems, Minneapolis, MN) at a single laboratory.
Data Collection
Perioperative data were prospectively collected using a standard case report form that included demographic information, preoperative medications, medical history, surgical characteristics, and postoperative course. Data regarding need for hospitalization during the 5 years following primary CABG surgery were obtained via questionnaires that were sent to patients 6 weeks, 6 months, and annually on postoperative years 1-5 as well as via follow-up telephone interviews and review of study institution electronic medical records. Hospital records were obtained for any subject who was identified as having postoperative hospitalizations for possible cardiac or pulmonary reasons. Deaths were identified using the Social Security Death Index. Cause of death information was obtained from death certificates or National Death Index (NDI) files for any subject that was found to have died during the study period. Hospitalization and death data was collected for the subject cohort through 5 years after surgery or through July 15, 2012 if that date came first (only 2 subjects had surgery dates that occurred less than 5 years before this study end date). Two study investigators who were blinded to plasma corin concentrations (AF and CB) independently reviewed hospital records, death certificates, and NDI files and determined HF hospitalization and HF death outcomes. Five subjects were lost to study follow-up and thus were not included in the analyses of HF outcomes.
Statistical Analysis
All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). Two-sided P values less than 0.05 were considered statistically significant. Spearman correlation coefficients were calculated to assess correlations between perioperative plasma biomarker concentrations. Generalized mixed-effects regression models were used to assess change in corin concentration over the perioperative time points. Perioperative corin concentration was assessed for the entire study group and then stratified by gender. Demographic, perioperative patient characteristics, and corin concentrations were compared between subjects who did and did not experience a postoperative HF event, with Student’s t-test or Wilcoxon Rank Sum tests used for continuous data as appropriate and Chi Squared tests used for categorical data. Relative difference in corin was defined as the difference between baseline preoperative corin concentration and nadir postoperative corin concentration divided by baseline preoperative corin concentration. Kaplan-Meier survival curves were created and log rank tests performed to compare time to first HF event in subjects with higher relative difference in corin concentration (top half) versus subjects with lower relative difference in corin concentration (lower half). Cox proportional-hazards regression analyses were then carried out to assess the association between dichotomized relative difference in perioperative corin concentration and time to first HF event, with additional adjustments made for gender and for the perioperative characteristics significantly associated with HF events in univariate analyses.
Results
Patient Characteristics
Perioperative characteristics of the 99 study subjects are shown in Table 1. Mean age at enrollment was 67.2 +/- SD 9.5 years, and 79% were male. Fifteen patients experienced postoperative HF events. 14 patients were hospitalized for HF and 2 patients died from HF (1 patient was hospitalized for HF prior to HF death). Five patients were lost to follow up (no contact after initial hospitalization). Perioperative characteristics of the 94 patients (15 patients who experienced a HF event and the 79 patients who did not) who were included in the analysis of HF outcome are provided in Table 2. Median time to first postoperative HF event was 1.6 years (range 13 days to 4.3 years). For the 79 study participants who did not experience a HF event, median follow up time was 5 years (range 48 days to 5 years). Patients with greater than 30 pack year smoking history were more likely to have a HF event than those who did not have such an extensive smoking history (P=0.0003). Patients who had a recent myocardial infarction within 2 weeks before surgery were more likely to have a HF event than those without a recent myocardial infarction (P =0.005). Otherwise, the baseline characteristics of the HF event and nonevent groups were similar.
Table 1.
Table of Patient Characteristics (n = 99)
| Variables | Mean, Median or Proportion |
|---|---|
| Demographic Characteristics | |
| Age [years (SD)] | 67.2 (9.5) |
| Gender [n (%)] | |
| Male | 78 (79%) |
| Female | 21 (21%) |
| Institution [n (%)] | |
| Brigham and Women’s Hospital | 69 (70%) |
| Texas Heart Institute | 30 (30%) |
|
| |
| Preoperative Medical Characteristics: | |
| Left ventricular ejection fraction [percent (SD)] | 60 (8) |
| Creatinine clearance (MDRD formula) [mL/min/1.73m2 (SD)] | 71.3 (18.5) |
| Diabetes mellitus (on insulin or oral medication) [n (%)] | 27 (28%) |
| Body mass index >30 kg/m2 [n (%)] | 37 (37%) |
| Greater than 30 pack year smoking history [n (%)] | 27 (28%) |
| Myocardial infarction within 2 weeks before surgery [n (%)] | 12 (12%) |
| Hypercholesterolemia [n (%)] | 67 (68%) |
| Hypertension [n (%)] | 86 (87%) |
| Coronary artery regions with >50% stenosis) [n (%)] | |
| 0-1 region | 7 (7%) |
| 2 regions | 26 (26%) |
| 3 regions | 66 (67%) |
|
| |
| Surgical Characteristics: | |
| Cardiopulmonary bypass time [minutes (SD)] | 97 (40) |
| Aortic cross clamp time [minutes (SD)] | 70 (31) |
| Number of coronary artery bypass grafts [n (%)] | |
| <3 grafts | 14 (14%) |
| 3 grafts | 45 (46%) |
| >3 grafts | 40 (40%) |
|
| |
| Perioperative Plasma Corin: | |
| Baseline (preoperative) corin [pg/mL median (IQR)] | 1365 (990-1747) |
| Minimum (perioperative) corin [pg/mL median (IQR)] | 889 (606-1146) |
| Relative difference corin [median (IQR)] | 0.4 (0.2-0.5) |
SD = standard deviation; MDRD = modification of diet in renal disease formula (creatinine clearance = 186 × [preop serum creatinine in mg/dL]-1.154 × Age-0.203 × [1.210 if Black] × [0.742 if Female]); IQR = interquartile range; relative difference = difference between baseline preoperative corin concentration and nadir postoperative corin concentration divided by baseline preoperative corin concentration
Table 2.
Comparison of Patient Characteristics and Plasma Corin Concentrations for Heart Failure Event and No Event Groups
| Variable | No HF Event Group (n = 79) Mean, Median or Proportion | HF Event Group (n= 15) Mean, Median or Proportion | P value |
|---|---|---|---|
| Demographic Characteristics | |||
| Age [years (SD)] | 66.4 (9.2) | 69.9 (9.5) | 0.17 |
| Gender [n (%)] | |||
| Male | 63 (80%) | 11 (73%) | 0.58 |
| Female | 16 (20%) | 4 (27%) | |
| Institution [n (%)] | |||
| Brigham and Women’s Hospital | 52 (66%) | 13 (87%) | 0.11 |
| Texas Heart Institute | 27 (34%) | 2 (13%) | |
|
| |||
| Preoperative Medical Characteristics: | |||
| Left ventricular ejection fraction [percent (SD)] | 60 (8) | 58 (8) | 0.37 |
| Creatinine clearance (MDRD formula) [mL/min/1.73m2 (SD) | 72.8 (18.3) | 65.2 (19.1) | 0.14 |
| 24 (31%) | 3 (20%) | 0.38 | |
| Diabetes mellitus (on insulin or oral medication) [n(%)] | 26 (33%) | 8 (53%) | 0.13 |
| 15 (19%) | 9 (69%) | 0.0003 | |
| Body mass index >30 kg/m2 [n(%)] | 6 (8%) | 5 (33%) | 0.005 |
| Greater than 30 pack year smoking history [n(%)] | 53 (67%) | 12 (80%) | 0.32 |
| 67 (85%) | 15 (100%) | 0.11 | |
| Myocardial infarction within 2 weeks [n(%)] | |||
| Hypercholesterolemia [n(%)] | 6 (8%) | 0 (0%) | 0.33 |
| Hypertension [n(%)] | 20 (25%) | 6 (40%) | |
| Coronary artery regions with >50% stenosis [n(%)] | 53 (67%) | 9 (60%) | |
| 0-1 region | |||
| 2 regions | |||
| 3 regions | |||
|
| |||
| Surgical Characteristics: | |||
| Cardiopulmonary bypass time [minutes (SD)] | 96 (41) | 112 (33) | 0.16 |
| Aortic cross clamp time [minutes (SD) | 69 (31) | 85 (27) | 0.06 |
| Number of coronary artery bypass grafts [n (%)] | |||
| <3 grafts | 12 (15%) | 2 (13%) | 0.65 |
| 3 grafts | 32 (41%) | 8 (53%) | |
| >3 grafts | 35 (44%) | 5 (33%) | |
|
| |||
| Perioperative Plasma Corin: | |||
| Baseline (preoperative) corin [pg/ml median (IQR)] | 1384 (1039-1815) | 1337 (854-1626) | 0.35 |
| 894 (610-1226) | 786 (484-914) | 0.08 | |
| Minimum (perioperative) corin [pg/mL median (IQR)] | 0.3 (0.2-0.5) | 0.4 (0.4-0.5) | 0.03 |
| Relative difference corin [median (IQR)] | |||
HF = heart failure; SD = standard deviation; MDRD = modification of diet in renal disease formula (creatinine clearance = 186 × [preop serum creatinine in mg/dL]-1.154 × Age-0.203 × [1.210 if Black] × [0.742 if Female]); IQR = interquartile range; relative difference = difference between baseline preoperative corin concentration and nadir postoperative corin concentration divided by baseline preoperative corin concentration
Perioperative Corin Concentrations
Among the 99 total study participants, median preoperative plasma corin concentration was 1365 pg/mL (interquartile range [IQR] 990-1747 pg/mL) and median lowest (nadir) postoperative corin concentration was 889 pg/mL (IQR 606-1146 pg/mL). Preoperative plasma corin concentration significantly correlated with nadir postoperative corin concentration (Rho = 0.83; P<0.0001). Mixed models analyses showed the perioperative plasma corin concentrations changed significantly over time with a decrease from preoperative baseline. This was true both in the overall cohort (P<0.0001; Figure 3) and when the cohort was stratified by gender (P<0.0001; Figure 4). Perioperative plasma corin was significantly higher in males than in females (P<0.0001; Figure 4). Given the initial patient selection was based upon genotype, rs12645164 (dominant genetic model) was additionally assessed in the mixed models analysis, and no significant effect of genotype on perioperative change in plasma corin concentration was identified (P=0.36).
Figure 3. Plasma corin concentrations.
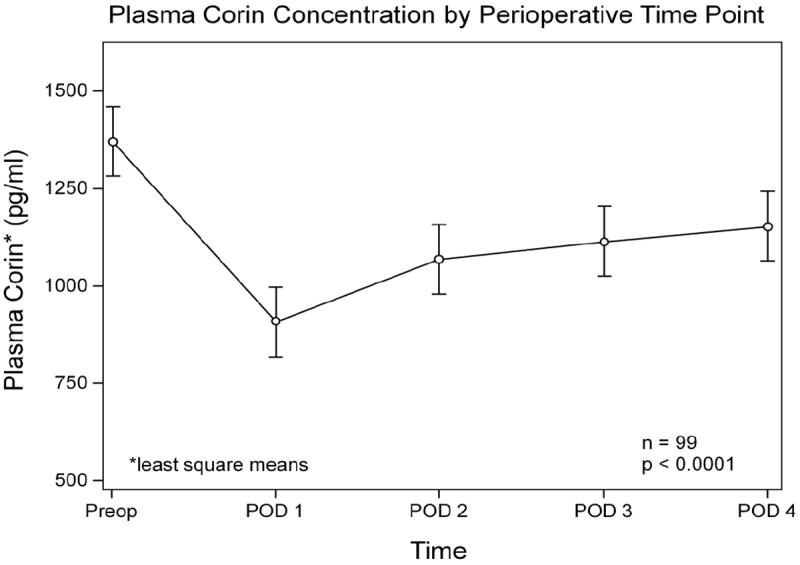
The time effect on perioperative corin concentration was significant (P<0.0001). Preop = patient preoperative baseline corin concentration; POD = postoperative day number. All corin concentrations values (pg/mL) represent least square means.
Figure 4. Plasma corin concentrations stratified by gender.

The time effect on perioperative corin concentration was significant for both males (P<0.0001) and females (P<0.0001) when the gender groups were considered separately. Males had significantly higher perioperative plasma corin concentrations than did females (P<0.0001). Preop = patient preoperative baseline corin concentration; POD = postoperative day number. All corin concentration values (pg/ml) represent least square means.
Perioperative Corin Concentration and Heart Failure Hospitalization or Heart Failure Death
The median nadir perioperative plasma corin concentration was 786 pg/mL (IQR 484-914 pg/mL) for the HF event group and 894 pg/mL (IQR 610-1226 pg/mL) for the non-HF event group (P = 0.08). The relative difference in plasma corin concentrations between preoperative baseline and postoperative nadir (i.e. preoperative plasma corin concentration minus nadir postoperative plasma corin divided by preoperative plasma corin concentration) differed significantly between the HF event and non-HF event groups (P= 0.03). Kaplan-Meier curves show significantly reduced HF event free survival in subjects in the top half for relative difference in perioperative corin concentration (i.e. bigger postoperative drop in plasma corin concentration) as compared to subjects in the lower half for relative difference in plasma corin concentration (i.e. smaller drop in plasma corin concentration) (P=0.02; Figure 5). Using Cox proportional hazards regression, relative difference in perioperative plasma corin concentration (dichotomized by top half versus lower half) was significantly associated with shorter time to first postoperative HF event (unadjusted Hazard Ratio = 3.94, 95% CI = 1.11-13.89, P=0.03). This remained true when additionally adjusting for gender, >30 pack year history of smoking, and myocardial infarction within two weeks before surgery (Hazard Ratio 8.41, 95% CI = 1.05-67.14; P=0.045).
Figure 5. Kaplan-Meier 5-year survival curves.
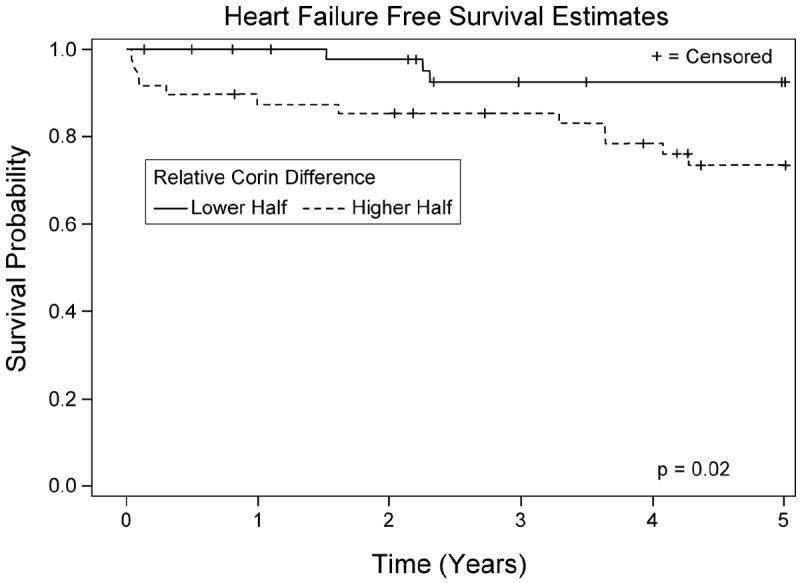
Curves represent heart failure (HF) event free survival and are stratified according to top versus bottom half of relative decline in perioperative plasma corin concentration. There was a significant difference in HF event free survival between the two groups (log rank P=0.02). Relative decline in perioperative plasma corin concentration is the difference between preoperative baseline plasma corin concentration and nadir postoperative plasma corin concentration divided by preoperative baseline corin concentration.
Discussion
In this exploratory study of 99 patients of European ancestry who underwent isolated primary CABG surgery with CPB, plasma corin concentrations decreased significantly after CABG surgery. Fifteen subjects developed HF severe enough to result in hospitalization or death during the 5 years following surgery. A bigger relative decrease from preoperative corin concentration to postoperative nadir corin concentration significantly associated with increased occurrence of this HF outcome. HF is a leading cause of mortality after CABG surgery5 and is accompanied by high morbidity and cost1, 2. The results of this initial study suggest that future larger studies may be warranted to explore the potential mechanistic role of corin in development of postoperative HF and to determine if perioperative corin concentrations can be used to aid in predicting development of postoperative HF. Improved understanding regarding CABG surgical patients at high risk for developing postoperative HF may ultimately allow health care providers and specifically perioperative physicians to better target patients for enhanced HF prevention and treatment.
The results of our study suggest that postoperative plasma corin concentrations decline significantly from preoperative baseline concentrations in response to CABG surgery performed with CPB and aortic cross-clamping. This finding has interesting parallels to studies that have observed lower plasma corin concentrations in ambulatory subjects with HF when compared with ambulatory controls11, 16.
Furthermore, studies of ambulatory patients have demonstrated significantly higher plasma corin concentrations in men versus women 22. While the general perioperative pattern of change in corin concentrations was similar in our study for male and female CABG surgery patients, we also observed significantly higher perioperative corin concentrations in men versus women. It remains unclear why men have higher plasma corin concentrations than women, but this difference may reflect a mechanism for gender differences in the biology of postoperative HF. Gender however was not a significant predictor of postoperative HF events in our present study cohort.
HF is a multifactorial disease process with complex underlying biology. It has been hypothesized that a deficiency in active functioning corin, a serine protease that converts the less active precursor proBNP to the more biologically active BNP, may contribute to the HF phenotype in some patients18. A recent study found that in mice with dilated cardiomyopathy, overexpression of corin was associated with both significantly reduced myocardial fibrosis and pulmonary edema, as well as enhanced ventricular fractional shortening and ejection fraction17. A prior study of corin deficient mice revealed significantly impaired ventricular function in response to increased cardiac afterload23. The role that corin may play in the development of postoperative HF has not been addressed. Our study is the first to assess perioperative corin concentrations. While our study does not address the specific mechanistic role of corin in the development of HF post-CABG surgery, our results suggest an association between the degree of diminution of postoperative plasma corin concentration and the development of postoperative HF.
The results of this exploratory study should be interpreted within the context of the study design. First, this study had a limited sample size of 99 patients of European ancestry who underwent isolated primary CABG surgery with CPB, of which only 15 experienced longer-term postoperative HF events. Based on our preliminary findings, larger studies focused on the value of corin as a HF biomarker, particularly when considered in conjunction with evaluation of concentration ratios of less biologically active precursor proBNP to more biologically active processed BNP may be warranted24, 25. Caution should be taken in extrapolating our findings regarding perioperative pattern of circulating plasma corin concentrations observed in patients undergoing isolated primary CABG surgery with CPB to patients undergoing other types of cardiac surgeries. Future studies should address potential impact of post-cardiopulmonary bypass hemodilution and other operative factors on absolute plasma corin concentrations by comparing perioperative plasma corin response between different cardiac surgical groups (e.g. CABG surgery with CPB versus off-pump CABG surgery versus open heart valve surgery). Larger studies that involve a greater number of HF outcome events will also allow for more extensive adjustment for clinical risk factors potentially associated with postoperative HF and should also enable exploration of the association between circulating perioperative plasma corin concentrations and additional HF related outcomes such as postoperative atrial fibrillation, acute right heart failure, and prolonged mechanical ventilation with appropriate adjustments for clinical risk factors. Second, our patients were initially selected based on whether they were carriers of single SNP genotype, with genotype carrier groups matched according to study institution, age, gender and preoperative left ventricular ejection fraction. While SNP rs12645164 was not associated with perioperative corin concentrations, additional validation studies that do not select subjects according to genotype or European ancestry are indicated. In addition, while corin has been more extensively explored in the ambulatory literature for its potential role in HF, it is important to note that furin, which we did not assess in this study, is also a convertase involved in human proBNP processing9, 26. Therefore, future studies of both furin and corin convertases and their roles in natriuretic peptide processing may be indicated in CABG surgery patients. Finally, this study established that corin concentrations decline after CABG surgery with CPB, but additional studies are needed to identify potential mechanisms by which such a decline might contribute to postoperative HF.
Conclusions
Plasma corin concentrations decrease in European ancestry patients after CABG surgery with CPB. Furthermore, perioperative change in plasma corin concentration associates with HF related hospitalization or HF death up to five years after CABG surgery. Additional studies are needed to better elucidate the role of corin in the biology of postoperative HF and to examine the utility of plasma corin concentration for predicting adverse cardiovascular outcomes after CABG surgery.
Acknowledgments
Funding sources: American Geriatrics Society Jahnigen Career Development Award (PI AAF); Society of Cardiovascular Anesthesiologists Mid-Career Grant (PI AAF); National Institutes of Health 1RO1HL098601 (PI SCB); Department of Anesthesiology, Perioperative, and Pain Medicine, Brigham and Women’s Hospital, Boston, MA; Division of Cardiovascular Anesthesia, Texas Heart Institute, Saint Luke’s Hospital, Houston, Texas.
We want to acknowledge the outstanding contributory efforts of the CABG Genomics Program’s research staff: Kutjim Bodinaku, M.D. (Research Assistant), Jonathan Gibbons, B.S. (Research Assistant), Svetlana Gorbatov, M.P.H. (Research Assistant), James Gosnell, R.N. (Research Nurse), Sejfudin Kavazovic, C.R.A. (Research Assistant), Sheila Moore, C.R.A. (Research Assistant), Adrienne Kicza, B.S. (Research Assistant), Carliss Ramos, R.N., B.S.N., C.C.R.C. (Research Nurse) and Charles Wellington, R.N., B.S.N., C.C.R.C. (Research Nurse). We want to thank James M. Bell (graphic designer) for his help in preparing the figures for this manuscript. We would like to credit the Texas Department of State Health Services as the source of death certificate information. Finally we thank all the individuals who have participated in the CABG Genomics Program.
Footnotes
Name of Department(s) and Institution(s): All work was performed at Brigham and Women’s Hospital, 75 Francis Street, Boston, Massachusetts 02115 and Texas Heart Institute, Saint Luke’s Hospital, 6770 Bertner Avenue, Houston, Texas 77030.
IRB: Partners Institutional Review Board, Boston, MA, USA; St. Luke’s Episcopal Hospital Institutional Review Board, Houston, TX, USA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) Circulation. 2004;110:e340–437. [PubMed] [Google Scholar]
- 4.Fox AA, Nascimben L, Body SC, et al. Increased perioperative B-type natriuretic peptide associates with heart failure hospitalization or heart failure death after coronary artery bypass graft surgery. Anesthesiology. 2013;119:284–294. doi: 10.1097/ALN.0b013e318299969c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor GT, Birkmeyer JD, Dacey LJ, et al. Results of a regional study of modes of death associated with coronary artery bypass grafting. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg. 1998;66:1323–1328. doi: 10.1016/s0003-4975(98)00762-0. [DOI] [PubMed] [Google Scholar]
- 6.Jhund PS, Macintyre K, Simpson CR, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009;119:515–523. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- 7.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 8.Liang F, O’Rear J, Schellenberger U, et al. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol. 2007;49:1071–1078. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 9.Semenov AG, Tamm NN, Seferian KR, et al. Processing of pro-B-type natriuretic peptide: furin and corin as candidate convertases. Clin Chem. 2010;56:1166–1176. doi: 10.1373/clinchem.2010.143883. [DOI] [PubMed] [Google Scholar]
- 10.Ichiki T, Huntley BK, Heublein DM, et al. Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation. Clin Chem. 2011;57:40–47. doi: 10.1373/clinchem.2010.153908. [DOI] [PubMed] [Google Scholar]
- 11.Dong N, Chen S, Yang J, et al. Plasma soluble corin in patients with heart failure. Circ Heart Fail. 2010;3:207–211. doi: 10.1161/CIRCHEARTFAILURE.109.903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang J, Wu S, Wang W, et al. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem. 2011;286:10066–10072. doi: 10.1074/jbc.M110.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luckenbill KN, Christenson RH, Jaffe AS, et al. Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: preliminary observations from the IFCC Committee for Standardization of Markers of Cardiac Damage. Clin Chem. 2008;54:619–621. doi: 10.1373/clinchem.2007.097998. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Rumayor A, Richards AM, Burnett JC, et al. Biology of the natriuretic peptides. Am J Cardiol. 2008;101:3–8. doi: 10.1016/j.amjcard.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Hawkridge AM, Heublein DM, Bergen HR, 3rd, et al. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci U S A. 2005;102:17442–17447. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibebuogu UN, Gladysheva IP, Houng AK, et al. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail. 2011;4:114–120. doi: 10.1161/CIRCHEARTFAILURE.109.895581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladysheva IP, Wang D, McNamee RA, et al. Corin overexpression improves cardiac function, heart failure, and survival in mice with dilated cardiomyopathy. Hypertension. 2013;61:327–332. doi: 10.1161/HYPERTENSIONAHA.112.193631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dries DL. Process matters: Emerging concepts underlying impaired natriuretic peptide system function in heart failure. Circ Heart Fail. 2011;4:107–110. doi: 10.1161/CIRCHEARTFAILURE.111.960948. [DOI] [PubMed] [Google Scholar]
- 19.Fox AA, Shernan SK, Collard CD, et al. Preoperative B-type natriuretic peptide is as independent predictor of ventricular dysfunction and mortality after primary coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2008;136:452–461. doi: 10.1016/j.jtcvs.2007.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox AA, Muehlschlegel JD, Body SC, et al. Comparison of the utility of preoperative versus postoperative B-type natriuretic peptide for predicting hospital length of stay and mortality after primary coronary artery bypass grafting. Anesthesiology. 2010;112:842–851. doi: 10.1097/ALN.0b013e3181d23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox AA, Marcantonio ER, Collard CD, et al. Increased peak postoperative B-type natriuretic peptide predicts decreased longer-term physical function after primary coronary artery bypass graft surgery. Anesthesiology. 2011;114:807–816. doi: 10.1097/ALN.0b013e31820ef9c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong N, Chen S, Wang W, et al. Corin in clinical laboratory diagnostics. Clin Chim Acta. 2012;413:378–383. doi: 10.1016/j.cca.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckley CL, Stokes AJ. Corin-deficient W-sh mice poorly tolerate increased cardiac afterload. Regul Pept. 2011;172:44–50. doi: 10.1016/j.regpep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macheret F, Boerrigter G, McKie P, et al. Pro-B-type natriuretic peptide(1-108) circulates in the general community: plasma determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2011;57:1386–1395. doi: 10.1016/j.jacc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dries DL, Ky B, Wu AH, et al. Simultaneous assessment of unprocessed ProBNP1-108 in addition to processed BNP32 improves identification of high-risk ambulatory patients with heart failure. Circ Heart Fail. 2010;3:220–227. doi: 10.1161/CIRCHEARTFAILURE.109.903153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichiki T, Boerrigter G, Huntley BK, et al. Differential expression of the pro-natriuretic peptide convertases corin and furin in experimental heart failure and atrial fibrosis. American journal of physiology. 2012;304:R102–109. doi: 10.1152/ajpregu.00233.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


