Abstract
Background
Ischemic stroke usually initiates inflammation and oxidative/nitrosative stress leading to neuronal death.
Aim
To investigate the existence of oxidative/nitrosativestress in rats subjected to focal cerebral ischemia/reperfusion and its effects on the consequent neurological deficits.
Material and Method
Experimental procedures were performed on 30 adult males Wister rats. In the test group, transient focal cerebral ischemia was induced in 15 rats by occlusion of the left common carotid artery (CCA) for 30 minutes followed by reperfusion for 24 hours. Another 15 rats underwent the surgery at the same neck region without occlusion of CCA and served as a control group. Neurobehavioral tests were evaluated, the levels of malondialdehyde (MDA), total antioxidant capacity (TAC) and nitric oxide (NO) metabolites were measured in the serum and brain tissue to detect the effect of surgery on in each group.
Result
The serum and brain tissue levels of MDA and NO in the test group were significantly higher compared to the control group (P < 0.001). In contrast, serum and brain tissue levels of TAC of rats subjected to ischemia reperfusion was significantly lower compared to the sham operated rats (P < 0.001). Neurological deficit of the test group correlated positively with serum TAC (CC = 0.937, P = 0.000) and brain tissue TAC (CC = 0.949, P = 0.000) and negatively with serum MDA (CC = −0.949, P = 0.000), brain tissue MDA (CC = −0.963, P = 0.000), serum NO (CC = −0.942, P = 0.000) and brain tissue NO (CC = −0.952, P = 0.000).
Conclusion
The study provided further evidence for the presence of oxidative/nitrosative stress in rats subjected to cerebral ischemia/reperfusion and demonstrates a relationship between oxidative/nitrosative biomarkers and the consequent neurological deficits.
Keywords: Cerebral, ischemia/reperfusion, L-NAME, malondialdehyde, nitric oxide, total antioxidant capacity
Introduction
Stroke is one of the leading causes of permanent disability worldwide and the second commonest cause of death. (1) Approximately 80% of strokes are ischemic in origin.(2) Advances intravascular techniques and thrombolytic agents have reduced functional deficits in stroke patients if intervention given within an optimal time window. However, reperfusion itself leads to reperfusion injury (RI).(3, 4) The pathophysiology regarding RI is still obscured; though involvement of oxidative stress mediators such as reactive oxygen species (ROS), nitric oxide (NO) and peroxynitrite anion (ONOO_) are potential arbitrators of brain damage. (5)
Localized cerebral ischemia is coupled with focal inflammatory reactions that ultimately end with cell death. (6) Pro-inflammatory mediators and ROS are released by microglial cells following tissue injury induced by ischemia. (7) Moreover, hypoxia may enhance formation of superoxide radicals and hydrogen peroxide by activating phospholipase C and increasing level of xanthine oxidase in the cerebral blood. (8, 9) Oxidative stress induced peroxidation of cell membrane lipids results in alterations in the biological properties of the membrane. Therefore, quantification of lipid peroxidation end products can faithfully reflect body oxidative damage. (10) Malondialdehyde (MDA) is frequently used as an indicator of lipid peroxidation and correlated well with the size of ischemic stroke as well as the clinical outcome. (11) In addition, measurement of the total antioxidant capacity (TAC) is believed to be a useful measure of the availability of the antioxidants which present to guard against oxidative cell damage. (12, 13)
The aim of this study was to evaluate oxidative/nitrosative stress in rats subjected to transient cerebral ischemia and to correlate the level of oxidative/nitrosative biomarkers with the resulting neurological deficit score.
Materials and Methods
Animals
The current study was approved by the Ethical Committee of the University of Alexandria, and the investigations conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). 30 Male Wistar rats, weighing 150–250 g were selected and preserved at a constant temperature of 22±2 °C with a fixed 12:12-h light-dark cycle. Nutritionally balanced pellets and water were freely available. The animals were randomly divided into two groups (15 rats in each group): test and control.
Cerebral ischemia induction
The animals were fasted overnight prior to surgery with free access to tap water. Anesthesia was induced by ether inhalation and maintained by thiopental sodium (2.5mg/kg). (14) Body temperature was kept constant at 36.5±0.5 °C using heating pad. A longitudinal cervical incision (2cm) was made lateral to the midline and the common carotid artery (CCA) was carefully dissected. Ischemia was induced by placing non traumatic microvascular clip on left CCA just prior to its bifurcation. (15) During ischemia rats were monitored for body temperature and respiration pattern. The vascular occlusion was maintained for 30 minutes, and then the clips were removed to resume blood flow to the ischemic region for 24 hours. (16) Finally, the incisions were sutured, the animal was allowed to recover from anesthesia, and returned to a warm cage for recuperation during reperfusion period.
In the control group (n = 15), the rats underwent the surgery at the neck region without occlusion of CCA and served as a control group. Alternatively, the ischemia reperfusion group (n = 15), brain ischemia was maintained for 30 minutes by internal CCA ligation followed by 24 hours reperfusion. The number of animals presented for each group is the number of rats that survived during 24 hour reperfusion period. The collected data of the animals that died during 24 hours reperfusion period were excluded.
Neurological and behavioral evaluation
Neurobehavioral tests of all experimental groups were assessed daily to determine the effect of ischemic injury on them. Neurobehavioral evaluations were performed three times: the day before surgery, the surgery day and just before killing the animals. Each rat was examined in the late afternoon hours so that rats that had been operated in the morning would fully recovered from the effects of anesthesia by the time of evaluation. The neurobehavioral study consisted of the following six tests: spontaneous activity, symmetry in the movement of the four limbs, forepaw outstretching, climbing, body proprioception and response to vibrissae touch. The score given to each rat at the end of the evaluation is the summation of all six individual test scores. The minimum neurological score was 3 and the maximum was 18. (15)
Laboratory investigations
At the end of experimental period, the rats were sacrificed by decapitation. Brains were rapidly removed from the skull and washed with cold saline and stored at −20 °C for further analysis. A small part of each brain from the affected hemisphere was dissected in to approximately 1–2mm pieces and they were homogenized in 7 ml of ice-cold extraction buffer contain: (1% Triton X-100, 10 mmol/l MgSO4, 1 mmol/l EDTA, 1 mmol/l dithiothreitol, 0.5 mol/l NaCl,1% protease inhibitor cocktail, 20 mmol/l HEPES (pH 7.5). (17) The homogenate was centrifuged; the supernatant was taken and stored at −20°C until being used. A modification of the method of Lowry was used for the determination of protein in the brain homogenate. (18) The level of the nitric oxide metabolites (nitrite and nitrate) (19, 20) and TAC concentrations were measured colorimetrically while Satoh method was used to measure serum and brain homogenate MDA levels. (21)
Data analysis
Statistical evaluation was performed using the Microsoft Office Excel (Microsoft Office Excel for windows; 2003) and SPSS (SPSS for windows version 19). Screening studied rats’ groups for significant difference in the mean of MDA, NO and TAC was performed using Student T-test while the relationships between these biomarkers and the resulting neurological deficit score of rats subjected to ischemia reperfusion were assessed using bivariate correlations. P < 0.05 was considered significant.
Results
As shown in figure 1, the concentrations of serum MDA and NO in rats subjected to ischemia reperfusion (14.88±1.14nmol/mL and 42.03±4.558 μmol/L respectively) were significantly higher compared to sham operated rats (5.43±0.44nmol/mL and 17.84±0.701 μmol/L respectively, P < 0.001). In contrast, the level of serum TAC of rats subjected to ischemia reperfusion (1.21±0.169 mM/L) was significantly lower compared to the sham operated rats (2.52±0.062 mM/L, P < 0.001).
Figure 1.
Serum level of MDA, NO and TAC in sham operated and in ischemia reperfusion rats.
*Significant with control group
There were significantly increased level of MDA and NO in brain tissue of rats subjected to ischemia reperfusion (8.56±0.66 nmol/mg protein and 8.88±0.572 μmol/mg protein respectively) compared to sham operated rats (3.24±0.25 nmol/mg protein and 3.48±0.228 μmol/mg protein, P< 0.001) (figure 2). The brain TAC level of rats subjected to ischemia reperfusion (0.0186±0.00373mmol/mg protein) was significantly lower compared to the sham operated rats (0.070±0.0085 mmol/mg protein, P < 0.001) (figure 2).
Figure 2.
Brain tissue level of MDA, NO and TAC in sham operated and in ischemia reperfusion rats.
*Significant with control group
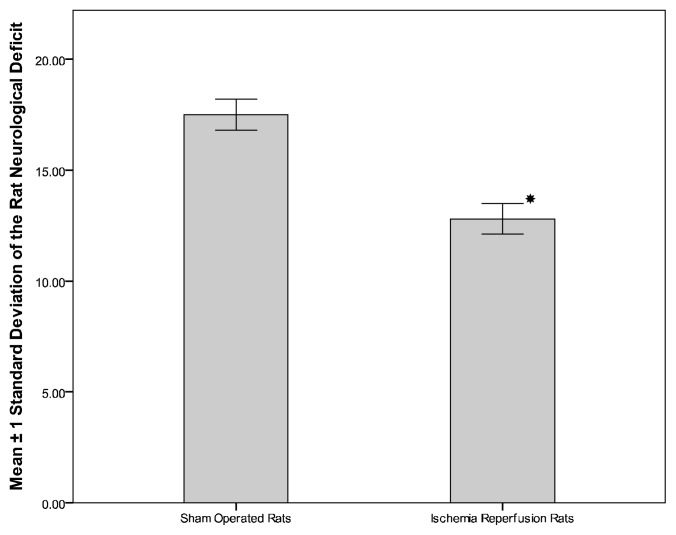
Figure 3 demonstrate the significant decrease in the neurological deficit score in rats subjected to ischemia reperfusion (12.798±0.689) compared to sham operated rats (17.50±0.707, P< 0.001). The relationships between MDA, NO, and TAC levels and neurological deficit score of rats subjected to ischemia reperfusion are given in table 1. In addition, MDA correlates negatively with TAC in the serum (CC = −0.967, P = 0.000) and brain tissue (CC = −0.966, P = 0.000).
Figure 3.
Neurological deficit score of sham operated and in ischemia reperfusion rats.
*Significant with control group
Table 1.
Correlations between neurological deficit score and the level of studied biomarkers
| The Biomarker | Correlation Coefficient | P | ||
|---|---|---|---|---|
| Tissue Level | MDA(nmol/ml) | −0.963 | .000 | |
| NO (umol/L) | −0.952 | .000 | ||
| Neurological deficit | TAC (mM/L) | 0.949 | .000 | |
| Serum Level | MDA(nmol/ml) | −0.949 | .000 | |
| NO (umol/L) | −0.942 | .000 | ||
| TAC (mM/L) | 0.937 | .000 | ||
Discussion
In the present study, it was observed that rats subjected to cerebral ischemia for 30 min then followed by reperfusion for 24 hours had significantly higher serum and brain tissue levels of MDA and NO compared to sham-operated rats. In contrast, TAC was significantly lower in the test group. These findings provide an evidence for the presence of oxidative stress in the studied model. The current results are comparable with previous studies conducted to evaluate oxidative stress in rats with transient focal or globalcerebral ischemia. (5, 8, 22) Moreover, the preceding researches demonstrated enhanced levels of oxidative stress biomarkers and reduced levels of antioxidants, (23) however, the pattern of changes in the levels of antioxidant enzymes is controversial. (12)
According to Liu et al data, there was significantly higher level of MDA in rats subjected to 90 minutes of ischemia followed by 12, 24, 72 hour and up to 7 days reperfusion of the middle cerebral artery. (23) These findings were further supported by Zhang et al who showed significantly higher level of MDA, NO, and reduced antioxidant enzymes in rats with cerebral ischemia. (24) Comparable ischemia/reperfusion studies in human are scarce; however, their findings are analogous with animal models of stroke. For example, an old study conducted by Soong et al in patients undergoing carotid end arterectomy revealed increased plasma level of MDA only 60 seconds after carotid clamp release. (25) Three years later, Weigand et al investigated cerebral formation of MDA as an index of lipid peroxidation in relation to different sources of ROS in patients undergoing carotid endarterectomy. A carotid artery shunt was placed in some patients after complete loss of somatosensory evoked potentials, indicating significant focal cerebral ischemia. The results suggested enhanced level of MDA before reperfusion and an additional rise 15 minutes after reperfusion. In addition, plasma total antioxidant status significantly decreased during carotid artery occlusion only in patients with carotid artery shunt. (26)
It’s worth mentioning that previous reports repeatedly suggest significant attenuation in TAC in cerebral ischemia. (27) The TAC was evaluated by Uzaret alina model of global cerebral ischemia/reperfusion induced by bilateral clamping of the CCA and through hypotension.(28) Ischemia/reperfusion produced a significant decrease in brain TAC, increase in the levels of total oxidant status and MDA levels. Similar finding was obtained by Simao et al in another experimental model of transient global cerebral ischemia due to four cerebral vessels occlusion. (29)
The current study showed a highly significant negative correlation between serum MDA levels and TAC in ischemia/reperfusion rats, which suggested increased utilization by ROS as a contributing factor to low TAC in rats with cerebral ischemia. (30) Also there is negative correlation between neurological deficit score and both serum and brain tissue levels of MDA and NO. These findings suggested the possibility that production of MDA and NO in the brain may persuade disturbance of neuronal networks leading to neurological dysfunction.
In this study the ratio of nitrite/nitrate was taken as an indirect marker of NO production. (31) Interestingly, the present data implied an overproduction of NO in both the rats’ serum and ischemic brain tissue. In addition, these results were well matched with the decrease in plasma and brain tissue of TAC in ischemic group. Therefore, the observed changes can be taken as indirect evidence that NO metabolism might contribute to ROS generation in rats subjected to ischemia/reperfusion. Moreover, the significant negative correlation between serum level of NO and neurological deficit score suggests potential toxic role NO to the brain. Although enhanced level of NO is a common reproducible outcome of many previous works, (32–35) a very recent report demonstrated lower level of NO in ischemia/reperfusion rats compared with the control group. (36) A possible explanation for this dispute is the type of nitric oxide synthase (NOS) responsible for production of NO measured by different studies. NO synthesized by the action of three different NOS isoforms; endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS). (36) NO derived from eNOS has been suggested to have neuroprotective effects during cerebral ischemia (37) while NO produced by nNOS, iNOS can be neurotoxic. (38) A shortcoming of this study is that it has not measured the enzymatic activity of different NOS isoforms. Additional researches are desirable to identify the offending cause of increased NO level during cerebral ischemia. Inhibition of such injurious NOS isoform(s) can be a potential protective measure following ischemic stroke.
In conclusion, the current study added further evidence for the presence of oxidative/nitrosative stress in rats subjected to cerebral ischemia/reperfusion and demonstrates a direct relationship between oxidative/nitrosative biomarkers and the consequent neurological deficits.
Acknowledgment
During this work we have collaborated with many colleagues in Alexandria University, Egypt, for whom I have great regard, and I wish to extend my sincere thanks to Dr. Ali. M. Kobil.
Footnotes
Competing interest
On behalf of all authors, the corresponding author states that there is no conflict of interest
References
- 1.Bi Q, Wang T, Zhang W. Frequency and etiological diagnosis of ischemic stroke in Chinese young adults. Neurol Res. May;34(4):354–8. doi: 10.1179/1743132812Y.0000000023. [DOI] [PubMed] [Google Scholar]
- 2.Kidd PM. Integrated brain restoration after ischemic stroke--medical management, risk factors, nutrients, and other interventions for managing inflammation and enhancing brain plasticity. Altern Med Rev. 2009 Mar;14(1):14–35. [PubMed] [Google Scholar]
- 3.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan J, Konstas AA, Bateman B, Ortolano GA, Pile-Spellman J. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology. 2007 Feb;49(2):93–102. doi: 10.1007/s00234-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15(1):1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- 6.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003 May;4(5):399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 7.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009 Feb 6;158(3):1021–9. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 8.Sudha K, Rao AV, Rao SN, Rao A. Oxidative damage and plasma antioxidants in cerebrovascular accident. Indian J Physiol Pharmacol. 2004 Oct;48(4):489–92. [PubMed] [Google Scholar]
- 9.Zhang L, Zhang ZG, Liu XS, Hozeska-Solgot A, Chopp M. The PI3K/Akt pathway mediates the neuroprotective effect of atorvastatin in extending thrombolytic therapy after embolic stroke in the rat. Arterioscler Thromb Vasc Biol. 2007 Nov;27(11):2470–5. doi: 10.1161/ATVBAHA.107.150748. [DOI] [PubMed] [Google Scholar]
- 10.Ferretti G, Bacchetti T, Masciangelo S, Nanetti L, Mazzanti L, Silvestrini M, et al. Lipid peroxidation in stroke patients. Clin Chem Lab Med. 2008;46(1):113–7. doi: 10.1515/CCLM.2008.011. [DOI] [PubMed] [Google Scholar]
- 11.Cherubini A, Ruggiero C, Polidori MC, Mecocci P. Potential markers of oxidative stress in stroke. Free Radic Biol Med. 2005 Oct 1;39(7):841–52. doi: 10.1016/j.freeradbiomed.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Gariballa SE, Hutchin TP, Sinclair AJ. Antioxidant capacity after acute ischaemic stroke. QJM. 2002 Oct;95(10):685–90. doi: 10.1093/qjmed/95.10.685. [DOI] [PubMed] [Google Scholar]
- 13.Ryan M, Grayson L, Clarke DJ. The total antioxidant capacity of human serum measured using enhanced chemiluminescence is almost completely accounted for by urate. Ann Clin Biochem. 1997 Nov;34(Pt 6):688–9. doi: 10.1177/000456329703400615. [DOI] [PubMed] [Google Scholar]
- 14.Keefer LK, Garland WA, Oldfield NF, Swagzdis JE, Mico BA. Inhibition of N-nitrosodimethylamine metabolism in rats by ether anesthesia. Cancer Res. 1985 Nov;45(11 Pt 1):5457–60. [PubMed] [Google Scholar]
- 15.Renolleau S, Aggoun-Zouaoui D, Ben-Ari Y, Charriaut-Marlangue C. A model of transient unilateral focal ischemia with reperfusion in the P7 neonatal rat: morphological changes indicative of apoptosis. Stroke. 1998 Jul;29(7):1454–60. doi: 10.1161/01.str.29.7.1454. discussion 61. [DOI] [PubMed] [Google Scholar]
- 16.Kuluz JW, Prado RJ, Dietrich WD, Schleien CL, Watson BD. The effect of nitric oxide synthase inhibition on infarct volume after reversible focal cerebral ischemia in conscious rats. Stroke. 1993 Dec;24(12):2023–9. doi: 10.1161/01.str.24.12.2023. [DOI] [PubMed] [Google Scholar]
- 17.Star RA. Treatment of acute renal failure. Kidney Int. 1998 Dec;54(6):1817–31. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–75. [PubMed] [Google Scholar]
- 19.Bories PN, Bories C. Nitrate determination in biological fluids by an enzymatic onestep assay with nitrate reductase. Clin Chem. 1995 Jun;41(6 Pt 1):904–7. [PubMed] [Google Scholar]
- 20.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 21.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978 Nov 15;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz M. Macrophages and microglia in central nervous system injury: are they helpful or harmful? J Cereb Blood Flow Metab. 2003 Apr;23(4):385–94. doi: 10.1097/01.WCB.0000061881.75234.5E. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Liu W, Sun X, Li R, Sun Q, Cai J, et al. Hydrogen saline offers neuroprotection by reducing oxidative stress in a focal cerebral ischemia-reperfusion rat model. Med Gas Res. 1(1):15. doi: 10.1186/2045-9912-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang JY, Jr, Si YL, Liao J, Yan GT, Deng ZH, Xue H, et al. Leptin administration alleviates ischemic brain injury in mice by reducing oxidative stress and subsequent neuronal apoptosis. J Trauma Acute Care Surg. Apr;72(4):982–91. doi: 10.1097/TA.0b013e3182405459. [DOI] [PubMed] [Google Scholar]
- 25.Soong CV, Young IS, Hood JM, Rowlands BJ, Trimble ER, Barros D’Sa AA. The generation of byproducts of lipid peroxidation following carotid endarterectomy. Eur J Vasc Endovasc Surg. 1996 Nov;12(4):455–8. doi: 10.1016/s1078-5884(96)80014-1. [DOI] [PubMed] [Google Scholar]
- 26.Weigand MA, Laipple A, Plaschke K, Eckstein HH, Martin E, Bardenheuer HJ. Concentration changes of malondialdehyde across the cerebral vascular bed and shedding of L-selectin during carotid endarterectomy. Stroke. 1999 Feb;30(2):306–11. doi: 10.1161/01.str.30.2.306. [DOI] [PubMed] [Google Scholar]
- 27.Jung HW, Mahesh R, Bae HS, Kim YH, Kang JS, Park YK. The antioxidant effects of Joongpoongtang 05 on brain injury after transient focal cerebral ischemia in rats. J Nat Med. Apr;65(2):322–9. doi: 10.1007/s11418-010-0497-3. [DOI] [PubMed] [Google Scholar]
- 28.Uzar E, Acar A, Evliyaoglu O, Firat U, Kamasak K, Gocmez C, et al. The anti-oxidant and anti-apoptotic effects of nebivolol and zofenopril in a model of cerebral ischemia/reperfusion in rats. Prog Neuropsychopharmacol Biol Psychiatry. Jan 10;36(1):22–8. doi: 10.1016/j.pnpbp.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Simao F, Matte A, Matte C, Soares FM, Wyse AT, Netto CA, et al. Resveratrol prevents oxidative stress and inhibition of Na(+)K(+)-ATPase activity induced by transient global cerebral ischemia in rats. J Nutr Biochem. Oct;22(10):921–8. doi: 10.1016/j.jnutbio.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Thanoon IA, Abdul-Jabbar HA, Taha DA. Oxidative Stress and C - reactive protein in Patients with Cerebrovascular Accident (Ischaemic Stroke): The role of Ginkgo biloba extract. Sultan Qaboos Univ Med J. May;12(2):197–205. doi: 10.12816/0003113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Archer S. Measurement of nitric oxide in biological models. FASEB J. 1993 Feb 1;7(2):349–60. doi: 10.1096/fasebj.7.2.8440411. [DOI] [PubMed] [Google Scholar]
- 32.Hirabayashi H, Takizawa S, Fukuyama N, Nakazawa H, Shinohara Y. Nitrotyrosine generation via inducible nitric oxide synthase in vascular wall in focal ischemia-reperfusion. Brain Res. 2000 Jan 10;852(2):319–25. doi: 10.1016/s0006-8993(99)02117-4. [DOI] [PubMed] [Google Scholar]
- 33.Hata R, Maeda K, Hermann D, Mies G, Hossmann KA. Evolution of brain infarction after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2000 Jun;20(6):937–46. doi: 10.1097/00004647-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Margaill I, Allix M, Charriaut-Marlangue C, Boulu RG, Plotkine M. Loss of NADPH-diaphorase containing neurones after reversible focal ischaemia in rats delayed by L-NAME. Br J Pharmacol. 1995 Nov;116(5):2344–5. doi: 10.1111/j.1476-5381.1995.tb15076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kader A, Frazzini VI, Solomon RA, Trifiletti RR. Nitric oxide production during focal cerebral ischemia in rats. Stroke. 1993 Nov;24(11):1709–16. doi: 10.1161/01.str.24.11.1709. [DOI] [PubMed] [Google Scholar]
- 36.Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009 Jun;20(4):223–30. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Cui X, Chopp M, Zacharek A, Zhang C, Roberts C, Chen J. Role of endothelial nitric oxide synthetase in arteriogenesis after stroke in mice. Neuroscience. 2009 Mar 17;159(2):744–50. doi: 10.1016/j.neuroscience.2008.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X, Haensel C, Araki E, Ross ME, Iadecola C. Gene-dosing effect and persistence of reduction in ischemic brain injury in mice lacking inducible nitric oxide synthase. Brain Res. 2000 Jul 28;872(1–2):215–8. doi: 10.1016/s0006-8993(00)02459-8. [DOI] [PubMed] [Google Scholar]