Abstract
Background
Patients with metabolic syndrome are at increased risk for cardiovascular disease. Combination lipid-lowering therapy is often needed in patients with metabolic syndrome and mixed dyslipidemia. The aim of this study was to compare the effect of statin combined with a new hypolipidemic agent, coenzyme A (CoA) with moderate-dose statin monotherapy in subjects with metabolic syndrome and mixed dyslipidemia by evaluating data from a subgroup of patients with metabolic syndrome and mixed dyslipidemia from a previously conducted randomized study.
Methods
In the present post hoc analysis, 212 patients were included, receiving statin monotherapy (n = 94) or statin combined with CoA 400 U/day (n = 118) for 8 weeks. The lipoprotein profile was determined at baseline and week 8 visits. Attainment of low-density lipoprotein-cholesterol (LDL-C) < 100 mg/dL, non-high-density lipoprotein-cholesterol (HDL-C) < 130 mg/dL, and the combined goal of these two parameters was also evaluated.
Results
The mean percent change was more prominent with CoA plus statin compared with placebo plus statin in triglyceride (TG) (-32.5% vs. -8.7%, respectively; P = 0.0002), total cholesterol (-9.6% vs. -3.6%, P = 0.013), LDL-C (-7.5% vs. 2.1%, P = 0.033), and non-HDL-C (-14.3% vs. -6.4%, P = 0.011). Treatment with CoA plus statin resulted in larger percentages of participants attaining lipid goals for LDL-C (70.3% vs. 56.4%, P = 0.044), non-HDL-C (60.2% vs. 45.7%, P = 0.039), and the combined goal of LDL-C and non-HDL-C (57.6% vs. 42.6%, P = 0.038) than statin monotherapy.
Conclusion
These results demonstrate that CoA plus statin therapy was more effective in improving lipoprotein parameters than statin alone in patients with metabolic syndrome and mixed hyperlipidemia.
Keywords: Coenzyme A, Hypertriglyceridemia, Dyslipidemia, Metabolic syndrome, Combination therapy
Introduction
Metabolic syndrome has become increasingly common in the world. Patients with metabolic syndrome often have raised triglycerides (TG), reduced high-density lipoprotein-cholesterol (HDL-C) and sometimes raised low-density lipoprotein-cholesterol (LDL-C) levels [1-3]. Although the definition of metabolic syndrome varies according to ethnicity, metabolic syndrome enhances the risk for cardiovascular disease and pharmacotherapies of the metabolic risk factors are often needed [4, 5].
Current guidelines recommend that patients with metabolic syndrome achieve a target LDL-C < 100 mg/dL as the primary goal of therapy and a target non-HDL-C < 130 mg/dL as the secondary goal of therapy if elevated TG is coexisting [5]. Although lifestyle intervention is critical and may be the initial treatment for patients with metabolic syndrome and dyslipidemia, pharmacological modification is often likely required to achieve lipid goals [6]. Statins are often chose for the initial therapy; however, even in maximally tolerable doses, it often fails to attain desirable lipid goals beyond LDL-C, and therapeutic regimen combining a statin with another hypolipidemic drug may be necessary [7].
Fibrates are often used in combination therapy with a statin in treating mixed dyslipidemia. However, safety issues, especially drug-induced hepatotoxicity, myositis and rhabdomyolysis, are also considered [8, 9]. Coenzyme A (CoA) was a new lipid-lowering agent functioning as an acyl group carrier and involving in the oxidation and catabolism of fatty acids [10, 11]. Animal and clinical studies have shown its normalizing activity on plasma lipids and good tolerability in the treatment of dyslipidemia [12-14].
In a previous study, we randomized 304 patients with mixed dyslipidemia to either moderate-dose statin monotherapy (S group) or statin with CoA 400 U/day (SC group) [14]. The SC combination was better at decreasing TG, total cholesterol (TC), LDL-C and non-HDL-C [14]. The present post hoc analysis only included participants with metabolic syndrome from the previous study [14]. We evaluated the efficacy of statin and CoA combination regimen on the changes in the levels and patterns of lipoproteins in patients with metabolic syndrome and mixed dyslipidemia.
Material and Methods
As a post hoc analysis, the present work includes subjects with metabolic syndrome from a randomized, placebo-controlled, phase 3 study that compared the effect and safety of statin plus CoA 400 U combination therapy with moderate dose of statin monotherapy in subjects with mixed dyslipidemia (NCT01928342) [14]. As previously described, men and non-pregnant women who had been receiving moderate dose of a statin (pitavastatin 4 mg/day, rosuvastatin 10 mg/day, atorvastatin 20 mg/day, pravastatin 40 mg/day, lovastatin 40 mg/day, simvastatin 40 mg/day, or fluvastatin 80 mg/day) for the control of raised LDL-C level for at least 8 weeks before screening and had a fasting TG level at 200 - 620 mg/dL on two consecutive visits were recruited [14].
Exclusion criteria were: 1) liver disease with elevations of alanine (ALT) and/or aspartate aminotransferase (AST) > 2 times upper limit of normal (ULN), 2) renal dysfunction as defined by serum creatinine levels ≥ 2.0 mg/dL, 3) poorly controlled hypertension (resting systolic blood pressure ≥ 180 mm Hg and/or diastolic blood pressure ≥ 110 mm Hg at two consecutive visits), 4) unexplained serum creatine phosphokinase (CPK) > 2 times ULN, 5) pregnancy, 6) breast-feeding, 7) women of childbearing age not using any contraception method, 8) established cardiovascular disease, 9) hypothyroidism, 10) heart failure with left ventricular ejection fraction < 30%, and 11) history of receiving therapies with other non-statin hypolipidemic treatment (e.g. fibrates, niacin, or fish oils) during the last 2 months before study entry.
In the present work, only subjects with metabolic syndrome were included. According to the Chinese Guidelines on Prevention and Treatment of Dyslipidemia in Adults, and National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) Criteria for Asian Americans, metabolic syndrome was identified by the diagnostic criteria of abdominal obesity defined by waist circumference ≥ 90 cm for men and ≥ 85 cm for women, TG ≥ 150 mg/dL, HDL-C < 40 mg/dL for men and < 50 mg/dL for women, fasting glucose ≥ 100 mg/dL and blood pressure ≥ 130/85 mm Hg [5, 15]. The diagnosis of metabolic syndrome was identified by the presence of three or more of these components [5, 15]. The study randomized patients at 10 sites in Mainland China. All subjects provided their written informed consent. The study protocol was reviewed and approved by the institutional ethics committee.
At baseline and week 8 visits, blood pressure was measured using a mercury sphygmomanometer. Body weight and height measurements were also performed. Body mass index (BMI) was calculated by body weight/height2. Waist circumference was measured in the narrowest part between the lower rib and the top of the hip. Patients were given individualized therapeutic lifestyle changes and dietary instructions, according to the NCEP-ATP III guidelines [5]. During the study period, all participants were asked to visit the clinic monthly for diet compliance assessment.
Patients were randomized to receive moderate-dose statin (the same open-label statin treatment as before with the dosage remaining stable) plus placebo group and moderate-dose statin plus CoA 400 U/day group at the randomization visit. Medication compliance was assessed at week 4 and 8 using a pill count.
Blood samples were obtained for lipoprotein profile and clinical chemistry (including glucose, ALT, AST, serum creatinine, and CPK) analyses at baseline, weeks 4 and 8 after fasting for at least 12 h. These analyses were performed in a central laboratory. Blood samples were mailed to the central laboratory located in First Affiliated Hospital, College of Medicine, Zhejiang University within 48 h in insulated container. Serum lipids and clinical chemistry were analyzed on a Hitachi 7600-210 analyzer (Hitachi High-Technologies, Tokyo, Japan). Pregnancy testing was assessed for women of childbearing potential (chemiluminometric immunoassay).
Statistical analysis
Data were collected for subjects with a diagnosis of metabolic syndrome at randomization. Of a total of 304 subjects randomized, 212 (69.7%) had metabolic syndrome.
The last observation carried forward method was adopted to impute missing post-baseline values for participants who discontinued the study after randomization. Mean percent changes from baseline to follow-up were assessed by an ANOVA with the baseline lipid parameter value as a covariate. The percentage and number of patients achieving therapeutic goals of LDL-C < 100 mg/dL, non-HDL-C < 130 mg/dL, and the combined therapeutic goal of these two parameters at the end of treatment were also evaluated. Statistics were calculated using SAS/STAT version 9.13 (SAS Institute, Inc., Cary, NC, USA).
Results
Two hundred twelve subjects with metabolic syndrome were randomized and treated in the study. Two hundred of 212 completed the whole study. The study populations for efficacy evaluation included 212 patients (94 in the S group and 118 in the SC group), receiving at least one dose of the study drug and had post-baseline efficacy data. Baseline clinical and demographic characteristics were similar between the two groups (Table 1). Overall, approximately 75% of participants were < 65 years old; about 60% of participants weighed over 70 kg. The most often used statins at baseline were atorvastatin (64.6%) and simvastatin (18.4%). There were no statistically significant differences between the two groups in the frequency of statins used.
Table 1. Demographics and Baseline Characteristics.
| Characteristic | Statin + CoA (n = 118) | Statin + placebo (n = 94) | P value |
|---|---|---|---|
| Age (years) | 55.6 ± 11.6 | 53.6 ± 13.1 | 0.25 |
| Male gender (n, %) | 65 (55.1) | 59 (62.8) | 0.27 |
| Current smokers (n, %) | 38 (32.2) | 36 (38.3) | 0.39 |
| BMI (kg/m2) | 26.0 ± 2.7 | 25.8 ± 2.7 | 0.52 |
| Waist circumference (cm) | 91.4 ± 10.6 | 90.9 ± 10.1 | 0.72 |
| SBP (mm Hg) | 134.8 ± 12.5 | 131.4 ± 14.5 | 0.07 |
| DBP (mm Hg) | 81.6 ± 9.7 | 81.6 ± 8.7 | 0.96 |
| Hypertension (n, %) | 56 (47.5) | 40 (42.6) | 0.49 |
| Diabetes mellitus (n, %) | 21 (17.8) | 23 (24.5) | 0.24 |
| ACEIs/ARBs (n, %) | 35 (29.7) | 24 (25.5) | 0.54 |
| β-blockers (n, %) | 25 (21.2) | 17 (18.1) | 0.61 |
| Calcium channel blockers (n, %) | 34 (28.8) | 18 (19.1) | 0.11 |
| Diuretics (%, n) | 7 (5.9) | 1 (1.1) | 0.079 |
| Platelet aggregation inhibitors (n, %) | 18 (15.3) | 14 (14.9) | 1.00 |
| Statins | 0.70 | ||
| Atorvastatin (n, %) | 74 (62.7) | 63 (67.0) | |
| Rosuvastatin (n, %) | 12 (10.2) | 5 (5.3) | |
| Simvastatin (n, %) | 22 (18.6) | 17 (18.1) | |
| Fluvastatin (n, %) | 9 (7.6) | 7 (7.4) | |
| Lovastatin (n, %) | 1 (0.8) | 0 (0.0) | |
| MetS components (n) | 3 (3 - 5) | 3 (3 - 5) | 0.37 |
| Waist criterion (n, %) | 79 (66.9) | 63 (67.0) | 1.00 |
| FPG criterion (n, %) | 82 (69.5) | 68 (72.3) | 0.76 |
| TG criterion (n, %) | 118 (100.0) | 94 (100.0) | 1.00 |
| HDL-C criterion (n, %) | 41 (34.7) | 43 (45.7) | 0.12 |
| BP criterion (n, %) | 93 (78.8) | 69 (73.4) | 0.42 |
CoA: coenzyme A; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; MetS: metabolic syndrome; FPG: fasting plasma glucose; BP: blood pressure.
The presence of metabolic syndrome components was well-balanced between the two groups at baseline. About 70% of patients in each group had increased blood pressure, and all had elevated TG levels. After treatment for 8 weeks, the percentage of patients who met the criteria of metabolic syndrome was significantly reduced in both groups. The proportion of participants who fulfilled the diagnostic criteria of metabolic syndrome was significantly lower in the SC group when compared with S group (80/118 (67.8%) vs. 76/94 (80.9%) respectively, P = 0.041). This was due to a larger reduction in the proportion of patients who met the diagnostic criteria of elevated TG level in this group.
The changes in serum lipoprotein levels are presented in Table 2. Statin plus CoA treatment reduced TC, LDL-C, and non-HDL-C levels significantly when compared with baseline (all, P < 0.01). Significant reduction in non-HDL-C level was also noted in S group (P < 0.05). The changes in these lipid levels were larger in the SC group when compared with the S group (P < 0.05, Fig. 1). So, there were more patients attained the LDL-C and non-HDL-C targets in the SC group (70.3 and 60.2% respectively) than in the S group (56.4 and 45.7% respectively) (both, P < 0.05, Fig. 2). The percentage of participants who attained the combined goal of LDL-C and non-HDL-C was significantly larger with statin plus CoA when compared with statin monotherapy (57.6% vs. 42.6%, P = 0.038, Fig. 2). Both therapeutic regimens reduced TG levels significantly (both, P < 0.05). The reduction was significantly larger in the SC compared with the S group (P = 0.0002, Fig. 1). No significant difference was noted in the percent changes of HDL-C levels between the two groups.
Table 2. Percentage Change From Baseline to Follow-Up in Levels of Serum Lipoproteins.
| Variable | Statin + CoA (n = 118) | Statin + placebo (n = 94) | P value |
|---|---|---|---|
| TG | |||
| Baseline mean (mg/dL) | 318.8 ± 114.2 | 305.5 ± 99.2 | |
| Final mean (mg/dL) | 215.2 ± 126.6 | 275.4 ± 172.7 | |
| Mean change, % ± SE (median) | -32.47 ± 28.30 (-36.81) | -8.69 ± 49.12 (-19.66) | 0.0002 |
| TC | |||
| Baseline mean (mg/dL) | 204.2 ± 42.9 | 192.2 ± 42.5 | |
| Final mean (mg/dL) | 181.7 ± 38.3 | 183.3 ± 44.9 | |
| Mean change, % ± SE (median) | -9.56 ± 17.85 (-9.86) | -3.58 ± 16.47 (-4.57) | 0.013 |
| HDL-C | |||
| Baseline mean (mg/dL) | 45.2 ± 12.4 | 42.2 ± 11.6 | |
| Final mean (mg/dL) | 48.3 ± 13.9 | 45.6 ± 14.7 | |
| Mean change, % ± SE (median) | 10.07 ± 30.08 (5.93) | 9.73 ± 27.12 (6.49) | 0.93 |
| LDL-C | |||
| Baseline mean (mg/dL) | 111.0 ± 35.6 | 103.2 ± 35.2 | |
| Final mean (mg/dL) | 99.4 ± 36.3 | 100.9 ± 35.2 | |
| Mean change, % ± SE (median) | -7.48 ± 31.40 (-11.36) | 2.08 ± 33.09 (-2.84) | 0.033 |
| Non-HDL-C | |||
| Baseline mean (mg/dL) | 159.3 ± 39.4 | 150.0 ± 41.0 | |
| Final mean (mg/dL) | 133.4 ± 37.5 | 137.7 ± 43.3 | |
| Mean change, % ± SE (median) | -14.31 ± 22.20 (-15.84) | -6.38 ± 22.40 (-7.38) | 0.011 |
CoA: coenzyme A; TG: triglyceride; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol.
Figure 1.
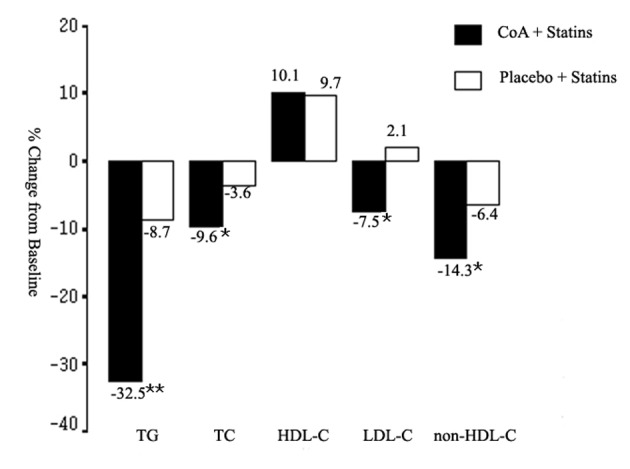
Mean percent change in triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and non-HDL-C from baseline to the end of study. CoA: coenzyme A. *P < 0.05; **P < 0.01.
Figure 2.
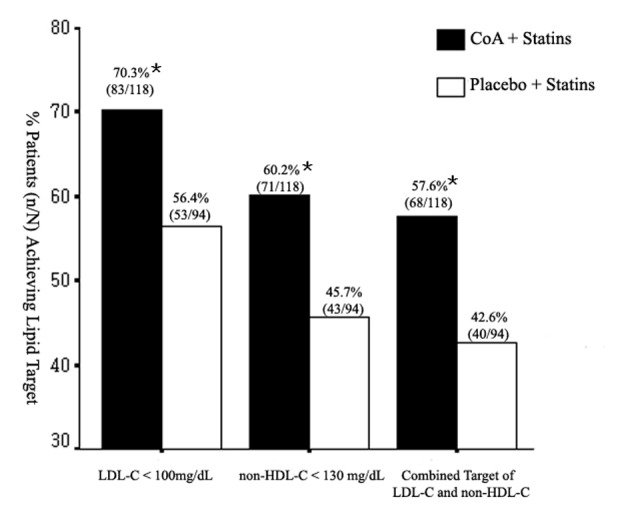
Rates of achievement of the NCEP ATPIII therapeutic goals at the end of treatment. *P < 0.05.
The safety profile of these two therapeutic regimens was similar. No significant difference was found between the two groups in the proportion of participants who experienced adverse events (Table 3). There were no symptoms leading to treatment discontinuation (e.g. myalgias, gastrointestinal symptoms) or clinically relevant increase of creatine kinase (> 5 × ULN) or AST/ALT (> 3 × ULN) in any group. No more than 3.2% of patients presented mild elevations of creatine kinase < 3 × ULN in each group (Table 3).
Table 3. Incidence of Adverse Events.
| Variable | Total (n = 212) | Statin + CoA (n = 118) | Statin + placebo (n = 94) |
|---|---|---|---|
| Any AE | 15 | 10 | 5 |
| Patients who experienced any AE | 15 | 10 | 5 |
| Most common AEs | |||
| Abdominal distention | 1 | 1 | 0 |
| Nausea | 1 | 1 | 0 |
| Elevated liver enzymes | 1 | 1 | 0 |
| Elevated creatine phosphokinase | 5 | 2 | 3 |
| Upper respiratory tract infection | 3 | 3 | 0 |
CoA: coenzyme A; AE: adverse event.
Discussion
This work is a post hoc analysis of a previously published phase 3, double-blinded, randomized, placebo-controlled study that assessed the effect and safety of statin plus CoA combination regimen in patients with mixed dyslipidemia [14]. In the previous study, 304 patients with mixed dyslipidemia were randomized to either moderate dose of statin monotherapy (S) or moderate-dose statin combined with coenzyme A (SC) [14]. The present post hoc analysis included only participants with metabolic syndrome. In this analysis, we found that SC reduced TG, LDL-C, and non-HDL-C levels more than statin monotherapy. These findings are consistent with our previous observation of lipid-altering effects in the whole population consisting of participants with mixed dyslipidemia independently of the presence of metabolic syndrome [14].
Because metabolic syndrome presents a string of various cardiometabolic risk factors, it is the main goal of hypolipidemic treatment targeting LDL-C and non-HDL-C levels (second therapeutic target, if TG > 200 mg/dL) in patients with metabolic syndrome and mixed hyperlipidemia according to the NCEP-ATPIII guidelines [5]. Although statins have been considered the first-line intervention for controlling elevated LDL-C levels, quite a lot of patients with high risk of cardiovascular disease cannot achieve optimal lipid goals and adding other hypolipidemic agents may provide additional benefits in optimizing the serum lipoprotein profile [16]. The achievement of LDL-C and/or non-HDL-C targets has already been assessed in lots of clinical studies with statin monotherapy or combinating with other hypolipidemic drugs [17-21]. It was reported that 24-92% of patients treated with different doses of statins monotherapy or combination therapy achieved LDL-C and/or non-HDL-C goals [17-21]. In this analysis, significantly greater percent changes in LDL-C, TG, and non-HDL-C with SC were noted in patients with metabolic syndrome and mixed dyslipidemia, when compared with statin monotherapy. Meanwhile, SC showed its better efficacy in attaining LDL-C, non-HDL-C targets and the combined goal of these two lipoprotein parameters than statin monotherapy. Although there were differences in duration of therapy and study design, the results of target attainment in these studies are similar to the percentage of participants who attained LDL-C and/or non-HDL-C goals with SC in the present analysis.
Combination therapy with statin plus CoA had generally fine tolerability in the subset of participants with metabolic syndrome, consisting with the safety profile of each monotherapy [13, 14]. No myalgia or rhabdomyolysis was reported in this analysis. Occurrences of elevated liver enzymes were infrequent and no abnormal creatine value was noted in this analysis.
CoA capsule was a newly developed lipid-lowering agent. The mechanism of lipid modifying effects with CoA has not been established thoroughly. As one of the most important compounds in the tricarboxylic acid cycle and cofactors for oxidative and biosynthetic reactions in metabolism, adding CoA may promote fat decomposition and normalize different patterns of lipoproteins [22].
The major limitations for this analysis are attributed to constraint of trial design. The duration of therapy was limited to 8 weeks in this study. Although it was sufficient to assess the efficacy of a lipid-lowering agent, it is necessary to know the long-term therapeutic profile of an anti-hyperlipidemic agent. Considering the safety of subjects, only moderate-dose of statins was used in this study, the full therapeutic effect of this regimen may not be accurately reflected in this study and remains unknown. Further studies with longer follow-up duration and higher dose of statin in combining with CoA will be required to fully establish the efficacy and tolerability of co-administration of CoA and statin.
Although these limitations exist, the present analysis clearly demonstrated the efficacy and safety profile of statin plus CoA combination therapeutic regimen in lipid modifying and in achieving individual and combined lipid goals in patients with metabolic syndrome and mixed dyslipidemia.
Conclusions
In the present work, the addition of CoA 400 U/day to ongoing moderate dose of statin was effective and safe in providing additional lowering of TG, TC, LDL-C, and non-HDL-C levels and achieving individual and combined lipid targets in patients with metabolic syndrome and mixed hyperlipidemia.
Acknowledgments
We gratefully thank Dr. Mingshuan Guo from Xuchang Central Hospital, Dr. Xiaoping Chen from West China Hospital of Sichuan University, Dr. Xiangqun Liu from Qilu Hospital of Shandong University, Dr. Xiaoqing Wu from the People’s Hospital of Wuxi, Dr. Shijuan Lu from the People’s Hospital of Haikou, Dr. Nan Wang from the People’s Hospital of Gansu, Bangning Wang from the First Hospital Affiliated to Anhui Medical College, Dr. Shuiping Zhao from Second Xiangya Hospital Affiliated to Central South University, and Dr. Shaocai Hong from Armed Police Guangxi Corps Hospital for giving assistance in collecting the clinical data.
Financial Disclosure/Funding
This study was supported financially by Public Technology Research and Social Development Project of Zhejiang Province (No. 2013C33118) and Institute for Herb Biomedical Engineering in Shanghai.
Competing Interests
The authors declare no conflict of interest.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CoA
coenzyme A
- CPK
creatine phosphokinase
- HDL-C
high-density lipoprotein-cholesterol
- LDL-C
low-density lipoprotein-cholesterol
- S group
statin monotherapy group
- SC group
statin-coenzyme A combination group
- TG
triglyceride
- TC
total cholesterol
- ULN
upper limit of normal
References
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ. et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Yamaoka K, Tango T. Effects of lifestyle modification on metabolic syndrome: a systematic review and meta-analysis. BMC Med. 2012;10:138. doi: 10.1186/1741-7015-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S. et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Reyes-Soffer G, Rondon-Clavo C, Ginsberg HN. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 6.Reyes-Soffer G, Rondon-Clavo C, Ginsberg HN. Combination therapy with statin and fibrate in patients with dyslipidemia associated with insulin resistance, metabolic syndrome and type 2 diabetes mellitus. Expert Opin Pharmacother. 2011;12(9):1429–1438. doi: 10.1517/14656566.2011.563506. [DOI] [PubMed] [Google Scholar]
- 7.Fruchart JC, Sacks FM, Hermans MP, Assmann G, Brown WV, Ceska R, Chapman MJ. et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in dyslipidaemic patient. Diab Vasc Dis Res. 2008;5(4):319–335. doi: 10.3132/dvdr.2008.046. [DOI] [PubMed] [Google Scholar]
- 8.McKeage K, Keating GM. Fenofibrate: a review of its use in dyslipidaemia. Drugs. 2011;71(14):1917–1946. doi: 10.2165/11208090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Guo J, Meng F, Ma N, Li C, Ding Z, Wang H, Hou R. et al. Meta-analysis of safety of the coadministration of statin with fenofibrate in patients with combined hyperlipidemia. Am J Cardiol. 2012;110(9):1296–1301. doi: 10.1016/j.amjcard.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 10.Consolo F, Di Tommaso G, Arrigo F, Trifiro A. [Coenzyme A and dyslipidemia. Note III. Action of coenzyme A on changes in the blood lipid pattern after administration of dietary lipids] Boll Soc Ital Biol Sper. 1975;51(24):1931–1936. [PubMed] [Google Scholar]
- 11.Daugherty M, Polanuyer B, Farrell M, Scholle M, Lykidis A, de Crecy-Lagard V, Osterman A. Complete reconstitution of the human coenzyme A biosynthetic pathway via comparative genomics. J Biol Chem. 2002;277(24):21431–21439. doi: 10.1074/jbc.M201708200. [DOI] [PubMed] [Google Scholar]
- 12.Dong Na, Pan Jing-Wei, Zhang Da, Su Ding-Feng, Chen Hong. The effects of an oral preparation of Coenzyme A on serum lipids of food-induced hyperlipidemia in rabbits and rats. Academic Journal of Second Military Medical University. 2004;25(6):255–257. [Google Scholar]
- 13.Zhao S, Chen Y, Chen J, Lai J. Effect and safety of coenzyme A on Hyperlipidemia. Chinese Journal of New Drugs and Clinical Remedies. 2012;5:242–245. [Google Scholar]
- 14.Lai J, Wu B, Xuan T, Liu Z, Chen J. Efficacy and tolerability of adding coenzyme A 400 U/d capsule to stable statin therapy for the treatment of patients with mixed dyslipidemia: an 8-week, multicenter, double-blind, randomized, placebo-controlled study. Lipids Health Dis. 2014;13:1. doi: 10.1186/1476-511X-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. Chinese Guidelines on Prevention and Treatment of Dyslipidaemia in Adults. Chinese Journal of Cardiology. 2007;35(5):390–419. [PubMed] [Google Scholar]
- 16.Kostapanos MS, Katsiki N, Elisaf MS, Mikhailidis DP. Editorial: reducing cardiovascular risk: is low-density lipoprotein-cholesterol (LDL-C) lowering enough? Curr Vasc Pharmacol. 2012;10(2):173–177. doi: 10.2174/157016112799305085. [DOI] [PubMed] [Google Scholar]
- 17.Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, Cain VA. et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial) Am J Cardiol. 2003;92(2):152–160. doi: 10.1016/S0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 18.Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. Am Heart J. 2005;149(3):464–473. doi: 10.1016/j.ahj.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Stalenhoef AF, Ballantyne CM, Sarti C, Murin J, Tonstad S, Rose H, Wilpshaar W. A comparative study with rosuvastatin in subjects with metabolic syndrome: results of the COMETS study. Eur Heart J. 2005;26(24):2664–2672. doi: 10.1093/eurheartj/ehi482. [DOI] [PubMed] [Google Scholar]
- 20.Agouridis AP, Tsimihodimos V, Filippatos TD, Tselepis AD, Elisaf MS. High doses of rosuvastatin are superior to low doses of rosuvastatin plus fenofibrate or n-3 fatty acids in mixed dyslipidemia. Lipids. 2011;46(6):521–528. doi: 10.1007/s11745-011-3538-0. [DOI] [PubMed] [Google Scholar]
- 21.Robinson JG, Ballantyne CM, Hsueh W, Rosen J, Lin J, Shah A, Lowe RS. et al. Achievement of specified low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol apolipoprotein B, and high-sensitivity C-reactive protein levels with ezetimibe/simvastatin or atorvastatin in metabolic syndrome patients with and without atherosclerotic vascular disease (from the VYMET study) J Clin Lipidol. 2011;5(6):474–482. doi: 10.1016/j.jacl.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 22.McCarty MF. Inhibition of acetyl-CoA carboxylase by cystamine may mediate the hypotriglyceridemic activity of pantethine. Med Hypotheses. 2001;56(3):314–317. doi: 10.1054/mehy.2000.1155. [DOI] [PubMed] [Google Scholar]


