Abstract
Objective
There is great interest in closed-loop neurostimulators that sense and respond to a patient’s brain state. Such systems may have value for neurological and psychiatric illnesses where symptoms have high intraday variability. Animal models of closed-loop stimulators would aid preclinical testing. We sought to demonstrate that rodents can directly control a closed-loop limbic neurostimulator via a brain-computer interface (BCI).
Approach
We trained rats to use an auditory BCI controlled by single units in prefrontal cortex (PFC). The BCI controlled electrical stimulation in the medial forebrain bundle (MFB), a limbic structure involved in reward-seeking. Rigorous offline analyses were performed to confirm volitional control of the neurostimulator.
Main Results
All animals successfully learned to use the BCI and neurostimulator, with closed-loop control of this challenging task demonstrated at 80% of PFC recording locations. Analysis across sessions and animals confirmed statistically robust BCI control and specific, rapid modulation of PFC activity.
Significance
Our results provide a preliminary demonstration of a method for emotion-regulating closed-loop neurostimulation. They further suggest that activity in prefrontal cortex can be used to control a BCI without pre-training on a predicate task. This offers the potential for BCI-based treatments in refractory neurological and mental illness.
Keywords: brain-computer interface, neuromodulation, mental disorders, closed-loop stimulation, deep brain stimulation
Introduction
Deep brain stimulators (DBS) have continued to demonstrate efficacy in degenerative movement disorders (Bronstein and Tagliati 2011), and have also shown promise in psychiatric illness. Multiple targets, including the subgenual cingulate gyrus (Kennedy et al 2011, Holtzheimer et al 2012), ventral striatum (Malone et al 2009, Goodman et al 2010), nucleus accumbens (Bewernick et al 2012), and medial forebrain bundle (MFB) (Schlaepfer et al 2013) have shown promise in treating depression. The striatal site has approval in the United States for treating obsessive-compulsive disorder (OCD) (Goodman et al 2010), and there is evidence for accumbens efficacy in OCD as well (Denys et al 2010). While these trials are encouraging, invasive psychiatric neurostimulation still has notable limitations. We present preliminary evidence that brain-computer interfaces (BCIs) may help address those limits by contributing to the next generation of closed-loop stimulators.
A cardinal limitation of psychiatric DBS is the stimulator’s inability to adapt to a patient’s needs. Depression and OCD fluctuate on timescales of days to weeks. Other illnesses, including posttraumatic stress disorder (PTSD) and various anxiety disorders, can flare and remit much more rapidly, in minutes to hours. Clinician adjustment of DBS parameters cannot track these rapid changes in brain state. While in theory severe symptoms could be controlled by increasing the stimulation “dose” (pulse width or amplitude), in practice this leads to side effects and rapid battery depletion (Haq et al 2010, Goodman et al 2010, Holtzheimer et al 2012). The latter drives symptom recurrence and surgical morbidity from replacements. Even “patient controlled” stimulators only offer a choice between a few pre-programmed settings, which may not be adequate for the heterogeneity of psychiatric illness. Furthermore, given that mental disorders still carry stigma, patients are unlikely to wish to frequently use a visible external controller in public (e.g., pressing a button to ward off an impending anxious episode).
Similar problems in other clinical domains are being addressed with closed-loop neurostimulators that sense and respond to patients’ needs in real-time. A DBS platform has been created by a major manufacturer to enable such devices (Afshar et al 2013). There have been encouraging results in suppressing epileptic seizures (Morrell 2011) and Parkinsonian tremor (Rosin et al 2011, Little et al 2013). Unfortunately, those algorithms are not promising for control of psychiatric symptoms. First, although some disorders (most notably depression) have candidate electrographic biomarkers (Ward and Irazoqui 2010, McLoughlin et al 2014), these markers change slowly and without tight correlation to symptoms or response (Baskaran et al 2012, McLoughlin et al 2014). Moreover, aside from a few well-established findings such as cingulate hyperactivity, biomarkers have usually been reported in small studies, and may not hold up to larger-scale validation (Nesse and Stein 2012, Whelan and Garavan 2013, Widge et al 2013). Finally, existing closed-loop brain stimulators operate on passive features of neural activity; they are not transparent to or modifiable by the patient. While modern psychosurgical research is conducted to high ethical standards, the field has a history of inappropriate paternalism (Feldman and Goodrich 2001). In that context, extraordinary transparency may be warranted, particularly as proponents encourage DBS for syndromes such as addiction or aggression (Pisapia et al 2013, Torres et al 2013).
BCI may offer a way past these dilemmas. BCIs also attempt to decode a patient’s instantaneous clinical need, but do so by requiring users to volitionally alter neural activity. This volitional control could have three major advantages in psychiatric brain stimulation. First, rather than requiring validation of a specific biomarker for each disorder, a single decoding strategy could address a broad set of conditions. Second, because a BCI is constantly under the user’s control, it achieves the desired transparency. Third, BCIs have already been demonstrated to provide volitional control over paralyzed body parts and artificial devices (Moritz et al 2008, Ethier et al 2012, Hochberg et al 2012, Collinger et al 2013). In the long run, this approach may give patients fine-grained control over stimulus parameters (and thus the window between relief and side effects), although care would be needed to prevent complications (Haq et al 2010).
We therefore envision a BCI-based, closed-loop neurostimulator for psychiatric disorders. In this system, patients would volitionally alter a recorded neural signal in response to uncontrolled symptoms or anticipation thereof. This would activate stimulation within the limbic circuit, which in turn would relieve symptoms. We believe such a device could increase patients’ sense of agency, reduce stimulator on-time compared to open-loop control (Little et al 2013), and mitigate undesired side effects.
Just as BCIs for paralysis use motor cortex as a “natural” source of control signals, a closed-loop neurostimulator for emotion regulation may be best controlled by signals from prefrontal cortex (PFC). Various PFC subdomains are implicated in emotion regulation, suggesting that the intention we seek to decode (desire to suppress pathologic emotional experiences) is already represented. Furthermore, there is growing evidence that descending pathways from PFC to limbic structures are a key anatomic substrate for that regulation. Functional imaging in multiple psychiatric disorders has shown hypoconnectivity specific to fronto-limbic tracts (Etkin and Wager 2007, Price and Drevets 2012). This raises an analogy to spinal cord injury, where motor cortex is disconnected from distal spinal cord and muscles. If we consider mental disorders as a similar connectivity deficit from PFC to the limbic loop, a BCI-based deep brain stimulator could play the same functional role as an idealized corticospinal prosthesis that treats paralysis.
PFC may be particularly suited for BCI control given the emerging finding that active plasticity and re-tuning of neurons routinely occurs during motor BCI use (Ganguly et al 2011, Koralek et al 2012). There is evidence that PFC neurons specialize in rapid re-configuration to meet task demands (Warden and Miller 2007, Cromer et al 2010). Controllable neurons have been found in primate PFC, although that work selected for neurons that were specifically responding to an existing BCI paradigm (Kobayashi et al 2010).
A rodent BCI platform would be particularly valuable for developing treatments targeting mental illness, as there are a wide variety of available rodent models and behavioral tasks (Kalueff et al 2007, Cohen et al 2012, Milad and Quirk 2012). Therefore, as a proof of concept, we developed a paradigm in which rats used PFC signals to control a BCI that in turn triggered deep-brain stimulation in the limbic circuit. By linking the PFC-controlled BCI to medial forebrain bundle (MFB) stimulation, we created an environment in which animals could use neural activity to electrically modulate limbic structures involved in pleasure and reward (Olds 1958, Carlezon and Chartoff 2007). That same structure is currently under trial as a stimulation site for human psychiatric patients (Schlaepfer et al 2013).
Methods
Female Long-Evans rats were trained to control implanted stimulating electrodes via a one-dimensional BCI based on single unit action potentials recorded from prefrontal cortex (PFC). Successful neural modulation in the BCI task triggered stimulation in medial forebrain bundle (MFB), a reinforcing limbic site. All experimental procedures were approved by the University of Washington Institutional Animal Care & Use Committee (IACUC).
Surgery
Animals were deeply anesthetized by isoflurane inhalation and implanted in prelimbic/infralimbic cortex with custom-built 2×8 arrays of tungsten microwire electrodes. Electrodes were stereotaxically placed with the medial row of electrode tips 3.5 mm anterior to bregma, 0.5 mm lateral from midline, and 4 mm below the dura (Paxinos and Watson 2009).
Deep brain stimulating electrodes were implanted in the same procedure. Twisted monopolar stimulating electrodes (Plastics One) were placed bilaterally targeting MFB (2.8 mm posterior to bregma, 1.7 mm lateral to midline, 7.8 mm below dura). MFB is a key pathway in the hedonic and appetite components of the limbic circuit, and stimulation produces a rewarding emotional experience (Olds 1958, Carlezon and Chartoff 2007). Animals recovered for at least a week postoperatively before the electrodes were tested.
Prefrontal Recording
After recovery, animals were connected via an active headstage and pre-amplifier to a Tucker-Davis Technologies (TDT) RZ5 biosignal processor controlled by a dedicated computer. Signals were filtered (1 to 8 kHz bandpass) and spikes identified with time-voltage windows. Detected spike events were streamed to a data acquisition system (DAQ, National Instruments, USB-6229), which synchronized neural recording with control of the experimental arena. The DAQ, computer, and TDT processor were simultaneously controlled by custom-written LabVIEW software (National Instruments).
Limbic Stimulation from Behavioral Triggers
All training and testing was conducted in the dark in a video-monitored acrylic arena, with a white noise source to mask environmental distractors. Sessions lasted a minimum of 60 minutes, but could extend up to 150 minutes if the animal was sustaining task performance.
Prior to implantation, all animals were trained to press two levers to deliver chocolate-flavored food pellets (BioServ). Beginning one week after surgery, animals were offered the same two levers, but one lever now triggered electrical stimulation of MFB via a stimulus isolator (A-M Systems Model 2200). Stimulus parameters were titrated until the animal reliably pressed the MFB-stimulation lever to the exclusion of food reward. Pulse trains could be monophasic or biphasic (cathodal leading). Stimulus parameters were adjusted at most weekly, if needed, to sustain behavior. Electrodes in both hemispheres were tested to determine the maximally reinforcing site, but stimuli were only delivered unilaterally in a given session.
Brain-Computer Interface
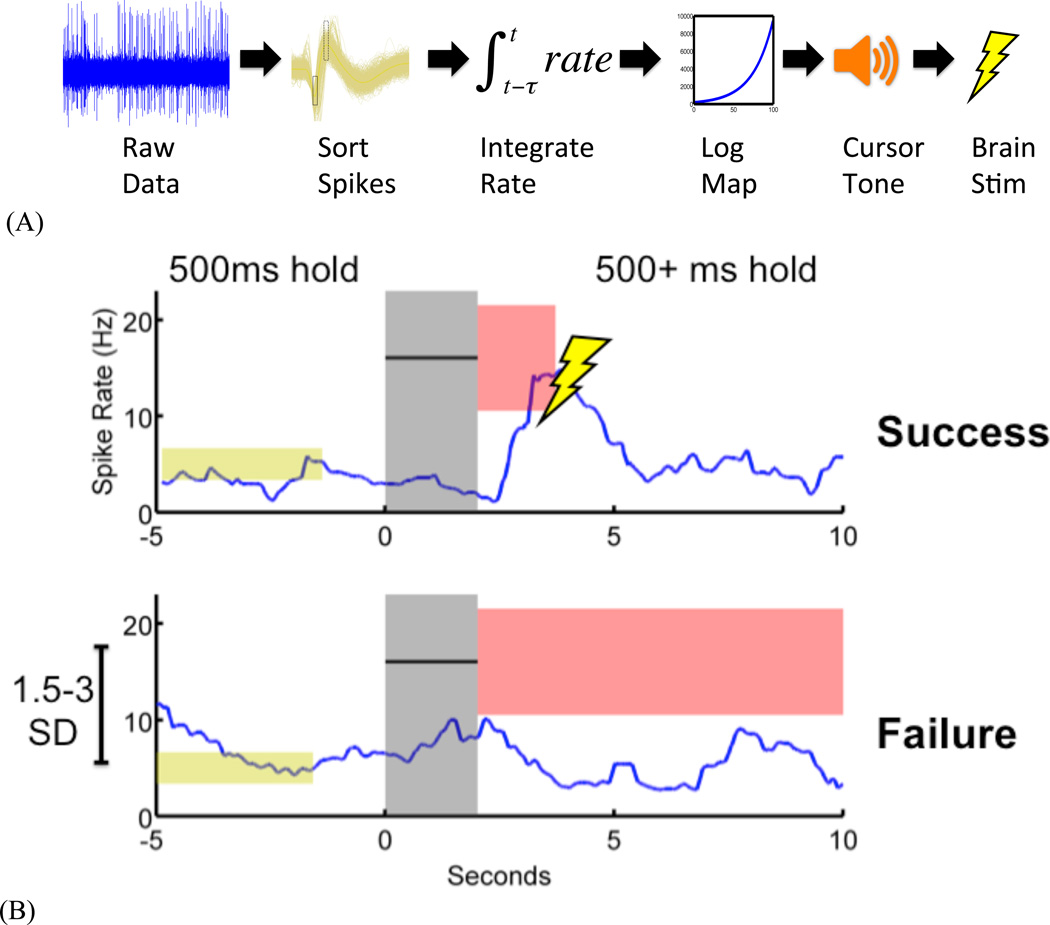
The BCI task was adapted from Gage et al. (2005), and is illustrated in Figure 1A. A single recorded unit directly controlled a single degree of freedom (the frequency of an audio tone). Neural firing rate, smoothed over the shorter of a 50-spike or 20-second window, was logarithmically mapped onto a frequency ladder. The tone was then played to the animal; the computational simplicity of the algorithm enabled updates every 10 ms.
Figure 1. Methods.
(A) BCI transform from brain activity to control an auditory cursor. Real-time sorted spikes from an isolated unit are transformed to an instantaneous rate, which is then linearly scaled into the 0–100 range. Cursor values from 0 to 100 are mapped onto a logarithmic frequency ladder from 200 to 10,000 Hz and continuously presented to the animal during trials.
(B) BCI trials for brain stimulation reward. The animal is required to maintain the controlled unit’s firing rate at a pre-established baseline for at least 500 ms. A cue tone then indicates a target that is at least 1.5 standard deviations (SD) from baseline. The auditory cursor must be controlled (by increasing firing rate) to remain within the target. Maintaining rate in target for at least 500 ms leads to trial “Success” and subsequent brain stimulation; failure to do during the trial duration (here, 10 seconds) leads to “Failure” and no stimulation. Trials are followed by a 5–10 second random duration timeout period.
Animals used the BCI in self-paced trials, illustrated in Figure 1B. Each BCI session began with identification of a single unit in PFC. The mean and standard deviation of that unit’s spike rate were measured for at least 3 minutes while the animal behaved freely in the arena. These parameters established the session baseline. Animals were then offered the BCI task. They were first required to maintain their firing rate within 0.3 standard deviations (SD) of the baseline mean for at least 500 ms. No cursor feedback occurred during the baseline-hold period. Once baseline rate was maintained, a tone played for 1–2 seconds, cueing the animal to increase the unit’s firing rate to match the cue tone frequency. The auditory cursor provided the animal continuous feedback of current neural activity during this period. The cursor was available for a 5–10 second trial window, and silenced if the animal acquired the target within that window. Dwelling rate within the target for at least 500 ms (success) was rewarded with a pulse train delivered to MFB. No stimulus occurred if the animal failed to reach the target before the trial expired (failure). A random-duration timeout followed each trial (5–10 s), after which the animal was once again required to hold baseline to initiate a new trial.
BCI Shaping and Testing Protocol
Animals were initially trained without the baseline hold, and were presented with targets that could easily be acquired by chance. Once an animal demonstrated success rates above 80% at any given difficulty, target parameters were titrated until she reliably could acquire targets at least 1.5 SD above baseline with a dwell time of at least 500 ms. The baseline-hold requirement was then added, and the animal allowed to learn this component through further operant sessions.
Once animals learned the task, they were only presented with targets whose minimum acceptable firing rate was at least 1.5 SD above baseline, with the highest targets having a floor of 3 SD. The tolerance (target width above the minimum) ranged from 1–2 SD. Dwell time for successful target acquisition was 500–1000 ms. Each day started with a target extending from 1.5–3.5 SD with a 500 ms dwell time, and the experimenter manually increased target distance and/or dwell time whenever the animal showed high performance (generally 80% or higher success rate over a 20-trial block). The modal increases of difficulty were +1.25 SD of required discharge rate and +500 ms of hold duration.
We selected the best-isolated single unit for training during each daily session. There was no bias toward using the same electrode or unit on successive days, and 73% of training sessions involved a unit recorded at a different electrode site than the previous day. This substantially increased the task difficulty, as the animals were required to learn to modulate a new pre-frontal unit on most testing sessions. Animals participated in the study until the PFC array no longer recorded discriminable units or the MFB stimulation failed to produce lever-pressing.
To verify that the animal was using the auditory BCI above chance performance, 20% of trials were randomly designated as “catch” trials. In catch trials, neural firing rate and target acquisition were tracked as in a regular BCI trial, but no cue or cursor was presented.
Statistical Methods
All animals in which we obtained both well-isolated PFC recordings and reinforcing MFB sites are reported (n=4). During each session, chance performance was assessed using randomly-inserted catch trials. Formal testing for BCI control was only performed if the animal’s trial success rate exceeded the catch success rate for at least 20 trials and 15 minutes; control was otherwise presumptively declared absent. As there was no other behavioral indication of engagement in this purely cognitive BCI task, we considered animals to have been potentially using the BCI and controlling the stimulator only once their on-line target acquisition rate gave evidence of exceeding the corresponding on-line chance estimate. Within-session statistical tests were only applied to these “task performance” periods, during which there were grounds to hypothesize that the animals’ neural activity differed from background fluctuations.
Within individual sessions, control was tested with a bootstrap analysis that randomly shuffled trial times across the recorded spike train. To increase stringency, we only tested time periods where the baseline-hold criterion had recently been satisfied, and laid a putative target over the record at an appropriate delay after the baseline hold. The set of task performance trials was randomly replicated 10,000 times, generating a distribution of the possible success rates that could be expected by chance. A one-tailed t-test was then performed for the hypothesis that the success rate actually observed during task performance was not contained within the chance distribution. If the observed success rate was greater than the bootstrapped chance distribution (p < 0.05, Z-score above 2), the animal was deemed to have successfully controlled the BCI and neurostimulator during that session.
In pooled analyses across all animals and sessions, paired-sample t-tests were used to test for differences between actual and bootstrapped data. For variables that were best represented as distributions, the two-sample Kolmogorov-Smirnov (K-S) test for equality of distributions was used.
Results
All Animals Controlled Pre-Frontal Cortex BCI to Trigger Limbic Stimulation
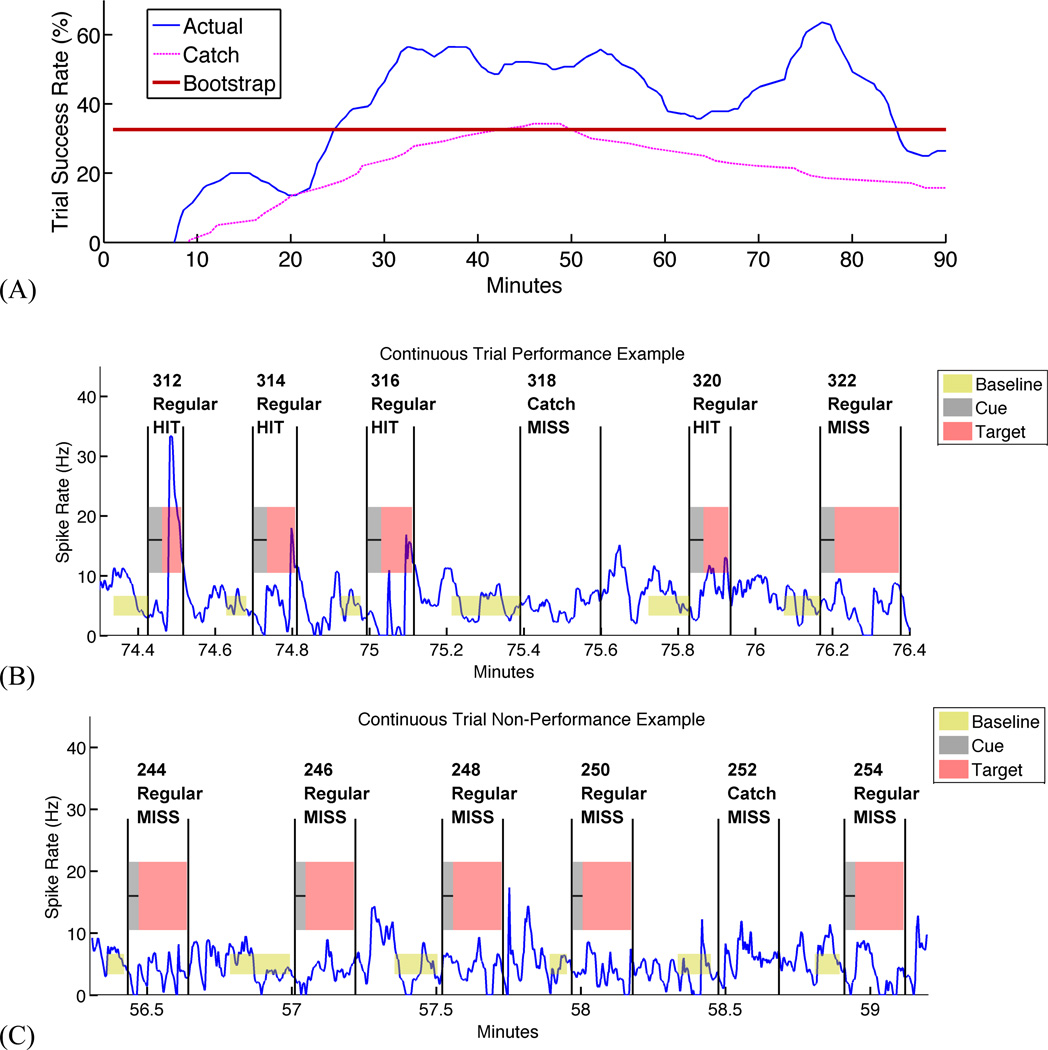
Figure 2 illustrates a typical day on which an animal successfully modulated PFC activity to receive MFB stimulation. Animals started at chance performance during early learning, then gained control of the PFC unit activity and increased their trial success rates well above chance. As seen in the Figure 2 example, this could occur quite rapidly in some cases. As the training session continued, the success rate generally rose to a sustained maximum, then declined toward chance levels as the animal either fatigued or was satiated by the reward (see Discussion). All animals successfully controlled the BCI, with task performance beginning after 34 ± 23 minutes (Mean ± SD) on average. This reflects the lead-in period associated with the selection of a new PFC unit for most sessions. Across animals, 25 electrode sites had discriminable PFC units, and 20 of these sites (80%) were controllable during at least one experimental session. Animals met stringent tests for BCI control in 21 of 51 experimental sessions (41%). Table 1 presents per-animal performance and average data.
Figure 2. Example of pre-frontal single-unit control for limbic reward.
(A) Smoothed success rate over a single testing session, compared to real-time (Catch) and offline (Bootstrap) measures of chance performance. In this session, the animal begins to acquire targets after approximately 10 minutes of practice, rapidly separating from chance levels. Performance continues to climb until approximately 30 minutes, then sustains until nearly the end of the 90-minute session.
(B) Spike rate during a string of successful trials from a subsection of (A), illustrating series of successes as well as an example of missed target during a catch trial.
(C) Spike rate during string of unsuccessful trials from an earlier subsection of (A), illustrating long period where animal held baseline but did not produce spike rate excursions of sufficient duration to satisfy the target.
Table 1.
Closed-loop neurostimulator control by individual animal and grand total. All animals achieved control of the system using single PFC units. Overall performance as measured by number of controllable sessions was variable, but animals achieved control at 80% of tested sites within PFC.
| Animal | Total Sessions |
Sessions with Control |
Total Sites |
Sites with Control |
|---|---|---|---|---|
| A | 7 | 4 (57%) | 4 | 4 (100%) |
| B | 11 | 3 (27%) | 6 | 3 (50%) |
| C | 9 | 4 (44%) | 6 | 5 (83%) |
| D | 24 | 10 (42%) | 9 | 8 (89%) |
| Total | 51 | 21 (41.18%) | 25 | 20 (80.00%) |
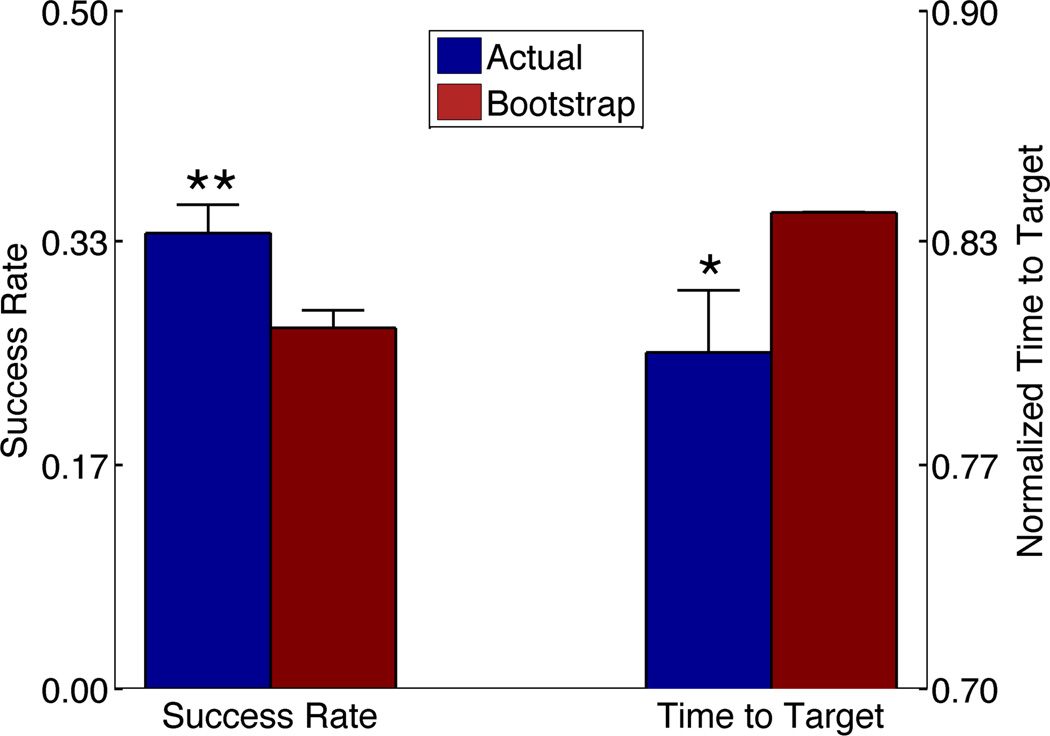
BCI control was verified in aggregate analyses across animals and sessions. In the aggregate analysis, we tested not only the task performance period, but all trials from the onset of task performance to the end of the testing session. The period before task onset was not tested, as it often represented animals’ first opportunity to learn control of the unit at a newly selected PFC recording site. We included data from all sessions, including those where animals’ success rates did not verify BCI control within that single day. We thus ensured that our analysis reflected overall performance, not merely times when the animal happened to be acquiring targets well. As shown in Figure 3, overall target acquisition rates using the BCI were significantly greater than bootstrapped chance (p = 0.0054, two-tailed paired-sample t-test).
Figure 3. Aggregate analysis of animals’ BCI performance.
(A) Trial success rates from onset of performance period to end of session, for all testing sessions. Error bars represent standard error of the mean. Trials with active neural cursor feedback (Actual) showed significantly higher hit rates than Bootstrap chance (**, p = 0.0054, paired-sample two-tailed t-test).
(B) Mean time to success during task performance in same trials and sessions as (A). Times are normalized by the maximum allowed duration (after which the trial times out). Error bars represent standard error of the mean, and the Bootstrap error bar is very small and thus not visible above the mean. Performance with active cursor is significantly faster than chance (*, p=0.017, two-sided K-S test).
Time-to-Target Analyses Verify Animals’ Use of BCI Feedback
When animals were actively attending to and controlling the BCI cursor to trigger the stimulator, they also reached targets faster than predicted by a chance distribution. Figure 3 also shows aggregate trial success times, normalized by the maximum allowed trial duration. Animals acquired the target significantly faster than bootstrapped chance (two-sided K-S test, p = 0.017), consistent with use of the BCI cursor feedback. Combined with the success rates, these findings support a conclusion that animals were volitionally controlling the PFC BCI to obtain brain stimulation.
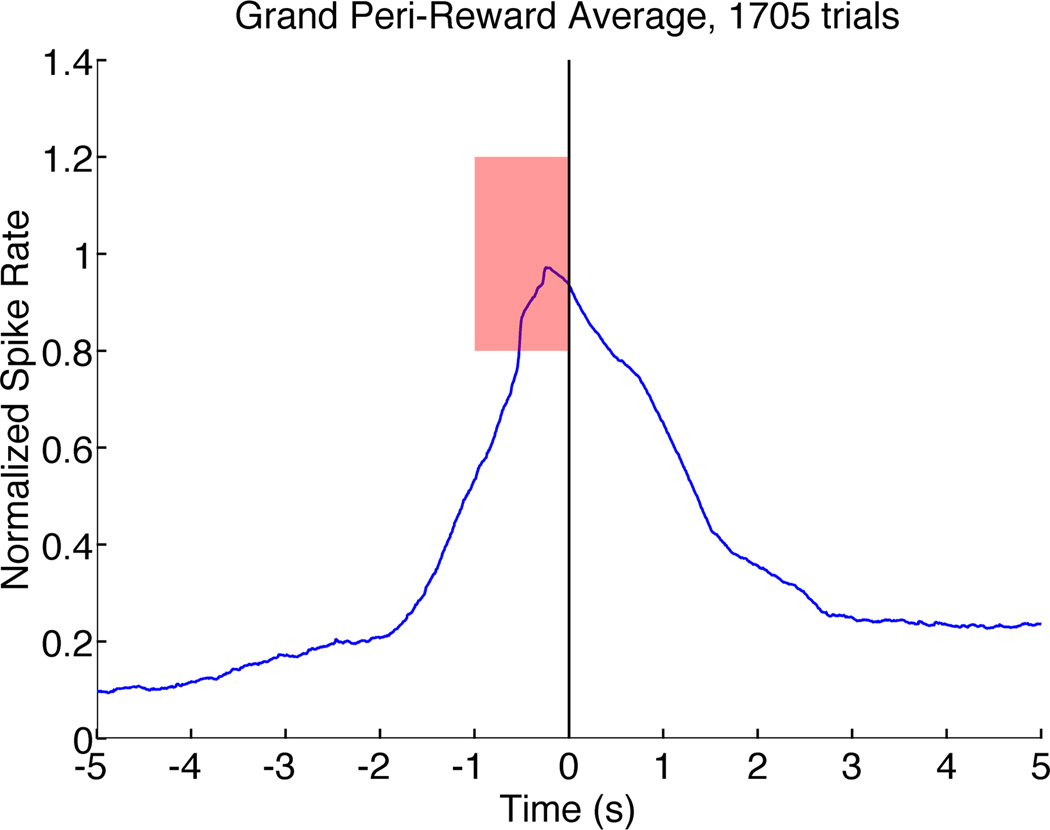
Peri-Reward Averages Demonstrate Smooth Control Without Feed-Forward
Agile control of a BCI cursor is indicated by a deliberate increase in firing rate to acquire the target when one is available, followed by an equally rapid decline once the target is satisfied. Figure 4 shows such a trajectory in the average peri-reward cursor trajectory of all successful trials after performance onset on all testing days. In addition, the peri-reward average verifies that there was no direct effect of MFB stimulation on PFC activity. We saw no sustained firing that would indicate “feed forward” activity induced by the stimulator. Similar averages on electrodes/units not involved in the BCI were flat (not shown), demonstrating that control was specific to the selected PFC unit.
Figure 4.
Grand peri-reward average of spike rate across all successful trials on all days, from onset of task performance to end of testing session. Rewarding brain stimulation occurs at t=0. Data have been normalized to the interval between baseline (0) and target (1). The mean trajectory is a smooth rise toward the center of the target, with a similarly rapid decline towards zero once target has been achieved. We do not observe persistently elevated activity post-reward, suggesting that MFB stimulation does not directly influence PFC firing.
Discussion
Rodents learned to modulate individual prefrontal cortex (PFC) units to control an implanted limbic stimulator via a BCI. Control of the closed-loop system was robust across days and animals. Time-to-target and peri-reward analyses provided additional evidence that the animals were aware of and volitionally controlling the neurostimulator via isolated PFC activity.
This preliminary demonstration is highly relevant to neural engineering in two ways. First, they represent an important step for BCI into the cognitive-emotional domain. Rather than controlling a computer or limb, these animals learned to control an element of their limbic circuit. The system demonstrated here could be a testbed for technologies targeting refractory mental illness. Second, our results indicate that even relatively unsophisticated animals can generate BCI control signals from non-motor cortex. This extends prior findings that a variety of cortical areas can be used for motor BCI control (Marzullo et al 2006, Kobayashi et al 2010), and may inform ongoing research on PFC plasticity and function. These results were also achieved without prior training on a motor task, suggesting that it is possible to directly train animals on a BCI.
An interesting observation from the present study was that BCI control often declined after a period of sustained performance. This was surprising, as MFB stimulation usually drives sustained performance of a simple motor task (Olds 1958, Carlezon and Chartoff 2007). This does not appear to be simple reward satiation; at effective parameters, our rats would lever-press to self-administer far more stimuli than they received during a typical BCI session. Depletion of the phasic dopamine pool is not generally seen with MFB stimulation, as this would lead to rapid loss of effect. Habituation to the rewarding effect is possible, but again would be inconsistent with the large MFB literature. We considered that the single decoded neuron might become metabolically exhausted, but saw no global change in firing rates over the course of a session. The closest explanation is the report that humans often find BCI use effortful and mentally fatiguing (Curran and Stokes 2003, Birbaumer 2006). We speculate that animals continued to desire the reward, but that continuous BCI use became increasingly effortful, to the point that the required effort exceeded MFB’s hedonic value. In the current experiment, we were not able to perform reward and task difficulty titrations to investigate this hypothesis. Its clinical significance, however, may not be great. We observed performance decline after requiring animals to perform the tasks multiple times per minute. A patient would likely not need to use a BCI to adjust emotion-regulating stimulation that frequently, and thus likely would not fatigue. Furthermore, we employed a challenging BCI task in which animals had to re-learn the decoder on a daily basis. A human implementation could use a more stable and sophisticated decoder that would likely place less burden on the patient.
The long-range prospect of human use raises some practical questions. First, mental illness has been viewed as a “hypofrontality”, suggesting that those patients may not be able to modulate frontal signals well (George et al 1994). However, there is also specific imaging evidence that even depressed patients increase PFC activity when asked to perform emotional tasks (Johnstone et al 2007, Greening et al 2013). The PFC BCI proposed here should therefore still be usable, as it is calibrated to changes relative to baseline. Second, because PFC is involved in many cognitive functions, these neurons frequently respond to complex stimuli (Rigotti et al 2013). One might worry that the BCI would somehow interfere with or be confounded by ongoing PFC activity. Motor cortical BCIs provide some reassurance. Recent work has found that the brain is readily able to readily learn novel and abstract associations between neural activity and BCI control, and to alternate between separate mappings of activity to manual and BCI control tasks (Ganguly et al 2011, Koralek et al 2012). This implies that there is sufficient neural capacity to devote a subset of cells to BCI control without impairing other performance. We would expect the same in PFC, which is evolutionarily newer and more flexible.
In summary, we have demonstrated a proof-of-concept closed-loop neurostimulator in which prefrontal neural activity can drive limbic stimulation. We have further demonstrated that effective BCI control signals can be found in anterior prefrontal regions. Numerous refinements can be envisioned in electrode placement, neural cursor algorithms, and stimulation parameters, but these results represent an important step towards closed-loop systems for treating psychiatric and neurological disorders.
Acknowledgements
We thank Amber Fechko, Michael Kasten, and Michael Sunshine for extensive technical assistance with electrode construction and surgeries. We are further grateful to Behnum Habibi for animal training and data collection. This work was supported by a seed grant from the Center for Sensorimotor Neural Engineering (National Science Foundation EEC-1028725) to ASW, as well as a grant from the National Institute for Neurological Disorders & Stroke to CTM (R01 NS066357). This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Footnotes
Conflicts of Interest: The authors affirm that we have no financial or other conflicts.
References
- Afshar P, Khambhati A, Carlson D, Dani S, Lazarewicz M, Cong P, Denison T. A translational platform for prototyping closed-loop neuromodulation systems. Front. Neural Circuits. 2013;6:117. doi: 10.3389/fncir.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran A, Milev R, McIntyre RS. The neurobiology of the EEG biomarker as a predictor of treatment response in depression. Neuropharmacology. 2012;63:507–513. doi: 10.1016/j.neuropharm.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-Term Effects of Nucleus Accumbens Deep Brain Stimulation in Treatment-Resistant Depression: Evidence for Sustained Efficacy. Neuropsychopharmacology. 2012;37:1975–1985. doi: 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N. Breaking the silence: Brain–computer interfaces (BCI) for communication and motor control. Psychophysiology. 2006;43:517–532. doi: 10.1111/j.1469-8986.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- Bronstein J, Tagliati M. Deep brain stimulation for Parkinson disease: An expert consensus and review of key issues. Arch. Neurol. 2011;68:165–165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat. Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kozlovsky N, Alona C, Matar MA, Joseph Z. Animal model for PTSD: From clinical concept to translational research. Neuropharmacology. 2012;62:715–724. doi: 10.1016/j.neuropharm.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, McMorland AJ, Velliste M, Boninger ML, Schwartz AB. High-performance neuroprosthetic control by an individual with tetraplegia. The Lancet. 2013;381:557–564. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer JA, Roy JE, Miller EK. Representation of Multiple, Independent Categories in the Primate Prefrontal Cortex. Neuron. 2010;66:796–807. doi: 10.1016/j.neuron.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran EA, Stokes MJ. Learning to control brain activity: A review of the production and control of EEG components for driving brain–computer interface (BCI) systems. Brain Cogn. 2003;51:326–336. doi: 10.1016/s0278-2626(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, Bosch A, Schuurman R. Deep Brain Stimulation of the Nucleus Accumbens for Treatment-Refractory Obsessive-Compulsive Disorder. Arch Gen Psychiatry. 2010;67:1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- Ethier C, Oby ER, Bauman MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature. 2012;485:368–371. doi: 10.1038/nature10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RP, Goodrich JT. Psychosurgery: a historical overview. Neurosurgery. 2001;48 doi: 10.1097/00006123-200103000-00041. Online: http://journals.lww.com/neurosurgery/Fulltext/2001/03000/Psychosurgery__A_Historical_Overview.41.aspx. [DOI] [PubMed] [Google Scholar]
- Gage GJ, Ludwig KA, Otto KJ, Ionides EL, Kipke DR. Naïve coadaptive cortical control. J. Neural Eng. 2005;2:52. doi: 10.1088/1741-2560/2/2/006. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Dimitrov DF, Wallis JD, Carmena JM. Reversible large-scale modification of cortical networks during neuroprosthetic control. Nat. Neurosci. 2011;14:662–669. doi: 10.1038/nn.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. Depression. 1994;2:59–72. [Google Scholar]
- Goodman WK, Foote KD, Greenberg BD, Ricciuti N, Bauer R, Ward H, Shapira NA, Wu SS, Hill CL, Rasmussen SA, Okun MS. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Inflamm. Alzheimers Dis. 2010;67:535–542. doi: 10.1016/j.biopsych.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening SG, Osuch EA, Williamson PC, Mitchell DGV. The neural correlates of regulating positive and negative emotions in medication-free major depression. Soc. Cogn. Affect. Neurosci. 2013 doi: 10.1093/scan/nst027. nst027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq IU, Foote KD, Goodman WK, Ricciuti N, Ward H, Sudhyadhom A, Jacobson CE, Siddiqui MS, Okun MS. A Case of Mania following Deep Brain Stimulation for Obsessive Compulsive Disorder. Stereotact. Funct. Neurosurg. 2010;88:322–328. doi: 10.1159/000319960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, Smagt P, van der and Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R, Moreines JL, Mewes K, Posse PR, Gutman DA, Mayberg HS. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch. Gen. Psychiatry. 2012;69:150–158. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to Regulate: Counterproductive Recruitment of Top-Down Prefrontal-Subcortical Circuitry in Major Depression. J. Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Wheaton M, Murphy DL. What’s wrong with my mouse model?: Advances and strategies in animal modeling of anxiety and depression. Behav. Brain Res. 2007;179:1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, Lozano AM. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6. years. Am. J. Psychiatry. 2011;168:502–510. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Schultz W, Sakagami M. Operant conditioning of primate prefrontal neurons. J. Neurophysiol. 2010;103:1843–1855. doi: 10.1152/jn.00173.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralek AC, Jin X, Ii JDL, Costa RM, Carmena JM. Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature. 2012;483:331–335. doi: 10.1038/nature10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, Foltynie T, Limousin P, Ashkan K, FitzGerald J, Green AL, Aziz TZ, Brown P. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 2013;74:449–457. doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol. Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzullo TC, Miller CR, Kipke DR. Suitability of the cingulate cortex for neural control. IEEE Trans. Neural Syst. Rehabil. Eng. 2006;14:401–409. doi: 10.1109/TNSRE.2006.886730. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Makeig S, Tsuang M. In search of biomarkers in psychiatry: EEG-based measures of brain function. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2014 doi: 10.1002/ajmg.b.32208. [to appear] [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu. Rev. Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456:639–643. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Stein DJ. Towards a genuinely medical model for psychiatric nosology. BMC Med. 2012;10:5. doi: 10.1186/1741-7015-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J. Self-stimulation of the brain: its use to study local effects of hunger, sex, and drugs. Science. 1958;127:315–324. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London, UK: Acade; 2009. [DOI] [PubMed] [Google Scholar]
- Pisapia JM, Halpern CH, Muller UJ, Vinai P, Wolf JA, Whiting DM, Wadden TA, Baltuch GH, Caplan AL. Ethical Considerations in Deep Brain Stimulation for the Treatment of Addiction and Overeating Associated With Obesity. AJOB Neurosci. 2013;4:35–46. doi: 10.1080/21507740.2013.770420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Rigotti M, Barak O, Warden MR, Wang X-J, Daw ND, Miller EK, Fusi S. The importance of mixed selectivity in complex cognitive tasks. Nature. 2013;497:585–590. doi: 10.1038/nature12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, Vaadia E, Bergman H. Closed-Loop Deep Brain Stimulation Is Superior in Ameliorating Parkinsonism. Neuron. 2011;72:370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Bewernick BH, Kayser S, Mädler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol. Psychiatry. 2013;73:1204–1212. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- Torres CV, Sola RG, Pastor J, Pedrosa M, Navas M, García-Navarrete E, Ezquiaga E, García-Camba E. Long-term results of posteromedial hypothalamic deep brain stimulation for patients with resistant aggressiveness: Clinical article. J. Neurosurg. 2013;119:277–287. doi: 10.3171/2013.4.JNS121639. [DOI] [PubMed] [Google Scholar]
- Ward MP, Irazoqui PP. Evolving refractory major depressive disorder diagnostic and treatment paradigms: toward closed-loop therapeutics. Front. Neuroengineering. 2010;3 doi: 10.3389/fneng.2010.00007. Online: www.frontiersin.org/neuroscience/neuroengineering/paper/10.3389/fneng.2010.00007/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, Miller EK. The representation of multiple objects in prefrontal neuronal delay activity. Cereb. Cortex. 2007;17:i41–i50. doi: 10.1093/cercor/bhm070. [DOI] [PubMed] [Google Scholar]
- Whelan R, Garavan H. When optimism hurts: inflated predictions in psychiatric neuroimaging. Biol. Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.05.014. Online: http://linkinghub.elsevier.com/retrieve/pii/S0006322313004575. [DOI] [PubMed] [Google Scholar]
- Widge AS, Avery DH, Zarkowski P. Baseline and treatment-emergent EEG biomarkers of antidepressant medication response do not predict response to repetitive transcranial magnetic stimulation. Brain Stimulat. 2013 doi: 10.1016/j.brs.2013.05.001. Online: http://www.sciencedirect.com/science/article/pii/S1935861X13001514. [DOI] [PMC free article] [PubMed] [Google Scholar]