Abstract
Screening of a plant-derived natural product library led to the observation of in vitro antileishmanial activity by three bisbenzyltetrahydroisoquinoline alkaloids (1-3) that were purified previously from Thalictrum alpinum. A spectroscopic study of the active compounds was conducted to confirm their identities. Of the compounds tested, northalrugosidine (1) showed the most potent in vitro activity against Leishmania donovani promastigotes (0.28 μM), and the highest selectivity (29.3–fold) versus its general cytotoxicity against HT-29 human colon adenocarcinoma cells. Northalrugosidine was tested in vivo using a murine model of visceral leishmaniasis, resulting in the observation of a dose-dependent reduction to the parasitic burden in the liver and spleen without overt toxicity effects at 2.8, 5.6, and 11.1 mg/kg per animal when administered intravenously. This represents the first report of a bisbenzyltetrahydroisoquinoline alkaloid with in vivo efficacy against visceral leishmaniasis.
Leishmaniasis is a neglected tropical disease that is caused by at least 20 species of parasites from the genus Leishmania (Trypanosomatidae), and an estimated 1.3 million new patients are infected annually in typically tropical and subtropical regions of the world.1–3 When these parasites infest the internal organs, this disease is categorized as visceral leishmaniasis, and approximately 300,000 new cases and 20,000 to 40,000 deaths per year are attributed to this cause.2–5 According to the World Health Organization, visceral leishmaniasis has a fatality rate of up to 100% when left untreated, so accurate diagnosis and therapy are of the utmost importance.5,6 The current drugs available for the clinical therapy of leishmaniasis have many drawbacks, including their toxicity, increasing parasite resistance, and routes of dose administration and costs that are suboptimal for, or inaccessible to, the infected populations.7 Pharmaceutical research on natural product compounds has yielded historically a significant number of new drugs, as well as lead molecules for drug discovery and development, in both general therapy8 and specifically for the parasitic disease leishmaniasis.9 During ongoing efforts toward the discovery of natural products with biological activity against leishmaniasis, over 200 compounds in a library including some alkaloids previously isolated from plants of the genus Thalictrum were screened in vitro against promastigotes of Leishmania donovani, the specific parasite responsible for a majority of visceral leishmaniasis cases and fatalities worldwide.
More than 100 species comprise the plant genus Thalictrum, which is a member of the buttercup family (Ranunculaceae).10–12 Many species of this genus have been used in traditional medicines for various indications in countries throughout the world, including mainland China, Japan, and Russia.10,11 A great deal of phytochemical investigation has been conducted on these species, and the non-alkaloidal11 and alkaloidal13–15 constituents reported have been reviewed thoroughly previously. Many researchers investigated for decades the phytochemistry of these plant materials, resulting in the discovery and characterization of numerous Thalictrum alkaloids. Perhaps the most structurally complex of the Thalictrum alkaloids are the bisbenzyltetrahydroisoquinoline alkaloids, and more than 400 of these have been reported to date from Thalictrum species as well as others.16 These compounds contain two subunits, distinct or identical, which each contain “head,” or tetrahydroisoquinoline, and “tail,” or benzyl, substructures. Although many research reports have been focused on the structural determination of these alkaloids, relatively few have described their biological activities. Some investigators have reported on the in vitro antileishmanial16,17 or antiplasmodial and cytotoxic18 activities of certain bisbenzyltetrahydroisoquinoline alkaloids. The classification and nomenclature of bisbenzyltetrahydroisoquinoline alkaloids is based on the diphenyl ether linkage of the two benzyltetrahydroisoquinoline derived subunits between various combinations of head and tail substructures, as well as the specific carbon positions of those linking substitutions.19 The biological activity of the type XII subclass of bisbenzyltetrahydroisoquinoline alkaloids, in particular, has gone largely unreported to date, most probably due to their relatively uncommon occurrence.14,15 Recently, however, several type XII bisbenzyltetrahydroisoquinoline alkaloids were isolated from Thalictrum flavum and reported to be inactive against Leishmania major promastigotes in vitro, with IC50 values of >20 μM.20 It is important to note that L. major is a causative parasite for cutaneous and not visceral leishmaniasis, and furthermore has a significant amount of unique genetic features when compared to L. donovani, and vice versa.21
In the present study, altogether 234 purified natural product compound library samples were screened against L. donovani promastigotes in vitro. Among those deemed to be active, having IC50 values below 10 μM, a series of type XII bisbenzyltetrahydroisoquinoline alkaloids stood out due to their structural inter-relationship, and the potency of compound 1. It has been suggested that a “lead” compound must lack overt toxicity at efficacious levels in diseased animals and that “hit” compounds exhibit in vitro selectivity of >20,22 so these compounds were also tested in vitro for cytotoxicity to HT-29 mammalian colon cancer cells. The selectivity index (SI) was then calculated as the ratio of antileishmanial activity to general cytotoxicity. The in vitro biological testing results for the type XII bisbenzyltetrahydroisoquinoline alkaloids examined are shown in Table 1. Since the in vitro activity observed for compound 1 was stronger than for compound 2, this seems to contradict the earlier interpretation of a structure-activity relationship (SAR) analysis reported for the antileishmanial activity of bisbenzyltetrahydroisoquinoline alkaloids in vitro against L. donovani promastigotes, which suggested that the nature of the amine functional group in the tetrahydroisoquinoline substructure led to activity following the order tertiary amine > secondary amine >> quaternary amine.16 Acknowledging that the formal charge carried by a quaternary amine would dramatically diminish drug uptake across membranes, it may be suggested herein that the SAR for bisbenzyltetrahydroisoquinoline alkaloids as antileishmanial agents has yet to be completely explored, and that the nature of the configuration, macrocyclic conformation, patterns of functional group substitution, and type of diphenyl linkages may play more important roles in the antileishmanial activity of bisbenzyltetrahydroisoquinoline alkaloids than does the substitution degree of the tetrahydroisoquinoline amines.
Table 1.
Results of in Vitro Biological Testing on Compounds 1–3
| compound | IC50 against L. donovani promastigotes (μM) | IC50 against HT-29 (μM) | selectivity index (SI) |
|---|---|---|---|
| 1 | 0.28 | 8.2 | 29.3 |
| 2 | 1.0 | 3.6 | 3.6 |
| 3 | 10.1 | 5.5 | 0.5 |
| miltefosinea | 3.7 |
Used as a positive control.
Compounds 1-3 are authentic natural product samples obtained from previously reported isolation work,23–25 which represented the initial discovery of compound 1 and the second isolation of 2, and thus they could not be readily compared to separate authentic samples for identity verification. Compound 1 was originally purified from Thalictrum alpinum L., compound 2 from T. rugosum Aiton, and compound 3 from T. dasycarpum Fisch. & Avé-Lall., as reported previously.23–25 At the time these compounds were discovered, only some details of their 1H NMR data were reported, as their structures were determined by several chemical degradation studies. No more recent report on their spectroscopic properties could be found, so to confirm the structures of 1-3, a detailed spectroscopic study of each was undertaken. The molecular formulas for 1-3 were confirmed as C37H40N2O7 [m/z 625.2908 (M + H)+ (calcd 625.2914)], C38H42N2O7 [m/z 639.3069 (M + H)+ (calcd 639.3070)], and C39H44N2O7 [m/z 653.3248 (M + H)+ (calcd 653.3227)], respectively, by HRESIMS and 13C NMR data. Based on correlations observed in their COSY, HSQC, HMBC, and NOESY spectra, as for example in compound 1 (Figure 1), the identity confirmation of compounds 1-3 was completed together with their first full NMR spectroscopic assignments (Tables 2 and 3). These NMR data were in general agreement with the limited information previously reported for 1-3, as were the specific rotation and UV absorption values for each.23–25
Figure 1.
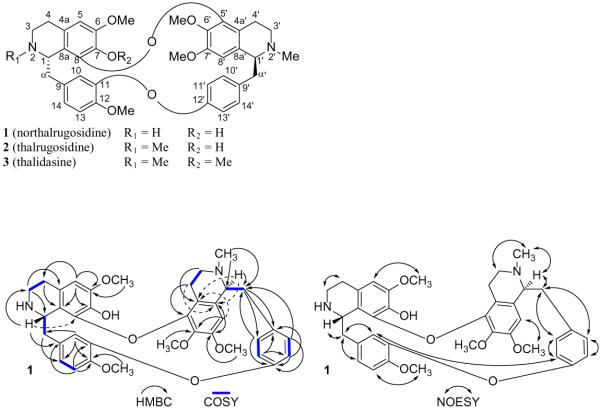
Selected spectroscopic correlations observed for northalrugosidine (1).
Table 2.
1H NMR Spectroscopic Data of Compounds 1–3 [δH, ppm, (J Hz)]a
| Proton | 1 | 2 | 3 |
|---|---|---|---|
| H-1 | 4.07, d (10.0) | 3.85, d (9.1) | 3.81, m |
| H-3 | 3.03, mb | 2.82, mb | 2.86, m |
| H-3 | 3.31, td (11.7, 4.2) | 3.41, m | 3.41, m |
| H-4 | 2.63, mb | 2.41, m | 2.44, m |
| H-4 | 2.80, mb | 2.87, m | 2.88, m |
| H-5 | 6.31, s | 6.30, s | 6.31, s |
| H-α | 2.67, mb | 2.62, m | 2.62, m |
| H-α | 3.09, mb | 3.02, m | 3.03, m |
| H-10 | 6.20 d, (2.0) | 6.33, mb | 6.35, mb |
| H-13 | 6.83, d (8.1) | 6.81, mb | 6.81, mb |
| H-14 | 6.72 dd, (8.1, 2.0) | 6.80, mb | 6.81, mb |
| H-1′ | 3.90, mb | 3.88, mb | 3.87, mb |
| H-3′ | 2.33, mb | 2.33, td (11.4, 1.8) | 2.33, mb |
| H-3′ | 2.86, m | 2.85, m | 2.86, m |
| H-4′ | 2.11, ddd (14.6, 11.3, 4.1) | 2.12, ddd (15.9, 11.4, 4.5) | 2.11, ddd (15.9, 12.5, 3.9) |
| H-4′ | 2.41, mb | 2.41, mb | 2.36, mb |
| H-8′ | 6.48, s | 6.49, s | 6.46, s |
| H-α′ | 2.70, mb | 2.68, dd (13.1, 4.1) | 2.69, dd (13.0, 4.0) |
| H-α′ | 3.24, dd (13.1, 2.1) | 3.21, db (13.1) | 3.20, db (12.6) |
| H-10′ | 6.39, dd (8.3, 2.2) | 6.35, dd (8.3, 2.2) | 6.33, db (2.1) |
| H-11′ | 6.59, dd (8.3, 2.6) | 6.56, dd (8.3, 2.6) | 6.54, dd (8.3, 2.5) |
| H-13′ | 6.97, dd (8.3, 2.6) | 6.98, dd (8.3, 2.6) | 6.98, dd (8.3, 2.5) |
| H-14′ | 7.57, dd (8.3, 2.2) | 7.53, dd (8.3, 2.2) | 7.52, dd (8.3, 2.1) |
| N-Me | - | 2.25, s | 2.25, s |
| 6-OMe | 3.78, s | 3.78, s | 3.76, s |
| 7-OH | 5.30, br | 5.44, s | - |
| 7-OMe | - | - | 3.26, s |
| 12-OMe | 3.92, s | 3.90, s | 3.90, s |
| N′-Me | 2.61, s | 2.60, s | 2.62, s |
| 6′-OMe | 3.52, s | 3.53, s | 3.49, s |
| 7′-OMe | 3.88, s | 3.88, s | 3.87, s |
Recorded in CDCl3 at 400 MHz and 300 K
Signal partially obscured
Table 3.
13C NMR Spectroscopic Data of Compounds 1–3 [δc, ppm]a
| carbon | 1 | 2 | 3 |
|---|---|---|---|
| C-1 | 54.4 | 61.6 | 61.8 |
| C-3 | 37.4 | 44.1b | 44.1b |
| C-4 | 29.1 | 22.5 | 23.1 |
| C-4a | 125.7 | 124.9 | 129.5 |
| C-5 | 105.1 | 105.0 | 105.8 |
| C-6 | 146.8 | 146.6 | 152.3 |
| C-7 | 133.6 | 133.9 | 137.1 |
| C-8 | 141.1 | 141.9 | 148.2 |
| C-8a | 123.2 | 122.6 | 121.6 |
| C-α | 38.4 | 39.6 | 39.3 |
| C-9 | 134.5 | 135.5 | 135.7 |
| C-10 | 115.4 | 115.0 | 115.0 |
| C-11 | 149.9 | 149.4 | 149.4 |
| C-12 | 147.4 | 147.2 | 147.3 |
| C-13 | 111.4 | 111.6 | 111.7 |
| C-14 | 122.2 | 123.0 | 123.1. |
| C-1′ | 65.2 | 65.2 | 65.2 |
| C-3′ | 51.2 | 51.1 | 51.4 |
| C-4′ | 24.2 | 24.1 | 24.2 |
| C-4a′ | 122.3 | 122.2 | 122.0 |
| C-5′ | 132.9 | 133.1 | 132.5 |
| C-6′ | 137.7 | 138.1 | 138.8 |
| C-7′ | 151.1 | 151.1 | 151.2 |
| C-8′ | 105.7 | 106.1 | 106.3 |
| C-8a′ | 146.5 | 147.0 | 147.4 |
| C-α′ | 40.8 | 41.0 | 41.2 |
| C-9′ | 134.5 | 134.1 | 134.1 |
| C-10′ | 131.1 | 131.0 | 131.1 |
| C-11′ | 120.3 | 120.0 | 119.9 |
| C-12′ | 153.4 | 153.9 | 153.9 |
| C-13′ | 120.3 | 120.0 | 120.0 |
| C-14′ | 133.0 | 132.9 | 132.7 |
| N-Me | - | 42.6 | 42.8 |
| 6-OMe | 56.3 | 56.3 | 56.0 |
| 7-OMe | - | - | 56.5 |
| 12-OMe | 56.2 | 56.3 | 56.2 |
| N′-Me | 44.1 | 44.1b | 44.1b |
| 6′-OMe | 60.6 | 60.8 | 60.6 |
| 7′-OMe | 56.2 | 56.2 | 56.5 |
Recorded in CDCl3 at 100 MHz and 300 K
Overlapped signals
After its identity was verified, northalrugosidine (1) was prioritized for in vivo testing in a murine model due to its in vitro potency and selectivity. Previously, there has been only one reported in vivo assessment of bisbenzyltetrahydroisoquinoline alkaloids relating to leishmaniasis,26 and these were tested by subcutaneous or local delivery to mice infected with cutaneous leishmaniasis by Leishmania amazonensis or Leishmania venezuelensis, two members of the Leishmania mexicana species complex. Thus, this report represents the first in vivo evaluation of a bisbenzyltetrahydroisoquinoline alkaloid in an animal model for visceral leishmaniasis. Since the target organs of visceral leishmaniasis are the liver and spleen, after the experiment was concluded these target organs were biopsied, and each was observed to have a dose-dependent reduction in parasitic burden between groups treated with 1 and a negative control (Figure 2). Additionally, there was no overt toxicity observed throughout the course of the experiment in any treatment group.
Figure 2.
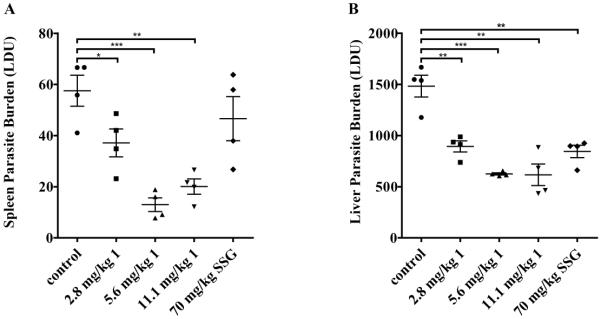
Results of in vivo testing of compound 1 using L. donovani infected mice. A. Parasite burden observed in spleen cells. B. Parasite burden observed in liver cells.
[LDU = organ weight (g) x number of L. donovani amastigotes per 1000 host cell nuclei; control = 10% DMSO in phosphate buffered serine; SSG = sodium stibogluconate, a standard antileishmanial drug (* p-value < 0.05; ** p-value < 0.005; *** p-value < 0.0005)].
The in vitro antileishmanial activity and selectivity over cytotoxicity to HT-29 mammalian cells observed for northalrugosidine (1) indicated it to be the most viable drug lead tested in the course of this study. The reduction in parasite burden to the murine spleen and liver after the in vivo testing of 1 supports the idea that it could be further investigated as a potential new drug candidate or lead molecule for drug discovery. As northalrugosidine (1) is the first bisbenzyltetrahydroisoquinoline alkaloid to demonstrate in vivo efficacy to treat visceral leishmaniasis, it may be suggested that the fairly large class of natural products it represents could be further explored for antileishmanial potential, and to determine a more profound understanding of their structure-activity relationships.
EXPERIMENTAL SECTION
General Experimental Procedures
A Perkin Elmer model 343 polarimeter (Perkin Elmer, Waltham, MA, USA) was used to measure optical rotations at 20 °C. Ultraviolet spectra were recorded using a Hitachi U-2910 UV/Vis double-beam spectrophotometer (Hitachi High-Technologies America, Schaumburg, IL, USA). A Bruker Avance DRX-400 spectrometer (Bruker, Billerica, MA, USA) was used to record NMR data at 300 K using standard Bruker pulse sequences. High-resolution electrospray ionization mass spectra (HRESIMS) were recorded using a Waters Q-TOF micro mass spectrometer (Waters, Milford, MA, USA) in the positive-ion mode, with NaI being used for mass calibration. Flow cytometry was conducted using a Fluorescence-Activated Cell Sorter (FACS) Calibur flow cytometer (BD Biosciences, San Jose, CA, USA). Deuterated NMR solvents were purchased from Cambridge Isotope Laboratories (Tewksbury, MA, USA). Cell culture media and supplements used for HT-29 cytotoxicity studies were obtained from Life Technologies, Inc. (Grand Island, NY, USA). Solvents, solutions, and standards for antileishmanial studies were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Plant Material
The roots of Thalictrum alpinum L. (Ranunculaceae) were collected and authenticated taxonomically as documented earlier.23
Extraction and Isolation
Compounds 1–3 were obtained from a library of natural product samples, provided by R.W.D., housed at the College of Pharmacy, The Ohio State University (Columbus, OH, USA). The method by which these compounds were extracted and isolated from T. alpinum has been described previously.23
Northalrugosidine (1): Orange-brown amorphous solid; 1H and 13C NMR data, see Tables 2 and 3; HRESIMS m/z 625.2908 (M + H)+ (calcd for C37H40N2O7, 625.2914). The [α]D and UV data were consistent with reported values.23
Thalrugosidine (2): Orange-brown amorphous solid; 1H and 13C NMR data, see Tables 2 and 3; HRESIMS m/z 639.3069 (M + H)+ (calcd for C38H42N2O7, 639.3070). The [α]D and UV data were consistent with reported values.24
Thalidasine (3): Orange-brown amorphous solid; 1H and 13C NMR data, see Tables 2 and 3; HRESIMS m/z 653.3248 (M + H)+ (calcd for C39H44N2O7, 653.3227). The [α]D and UV data were consistent with reported values.25
In Vitro Cytotoxicity Test Protocol
As published,27 human colorectal cancer (HT-29) cells were placed into 96-well plates (9500 cells/190 μL) and exposed to different concentrations of drugs or test samples in triplicate for 72 h. Cells treated only with 10% DMSO in H2O and those treated with paclitaxel (taxol) in the same carrier were kept as negative and positive controls, respectively. The sample-induced cytotoxicity was quantified by fluorescence detection after application of sulforhodamine B. IC50 values (concentrations that resulted in 50% inhibition of cell survival) were determined by Table Curve2Dv4 software using non-linear regression (log inhibitor vs. response on a variable slope).
In Vitro Killing of L. donovani Promastigotes Test Protocol
Previously developed transgenic DsRed2 L. donovani (strain LV 82) promastigotes,28 expressing red fluorescent protein, were grown in Medium 199 supplemented with 10% fetal bovine serum (FBS), 1% penicillin and streptomycin. The parasites were obtained from previously infected STAT4 knockout (immunocompromised) mice. During alternate passages, 50 μg/mL nourseothricin (clonNAT) was added to the growth medium to ensure selection of the fluorescent L. donovani promastigotes only. According to a published method,29 these promastigotes were placed into 24-well culture plates (1×106 cells/mL/well) and exposed to different concentrations of drugs or test samples in duplicate for 72 hours at 23 °C. Untreated promastigotes and those treated for 1 h with 1 mg/mL saponin (from Quillaja saponaria) were kept as negative and positive controls for the experiment, respectively. Miltefosine was used as a standard antileishmanial drug in this study. The drug-induced promastigote killing was quantified by flow cytometry, and IC50 values were determined by GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). IC50 calculations were made using non-linear regression (log inhibitor vs. response on a variable slope).
In Vivo Antileishmanial Test Protocol
Following a published protocol,30 BALB/c mice were infected with 107 L. donovani (strain LV 82) amastigotes by tail vein injection. Mice weighing an average of 18 g each were treated two weeks post-infection by 100 μL intravenous injections of DMSO (10% v/v) in phosphate buffered saline (PBS; 90% v/v) as a negative control, compound 1 (2.8, 5.6, or 11.1 mg per kg body weight in 10% DMSO/PBS), or sodium stibogluconate (SSG; 70 mg per kg body weight in 10% DMSO/PBS) as a positive control. Each treatment group comprised four animals. This protocol has been approved by The Ohio State University Institutional Review Board (IRB) and Institutional Animal Care and Use Committee (IACUC) as Protocol #2010A0048-R1, and the drug candidate was approved under amendment AM-7. These experiments were performed in accordance with all local and national guidelines and regulations. Mice were sacrificed two weeks post-injection. The liver and spleen of each euthanized mouse were collected and weighed. Tissue samples of each organ were used for taking impressions on glass slides followed by MeOH fixation, Giemsa staining, and examination under a light microscope for enumerating the number of amastigotes per 1000 host cell nuclei. The parasite burden was calculated in Leishman Donovan units (LDU) by the equation LDU = organ weight (g) × number of amastigotes per 1000 host cell nuclei. Statistical analysis was conducted using GraphPad Prism 5.00 (GraphPad Software, La Jolla, CA, USA) by unpaired t-tests between the LDU values of control and test groups.
Supplementary Material
ACKNOWLEDGMENT
This work was partially supported by NIH grant RC4 - AI 076309 (to A.R. Satoskar and A.D. Kinghorn). C. B. Naman acknowledges financial support provided by pre-doctoral fellowships from the American Foundation for Pharmaceutical Education (AFPE), College of Pharmacy, The Ohio State University, and an NIH Chemistry-Biology Interface Training Program (T32 GM08512). We wish to thank Mr. J. Fowble and Dr. C. A. McElroy, College of Pharmacy, The Ohio State University, for their diligent support and maintenance of the analytical instrumentation used in this study. We acknowledge Mr. Anthony D. Gromovsky, College of Pharmacy, The Ohio State University, for assistance in organizing and sampling the compound library.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Specific absorption data, UV, 1H NMR, 13C NMR, 13C DEPT 135, 1H-1H COSY, HSQC, HMBC, and NOESY spectra for compounds 1-3, along with HRESIMS data are available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
DEDICATION Dedicated to Dr. William Fenical of Scripps Institution of Oceanography, University of California–San Diego, for his pioneering work on bioactive natural products.
REFERENCES
- (1).Dawit G, Girma Z, Simenew K. J. Bacteriol. Parasitol. 2013;4:1000166. [Google Scholar]
- (2).WHO . In: Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases. Crompton DWT, editor. World Health Organization; Geneva: 2013. pp. 67–71. [Google Scholar]
- (3).Kaye P, Scott P. Nat. Rev. Microbiol. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- (4).McCall L-I, Zhang W-W, Matlashewski G. PLoS Pathog. 2013;9:e1003053. doi: 10.1371/journal.ppat.1003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, the WHO Leishmaniasis Control Team PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Gupta G, Oghumu S, Satoskar AR. In: Advances in Applied Microbiology. Sariaslani S, Gadd GM, editors. Vol. 82. Elsevier; New York: 2013. pp. 155–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).den Boer M, Argaw D, Jannin J, Alvar J. Clin. Microbiol. Infect. 2011;17:1471–1477. doi: 10.1111/j.1469-0691.2011.03635.x. [DOI] [PubMed] [Google Scholar]
- (8).Newman DJ, Cragg GM. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Salem MM, Werbovetz KA. Curr. Med. Chem. 2006;13:2571–2598. doi: 10.2174/092986706778201611. [DOI] [PubMed] [Google Scholar]
- (10).Chen S-B, Chen S-L, Xiao P-G. J. Asian Nat. Prod. Res. 2003;5:263–271. doi: 10.1080/1028602031000111941. [DOI] [PubMed] [Google Scholar]
- (11).Khamidullina EA, Gromova AS, Lutsky VI, Owen NL. Nat. Prod. Rep. 2006;23:117–129. doi: 10.1039/b504014k. [DOI] [PubMed] [Google Scholar]
- (12).Kuzmanov B, Dutschewska H. J. Nat. Prod. 1982;45:766–771. [Google Scholar]
- (13).Schiff PL., Jr. J. Nat. Prod. 1983;46:1–43. doi: 10.1021/np50026a018. [DOI] [PubMed] [Google Scholar]
- (14).Schiff PL., Jr. In: Alkaloids, Chemical and Biological Perspectives. Pelletier SW, editor. Vol. 5. New York; John Wiley and Sons: 1987. pp. 271–638. [Google Scholar]
- (15).Schiff PL., Jr. In: Alkaloids, Chemical and Biological Perspectives. Pelletier SW, editor. Vol. 11. Pergamon; London: 1996. pp. 1–236. [Google Scholar]
- (16).del Rayo Camacho M, Phillipson JD, Croft SL, Rock P, Marshall SJ, Schiff PL., Jr. Phytother. Res. 2002;16:432–436. doi: 10.1002/ptr.929. [DOI] [PubMed] [Google Scholar]
- (17).Fournet A, Muñoz V, Manjón AM, Angelo A, Hocquemiller R, Cortés D, Cavé A, Bruneton J. J. Ethnopharmacol. 1988;24:327–335. doi: 10.1016/0378-8741(88)90162-6. [DOI] [PubMed] [Google Scholar]
- (18).Angerhofer CK, Guinaudeau H, Wongpanich V, Pezzuto JM, Cordell GA. J. Nat. Prod. 1999;62:59–66. doi: 10.1021/np980144f. [DOI] [PubMed] [Google Scholar]
- (19).Schiff PL., Jr. In: Alkaloids, Chemical and Biological Perspectives. Pelletier SW, editor. Vol. 14. Elsevier; Oxford, UK: 1999. pp. 1–284. [Google Scholar]
- (20).Ropivia J, Derbré S, Rouger C, Pagniez F, Le Pape P, Richomme P. Molecules. 2010;15:6476–6484. doi: 10.3390/molecules15096476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Rogers MB, Hilley JD, Dickens NJ, Wilkes J, Bates PA, Depledge DP, Harris D, Her Y, Herzyk P, Imamura H, Otto TD, Sanders M, Seeger K, Dujardin J-C, Berriman M, Smith DF, Hertz-Fowler C, Mottram JC. Genome Res. 2011;21:2129–2142. doi: 10.1101/gr.122945.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Nwaka S, Hudson A. Nat. Rev. Drug Discov. 2006;5:941–955. doi: 10.1038/nrd2144. [DOI] [PubMed] [Google Scholar]
- (23).Wu W-N, Beal JL, Doskotch RW. J. Nat. Prod. 1980;270:372–381. [Google Scholar]
- (24).Mitscher LA, Wu W-N, Doskotch RW, Beal JL. Chem. Commun. 1971:589–590. [Google Scholar]
- (25).Kupchan SM, Yang T-H, Vasilikiotis GS, Barnes MH, King ML. J. Am. Chem. Soc. 1967;1019:3075–3076. [Google Scholar]
- (26).Fournet A, Fayette L, Barrios AA, Muñoz V, Hocquemiller R, Cavé A. Phytother. Res. 1993;7:281–284. [Google Scholar]
- (27).Pan L, Kardono LBS, Riswan S, Chai H, Carcache de Blanco EJ, Pannell CM, Soejarto DD, McCloud TG, Newman DJ, Kinghorn AD. J. Nat. Prod. 2010;73:1873–1878. doi: 10.1021/np100503q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lezama-Dávila CM, Isaac-Márquez AP, Kapadia G, Owens K, Oghumu S, Beverley S, Satoskar AR. Biol. Pharm. Bull. 2012;35:1761–1764. doi: 10.1248/bpb.b12-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Singh N, Gupta R, Jaiswal AK, Sundar S, Dube A. J. Antimicrob. Chemother. 2009;64:370–374. doi: 10.1093/jac/dkp206. [DOI] [PubMed] [Google Scholar]
- (30).Peine KJ, Gupta G, Brackman DJ, Papenfuss TL, Ainslie KM, Satoskar AR, Bachelder EM. J. Antimicrob. Chemother. 2014;69:168–175. doi: 10.1093/jac/dkt320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


