Abstract
Importance
Observational studies suggest a role for dietary nutrients such as vitamin E and selenium in cataract prevention. However, the results of randomized trials of vitamin E supplements and cataract have been disappointing, and are not yet available for selenium.
Objective
To test whether long-term supplementation with selenium and vitamin E affects the incidence of cataract in a large cohort of men.
Design, Setting, and Participants
The SELECT Eye Endpoints (SEE) study was an ancillary study of the SWOG-coordinated Selenium and Vitamin E Cancer Prevention Trial (SELECT), a randomized, placebo-controlled, four arm trial of selenium and vitamin E conducted among 35,533 men aged 50 years and older for African Americans and 55 and older for all other men, at 427 participating sites in the US, Canada, and Puerto Rico. A total of 11,267 SELECT participants from 128 SELECT sites participated in the SEE ancillary study.
Intervention
Individual supplements of selenium (200 µg/d from L-selenomethionine) and vitamin E (400 IU/d of all rac-α-tocopheryl acetate).
Main Outcome Measures
Incident cataract, defined as a lens opacity, age-related in origin, responsible for a reduction in best-corrected visual acuity to 20/30 or worse based on self-report confirmed by medical record review, and cataract extraction, defined as the surgical removal of an incident cataract.
Results
During a mean (SD) of 5.6 (1.2) years of treatment and follow-up, 389 cases of cataract were documented. There were 185 cataracts in the selenium group and 204 in the no selenium group (hazard ratio [HR], 0.91; 95 percent confidence interval [CI], 0.75 to 1.11; P=.37). For vitamin E, there were 197 cases in the treated group and 192 in the placebo group (HR, 1.02; CI, 0.84 to 1.25; P=.81). Similar results were observed for cataract extraction.
Conclusions and Relevance
These randomized trial data from a large cohort of apparently healthy men indicate that long-term daily supplementation with selenium and/or vitamin E is unlikely to have a large beneficial effect on age-related cataract.
Basic research and animal studies suggest a potential role for dietary nutrients in cataract onset and progression.1–3 Among examined nutrients, vitamin E and selenium are of particular interest because both are found in the human lens4, 5 and plausible mechanisms have been described. Vitamin E is a lipid soluble antioxidant concentrated in lens fibers and membranes and may inhibit cataract formation by reducing photo-peroxidation of lens lipids and stabilizing lens cell membranes.6–8 Selenium is a trace element incorporated into the endogenous antioxidant enzyme glutathione peroxidase which is found in high concentrations in the lens, particularly in peripheral lens fiber cells where glutathione is synthesized.9 Glutathione peroxidase acts to protect membrane lipids and macromolecules from peroxide-induced oxidative damage,1, 10–12 and its activity is positively correlated with selenium concentration.13–15 Selenium has also been shown to inhibit oxidative stress-induced apoptosis in lens epithelial cells.16
Observational studies in humans generally indicate lower rates of cataract in persons with higher dietary intake or blood levels of selenium (or glutathione peroxidase) and, in particular, vitamin E.4, 17–21 However, the results of randomized trials have been disappointing. Seven trials testing high-dose vitamin E supplements, either alone or in combination with other vitamin supplements, in generally well-nourished populations have found little benefit on cataract for treatment durations as long as 8 years in men and 10 years in women.22–28 For selenium, randomized trial data are limited. Selenium was included in a multivitamin/mineral combination found to be associated with a lower risk of cataract in a nutritionally-deficient population in China,23 and in well-nourished populations in Italy29 and the United States.30 However, the individual effect of selenium on cataract occurrence could not be determined in those trials.
Herein, we report the findings for cataract from the SELECT Eye Endpoints (SEE) Study. SEE was an ancillary study of the Selenium and Vitamin E Cancer Prevention Trial (SELECT), a randomized, placebo-controlled, four arm trial of selenium, vitamin E and the combination in prostate cancer prevention.. To our knowledge, the data reported here represent the first trial data for the individual effect of selenium in cataract prevention.
METHODS
Study Design
Selenium and Vitamin E Cancer Prevention Trial (SELECT)
Detailed descriptions of the rationale, design, and conduct of SELECT have been previously published.31, 32 SELECT was a phase 3 randomized, placebo-controlled, four arm trial of selenium (200 µg/d from L-selenomethionine), vitamin E (400 IU/d of all rac-α-tocopheryl acetate) and the combination for the prevention of prostate cancer among 35,533 men aged 50 years and older for African American men and 55 and older for all other men.32 Eligible men had no prior diagnosis of prostate cancer, 4 ng/mL or less of PSA in serum, and a digital rectal examination not suspicious for cancer. Eligible men also reported no current use of anticoagulant therapy other than 175 mg/d or less of acetylsalicylic acid or 81 mg/d or less of acetylsalicylic acid with clopidogrel bisulfate, no history of hemorrhagic stroke, and normal blood pressure. Men were offered a free multivitamin containing no selenium or vitamin E (to increase adherence), and were required to avoid over-the-counter supplements of selenium and vitamin E throughout the study. Men were randomized in a randomized block scheme, in which the block was the study site. This ensured a balance of the 4 intervention groups within each study site. All men provided written informed consent for participation in SELECT, and the local institutional review board of each study site approved the SELECT trial for activation and reviewed its progress annually.
SELECT was activated in July, 2001, with planned follow-up of 7 to 12 years. Participant clinic visits were scheduled every 6 months throughout the trial. Upon diagnosis of prostate cancer, participants were followed at annual clinic visits. At study visits, men were asked about new medical events including a diagnosis of cataract or cataract surgery in the previous 6 months. Adherence and adverse events were monitored every 6 months and a limited physical examination including assessments of blood pressure, weight, and smoking status was conducted annually. The trial was terminated early, on October 23, 2008, because of lack of efficacy in prostate cancer prevention and the observation of possible adverse events (ie, small and not statistically significant increases in type 2 diabetes in the selenium [alone] group and prostate cancer incidence in the vitamin E [alone] group); with longer follow-up those taking vitamin E alone had a 17% increased risk of prostate cancer.33
SELECT Eye Endpoints (SEE) study
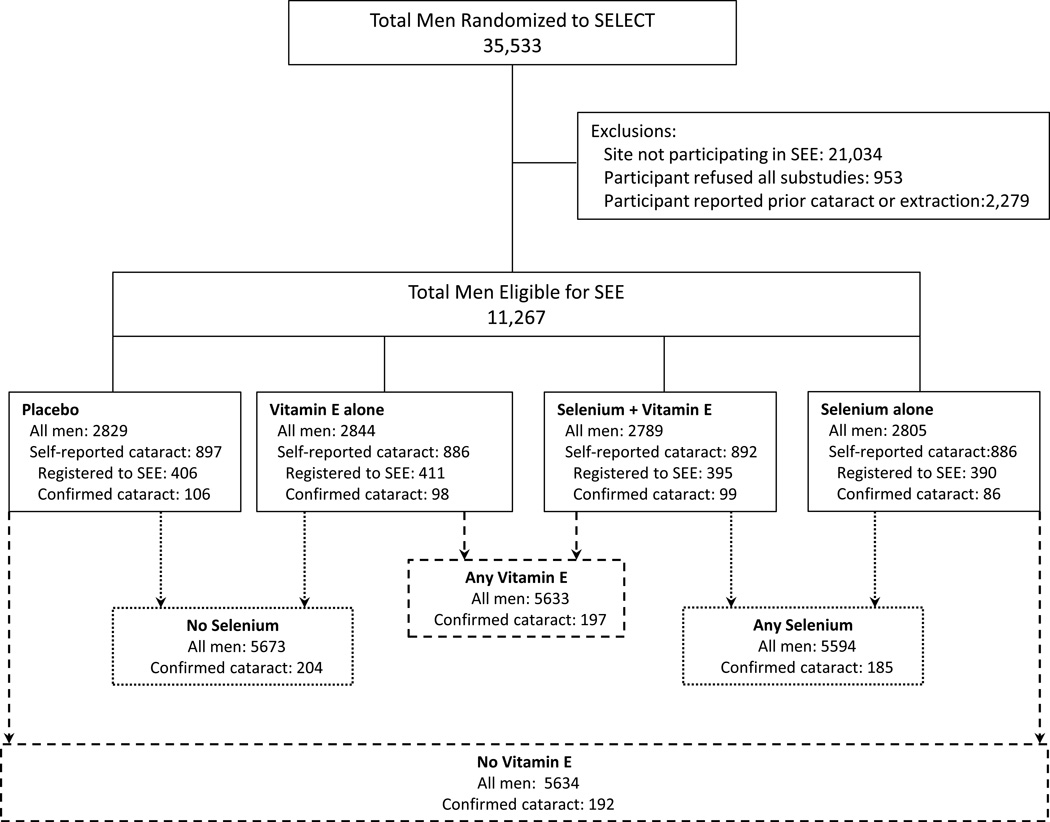
The SEE study was an ancillary study of SELECT begun in September, 2003, with funding from the National Eye Institute. Participation in the SEE ancillary study was voluntary. Of 427 SELECT study sites in the United States, Canada, and Puerto Rico, 148 sites obtained local review board approval to participate in SEE. Of these, 104 sites registered at least one participant to SEE. Twenty-four of the remaining 44 sites did not register any participants to SEE but maintained current IRB approval and are included in the denominator Thus, this report includes data from 128 sites comprising 11,267 SELECT participants (Figure 1). The characteristics of men who participated in SEE did not differ from those of the overall SELECT population (data not shown).
Figure 1.
The CONSORT flow diagram for the SELECT Eye Endpoints (SEE) Study. Primary comparisons were to examine the main effects of the SELECT study supplements (selenium vs. no selenium; vitamin E vs. no vitamin E) on occurrence of cataract.
End Point Assessment
Men who reported a prior diagnosis of cataract at baseline in SELECT were not eligible and were excluded from analysis. At their first visit after approval and activation at their site, men were asked to report new events including cataract diagnosis or extraction since entering the SELECT trial. In subsequent 6-month visits, men reported new events since their last visit. Following a report of cataract, the participant was asked to provide 1) written informed consent for participation in SEE, and 2) written consent to obtain relevant medical records. The completed medical record release form was faxed to the SEE Center in Boston which then contacted ophthalmologists and optometrists by mail and requested them to complete a cataract questionnaire or, alternatively, to supply copies of the relevant medical records.
The cataract questionnaire asked about the presence of lens opacities, diagnosis date, visual acuity loss, cataract extraction, other ocular abnormalities, and cataract type (eg. nuclear sclerosis, cortical, posterior subcapsular [PSC]) and origin (including age-related, traumatic, congenital, inflammatory, or surgery- or steroid-induced).
The primary endpoint was incident cataract, defined as a lens opacity diagnosed after randomization but before October 23, 2008, age-related in origin, and best-corrected visual acuity of 20/30 or worse attributable to the opacity. Cataract extraction was a secondary endpoint and was defined as the surgical removal of an incident cataract.
Statistical Analysis
SEE was designed with 4 pre-specified comparisons to assess the main effects of the two study agents (selenium ± vitamin E vs no selenium ± vitamin E; vitamin E ± selenium vs no vitamin E ± selenium) on the primary endpoints of incident cataract and visually-significant AMD. For each comparison, the pre-specified significance level was set at p<0.05. To adjust for multiple comparisons, a simple solution is to apply the Bonferroni correction and consider 0.0125 (0.05/4) statistically significant.
Men were classified according to their randomized selenium or vitamin E treatment assignment and were followed until the occurrence of cataract, death, or loss to follow-up. Men who reported cataract but did not register to SEE were censored as of the date of their cataract report. With 389 confirmed events, the study had adequate power (80%) to detect a 25% reduction in the hazard of incident cataract.
We compared baseline characteristics in the selenium and vitamin E groups using 2-sample t tests, χ2 tests for proportions, and tests for trend for ordinal categories. Kaplan-Meier curves estimated cumulative incidence over time by randomized group, and curves were compared using the log-rank test. Cox proportional-hazards models were used to estimate the hazard ratio (HR) of cataract comparing treated and non-treated groups after adjustment for the other treatment assignment (selenium or vitamin E). Models were also fit separately within four age groups; 50–54, 55–64, 65–74, ≥75 years. Tests of trend were calculated by including a term for the interaction of the antioxidant and age (expressed as a continuous variable with values 1 to 4 corresponding to the four age groups) in a proportional hazards model. Interaction terms were used to test for additivity of the two antioxidant agents using multiplicative terms in the Cox model. We tested the proportional hazards assumption by modeling interaction terms separately for selenium or vitamin E with the logarithm of time, and these assumptions were not violated (P>.05). For each HR, the 95 percent confidence interval (CI) and two-sided P value were calculated.
We also analyzed subgroup data by categories of baseline variables that are possible risk factors for cataract. We explored possible effect modification by using interaction terms between subgroup indicators and treatment assignment, and we tested for trend when subgroup categories were ordinal.
In secondary analyses, Cox models were used to calculate HRs of cataract comparing event rates in the active treatment arms (selenium alone, vitamin E alone, selenium + vitamin E) to the rate in the placebo group.
Individuals were the unit of analysis because eyes were not examined independently and we classified individuals according to the status of the worse eye as defined by disease severity. When the worse eye was excluded because of visual acuity loss attributed to other ocular abnormalities, the fellow eye was considered for classification. Data were analyzed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina).
RESULTS
The distribution of baseline characteristics of SEE participants that are possible risk factors for cataract is shown in Table 1. As expected in this large randomized trial, the characteristics were distributed evenly between active and placebo groups for selenium and vitamin E.
Table 1.
Baseline Characteristics by Randomized Groups in the SELECT Eye Endpoints (SEE) Study.a
| Selenium | Vitamin E | ||||
|---|---|---|---|---|---|
| Characteristic |
No. of Participants |
Active (n=5,594) |
Placebo (n=5,673) |
Active (n=5,633) |
Placebo (n=5,634) |
| Age (median [IQR], y | 61 [57–66] | 61 [57–66] | 61 [57–66] | 61 [57–66] | 61 [57–66] |
| Age | |||||
| 50–54 | 583 | 5.0 | 5.3 | 5.5 | 4.9 |
| 55–64 | 7,186 | 63.6 | 63.9 | 64.3 | 63.2 |
| 65–74 | 3,084 | 27.8 | 26.9 | 26.7 | 28.0 |
| >=75 | 414 | 3.5 | 3.8 | 3.5 | 3.9 |
| Race/Ethnicity | |||||
| White | 9,123 | 81.1 | 80.8 | 81.0 | 80.9 |
| Non-white | 2,144 | 18.9 | 19.2 | 19.0 | 19.1 |
| Education (highest level) | |||||
| <=High school graduate or GED | 2,149 | 18.4 | 19.8 | 18.7 | 19.5 |
| Some college/vocational school | 3,176 | 27.9 | 28.5 | 28.4 | 28.0 |
| >=College graduate | 5,883 | 53.1 | 51.4 | 52.4 | 52.0 |
| Unknown/missing | 59 | 0.7 | 0.4 | 0.6 | 0.5 |
| Cigarette smoking | |||||
| Never | 4,875 | 43.8 | 42.7 | 44.2 | 42.4 |
| Current | 969 | 8.0 | 9.1 | 8.4 | 8.8 |
| Former | 5,402 | 48.0 | 47.9 | 47.3 | 48.6 |
| Unknown | 21 | 0.2 | 0.2 | 0.2 | 0.2 |
| Alcohol use | |||||
| Rarely/never | 3,757 | 33.4 | 33.3 | 33.4 | 33.3 |
| >=1 drink/month | 7,011 | 62.6 | 61.8 | 62.3 | 62.2 |
| Unknown | 499 | 4.0 | 4.8 | 4.3 | 4.5 |
| Body mass index (kg/m2) | |||||
| <25 | 2,172 | 19.6 | 19.0 | 19.0 | 19.5 |
| 25-<30 | 5,351 | 47.5 | 47.5 | 48.0 | 47.0 |
| >=30 | 3,744 | 32.9 | 33.5 | 33.0 | 33.5 |
| History of hypertensionb | |||||
| Yes | 4,252 | 37.6 | 37.9 | 37.2 | 38.3 |
| No | 7,015 | 62.4 | 62.1 | 62.8 | 61.7 |
| Aspirin use | |||||
| Yes | 4,741 | 41.7 | 42.4 | 42.0 | 42.2 |
| No | 6,526 | 58.3 | 57.6 | 58.0 | 57.8 |
| Statin usec | |||||
| Yes | 2,867 | 25.3 | 25.6 | 25.8 | 25.1 |
| No | 8,318 | 73.9 | 73.7 | 73.4 | 74.2 |
| Unknown | 82 | 0.8 | 0.7 | 0.8 | 0.7 |
| History of diabetes | |||||
| Yes | 1,045 | 9.1 | 9.4 | 9.0 | 9.5 |
| No | 10,222 | 90.9 | 90.6 | 91.0 | 90.5 |
Abbreviations: IQR, interquartile range; GED, General Educational Development.
Data are given as percentage unless otherwise noted.
History of hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, reported hypertension, or reported use of hypertension medication.
Collection of statin use added to study forms partway through randomization, includes some information collected at six months, one year, or 1.5 years. (baseline statins: 7,491 men; six months: 1,904 men; one year: 2,097 men; 1.5 years: 37 men)
During a mean (SD) follow-up of 5.6 (1.2) years, a total of 389 cataracts and 219 cataract extractions were confirmed through medical record review.
Selenium
There were 185 cataracts in the selenium group and 204 in the no selenium group (HR, 0.91, 95% confidence interval [CI], 0.75 to 1.11, P=.37) (Table 2). In age-stratified analyses, HRs tended to be lowest in the older age groups although a test of trend did not attain statistical significance. For cataract extraction, there were 99 cases in the selenium group and 120 in the no selenium group (HR, 0.84, 95% CI, 0.64 to 1.09, P=.19) (Table 2). As observed for diagnosed cataract, HRs for extraction tended to be lowest in the older age groups although the test of trend was not significant.
Table 2.
Confirmed Cases of Age-related Cataract and Cataract Extraction According to Randomized Treatment Assignment in Four Age Groups.
| Selenium | Vitamin E | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Active (n=5,594) |
Placebo (n=5,673) |
HRa | 95% CI | P value |
P for Trendc |
Active (n=5,633) |
Placebo (n=5,634) |
HRb | 95% CI | P value |
P for Trendc |
|
| Cataract | ||||||||||||
| 50–54 years | 8 | 5 | 1.74 | 0.57–5.33 | 0.33 | 0.21 | 6 | 7 | 0.77 | 0.26–2.28 | 0.63 | 0.75 |
| 55–64 years | 81 | 85 | 0.97 | 0.71–1.31 | 0.83 | 83 | 83 | 0.98 | 0.72–1.33 | 0.91 | ||
| 65–74 years | 79 | 89 | 0.85 | 0.63–1.15 | 0.29 | 90 | 78 | 1.22 | 0.90–1.65 | 0.21 | ||
| >=75 years | 17 | 25 | 0.74 | 0.40–1.38 | 0.35 | 18 | 24 | 0.84 | 0.46–1.55 | 0.58 | ||
| Total | 185 | 204 | 0.91 | 0.75–1.11 | 0.37 | 197 | 192 | 1.02 | 0.84–1.25 | 0.81 | ||
| Cataract Extraction | ||||||||||||
| 50–54 years | 3 | 2 | 1.59 | 0.27–9.52 | 0.61 | 0.28 | 2 | 3 | 0.61 | 0.10–3.65 | 0.59 | 0.53 |
| 55–64 years | 47 | 53 | 0.91 | 0.61–1.34 | 0.63 | 53 | 47 | 1.09 | 0.74–1.62 | 0.66 | ||
| 65–74 years | 38 | 46 | 0.80 | 0.52–1.22 | 0.30 | 49 | 35 | 1.49 | 0.96–2.29 | 0.07 | ||
| >=75 years | 11 | 19 | 0.66 | 0.31–1.38 | 0.27 | 10 | 20 | 0.56 | 0.26–1.20 | 0.14 | ||
| Total | 99 | 120 | 0.84 | 0.64–1.09 | 0.19 | 114 | 105 | 1.08 | 0.83–1.41 | 0.58 | ||
Abbreviations: HR, hazard ratio; CI, confidence interval.
Adjusted for vitamin E treatment assignment.
Adjusted for selenium treatment assignment.
Test for trend of the effect of age on the association between randomized treatment assignment and cataract (cataract extraction).
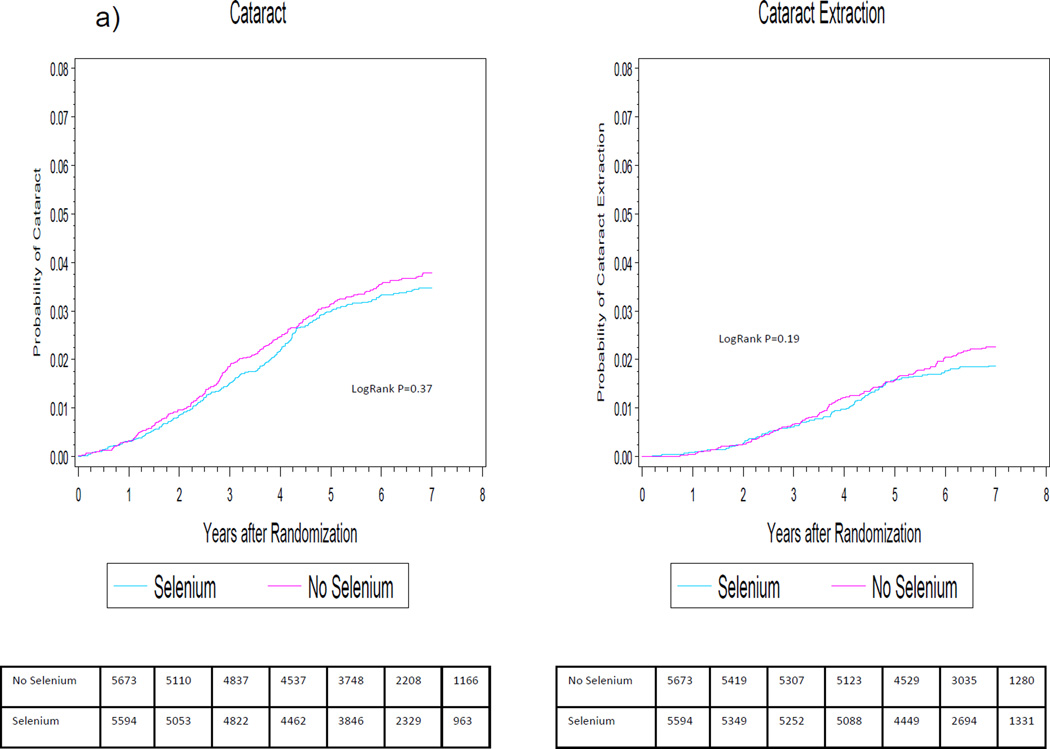
Cumulative incidence rate of cataract and cataract extraction according to year of follow-up are shown in Figure 2a. For the primary endpoint of cataract, there was no apparent benefit of selenium at any point during the trial. For cataract extraction, curves appeared to diverge at 5–6 years of follow-up but never attained statistical significance.
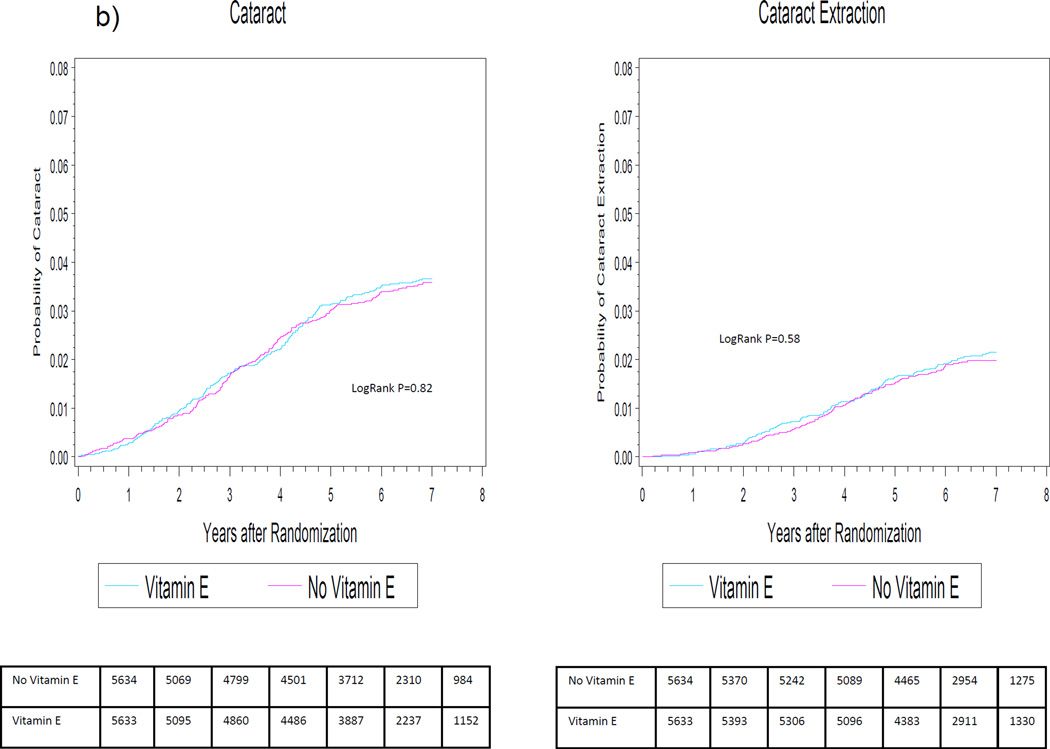
Figure 2.
Cumulative incidence rates of cataract and cataract extraction by randomized selenium (2a) and vitamin E (2b) assignment in the SELECT Eye Endpoints (SEE) Study.
The effect of selenium on cataract diagnosis did not differ markedly within categories of known or possible risk factors (see eTable 1).
Vitamin E
There were 197 cataracts in the vitamin E group and 192 in the no vitamin E group (HR, 1.02, 95% CI, 0.84 to 1.25, P=.81) (Table 2). HRs did not vary significantly among the four age groups. For cataract extraction, there were 114 cases in the vitamin E group and 105 in the no vitamin E group (HR, 1.08, 95% CI, 0.83 to 1.41, P=.58) (Table 2). HRs did not vary significantly among the four age groups.
Cumulative incidence curves for cataract and cataract extraction are shown in Figure 2b. For both endpoints, there was no apparent effect of vitamin E at any point during the trial.
The lack of effect of vitamin E on cataract diagnosis did not differ markedly within categories of known or possible risk factors (eTable 1).
Secondary Comparisons
There were no significant differences in the rates of cataract or cataract extraction between the 4 treatment groups. Compared with placebo, HRs for cataract were 0.82 (95 % CI, 0.62 to 1.09, P=.18) in the selenium alone group, 0.93 (95 % CI, 0.71 to 1.22, P=.61) in the vitamin E alone group, and 0.94 (95 % CI, 0.71 to 1.23, P=.65) in the selenium + vitamin E group (P for interaction=.32). For cataract extraction, HRs were 0.73 (95 % CI, 0.50 to 1.08, P=.11) in the selenium alone group, 0.96 (95 % CI, 0.67 to 1.38, P=.83) in the vitamin E alone group, and 0.91 (95 % CI, 0.63 to 1.31, P=.60) in the selenium + vitamin E group (P for interaction=.35).
DISCUSSION
In this large randomized trial of middle aged and older, apparently healthy men, daily supplementation with selenium and/or vitamin E for an average of 5.6 years had no significant effect on diagnosed cataract, the primary study endpoint, or on cataract extraction. In our primary main-effects analysis, men assigned selenium (± vitamin E) had a non-significant 9% reduction in diagnosed cataract, and a non-significant 16% reduction in cataract extraction. The 95% CIs around these estimates excluded with reasonable certainty beneficial effects of 25% or greater for diagnosed cataract, and 36% or greater for cataract extraction. For vitamin E (± selenium), HRs for both diagnosed cataract and extraction were near the null value of 1.0, and the 95% CIs excluded with reasonable certainty beneficial effects greater than 17% for each endpoint.
To our knowledge, SEE is the first randomized trial to assess the separate effect of selenium supplementation in cataract prevention. While the overall main-effects analysis indicated a non-significant 9% lower risk of cataract for men in the selenium (± vitamin E) group, the reduction was somewhat greater (18%), though still non-significant, when we compared men in the selenium alone arm to the placebo arm. Three previous trials examined selenium as a component of a multivitamin/multimineral supplement in cataract prevention, but the separate effect of selenium could not be determined in those trials. In the Linxian Cataract Study, end-of-trial eye examinations conducted among a subset of participants with esophageal dysplasia indicated a reduced prevalence of nuclear cataract in participants aged 65 to 74 years who were randomly assigned to a daily vitamin/mineral supplement that included selenium (50 µg) compared to placebo (OR, 0.57; 95% CI, 0.36–0.90).23 In a second trial, a double-blind, single center, clinical trial of 1,020 participants aged 55–75 years, persons randomized to a daily multivitamin that included selenium (25 µg), compared with placebo, had a significant 18% reduction in cataract development or progression after 9-years of treatment.29 Finally, recent results from Physicians’ Health Study II indicated a significant 9% lower risk of cataract (HR, 0.91; 95% CI, 0.83–0.99) for men assigned a daily multivitamin that included selenium (20 µg), compared to placebo, after 11 years of treatment.30 Our findings in SEE extend these prior findings by showing that selenium, alone or in combination with vitamin E, is not likely to have a large beneficial effect on cataract, although a smaller, but potentially important, benefit cannot be ruled out.
Our null findings for vitamin E in cataract prevention are consistent with the overall negative findings in previous randomized trials, and in particular with four trials designed to estimate the individual effect of vitamin E supplementation. These include a trial of 29,133 Finnish male smokers treated with daily vitamin E (50 mg) for 5.7 years,22 a trial of 1,193 men and women treated with daily vitamin E (500 IU) for 4 years,26 a trial of 39,876 female health professionals treated with alternate day vitamin E (600 IU) for 10 years,27 and a trial of 11,545 male physicians treated with alternate day vitamin E (400 IU) for 8 years.28 Our findings in SEE, based on an average of 5.5 years of treatment and follow-up, are consistent with these prior findings and add to the total body of evidence showing that long-term supplementation with high-dose vitamin E has no material impact on cataract development or progression in men or women.
Previous studies have shown that vitamin E interacts with selenium and glutathione peroxidase to prevent the formation of oxidative products of polyunsaturated fatty acids and oxidative damage.34, 35 Other studies have shown that selenium-dependant glutathione peroxidase reduces hydroperoxides formed by the scavenging action of vitamin E,36 and may function indirectly by maintaining vitamin E (and vitamin C) in its reduced and functional form.37 However, we found no evidence of a significant interaction of selenium and vitamin E on risks of cataract or cataract extraction.
Several possible limitations of the trial need to be considered, particularly in view of the negative findings. An inadequate dosage of study agents seems unlikely. The dose of vitamin E (400 IU/d [equivalent to 400 mg/d] of all rac-α-tocopheryl acetate) was more than 26 times the recommended daily intake of 15 mg,38 and previous observational studies reported benefits with a median intake of 12 mg of vitamin E.39 The dose of selenium (200 µg/d from L-selenomethionine) was based on efficacy and safety data31, 32 and was approximately four times the recommended dietary allowance for adult North Americans.40 Inadequate duration of treatment also seems unlikely, particularly for vitamin E, since results from the WHS27 and PHS II28 indicated no benefit of vitamin E on cataract even with treatment durations 3–4 years longer than that in SEE. Random misclassification of cataract was reduced by the use of medical record data to confirm self-reports. Non-random or differential misclassification was unlikely since medical records were reviewed without knowledge of treatment assignment. Informative censoring, which would have occurred if dropouts or missing data were related to occurrence of cataract, also seems unlikely. Adherence is a concern in any long-term trial, but adherence as determined by pill count remained consistently good during follow-up averaging 83% at year 1 and 65% at year 5. Finally, these findings in men may not be applicable to women.
In summary, these randomized trial data from a large cohort of apparently healthy men indicate that long-term daily supplemental use of vitamin E has no material impact on cataract incidence. The data also exclude any large beneficial effect on cataract for long-term supplemental use of selenium, with or without vitamin E, although a smaller, but potentially important, beneficial effect could not be ruled out.
Supplementary Material
Acknowledgements
This work was supported by grant EY014418, and in part by Public Health Service Cooperative Agreement grant CA37429 awarded by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and by the National Center for Complementary and Alternative Medicine (National Institutes of Health). Study agents and packaging were provided by Sabinsa Corporation (Piscataway, New Jersey), Tishcon Corporation (Westbury, New York), and DSM Nutritional Products Inc (Parsipanny, New Jersey). Optional study multivitamins were provided by Perrigo Company (Allegan, Michigan).
Role of the Funding Organization or Sponsor:
Design and Conduct of the Study: none
Collection, Management, Analysis, and Interpretation of the Data: none
Preparation, Review, or Approval of the Manuscript: none
Decision to Submit the Manuscript for Publication: none
Footnotes
Trial registration: ClinicalTrials.gov Identifier: NCT00784225
Author Contributions:
Dr. Christen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Christen, Glynn, Gaziano, Crowley, P. Goodman, Lippman, Minasian, Klein.
Acquisition of data: Christen, Darke, P. Goodman.
Analysis and interpretation of data: Christen, Glynn, Gaziano, Darke, Crowley, P. Goodman.
Drafting of the manuscript: Christen.
Critical revision of the manuscript for important intellectual content: Christen, Glynn, Gaziano, Darke, Crowley, P. Goodman, Lippman, Lad, Bearden, G. Goodman, Minasian, Thompson, Blanke, Klein.
Statistical analysis: Christen, Glynn, Darke, Crowley, P. Goodman.
Obtained funding: Christen, Glynn, Gaziano.
Administrative, technical, or material support: Christen, Glynn, Gaziano, Darke, Crowley, P. Goodman, Minasian, Klein.
Study supervision: Christen, Darke, Crowley, P. Goodman, Minasian, Blanke, Klein.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Gaziano reported receiving study support and a speaking honorarium from Pfizer related to vitamins. No other authors reported financial disclosures.
REFERENCES
- 1.Gerster H. Antioxidant vitamins in cataract prevention. Z Ernahrungswiss. 1989 Mar;28(1):56–75. doi: 10.1007/BF02025566. [DOI] [PubMed] [Google Scholar]
- 2.Bunce GE, Kinoshita J, Horwitz J. Nutritional factors in cataract. Annu Rev Nutr. 1990;10:233–254. doi: 10.1146/annurev.nu.10.070190.001313. [DOI] [PubMed] [Google Scholar]
- 3.Varma SD, Devamanoharan PS, Morris SM. Prevention of cataracts by nutritional and metabolic antioxidants. Crit Rev Food Sci Nutr. 1995 Jan;35(1–2):111–129. doi: 10.1080/10408399509527691. [DOI] [PubMed] [Google Scholar]
- 4.Rasi V, Costantini S, Moramarco A, Giordano R, Giustolisi R, Balacco Gabrieli C. Inorganic element concentrations in cataractous human lenses. Ann Ophthalmol. 1992 Dec;24(12):459–464. [PubMed] [Google Scholar]
- 5.Yeum KJ, Shang FM, Schalch WM, Russell RM, Taylor A. Fat-soluble nutrient concentrations in different layers of human cataractous lens. Curr Eye Res. 1999 Dec;19(6):502–505. doi: 10.1076/ceyr.19.6.502.5282. [DOI] [PubMed] [Google Scholar]
- 6.Varma SD, Beachy NA, Richards RD. Photoperoxidation of lens lipids: prevention by vitamin E. Photochem Photobiol. 1982 Dec;36(6):623–626. doi: 10.1111/j.1751-1097.1982.tb09481.x. [DOI] [PubMed] [Google Scholar]
- 7.Libondi T, Menzione M, Auricchio G. In vitro effect of alpha-tocopherol on lysophosphatidylcholine-induced lens damage. Exp Eye Res. 1985 May;40(5):661–666. doi: 10.1016/0014-4835(85)90135-6. [DOI] [PubMed] [Google Scholar]
- 8.Karslioglu I, Ertekin MV, Kocer I, et al. Protective role of intramuscularly administered vitamin E on the levels of lipid peroxidation and the activities of antioxidant enzymes in the lens of rats made cataractous with gamma-irradiation. Eur J Ophthalmol. 2004 Nov-Dec;14(6):478–485. [PubMed] [Google Scholar]
- 9.Giblin FJ. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther. 2000 Apr;16(2):121–135. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- 10.Yung LM, Leung FP, Yao X, Chen ZY, Huang Y. Reactive oxygen species in vascular wall. Cardiovasc Hematol Disord Drug Targets. 2006 Mar;6(1):1–19. doi: 10.2174/187152906776092659. [DOI] [PubMed] [Google Scholar]
- 11.Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000 Dec;57(13–14):1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patching SG, Gardiner PH. Recent developments in selenium metabolism and chemical speciation: a review. J Trace Elem Med Biol. 1999 Dec;13(4):193–214. doi: 10.1016/s0946-672x(99)80037-6. [DOI] [PubMed] [Google Scholar]
- 13.Alfthan G, Aro A, Arvilommi H, Huttunen JK. Selenium metabolism and platelet glutathione peroxidase activity in healthy Finnish men: effects of selenium yeast, selenite, and selenate. Am J Clin Nutr. 1991 Jan;53(1):120–125. doi: 10.1093/ajcn/53.1.120. [DOI] [PubMed] [Google Scholar]
- 14.Neve J. Human selenium supplementation as assessed by changes in blood selenium concentration and glutathione peroxidase activity. J Trace Elem Med Biol. 1995 Jul;9(2):65–73. doi: 10.1016/S0946-672X(11)80013-1. [DOI] [PubMed] [Google Scholar]
- 15.Levander OA, Alfthan G, Arvilommi H, et al. Bioavailability of selenium to Finnish men as assessed by platelet glutathione peroxidase activity and other blood parameters. Am J Clin Nutr. 1983 Jun;37(6):887–897. doi: 10.1093/ajcn/37.6.887. [DOI] [PubMed] [Google Scholar]
- 16.Zhu X, Guo K, Lu Y. Selenium effectively inhibits 1,2-dihydroxynaphthalene-induced apoptosis in human lens epithelial cells through activation of PI3-K/Akt pathway. Mol Vis. 2011;17:2019–2027. [PMC free article] [PubMed] [Google Scholar]
- 17.Knekt P, Heliovaara M, Rissanen A, Aromaa A, Aaran RK. Serum antioxidant vitamins and risk of cataract. Bmj. 1992 Dec 5;305(6866):1392–1394. doi: 10.1136/bmj.305.6866.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacques PF, Chylack LT, Jr, McGandy RB, Hartz SC. Antioxidant status in persons with and without senile cataract. Arch Ophthalmol. 1988 Mar;106(3):337–340. doi: 10.1001/archopht.1988.01060130363022. [DOI] [PubMed] [Google Scholar]
- 19.Jacques PF, Hartz SC, Chylack LT, Jr, McGandy RB, Sadowski JA. Nutritional status in persons with and without senile cataract: blood vitamin and mineral levels. Am J Clin Nutr. 1988 Jul;48(1):152–158. doi: 10.1093/ajcn/48.1.152. [DOI] [PubMed] [Google Scholar]
- 20.Chiu CJ, Taylor A. Nutritional antioxidants and age-related cataract and maculopathy. Exp Eye Res. 2007 Feb;84(2):229–245. doi: 10.1016/j.exer.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Karakucuk S, Ertugrul Mirza G, Faruk Ekinciler O, Saraymen R, Karakucuk I, Ustdal M. Selenium concentrations in serum, lens and aqueous humour of patients with senile cataract. Acta Ophthalmol Scand. 1995 Aug;73(4):329–332. doi: 10.1111/j.1600-0420.1995.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 22.Teikari JM, Rautalahti M, Haukka J, et al. Incidence of cataract operations in Finnish male smokers unaffected by alpha tocopherol or beta carotene supplements. J Epidemiol Community Health. 1998 Jul;52(7):468–472. doi: 10.1136/jech.52.7.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sperduto RD, Hu TS, Milton RC, et al. The Linxian cataract studies. Two nutrition intervention trials. Arch Ophthalmol. 1993 Sep;111(9):1246–1253. doi: 10.1001/archopht.1993.01090090098027. [DOI] [PubMed] [Google Scholar]
- 24.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001 Oct;119(10):1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chylack LT, Jr, Brown NP, Bron A, et al. The Roche European American Cataract Trial (REACT): a randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract. Ophthalmic Epidemiol. 2002 Feb;9(1):49–80. doi: 10.1076/opep.9.1.49.1717. [DOI] [PubMed] [Google Scholar]
- 26.McNeil JJ, Robman L, Tikellis G, Sinclair MI, McCarty CA, Taylor HR. Vitamin E supplementation and cataract: randomized controlled trial. Ophthalmology. 2004 Jan;111(1):75–84. doi: 10.1016/j.ophtha.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Christen WG, Glynn RJ, Chew EY, Buring JE. Vitamin E and age-related cataract in a randomized trial of women. Ophthalmology. 2008 May;115(5):822–829. e821. doi: 10.1016/j.ophtha.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 28.Christen WG, Glynn RJ, Sesso HD, et al. Age-related cataract in a randomized trial of vitamins E and C in men. Arch Ophthalmol. 2010 Nov;128(11):1397–1405. doi: 10.1001/archophthalmol.2010.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maraini G, Williams SL, Sperduto RD, et al. A randomized, double-masked, placebo-controlled clinical trial of multivitamin supplementation for age-related lens opacities. Clinical trial of nutritional supplements and age-related cataract report no. 3. Ophthalmology. 2008 Apr;115(4):599–607. e591. doi: 10.1016/j.ophtha.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014 Feb;121(2):525–534. doi: 10.1016/j.ophtha.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2009 Jan 7;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lippman SM, Goodman PJ, Klein EA, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005 Jan 19;97(2):94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 33.Klein EA, Thompson IM, Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2011 Oct 12;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr. 1991 Apr;53(4 Suppl):1050S–1055S. doi: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- 35.Chow CK, Reddy K, Tappel AL. Effect of dietary vitamin E on the activities of the glutathione peroxidase system in rat tissues. J Nutr. 1973 Apr;103(4):618–624. doi: 10.1093/jn/103.4.618. [DOI] [PubMed] [Google Scholar]
- 36.Costagliola C, Menzione M. Effect of vitamin E on the oxidative state of glutathione in plasma. Clin Physiol Biochem. 1990;8(3):140–143. [PubMed] [Google Scholar]
- 37.Reed DJ. Glutathione: toxicological implications. Annu Rev Pharmacol Toxicol. 1990;30:603–631. doi: 10.1146/annurev.pa.30.040190.003131. [DOI] [PubMed] [Google Scholar]
- 38.Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000 Jun;100(6):637–640. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 39.Mares-Perlman JA, Brady WE, Klein BE, et al. Diet and nuclear lens opacities. Am J Epidemiol. 1995 Feb 15;141(4):322–334. doi: 10.1093/aje/141.4.322. [DOI] [PubMed] [Google Scholar]
- 40.Institute of Medicine. Panel on Dietary Antioxidants and Related Compounds. Dietary reference intakes for vitamin C, vitamin E, and carotenoids. Washingon (DC): National Academy Press; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.