Abstract
Ischemic colitis is a rare, life-threatening, consequence of mesenteric arteriovenous malformations. Ischemia ensues from a steal phenomenon through shunting, and may be compounded by the resulting portal hypertension. Computed tomographic angiography is the most common first-line test because it is quick, non-invasive, and allows for accurate anatomic characterization. Also, high-resolution three-dimensional images can be created for treatment planning. Magnetic resonance angiography is similarly sensitive for vascular mapping. Conventional angiography remains the gold standard for diagnosis and also allows for therapeutic endovascular embolization. Our patient underwent testing using all three of these modalities. We present the first reported case of this entity in a patient with a vascular connective tissue disorder.
Keywords: Ischemic colitis, Arteriovenous malformation, Connective Tissue Disorder, CT, Computed Tomography
CASE REPORT
A 38-year-old woman presented to an outside hospital with a 2-month history of worsening left lower quadrant abdominal pain, hematochezia, and 25-pound weight loss. She had a history of presumed Marfan’s syndrome with pulmonary artery dilation and ascending aortic aneurysm, status post aortic root replacement. In the emergency department she was writhing in pain. Her vital signs were normal and she was afebrile. On physical exam she was tender to palpation in the left lower quadrant. Her white blood cell count was elevated at 17.3 K/μL (normal <11 K/μL). Her lactate was 1.5 mmol/L (normal <3).
Imaging Findings
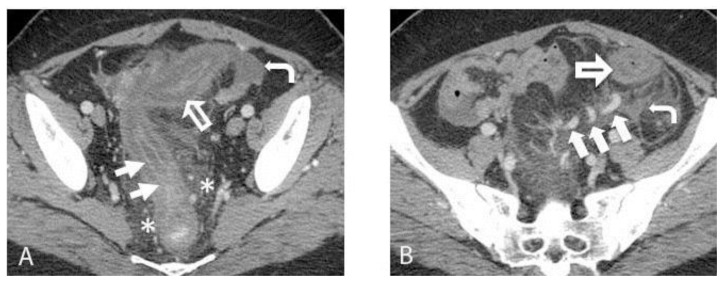
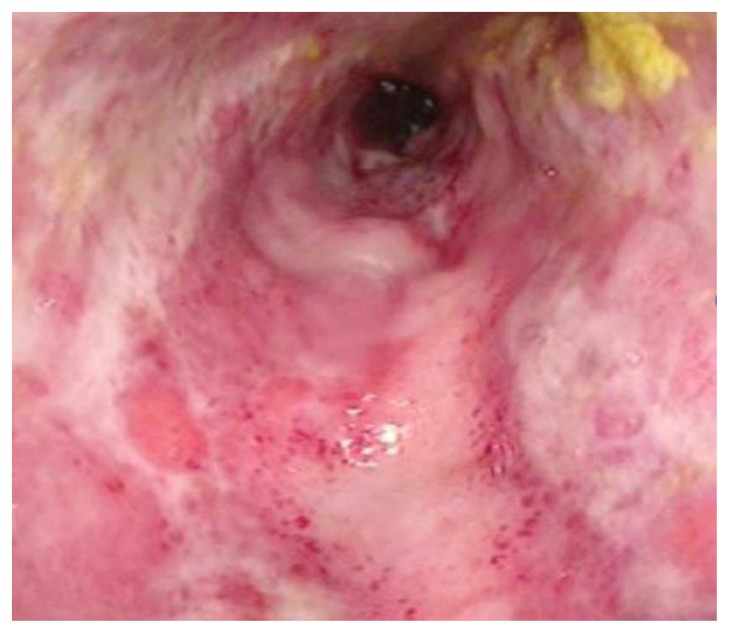
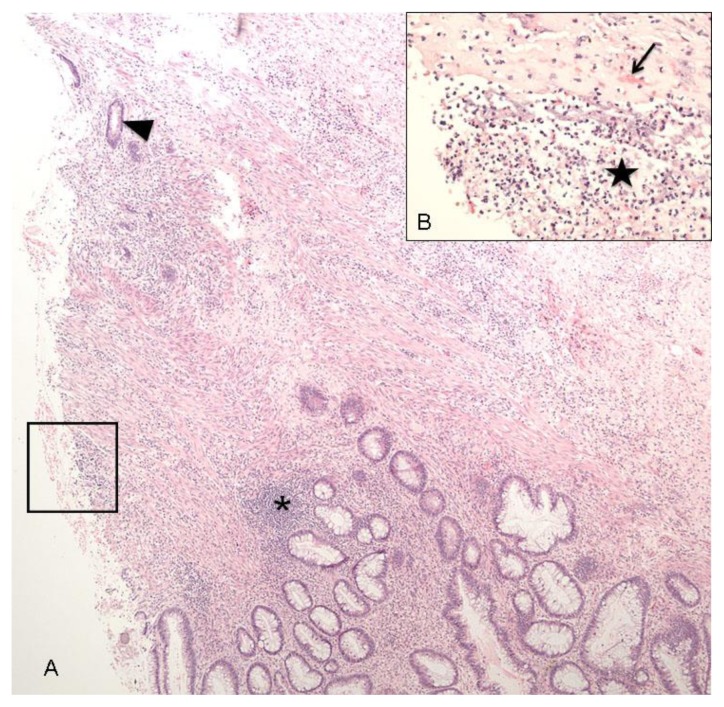
A contrast-enhanced Computed Tomography (CT) scan of the abdomen and pelvis demonstrated marked sigmoid colonic wall thickening with submucosal edema and mucosal hyperemia, as well as surrounding inflammatory changes (Figures 1A and 1B). The differential diagnosis given was ischemic, infectious, or inflammatory colitis. Characteristic findings of an arteriovenous malformation (AVM) were present, but not appreciated at that time. These included small corkscrew vessels of the nidus surrounding several hypertrophied sigmoid branches of the Inferior Mesenteric Artery (IMA). The radiologist attributed dilated vessels within the edematous sigmoid mesocolon simply to the sequelae of inflammation and hyperemia. A flexible sigmoidoscopy was performed, which showed diffuse ulceration, edema, and exudate from the rectum to the descending colon, with luminal narrowing (Figure 2). Biopsies demonstrated atrophy of crypts and ulceration, hemorrhage, and fat necrosis consistent with ischemic colitis (Figure 3).
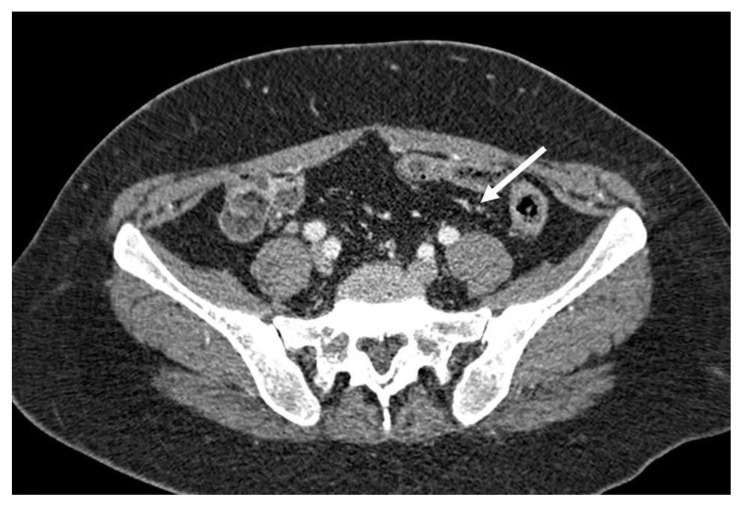
Figure 1.
A 38-year old woman with history of an unknown connective tissue disorder who presents with ischemic colitis secondary to inferior mesenteric arteriovenous malformation.
FINDINGS: (A) Axial contrast enhanced CT image obtained in the portal venous phase demonstrates severe colonic wall thickening and submucosal edema (large open arrow) with mucosal hyperenhancement (arrows). Pericolonic fat stranding (asterisk) and a small amount of free fluid (curved arrow) are also seen. (B) Axial contrast enhanced CT image demonstrates dilated veins within the edematous sigmoid mesocolon (large solid arrows). There is colonic wall thickening (large open arrow). Free fluid (curved arrow) is also seen.
TECHNIQUE: Axial contrast-enhanced CT. mA: 380. kvp: 120. Slice thickness: 2.5 mm. Contrast agents: 500 mL Gastrografin oral contrast and 100 mL of Isovue 370 IV contrast.
Figure 2.
A 38-year old woman with history of an unknown connective tissue disorder who presents with ischemic colitis secondary to inferior mesenteric arteriovenous malformation.
FINDINGS: Photograph of the sigmoid colon obtained during flexible sigmoidoscopy demonstrates diffuse ulceration, edema, and exudate with luminal narrowing. The mucosa does not appear necrotic.
TECHNIQUE: Flexible sigmoidoscopy.
Figure 3.
A 38-year old woman with history of an unknown connective tissue disorder who presents with ischemic colitis secondary to inferior mesenteric arteriovenous malformation.
FINDINGS: Acute ischemic necrosis. (A) Colonic ulceration (the left side of the image) characterized by denuded superficial epithelium and mixed inflammatory infiltrate within the submucosa (asterisk). There is also superficial epithelial necrosis that spares the deep portions of the crypts (arrowhead), a pattern characteristic of ischemic colitis. Viable surface epithelium is seen at the bottom of the image.
(B) Magnified view of the ulcer within the box shows hemorrhage (arrow), identified by extravascular red blood cells present within the lamina propria. A thin layer of acute neutrophilic inflammation (star) overlies the ulcerated mucosa.
TECHNIQUE: H&E stain; (A) Power 40x, (B) Power 200x
Magnetic resonance angiography (MRA) (Figure 4) was ordered at the outside hospital to “rule out mesenteric ischemia.” A large vein could be seen in the pelvis draining towards the portal splenic confluence. However, because of the poor spatial resolution of this exam, smaller arterial abnormalities were not seen, and the significance of the draining veins was not elucidated. The patient was transferred to our hospital for further workup.
Figure 4.
A 38-year old woman with history of an unknown connective tissue disorder who presents with ischemic colitis secondary to inferior mesenteric arteriovenous malformation.
FINDINGS: Thick slab maximum intensity projection (MIP) image from a contrast-enhanced MR angiogram is of suboptimal technical quality. It demonstrates a large draining vein (arrows) originating in the sigmoid mesocolon. One can also appreciate the draining veins (curved open arrows) extending from the pelvis towards the portosplenic confluence. The IMA does not project well on the MIP, but can be seen faintly on the source images. There is a hint of a tangle of vessels around the superior aspect of the draining vein, but this would not be possible to call prospectively.
TECHNIQUE: Contrast-enhanced 3-D MR Angiogram. Magnet strength: 1.5 Tesla. TR: 5.256. TE: 1.128. Slice thickness: 3 mm. Slice spacing: 1.5 mm. Field of view: 36 cm. Matrix: 0/256/128/0. Contrast agent: 30 mL of gadolinium.
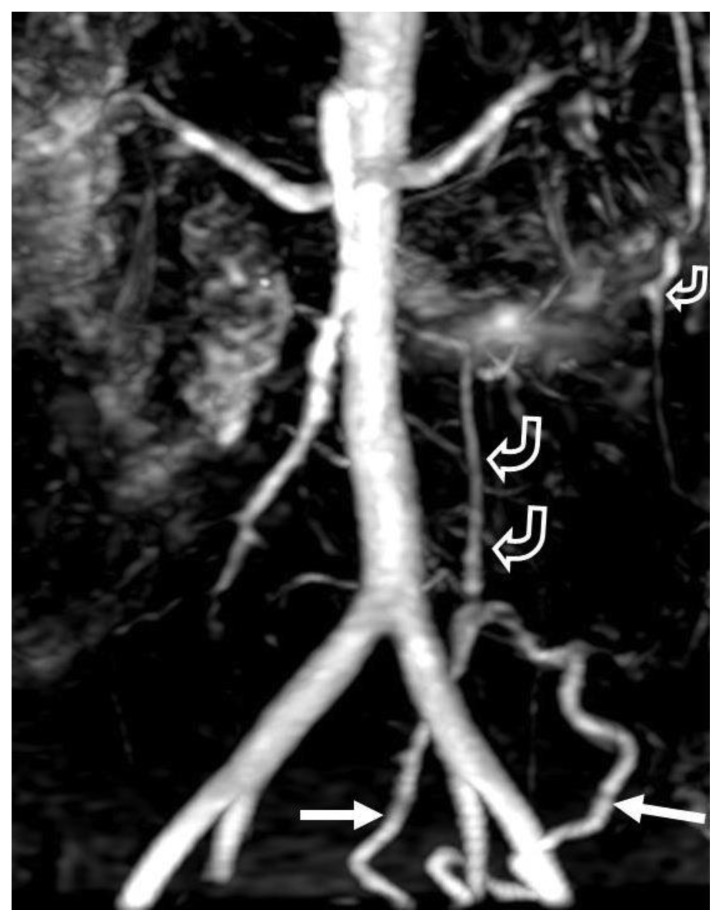
Initial management was conservative with intravenous antibiotics, fluids, and total parenteral nutrition. CT angiography (CTA) of the abdomen and pelvis was obtained (Figures 5A and B). But once again the diagnosis of AVM was missed. Over the ensuing months she was hospitalized and imaged with CT two more times, but the etiology of her ischemia remained undiagnosed. She clinically worsened until surgery was deemed necessary.
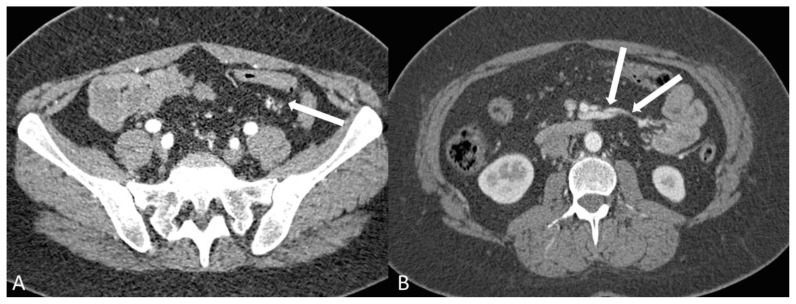
Figure 5.
A 38-year old woman with history of an unknown connective tissue disorder who presents with ischemic colitis secondary to inferior mesenteric arteriovenous malformation.
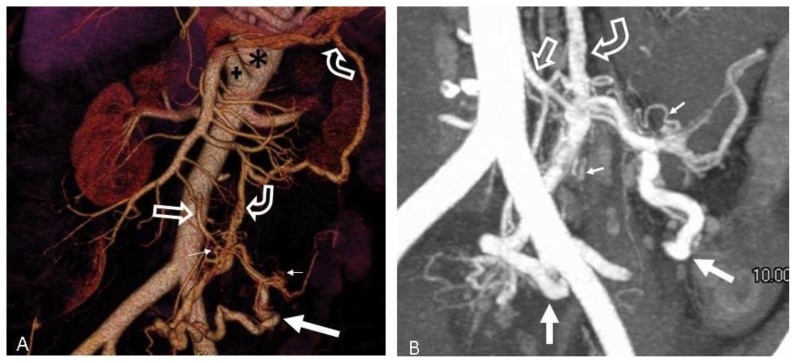
FINDINGS: 3-D volume rendered images from a CTA (A) and a thick slab MIP (B) both demonstrate a hypertrophied IMA and branches (open arrow) feeding the AVM. There are extensive small corkscrew vessels (tiny arrows) of the AVM nidus surrounding the sigmoid branches of the IMA. Large veins (solid arrows) in the mesocolon drain via early filling of a large draining vein (curved arrows) extending towards the portosplenic confluence. There is also ectasia of the celiac (asterisk) and SMA (cross) measuring 15 mm and 12 mm respectively.
TECHNIQUE: (A) 3-D volume rendered image from CTA obtained in the arterial phase. (B) Thick slab (1 cm) MIP from CTA obtained in the arterial phase. mA: 585. kvp: 120. Slice thickness: 0.625 mm. Contrast agents: 120 mL of Isovue 370 IV contrast.
Preoperatively, the patient was referred to interventional radiology to perform a conventional angiogram (Figures 6A and B), both to exclude underlying vasculitis and to help with surgical planning. At that point, the AVM was finally diagnosed. One additional finding only appreciated on angiography was sluggish flow within several jejunal branches, suggesting high portal venous pressure from the arteriovenous shunting. There was no mesenteric arterial stenosis, dissection, pseudoaneurysm or thromboembolic arterial occlusion.
Figure 6.
A 38-year old woman with history of an unknown connective tissue disorder who presents with ischemic colitis secondary to inferior mesenteric arteriovenous malformation.
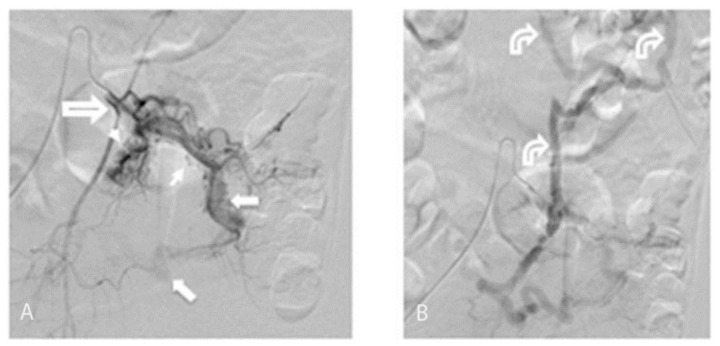
FINDINGS: (A) Selective inferior mesenteric sigmoid branch arteriogram demonstrates extensive small corkscrew vessels (small arrows) of the AVM nidus surrounding the sigmoid branches of the inferior mesenteric artery fed from the IMA (open arrow). There is early filling of a large draining vein (larger solid arrows) in the sigmoid mesocolon. (B) Selective inferior mesenteric arteriogram seconds later than in “6A” demonstrates filling of large draining veins (curved arrows) in the sigmoid mesocolon extending towards the portosplenic confluence.
TECHNIQUE: Conventional Angiogram. mA: 324. kvp: 70.
With knowledge of the conventional angiographic findings, her prior imaging was re-reviewed. Tiny corkscrew vessels of the nidus around a hypertrophied IMA and branches, with filling of large draining veins extending towards the portosplenic confluence, were now appreciated on all the prior CTs, but best on the CT angiogram (Figures 5A and B). Additionally, the celiac and superior mesenteric artery (SMA) were ectatic, measuring 15 mm (normal <8 mm) and 12 mm (normal <7 mm) respectively, which was compatible with her known systemic connective tissue vasculopathy. The colonic findings were stable.
Management
During surgery, the sigmoid colon was found to be extremely fibrotic, thickened and edematous. The rectum was also involved, albeit to a lesser degree. An open sigmoid colectomy was performed, along with resection of the large AVM. Histopathology of the resected recto-sigmoid colon revealed ulceration, hemorrhage, acute and chronic inflammation, and fat necrosis (Figure 7) consistent with ischemia. Grossly, within the pericolic adipose tissue there was a tight cluster of disorganized vessels of various sizes. Microscopically, a tangle of abnormal vessels interposed between large muscular arteries and draining veins was identified, consistent with AVM (Figure 8).
Figure 7.
A 38-year old woman with history of an unknown connective tissue disorder who presents with ischemic colitis secondary to inferior mesenteric arteriovenous malformation.
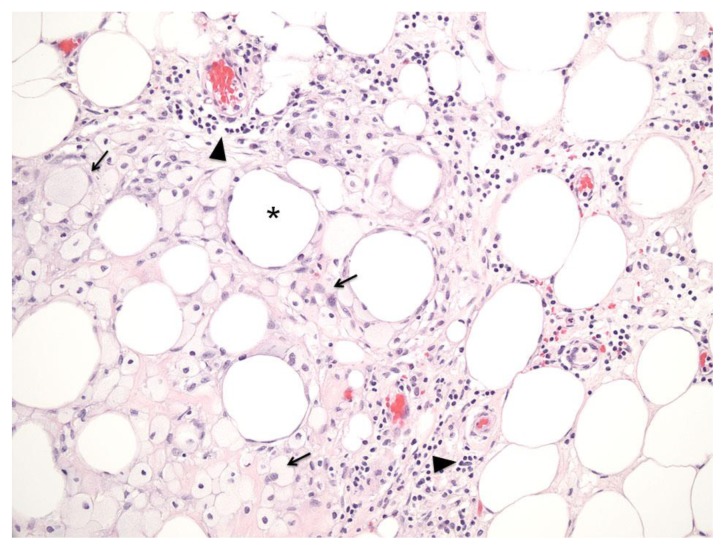
FINDINGS: Fat necrosis. Necrotic adipose tissue is characterized by foamy macrophages (arrows) and chronic inflammatory infiltrate predominantly composed of lymphocytes (arrow head), which surround irregular fatty spaces (asterisk) formed by adipocytes.
TECHNIQUE: H&E stain; Power 200x
Figure 8.
A 38-year old woman with history of an unknown connective tissue disorder who presents with ischemic colitis secondary to inferior mesenteric arteriovenous malformation.
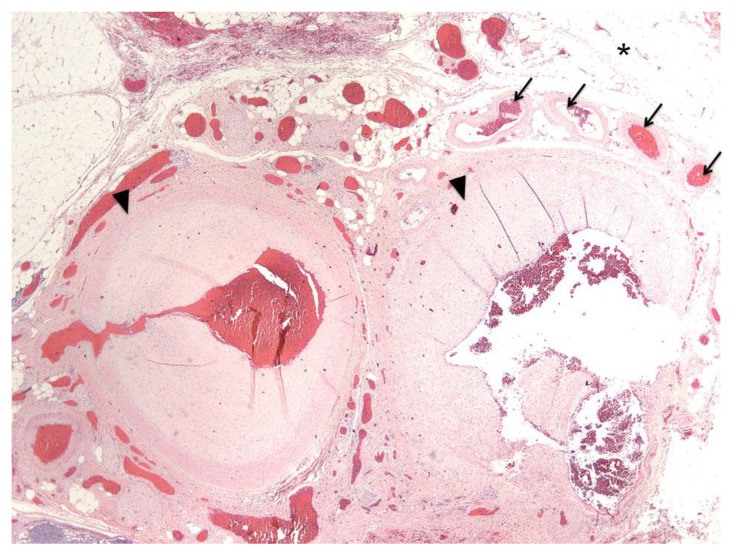
FINDINGS: Arteriovenous malformation. A tangle of abnormal vessels interposed between feeding arteries (arrowheads) and draining veins (arrows) in the pericolic adipose tissue (asterisk).
TECHNIQUE: H&E stain; Power 40x
Follow-up
Six months postoperatively, the patient underwent colostomy takedown with re-anastomosis. Intraoperative proctoscopy demonstrated normal rectum and colon. Subsequent genetic testing for fibrillin-1 (Marfan’s syndrome), collagen 3A1 (Ehlers-Danlos Syndrome, type IV), Transforming Growth Factor-Beta receptor 1 and 2 (Loeys Dietz Syndrome), and “actin, alpha 2, smooth muscle, aorta” or ACTA2 gene (Familial Aortic Aneurysm and/or Dissection (AAD)) was negative, as was her Antinuclear Antibody test. Thus, the etiology of the patient’s connective tissue disorder (CTD) remains unknown. She continues to undergo periodic surveillance, and has not had recurrence for three years.
DISCUSSION
Etiology & demographics
AVMs and arteriovenous fistulas (AVFs) are abnormal direct connections between arteries and veins that result in direct arteriovenous shunting without an intervening capillary bed [1, 2]. Because the physiologic consequences are the same, AVMs and AVFs are often grouped together in clinical studies. However, there are important distinctions. AVFs are usually acquired, and may result from penetrating trauma, arterial catheterization, surgical procedures, or rupture of a preexisting aneurysm into a nearby vein [3, 4]. There is usually a single communication between the artery and vein in an AVF. AVMs, on the other hand, can be distinguished by a dilated feeding artery, a large tangle of vessels representing the AVM nidus with multiple arteriovenous connections, and a densely opacified early draining vein or veins [5]. They are usually congenital, caused by disruptions in vascular morphogenesis that result in persistent embryonic vessels that fail to differentiate properly into arteries and veins [2, 3].
Connective tissue disorders of collagen and elastin can lead to the development of AVMs, as well as aneurysms, pseudoaneurysms, and dissections [6]. Our patient had abnormal dilation of the pulmonary, celiac, and superior mesenteric arteries, as well as an ascending aortic aneurysm. On histopathology, her aortic aneurysm demonstrated myxoid medial degeneration, the same as that characteristic of Marfan’s syndrome. Genetic testing is available for only a limited number of CTDs, none of which our patient had. To our knowledge this is the first reported case of colonic ischemia due to an AVM in a patient with a vascular connective tissue disorder. Another case of AVMs associated with an unknown CTD with Marfanoid features was reported [7]. That patient had multiple AVMs in the chest and neck, and there was no mention of abdominal symptoms or abdominal AVMs.
Visceral AVMs are poorly understood due to their rarity, and are often misdiagnosed, as was the case with our patient [8]. AVFs and AVMs of the splanchnic circulation are particularly rare with approximately 200 cases reported [9]. They usually involve the hepatic, splenic, or superior mesenteric vessels [4, 10]. Those involving the inferior mesenteric vessels are very rare, with only 24 cases reported in the literature [1, 2, 4, 5, 9–26].
The use of terminology in the literature is inconsistent, which complicates our ability to distinguish reported cases of AVMs from AVFs. Of the 24 reported cases of inferior mesenteric AVMs/AVFs, 13 were of congenital origin [1, 2, 4, 5, 9, 11, 12, 18, 19, 21, 23–25], and one was acquired [16]. The other 10 were iatrogenic, usually the result of postoperative complications from abdominal surgery [10, 13–15, 17, 20, 22, 26, 27]. Our patient had no history of abdominal trauma or abdominal surgery. She had been annually followed with CT angiography of the chest, abdomen, and pelvis for the five years prior to her current admission. Retrospectively reviewing these, the AVM is not distinctly visible until two years prior, at which point it was much smaller and less noticeable (Figure 9, Figure 10A and 10B). This is consistent with the normal natural history of AVMs, in that although they are congenital, they usually do not present clinically until young adulthood. It is probable that our patient’s AVM developed during angiogenesis and worsened progressively, as did her underlying aortic vasculopathy.
Figure 9.
A 38-year old woman with history of an unknown connective tissue disorder who presents with ischemic colitis secondary to inferior mesenteric arteriovenous malformation.
FINDINGS: Axial image from a CTA performed four years prior to the current admission demonstrates normal appearing vessels (arrow) in the sigmoid mesocolon without evidence of AVM.
TECHNIQUE: Axial image from CTA obtained in the angiographic phase. mA: 283. kvp: 120. Slice thickness: 1.0 mm. Contrast agents: 120 mL of Isovue 370 IV contrast.
Figure 10.
A 38-year old woman with history of an unknown connective tissue disorder who presents with ischemic colitis secondary to inferior mesenteric arteriovenous malformation.
FINDINGS: (A) Axial image from a CTA performed 2 years prior to the current admission demonstrates small corkscrew vessels (arrow) starting to appear in the sigmoid mesocolon, the earliest demonstrable evidence of AVM. (B) Axial image from the same scan as (A) at a location more superior demonstrates early filling of a draining vein coursing toward the SMV. Note that this was obtained in the arterial phase, and no other mesenteric veins are enhancing at this time point.
TECHNIQUE: (A and B) Axial image from CTA obtained in the angiographic phase mA: 219. kvp: 120. Slice thickness: 1.0 mm. Contrast agents: 120 mL of Isovue 370 IV contrast.
Clinical & Imaging Findings
Reported manifestations of splanchnic AVMs/AVFs include abdominal pain, portal hypertension, colonic ischemia, ascites, bleeding esophageal varices, diarrhea, malabsorption, and lower gastrointestinal bleeding [4, 9, 10, 24]. However, some patients are asymptomatic, with incidental detection of AVM/AVF on imaging or as an abdominal bruit on physical exam [9].
Ischemic colitis is one of the more serious manifestations of inferior mesenteric AVM/AVF, present in 12 of the 24 cases reported in the literature [2, 9, 10, 12, 13, 15–17, 24–27]. The classic clinical triad includes diarrhea or change in bowel habits, hematochezia, and abdominal pain [28]. Wall thickening is the most common CT manifestation. The wall is usually hypodense from mural edema, although hemorrhage or superinfection can cause it to be hyperattenuating. The presence of wall thickening is nonspecific, and there is a wide degree of overlap with other colonic diseases such as inflammatory bowel disease and infectious colitis. Mesenteric fat stranding and ascites are also common but nonspecific. When ischemia progresses to infarction, one may see perforation, peritonitis, pneumatosis, luminal dilatation, or portomesenteric gas [29]. Direct colonoscopic visualization of a dusky bowel wall with submucosal edema or hemorrhage, necrotic mucosa with ulcers, or infarction can confirm the diagnosis [30].
A distinctive feature of visceral AVMs/AVFs is their tendency to cause so-called “forward” or “hyperkinetic” portal hypertension, which likely results from increased blood flow to the portal system with subsequent increased vascular resistance [1, 5, 10, 24]. It is reported to occur in approximately 50 percent of patients with AVM/AVF of the splanchnic circulation, and 29 percent of patients with AVM/AVF of the mesenteric circulation [2]. Of the 24 reported cases of inferior mesenteric AVMs/AVFs to date, 11 had portal hypertension [1, 4, 5, 10, 11, 14, 17, 19, 20, 24, 27]. Our patient lacked typical signs such as ascites or splenomegaly. However, sluggish filling of jejunal branches of the superior mesenteric artery was noted during angiography, suggestive of portal hypertension.
The typical appearance of the AVM itself on angiography, multiphasic contrast-enhanced CT (CECT) and dynamic MR angiography, consists of serpentine and enlarged feeding arteries, a central nidus with tangled vessels, and early enhancement of enlarged draining veins [9, 31]. Noncontrast T1-GRE may demonstrate low or high signal vessels, the latter of which may represent hemorrhage, intravascular thrombosis, or flow-related enhancement [31, 32]. T2 weighted imaging (T2WI) may show vascular flow voids [31]. Multiple anechoic spaces are seen on gray-scale ultrasound, with turbulent flow and flow reversals demonstrated on Doppler [33, 34].
Diagnosis of AVMs/AVFs is best accomplished with CTA, followed by conventional angiography if necessary, though MRA is also used on occasion [1, 9]. CT angiography is the first-line test because it is noninvasive, allows for the assessment of arterial inflow and venous drainage, and has the ability to create high-resolution three-dimensional images that can be helpful in surgical planning. It has several advantages over MRA. Faster image acquisition not only means that there are fewer motion artifacts from breathing or peristalsis, but also makes it more appropriate for patients who are acutely ill with significant gastrointestinal (G.I.) bleeding or sepsis. CTA is also less expensive and more readily available in the emergency setting [9, 35].
Conventional angiography is considered the gold standard in vascular imaging due to its superior spatial resolution, real-time flow depiction, and the ability to perform concurrent interventions [1]. Embolization can be performed to decrease arterial inflow to the AVM/AVF preoperatively. Coil embolization of feeding arteries can also be the definitive treatment [9, 18]. Despite these advantages, it is an invasive procedure with associated risks, such as bleeding, arterial dissection, and bowel ischemia.
MRA shares several advantages with CTA over angiography. It offers comparable vascular evaluation, plus the advantage of primary 3-D data acquisition that can be visualized with multiplanar reconstruction. Three-dimensional, T1-weighted images (T1WI) with gradient echo (GRE) during dynamic gadolinium-enhanced MRA are particularly useful to evaluate visceral AVMs/AVFs [33]. Recent gains in temporal resolution allow for nearly real time depiction of vascular flow. Due to the absence of ionizing radiation, MRA may also be more suitable for children than CTA [35]. A notable drawback is that metallic clips and embolization materials, as well as motion from peristalsis and breathing, can create artifacts on MR imaging [33, 36]. As was seen in our case, the tiny corkscrew vessels of the nidus can be difficult to visualize using a body coil, especially at low field strength.
Differential Diagnosis
The differential diagnosis of splanchnic AVMs is broad and includes the following entities.
Venous malformations are mostly located in the skin and subcutaneous tissues, but can involve abdominal viscera. On multiphasic contrast enhanced CT and TI-GRE they demonstrate contrast enhancement that slowly progresses from the periphery to the center. Drainage is via enlarged veins, but feeding arteries are notably absent. On T1-GRE venous malformations are heterogeneous with high signal thrombosis or hemorrhage, and on T2WI they are hyperintense and may appear septated. They may also demonstrate fatty components. Phleboliths are common, evident as round hyperdensities with central lucency on NECT, or as signal voids on Magnetic Resonance Imaging (MRI) [31, 37]. Most venous malformations are hypoechoic or heterogeneous on ultrasound, with low velocity flow on spectral Doppler [37].
Peritoneal hypervascular metastases can implant on the sigmoid mesocolon and pelvic peritoneal reflections and appear as discrete or confluent soft tissue nodules or masses, with or without ascites. Carcinoid metastases may demonstrate coarse central calcifications most evident on NECT. They show marked contrast enhancement on multiphasic contrast-enhanced CT or TI-GRE [38, 39]. Larger tumors may also demonstrate parasitization and hypertrophy of feeding arteries with enlarged draining veins. However, central necrosis, notably absent in AVMs, can be a helpful distinguishing feature. On ultrasound their echogenicity is variable relative to the surrounding tissue, with hypervascularity evident on Doppler and high velocity waveforms seen in cases of arteriovenous shunting [40].
Pelvic Solitary Fibrous Tumor (SFT) is a rare tumor characterized on multiphasic contrast-enhanced CT and T1-GRE MRI as a well-defined mass with intense heterogeneous enhancement that progresses on delayed phase images due to the fibrous component. Depending on the cellular content, the signal intensity is variable on T2WI, sometimes heterogeneous, with multiple flow voids representing large vascular channels [41]. Ultrasound shows a well-defined mass with mixed echogenicity and internal blood flow on Doppler [42].
Extraadrenal pheochromocytomas are well-defined hypervascular masses, sometimes with cystic or necrotic areas, that may occur anywhere in the sympathetic chain along the aorta and iliac vessels. On NECT they may appear hypo- or isodense with or without cystic or necrotic areas [41]. Hemorrhage appears hyperdense and calcifications are present in 10% of patients. On non-contrast T1-GRE, signal intensity is variable and may be heterogeneous if there is necrosis or hemorrhage. Signal intensity is very high on T2WI. Multiphasic CT and MRI demonstrate intense arterial enhancement that may be homogeneous or heterogeneous. A unique characteristic is a “salt and pepper” appearance on enhanced T1-GRE. Gray-scale ultrasound may show a circumscribed mass with cystic areas that may be anechoic or demonstrate echogenic internal debris due to hemorrhage. Metaiodobenzylguanidine (MIBG) scintigraphy is highly specific for lesion detection [43].
Mild mesenteric trauma may present on NECT as mesenteric fat stranding. With more severe trauma, significant hemorrhage may occur resulting in formation of a hyperdense (>60 Hounsfield Units (HU)) hematoma or hemoperitoneum. Identification of the “sentinel clot,” the intraperitoneal blood with highest attenuation, can localize the site of bleeding [44]. If the bowel is involved, hyperdense wall thickening may be seen. Extravasation of IV contrast material indicates arterial injury, usually from an avulsion injury, and is seen as a progressively enhancing extravascular contrast blush that follows density of the blood pool. A pseudoaneurysm may also be seen. MRI is not usually performed in the setting of trauma. However, on T1-GRE, one would see mesenteric hematoma or hemorrhagic ascites as high signal intensity. Active arterial extravasation has the same pattern of contrast enhancement as on CECT. On T2WI, signal intensity of hemorrhage is variable, but is usually low acutely. Bowel wall thickening appears as high signal on T1-GRE sequences. Focused abdominal ultrasound may show abdomino-pelvic free fluid on grayscale [45], and a “yin-yang” pseudoaneurysmal sac on Doppler.
Treatment & Prognosis
Surgical resection of the AVM or AVF, with or without resection of the affected bowel remains the primary treatment for inferior mesenteric lesions, and has an excellent prognosis. Minimally invasive alternatives to surgery, such as endovascular embolization of the feeding artery, are also increasingly discussed in the literature either as a primary treatment or as a pre-operative treatment [1, 9, 10, 13, 27]. In our patient the sigmoid colon had to be removed along with the AVM because there was extensive fibrotic structuring from chronic ischemia. The patient had no post-operative complications and has remained free of symptoms for three years.
TEACHING POINT
In patients who present with ischemic colitis without an identifiable cause, mesenteric AVM or AVF should be considered in the differential diagnosis, and CT or MR angiography should be performed as the initial imaging test, followed by conventional angiography if necessary. Characteristic imaging findings include a dilated feeding artery, a large tangle of vessels representing the AVM nidus, and a densely opacified, early draining vein.
Table 1.
Summary table for splanchnic arteriovenous malformations (AVMs)
| Etiology | Usually congenital, caused by disruptions in vascular morphogenesis that result in persistent embryonic vessels that fail to differentiate properly into arteries and veins. |
| Incidence | AVMs and AVFs of the splanchnic circulation are reported together in the literature due to their rarity and similar physiologic consequences. There are approximately 200 cases reported, but only 21 cases involving the inferior mesenteric vessels. |
| Gender ratio | Twice as common in women as in men. |
| Age predilection | Present clinically in early adulthood. |
| Risk factors | Possibly family history. Connective tissue disorders, including Marfan’s syndrome and Ehlers-Danlos syndrome Type IV. |
| Treatment | Angiographic embolization and/or surgical resection. |
| Prognosis | Depends on clinical presentation. Excellent with early identification and treatment. |
| Findings on Imaging | Dilated feeding arteries, a large tangle of disordered vessels representing the nidus, and a densely opacified, early draining vein. |
Table 2.
Differential table for splanchnic arteriovenous malformations (AVMs)
| Entity | General features | Computed Tomography | Magnetic Resonance Imaging | Ultrasound |
|---|---|---|---|---|
| Arteriovenous Malformation |
|
|
|
|
| Venous malformation |
|
|
|
|
| Hypervascular Peritoneal Metastases |
|
|
|
|
| Pelvic Solitary Fibrous Tumor (SFT) |
|
|
|
|
| Extraadrenal Pheochromocytoma |
|
|
|
|
| Mesenteric trauma with hemorrhage |
|
|
|
|
ABBREVIATIONS
- AAD
Familial Aortic Aneurysm and/or Dissection
- ACTA2
actin, alpha 2, smooth muscle, aorta gene
- AVM
Arteriovenous Malformation
- AVF
Arteriovenous Fistula
- CECT
Contrast Enhanced Computed Tomography
- CT
Computed Tomography
- CTA
Computed Tomographic Angiography
- CTD
Connective Tissue Disorder
- GI
Gastrointestinal
- HU
Hounsfield Units
- IMA
Inferior Mesenteric Artery
- IV
Intravenous
- MIBG
Metaiodobenzylguanidine
- MIP
Maximum Intensity Projection
- MRA
Magnetic Resonance Angiography
- MRI
Magnetic Resonance Imaging
- NECT
Non-enhanced Computed Tomography
- SFT
Solitary Fibrous Tumor
- T1-GRE
T1 Gradient Echo
- T2WI
T2 Weighted Imaging
- US
Ultrasound
REFERENCES
- 1.Bettenworth D, Rijcken E, Muller KM, Mosch-Messerich A, Heidemann J. Rare cause of upper gastrointestinal bleeding in a 27-year-old male patient. Gut: An International Journal of Gastroenterology and Hepatology. 2012;61(9):1367. doi: 10.1136/gutjnl-2012-302034. [DOI] [PubMed] [Google Scholar]
- 2.Metcalf DR, Nivatvongs S, Andrews JC. Ischemic colitis: an unusual case of inferior mesenteric arteriovenous fistula causing venous hypertension. Report of a case. Dis Colon Rectum. 2008;51(9):1422–4. doi: 10.1007/s10350-008-9377-2. [DOI] [PubMed] [Google Scholar]
- 3.Szilagy DE, Elliott JP. Peripheral congenital arteriovenous fistulae. Observations in 45 cases. J Cardiovasc Surg. 1965(Suppl):246–50. [PubMed] [Google Scholar]
- 4.Van Way CW, III, Crane JM, Riddell DH, Foster JH. Arteriovenous fistula in the portal circulation. Surgery. 1971;70(6):876–90. [PubMed] [Google Scholar]
- 5.Manns RA, Vickers CR, Chesner IM, McMaster P, Elias E. Portal hypertension secondary to sigmoid colon arteriovenous malformation. Clin Radiol. 1990;42(3):203–4. doi: 10.1016/s0009-9260(05)81935-x. [DOI] [PubMed] [Google Scholar]
- 6.Vaz CC, Couto M, Medeiros D, et al. Undifferentiated connective tissue disease: a seven-center cross-sectional study of 184 patients. Clin Rheumatol. 2009;28(8):915–21. doi: 10.1007/s10067-009-1175-2. [DOI] [PubMed] [Google Scholar]
- 7.Skinner JS, Adams PC. Multiple arteriovenous malformations in a man with a connective tissue disorder. European Heart Journal. 1994;15(12):1725–1726. doi: 10.1093/oxfordjournals.eurheartj.a060458. [DOI] [PubMed] [Google Scholar]
- 8.Fishman SJ, Burrows PE, Leichtner AM, Mulliken JB. Gastrointestinal manifestations of vascular anomalies in childhood: varied etiologies require multiple therapeutic modalities. J Pediatr Surg. 1998;33(7):1163–7. doi: 10.1016/s0022-3468(98)90552-8. [DOI] [PubMed] [Google Scholar]
- 9.Turkvatan A, Ozdemir AP, Akdogan M, Cumhur T, Olcer T, Parlak E. Inferior mesenteric arteriovenous fistula with ischemic colitis: multidetector computed tomographic angiography for diagnosis. Turk J Gastroenterol. 2009;20(1):67–70. [PubMed] [Google Scholar]
- 10.Capron JP, Gineston JL, Remond A, et al. Inferior mesenteric arteriovenous fistula associated with portal hypertension and acute ischemic colitis. Successful occlusion by intraarterial embolization with steel coils. Gastroenterology. 1984;86(2):351–5. [PubMed] [Google Scholar]
- 11.Baranda J, Pontes JM, Portela F, et al. Mesenteric arteriovenous fistula causing portal hypertension and bleeding duodenal varices. Eur J Gastroenterol Hepatol. 1996;8(12):1223–5. doi: 10.1097/00042737-199612000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Doppl WE, Kuttner D, Stolte M, et al. Clinical challenges and images in GI. Segmental ischemic colitis owing to vascular malformation of the inferior mesenteric artery. Gastroenterology. 2007;133(1):15, 373. doi: 10.1053/j.gastro.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 13.Fabre A, Abita T, Lachachi F, et al. Inferior mesenteric arteriovenous fistulas. Report of a case. Ann Chir. 2005;130(6–7):417–20. doi: 10.1016/j.anchir.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Hirner A, Haring R, Bost H, Sorensen R. Hyperkinetic portal hypertension. Arterioportal fistula: problems--case reports--review of the literature. Chirurg. 1978;49(5):303–10. [PubMed] [Google Scholar]
- 15.Houdard C, Helenon C, Carles JF, et al. Inferior mesenteric arteriovenous fistula and ulcerative rectocolitis. Arch Fr Mal App Dig. 1970;59(7):463–74. [PubMed] [Google Scholar]
- 16.Kamo M, Huwa S, Ishiyama M, Nozaki T, Onoda Y, Oikado K. A case of ischemic colitis caused by inferior mesenteric arteriovenous fistula. Japanese Journal of Clinical Radiology. 2012;57(2):309. [Google Scholar]
- 17.Kim IH, Kim DG, Kwak HS, Yu HC, Cho BH, Park HS. Ischemic colitis secondary to inferior mesenteric arteriovenous fistula and portal vein stenosis in a liver transplant recipient. World J Gastroenterol. 2008;14(26):4249–52. doi: 10.3748/wjg.14.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui A, Iwai K, Kawasaki R, Wada T, Mito Y, Doi T. Transcatheter embolization of an inferior mesenteric arteriovenous fistula with frequent mucous diarrhea. Nihon Shokakibyo Gakkai Zasshi. 2007;104(2):194–9. [PubMed] [Google Scholar]
- 19.Nemcek AA, Jr, Yakes W. SIR 2005 Annual Meeting Film Panel case: inferior mesenteric artery-to-inferior mesenteric vein fistulous connection. J Vasc Interv Radiol. 2005;16(9):1179–82. doi: 10.1097/01.RVI.0000175327.10770.40. [DOI] [PubMed] [Google Scholar]
- 20.Okada K, Furusyo N, Sawayama Y, et al. Inferior mesenteric arteriovenous fistula eight years after sigmoidectomy. Intern Med. 2002;41(7):543–8. doi: 10.2169/internalmedicine.41.543. [DOI] [PubMed] [Google Scholar]
- 21.Oyama K, Hayashi S, Kogure T, Kirakawa K, Akaike A. Inferior mesenteric arteriovenous fistula. -Report of a case and review of the literature. Nihon Igaku Hoshasen Gakkai Zasshi. 1980;40(10):944–50. [PubMed] [Google Scholar]
- 22.Peer A, Slutzki S, Witz E, Abrahmsohn R, Bogokowsky H, Leonov Y. Transcatheter occlusion of inferior mesenteric arteriovenous fistula: a case report. Cardiovasc Intervent Radiol. 1989;12(1):35–7. doi: 10.1007/BF02577124. [DOI] [PubMed] [Google Scholar]
- 23.Sabatier JC, Bruneton JN, Drouillard J, Elie G, Tavernier J. Inferior mesenteric arteriovenous fistula of congenital origin. A report on one case and review of the published literature. J Radiol Electrol Med Nucl. 1978;59(12):727–9. [PubMed] [Google Scholar]
- 24.Akgun V, Sari S, Verim S, Bozlar U. Arteriovenous malformation of the inferior mesenteric artery in a patient with ischemic colitis. BMJ Case Rep. 2013:1–2. doi: 10.1136/bcr-2013-009565. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Muhtaseb MS, Gorgun E, Liu M. Arteriovenous malformation: a potential cause of ischaemic colitis. ANZ J Surg. 2013;83(1–2):93–94. doi: 10.1111/j.1445-2197.2012.06322.x. [DOI] [PubMed] [Google Scholar]
- 26.Gorospe EC, Leggett CL, Sun G. Inferior mesenteric arteriovenous malformation: an unusual cause of ischemic colitis. Ann Gastroenterol. 2012;25(2):165. [PMC free article] [PubMed] [Google Scholar]
- 27.Pietri J, Remond A, Reix T, Abet D, Sevestre H, Sevestre MA. Arterioportal fistulas: twelve cases. Ann Vasc Surg. 1990;4(6):533–9. doi: 10.1016/S0890-5096(06)60834-0. [DOI] [PubMed] [Google Scholar]
- 28.Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum. 1996;39(1):88–100. doi: 10.1007/BF02048275. [DOI] [PubMed] [Google Scholar]
- 29.Wiesner W, Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226(3):635–50. doi: 10.1148/radiol.2263011540. [DOI] [PubMed] [Google Scholar]
- 30.Taourel P, Aufort S, Merigeaud S, Doyon FC, Hoquet MD, Delabrousse E. Imaging of ischemic colitis. Radiol Clin North Am. 2008;46(5):909–24. doi: 10.1016/j.rcl.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Flors L, Leiva-Salinas C, Maged IM, et al. MR imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics. 2011;31(5):1321–40. doi: 10.1148/rg.315105213. discussion 1340–1. [DOI] [PubMed] [Google Scholar]
- 32.Abernethy LJ. Classification and imaging of vascular malformations in children. Eur Radiol. 2003;13(11):2483–97. doi: 10.1007/s00330-002-1773-8. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal S, Magu S, Goyal M. Pelvic arteriovenous malformation: an important differential diagnosis of a complex adnexal mass. J Ultrasound Med. 2009;28(8):1111–4. doi: 10.7863/jum.2009.28.8.1111. [DOI] [PubMed] [Google Scholar]
- 34.Huang MW, Muradali D, Thurston WA, Burns PN, Wilson SR. Uterine arteriovenous malformations: gray-scale and Doppler US features with MR imaging correlation. Radiology. 1998;206(1):115–23. doi: 10.1148/radiology.206.1.9423660. [DOI] [PubMed] [Google Scholar]
- 35.Shih MC, Hagspiel KD. CTA and MRA in mesenteric ischemia: part 1, Role in diagnosis and differential diagnosis. AJR Am J Roentgenol. 2007;188(2):452–61. doi: 10.2214/AJR.05.1167. [DOI] [PubMed] [Google Scholar]
- 36.Keogan MT, Edelman RR. Technologic advances in abdominal MR imaging. Radiology. 2001;220(2):310–20. doi: 10.1148/radiology.220.2.r01au22310. [DOI] [PubMed] [Google Scholar]
- 37.Dubois J, Soulez G, Oliva VL, Berthiaume MJ, Lapierre C, Therasse E. Soft-tissue venous malformations in adult patients: imaging and therapeutic issues. Radiographics. 2001;21(6):1519–31. doi: 10.1148/radiographics.21.6.g01nv031519. [DOI] [PubMed] [Google Scholar]
- 38.Hamrick-Turner JE, Chiechi MV, Abbitt PL, Ros PR. Neoplastic and inflammatory processes of the peritoneum, omentum, and mesentery: diagnosis with CT. Radiographics. 1992;12(6):1051–68. doi: 10.1148/radiographics.12.6.1439011. [DOI] [PubMed] [Google Scholar]
- 39.Ricke J, Sehouli J, Hach C, Hanninen EL, Lichtenegger W, Felix R. Prospective evaluation of contrast-enhanced MRI in the depiction of peritoneal spread in primary or recurrent ovarian cancer. Eur Radiol. 2003;13(5):943–9. doi: 10.1007/s00330-002-1712-8. [DOI] [PubMed] [Google Scholar]
- 40.Testa AC, Ludovisi M, Savelli L, et al. Ultrasound and color power Doppler in the detection of metastatic omentum: a prospective study. Ultrasound Obstet Gynecol. 2006;27(1):65–70. doi: 10.1002/uog.2673. [DOI] [PubMed] [Google Scholar]
- 41.Shanbhogue AK, Fasih N, Macdonald DB, Sheikh AM, Menias CO, Prasad SR. Uncommon primary pelvic retroperitoneal masses in adults: a pattern-based imaging approach. Radiographics. 2012;32(3):795–817. doi: 10.1148/rg.323115020. [DOI] [PubMed] [Google Scholar]
- 42.Wat SY, Sur M, Dhamanaskar K. Solitary fibrous tumor (SFT) of the pelvis. Clin Imaging. 2008;32(2):152–6. doi: 10.1016/j.clinimag.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Leung K, Stamm M, Raja A, Low G. Pheochromocytoma: the range of appearances on ultrasound, CT, MRI, and functional imaging. AJR Am J Roentgenol. 2013;200(2):370–8. doi: 10.2214/AJR.12.9126. [DOI] [PubMed] [Google Scholar]
- 44.LeBedis CA, Anderson SW, Soto JA. CT imaging of blunt traumatic bowel and mesenteric injuries. Radiol Clin North Am. 2012;50(1):123–36. doi: 10.1016/j.rcl.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Richards JR, McGahan JP, Simpson JL, Tabar P. Bowel and mesenteric injury: evaluation with emergency abdominal US. Radiology. 1999;211(2):399–403. doi: 10.1148/radiology.211.2.r99ma54399. [DOI] [PubMed] [Google Scholar]