Abstract
Neurocutaneous melanosis is a rare congenital disorder which presents with congenital cutaneous nevi and involvement of the central nervous system. We herein present a rare case of a 2-year-old girl who had central nervous system melanosis and giant congenital melanocytic nevi. Magnetic resonance imaging, especially precontrast T1 images play a crucial role in making the diagnosis combined with the skin findings of physical examination.
Keywords: Neurocutaneous melanosis, computed tomography, magnetic resonance imaging
CASE REPORT
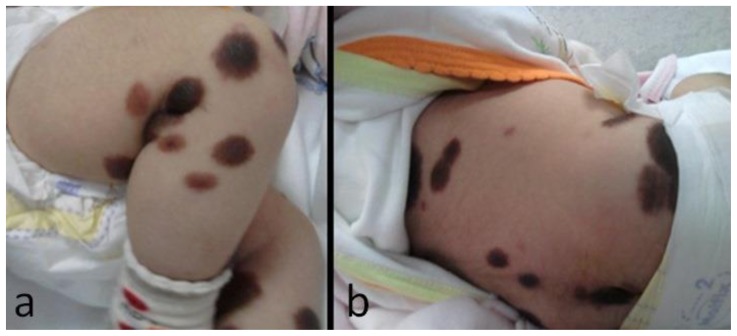
A female infant was delivered by cesarean section after a full-term, uneventful pregnancy. She was born at term with normal weight and length. The patient had a slight bulging on anterior fontanelle and 41.5 centimeter head circumference (above percentile 97) at the age of one month. Family history revealed that the parents were first degree consanguineous. She was admitted to emergency service due to projectile vomiting at the age of one month. She had no history of seizure or loss of consciousness. On physical examination, the patient had normal sucking, Moro and palmar grasp reflexes. On dermatologic exam, she had large, numerous hairy pigmented nevi on the extremities and trunk (Fig. 1a, b). Neurologic examination was normal except for horizontal nystagmus.
Figure 1.
2-year-old girl diagnosed with neurocutaneous melanosis (NCM). Pictures demonstrate multiple hairy pigmented nevi on the lower extremities (a) and trunk (b).
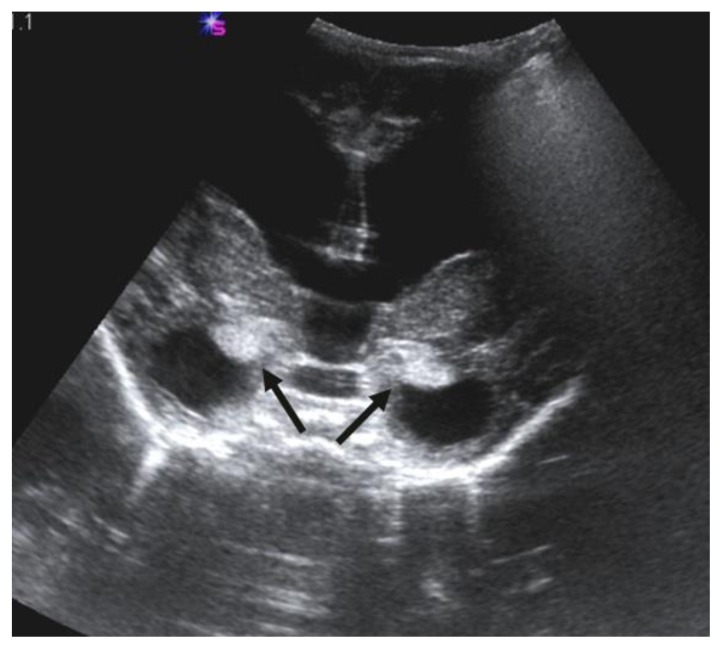
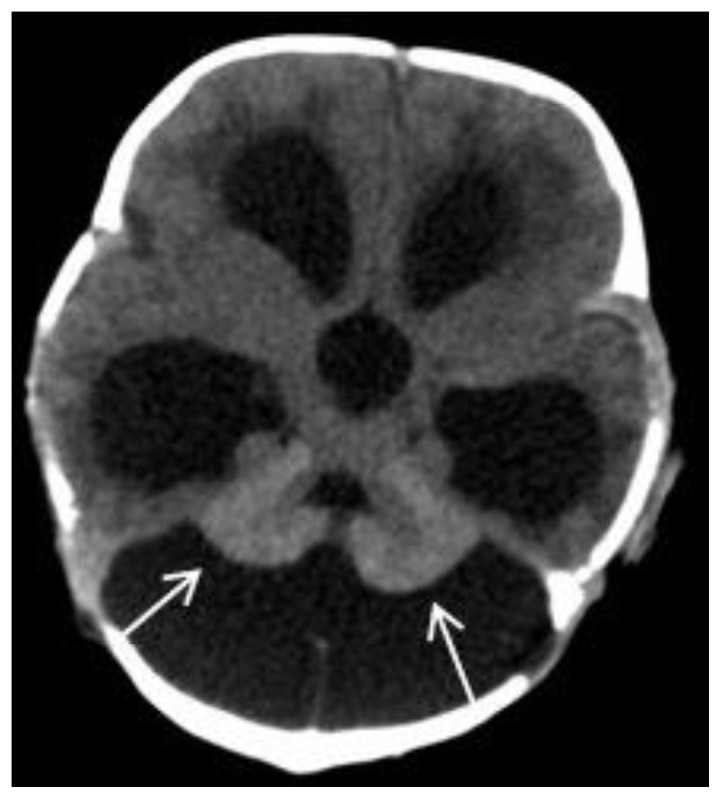
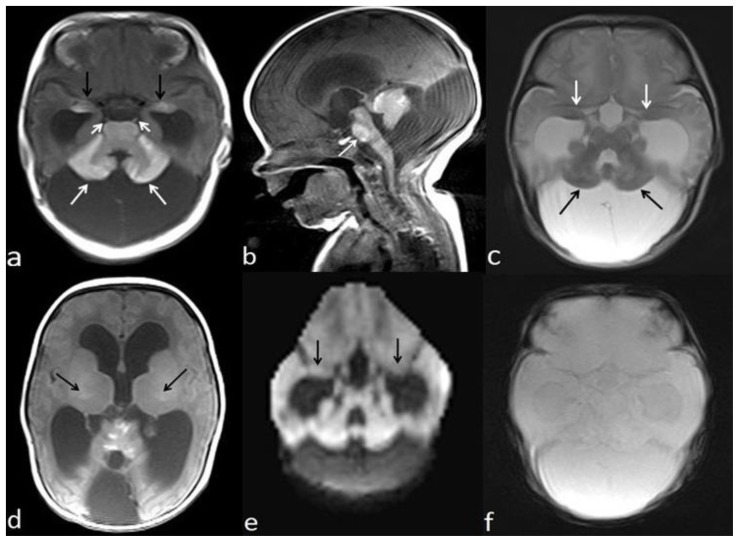
Transfontanellar ultrasonography demonstrated a slightly increased parenchymal echogenicity of the bilateral amygdalae (Fig. 2). A computed tomography (CT) scan showed enlargement of cisterna magna and mild hyperattenuation within the cerebellum (Fig. 3). A brain magnetic resonance imaging (MRI) demonstrated mega cisterna magna (Fig. 4a–c). Additionally, the both lateral ventricles and third ventricle were dilated (Fig. 4a–c). Unenhanced T1-weighted (W) images showed hyperintense areas within the bilateral amygdala, thalami, pons, cerebellar folia, and bilateral sixth cranial nerves (Fig. 4a–d). The cerebellum and amygdalae have slightly hypointense signal alteration on T2W images (Fig. 4c). Diffusion-weighted imaging and gradient-echo T2* images show no signal alterations in the cerebellum and amygdalae (Fig. 4e, f). The diagnosis of NCM was made upon typical imaging and skin findings. A ventricular tap was performed at the emergency service to reduce intracranial pressure three weeks after a ventriculoperitoneal shunt (VPS) was placed. The symptoms due to hydrocephalus were resolved, and the patient was discharged two days after the operation. Four months after the operation, she was re-operated to revise wound surface due to infected tissue debridement. Two months after that, she presented with vomiting and fever. Lumbar puncture findings were in accordance with meningitis. Appropriate antibiotic therapy was started. The VPS infection was claimed to be responsible for the meningitis. Two months later, patient underwent a shunt revision because of shunt dysfunction. After the procedure, she was given intravenous antibiotic therapy owing to symptoms of infection. Because of persistent infections, she underwent third ventriculostomy. Fifteen days later, a VPS was replaced to control hydrocephalus. The VPS works effectively and up-to date, patient has been on follow-up for 23 months since the diagnosis of NCM. When the patient was 18 months old, excisional biopsies of pigmented nevi from 12 different spots were performed. Pathology results confirmed dermal and compound nevi. She didn’t have any further problems, and there was no sign of malignant transformation.
Figure 2.
Two-year-old girl diagnosed with neurocutaneous melanosis (NCM). Transfontanellar ultrasonography image shows a mildly increased echogenicity of bilateral amygdalae (arrows). (Siemens SONOLINE Elegra, 2.5PL20/2.6 - Probe/frequency [MHz]).
Figure 3.
Two-year-old girl diagnosed with neurocutaneous melanosis (NCM). Axial non-enhanced CT image (Siemens Emotion Duo, Forchheim, Germany. Slice thickness (ST): 2mm, kVP:130, mAS:200 with total DLP: 687 mGy cm) which was obtained when the patient was one month old, demonstrates mild hyperattenuation in the cerebellum (arrows), enlargement of cisterna magna, and dilatation of the lateral ventricles and the third ventricle.
Figure 4.
Two-year-old girl diagnosed with neurocutaneous melanosis (NCM). Precontrast axial T1-weighted (W) MRI (ST:5mm, TR/TE: 488/14.3ms) (a) shows T1 shortening in bilateral amygdalae (black arrows), pons, cerebellar cortex (white arrows), and bilateral sixth cranial nerves (short arrows). Precontrast sagittal T1W image (TR/TE: 501/14.3ms, ST:5 mm) (b) demonstrates T1 shortening in the cerebellum, pons (arrow), and anterior mesencephalon. Cisterna magna is enlarged. The both lateral ventricles and third ventricle are dilated. Axial T2W image (TR/TE:5200/122.4, ST:5 mm) (c) demonstrates that lesions in bilateral amygdalae (white arrows) and cerebellum (black arrows) are slightly hypointense. Precontrast axial T1W image (TR/TE: 488/14.3ms, ST:5mm) (d) shows mild hyperintensity of bilateral ventral aspect of thalami (arrows). Axial diffusion-weighted image (is volumetric, maximum b value: 700 s/mm2) (e) and gradient-echo T2* image at the same level with fig. 4a (f) show no signal alterations in the cerebellum and amygdalae. (1.5T, Philips, Achieva, Netherlands).
DISCUSSION
Etiology/Demography
NCM is a rare phakomatosis characterized by pigmented nevi that are large or multiple or both, and leptomeningeal melanosis or melanoma, without evidence of malignancy in the skin lesions, and without the involvement of nonmeningeal organs (1). It was first described by Rokitanski (2) in 1861 and to date, about 100 cases of neurocutaneous melanosis have been reported. Prevalence is estimated at 1/50,000–1/200,000. The incidence of symptomatic NCM appears to be approximately a third to a half of these. Only sporadic cases of NCM have been reported to date and no predisposition according to sex is noted. The pathogenesis is believed to be due to dysplasia of the neuroectodermal melanocyte precursor cells, leading to the proliferation of melanin producing cells in the skin and leptomeninges, but the pathogenic mechanism is still unclear (3, 4). The patients with neurocutaneous melanosis are also at risk for malignant transformation to leptomeningeal melanoma and cutaneous melanoma. Malignant transformation of leptomeningeal tumors occurs in 40 to 60% of symptomatic cases (5).
Clinical Presentation
It has been reported to manifest itself most commonly within the first two years of life. The children with this entity have been born as stillbirths or have been reported at as early as one month of age. Clinically, patients present with seizure, cranial nerve palsy, hydrocephalus, stillbirth, and radiculopathy (1–4, 7). The most common neurological complications are hydrocephalus, seizures, cranial nerve dysfunction, and signs of spinal cord and root involvement (1–4, 7). The pathogenesis for this association is not exactly known. NCM is associated with other neurocutaneous syndromes and Dandy-Walker malformation. Dandy-Walker complex has been reported in 10% of patients with NCM 3. However, in our case the findings did not suggest Dandy- Walker malformation because the cerebellar vermis development was normal on MRI. Three theories for the association of NCM with Dandy-Walker malformation include: 1) obstruction of the 4th ventricle by melanocytes, 2) leptomeningeal disease leads to abnormal development of the 4th ventricle, 3) leptomeningeal melanosis interferes with inductive effects to extracellular matrix, neuronal migration, and the formation of cerebrospinal fluid pathways (7, 9). The diagnostic criteria of neurocutaneous melanosis were first described in 1972 by Fox (9) where it was defined as having meningeal melanosis in patients with large or multiple congenital nevi without evidence of cutaneous melanoma. Kadonaga et al. revised the criteria, which are as follows: large or multiple congenital nevi in association with meningeal melanosis or melanoma; no evidence of cutaneous melanoma except in patients in whom the examined areas of the meningeal lesions are histologically benign; and no evidence of meningeal melanoma except in patients in whom the examined areas of the cutaneous lesions are histologically benign (8).
Imaging Findings
CT is generally not adequate to detect melanocyte accumulations. Occasionally, the foci of melanocyte accumulation may appear only as subtle areas of increased density. MRI is the modality of choice for imaging of NCM. The melanocytic deposits tend to be hyperintense or isointense from T1 shortening on precontrast T1W images, of the paramagnetic properties of melanin. The anterior temporal lobe, especially the amygdala is the most common location of melanocytic deposits in NCM. The other frequent areas of melanocytic cell infiltration are inferior surface of the cerebellum, ventral aspect of the medulla, pons, cerebral peduncles, and upper cervical spinal cord (1–4, 6). The dura is generally spared. The cerebral parenchyma may be primarily or secondarily involved. Primary involvement may be caused by melanin-containing macrophages and melanocytes. Secondary involvement of the cerebral parenchyma occurs from spread via the Virchow-Robin spaces; the deep cerebral parenchyma is usually spared (3, 6). In our case, both the parenchymal and leptomeningeal involvement simultaneously were observed. The patients with neurocutaneous melanosis are also at risk for malignant transformation to leptomeningeal melanoma and cutaneous melanoma. Malignant transformation of leptomeningeal tumors occurs in 40 to 60% of symptomatic cases (10). Differentiation between benign and malignant parenchymal melanocytosis on MRI is difficult. But, on CT/MRI, presence of a mass lesion, nodular or thick plaque-like contrast enhancement, edema, growth, necrosis, and hemorrhage suggest malignant transformation. In our case, since these findings were not demonstrated in MRI and follow-up CT scans, malignant transformation was not suggested. Transfontanellar ultrasonography is usually not a helpful tool in the diagnosis in NCM. However, ultrasonography may demonstrate a hyperechoic amygdala as in our case. Additionally, it can reveal hydrocephalus and Dandy-Walker malformation that may be associated with NCM. Also, it can be used for monitoring of hydrocephalus.
Treatment/Prognosis
There is no effective treatment. Chemotherapy and radiotherapy have been shown to be ineffective in cases of NCM where malignancy is present. Additionally, due to the total infiltration of the central nervous system (CNS) by these lesions, surgical resection is not a viable treatment option (7). Most therapies are designed to treat the symptoms associated with the disorder, mainly those related to hydrocephalus. The presence of a Dandy-Walker malformation along with neurocutaneous melanosis further deteriorates prognosis. A VPS to relieve intracranial pressure is the preferred method (10). The majority of patients with NCM is asymptomatic and therefore has a good prognosis with few complications. Unfortunately, the prognosis of patients with associated neurologic findings is extremely poor. The majority of patients die within three years from benign overgrowth of melanocytic cells or development of malignant transformation (3). Close dermatologic, neurologic follow-up and imaging studies are advised due to the risk of malignant transformation. Dermatologists in their follow-up of patients with large or multiple congenital melanocytic nevi should be aware of this condition, to aid in prompt diagnosis, because the treatment of cutaneous lesions may be altered in the presence of symptomatic neurocutaneous melanosis.
Differential Diagnosis
The cutaneous lesions played a crucial role in establishing a correct diagnosis on the basis of the imaging findings. Radiologic differential diagnosis includes the intrinsically high T1 signal intensity at MRI such as melanin, lipid, protein, methemoglobin, calcium, copper, and manganese. The location and extent of a region of abnormal signal hyperintensity may be helpful for identifying rare diseases such as involvement of the anterior temporal lobe and cerebellum in neurocutaneous melanosis, diffuse leptomeningeal involvement in leptomeningeal melanomatosis. Many of these substances have physical properties that lead to other specific imaging features as well. For instance, lipid-containing lesions produce chemical shift artifact, and usually melanin-containing lesions exhibit a combination of high signal intensity on T1W images and low signal intensity on T2W images.
It is important to establish that the cutaneous lesions are benign in NCM. If not, then the melanocytic deposits in the CNS may be the result of metastasis of cutaneous melanoma and not neurocutaneous melanosis. NCM is different from leptomeningeal melanomatosis (LM). In NCM; the dura is usually spared, but not in LM, and typically the cerebral parenchyma, choroid plexus and ependyma are affected (3). The parenchymal localization is related to tracking along the meningeal penetration of the perivascular spaces. The amygdala is the most common site of involvement; other sites include the cerebellum and thalami. LM is disseminated on MRI and it has no predilection. There is no associated skin melanoma in LM. In contrast to neurocutaneous melanosis, melanomas also often show thickening and contrast enhancement of the involved leptomeninges. Urbach-Wiethe disease (also known as lipoid proteinosis) is a rare recessive genetic disorder, characterized by multisystem involvement due to intracellular deposition of an amorphous hyaline material (11). They may include a hoarse voice, dermatological manifestations, particularly the beaded papules on the eyelids. The hallmark findings are calcifications, mostly occurring in the amygdala, hippocampus, parahippocampal gyrus, or even the striatum. On MRI, such lesions are hypointense in all pulse sequences, especially in GRE T2*W images. Amygdala calcification on CT is considered pathognomonic.
TEACHING POINT
Neurocutaneous melanosis (NCM) is a rare phakomatosis characterized by pigmented nevi that are large or multiple or both, and leptomeningeal melanosis or melanoma, without evidence of malignancy in the skin lesions, and without an involvement of nonmeningeal organs. The cutaneous lesions play a crucial role in establishing a correct diagnosis on the basis of the imaging findings. MRI is the modality of choice for detecting foci of melanocyte accumulation in NCM: the anterior temporal lobe, especially the amygdala, and cerebellum are the most common locations of T1 hyperintensity corresponding to melanocytic deposits.
Table 1.
Summary table of neurocutaneous melanosis (NCM)
| Etiology | Non-familial. Pathogenic mechanism is still unclear; it is believed to be due to dysplasia of the neuroectodermal melanocyte precursor cells. |
| Incidence | Rare, about 100 cases reported in the English literature. Prevalence is estimated at 1/50,000–1/200,000. |
| Gender ratio | No predisposition according to gender |
| Age predilection | Most commonly within the first two years of life |
| Risk factors | Unknown |
| Treatment | There is no effective treatment. Chemotherapy and radiotherapy have been shown to be ineffective or little effective. Most therapies are designed to treat the symptoms associated with the disorder, mainly those related to hydrocephalus. |
| Prognosis | Asymptomatic NCM shows a normal life expectancy. Symptomatic NCM has an extremely poor prognosis. |
| Findings on imaging | In fact, as melanin pigment is inherently paramagnetic, the typical NCM lesions usually exhibit high intensity on T1W images and low intensity to iso-intensity on T2W images. The amygdala is the most common location of melanocytic deposits. The other frequent areas of melanocytic cell infiltration are cerebellum, medulla, pons, cerebral peduncles, and upper cervical spinal cord. |
Table 2.
Differential diagnosis table of T1 hyperintense lesions of central nervous system
| MRI | CT | |
|---|---|---|
| Neurocutaneous melanosis |
|
|
| Primary/secondary central nervous system melanoma |
|
|
| Leptomeningeal melanomatosis |
|
|
| Urbach–Wiethe disease (lipoid proteinosis) |
|
|
ABBREVIATIONS
- CNS
Central nervous system
- CT
Computed tomography
- MR
Magnetic resonance
- NCM
Neurocutaneous melanosis
- W
Weighted
REFERENCES
- 1.Barkovich AJ, Frieden IJ, Williams ML. MR of neurocutaneous melanosis. AJNR Am J Neuroradiol. 1994 May;15(5):859–67. [PMC free article] [PubMed] [Google Scholar]
- 2.Rokitanski J. Ein ausgezeichneter Fall von Pigment-Mal mit ausgebreiteter Pigmentierung der inneren Hirnund Rüchenmarkhaute. Ally Wien Med Z. 1861(6):113–16. [Google Scholar]
- 3.Barkovich AJ. Pediatric neuroimaging. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 4.Burstein F, Seier H, Hudgins PA, Zapiach L. Neurocutaneous melanosis. J Craniofac Surg. 2005 Sep;16(5):874–6. doi: 10.1097/01.scs.0000181050.06696.4d. [DOI] [PubMed] [Google Scholar]
- 5.Ginat DT, Meyers SP. Intracranial lesions with high signal intensity on T1-weighted MR images: differential diagnosis. Radiographics. 2012 Mar-Apr;32(2):499–516. doi: 10.1148/rg.322105761. [DOI] [PubMed] [Google Scholar]
- 6.Smith AB, Rushing EJ, Smirniotopoulos JG. Pigmented lesions of the central nervous system: radiologic-pathologic correlation. Radiographics. 2009 Sep-Oct;29(5):1503–24. doi: 10.1148/rg.295095109. [DOI] [PubMed] [Google Scholar]
- 7.Pavlidou E, Hagel C, Papavasilliou A, Giouroukos S, Panteliadis C. Neurocutaneous Melanosis: Report of Three Cases and Up-to-date Review. J Child Neurol. 2008 Dec;23(12):1382–91. doi: 10.1177/0883073808319069. [DOI] [PubMed] [Google Scholar]
- 8.Kadonaga JN, Frieden IJ. Neurocutaneous melanosis: definition and review of the literature. J Am Acad Dermatol. 1991 May;24(5 Pt 1):747–55. doi: 10.1016/0190-9622(91)70115-i. [DOI] [PubMed] [Google Scholar]
- 9.Fox H. Neurocutaneous melanosis. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 14. Amsterdam: Elsevier; 1972. pp. 414–428. [Google Scholar]
- 10.Alikhan A, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: Where are we now? Part I. Clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 2012 Oct;67(4):495.e1–17. doi: 10.1016/j.jaad.2012.06.023. quiz 512-4. [DOI] [PubMed] [Google Scholar]
- 11.Arkadir D, Lerer I, Klapholz L, et al. Lipoid proteinosis with bilateral amygdalae calcifications, headache, and cognitive impairments. Neurology. 2013 Jul 16;81(3):303–4. doi: 10.1212/WNL.0b013e31829bfe1c. [DOI] [PubMed] [Google Scholar]