Abstract
Background
This study aimed to determine the effects of long-term running exercise on spatial learning, spatial memory, and cortical capillaries in aged rats.
Material/Methods
Fourteen-month-old female and male Sprague-Dawley rats were randomly divided into an exercised group (EG) and a non-exercised group (NG). The EG rats were trained on treadmill running for 4 or 14 months. The NG rats were housed under identical conditions without running. Spatial learning and memory were assessed with the Morris water maze. The cortical capillary parameters were quantitatively investigated using immunohistochemical and stereological methods.
Results
The escaped latencies of the EG were significantly different from those of the NG in 18-month-old females and 28-month-old males (p<0.05). However, 28-month-old females and 18-month-old males showed no differences in escape latency between the EG and NG (p>0.05). In 28-month-old female rats, stereological techniques showed significant differences between the EG and NG in the cortical capillary volume (median, 22.55 vs. 11.42, p<0.05) and the cortical capillary surface area (median, 7474.13 vs. 3935.90, p<0.05). In 28-month-old male rats, the EG had a significantly longer total cortical capillary length (median, 530.35 vs. 156.27, p<0.05), significantly larger cortical capillary volume (median, 16.47 vs. 3.65, p<0.01), and a significantly larger cortical capillary total surface area (median, 7885.79 vs. 1957.16, p<0.01) compared with the NG group.
Conclusions
These data demonstrate that exercise improved spatial learning, memory capacity and cortical capillaries in aged rats.
MeSH Keywords: Cerebral Cortex, Exercise, Spatial Behavior
Background
Many studies have indicated that brain functions and structures in humans and other mammals can be maintained and promoted by exercise [1–4]. Colcombe et al. (2006) demonstrated that gray and white matter volumes increased in older humans who participated in aerobic exercise [1]. Previous studies have demonstrated that exercise intervention promotes neurogenesis in the hippocampus [5,6], and the number of newborn neurons in the hippocampi of both young adult and aged mice have also been shown to be increased by running [6,7]. Kronenberg et al. (2006) determined that exercise reduces the typical age-dependent decline in neurogenesis that occurred in sedentary adult animals [8]. The fundamental function of cerebral perfusion is sustained by capillaries, and brain blood vessels are influenced by exercise in many ways. Exercise has been shown to reduce stroke risk factors, such as hypertension, diabetes, and dyslipidemia [9], improve cerebral blood flow [10,11], promote angiogenesis [12] and improve cerebral blood volume [1]. Isaacs et al. (1992) demonstrated that running rats had more blood vessels than control-housed rats [13]. The cerebral cortex is one of the most important brain structures, and exercise plays an important role in maintaining brain function. What are the effects of aerobic running on cortical capillaries? Using the noninvasive technique functional magnetic imaging (fMRI), Swain et al. (2003) determined that angiogenesis occurs in the motor areas of the cerebral cortex as a strong adaptation to prolonged exercise [14]. Previous studies in rodent models have found that the structure and function of the brain peaks during middle age and then starts to slowly decline. In those previous studies, 14 months old was considered middle aged, 18 months old was considered the beginning of old age, and 28 months old was considered old age in rats[15,16]. As a lifestyle, exercise running is always a long-term repeated process. To better determine the effects of long-term running exercise on spatial learning, memory and cortical capillaries in aged rats, we allowed rats to run for 4 months or 14 months, starting at 14 months old and ending at 18 or 28 months old, respectively. Our group Huang et al. (2013) has previously investigated the effects of 4 months of exercise on the cortical capillaries of middle-aged rats (from 14 to 18 months old) using stereological techniques, they found that the volume, length and surface area of cortical capillaries in the exercised group (EG) were significantly increased in the females but unchanged in the males compared with the non-exercised group (NG) [17]. However, to date, researchers have not examined the effects of long-term exercise on cognitive ability and cortical capillaries in aged rats. Additionally, it is unknown whether there is a sex difference in the effects of long-term aerobic running exercise on cognitive ability and cortical capillaries in aged rats. Therefore, in the present study, the Morris water maze was used to test spatial learning and memory, and immunohistochemical and stereological methods were used to examine the effects of 14 months of running exercise on the cortical capillaries in aged male and female rats.
Material and Methods
Animals
Forty male and 40 female 14-month-old Sprague Dawley rats were obtained from the laboratory animal center of Chongqing Medical University, P. R. China. The animals were housed at the laboratory animal center of Chongqing Medical University and maintained with a controlled temperature (22±2°C) and a 12-h light/dark cycle. Each standard plastic cage housed 3 or 4 rats, and the rats were given food and water ad libitum. The male and female Sprague Dawley rats were randomly divided into the EG (n=20) and the NG (n=20).
The 14-month-old male and female rats were randomly divided into the following groups. In 1 group, the rats ran for 4 months, from 14 to 18 months of age (18 m EG, n=10). Another group of rats ran for 14 months, from 14 to 28 months of age (28 m EG, n=10). After the running exercise was completed, the rats were tested in the Morris water maze. The male and female NG rats were randomly divided into the 18 m NG group (n=10) and the 28 m NG (n=10). All of the NG rats were housed under conditions identical to those of the EG but without running. The numbers of rats in each group that were excluded from the analysis due to tumors or other causes at 28 months old were as follows: 1 male EG (10%), 2 male NG (20%), 2 female EG (20%), and 1 female NG (10%). All procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Exercise training
A straight 6-lane tread-mill (600×95×115 per lane) with an electrical stimulator connected to a manual control system (Beijing Sunny Instruments Co. Ltd., Beijing, China) was used to exercise the rats. In the EG, the rats were trained to run on the treadmill for 4 months or 14 months, as indicated. The first 2 weeks represented an adaptation period. All of the rats in the EG were acclimated to the treadmill by sitting on it during the first day of training. These rats subsequently learned to run on the treadmill, and the speed was slowly increased from 5 to 20 m/min for 20 min/day. Each rat ran at the speed of 20 m/min for 5 days/week from the third week until the end of the experiment [7,18–20]. The NG rats were housed under identical conditions but without running.
Morris water maze test
To examine the changes in learning and memory ability of the rats during the period of exercise training, the 18-month-old and 28-month-old EG rats were tested using the Morris water maze after 4 or 14 months of running, respectively, along with the NG rats of the corresponding ages. A circular pool (1.5 m in diameter) connected to an automatic tracking and analysis system (SLY-R01 & WMS, Beijing Sunny Instruments Co. Ltd., Beijing, China) was used to complete the experiment. The water was darkened with ink, and a controlled temperature (22±2°C) was maintained during the test. Each rat was tested in 4 different quadrants – labeled A, B, C and D – for 6 days, with 4 trials/day. The starting position of the 4 experiments was the same each day, and the order of the starting position in the 4 experiments for all animals was the same on the same day. However, the order of the start position was randomly determined each day. The rats faced the wall of the pool while they were slowly placed in the water. A visible platform was used on the first day, and a hidden platform was used on the next 5 days. The platform remained in the same quadrant for 6 days. In each experiment, the animal was allowed to swim for up to 120 s until it located the platform and then remained on the platform for 15 s. If the rat did not locate the platform within 120 s, it was guided to the platform and remained on the platform for 15 s. The inter-trial interval for each animal was more than 15 min. The distance and time required to reach the platform (escape latency) were automatically recorded by a video tracking system [21–23].
Perfusion and estimation of cortex volume
After the tests of spatial learning and memory, 5 male and 5 female rats were randomly selected from the 28 m EG and NG rats, respectively. The rats were deeply anaesthetized via an intraperitoneal injection of 1% pentobarbital sodium (Sigma, Germany) 0.4 ml/100 g. The rats were subsequently perfused with 100 ml of 4% paraformaldehyde in 0.6 M phosphate-buffered saline (pH 7.4). The cerebellum, brain stem, and cranial nerves, which are under the pavimentum cerebri, were removed, and the cerebral hemispheres were removed after the perfusion. The hemispheres were embedded in 6% agar and cut into 1-mm-thick coronary slabs randomly starting at the rostral pole (Figure 1).
Figure 1.
Sequential 1-mm cerebral slabs.
The slabs were photographed under a dissecting microscope at a magnification of 10×. A transparent plastic sheet marked with equidistant points was randomly placed on the caudal surface of each photographed slab, and the points that hit the cortex were then counted (Figure 2). The cortex volume was calculated according to Cavaleiri’s principle [24,25]:
Figure 2.
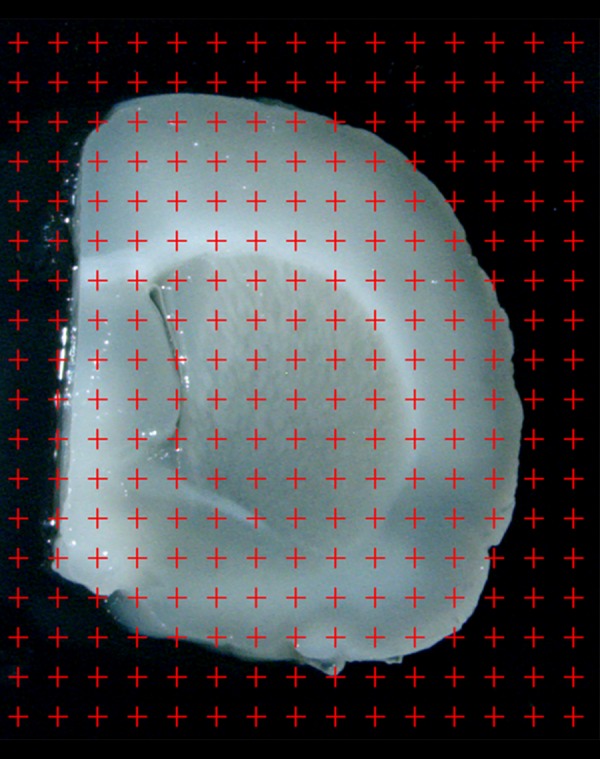
The slab is photographed under an anatomical microscope at a magnification of 10×. A point grid is randomly superimposed on the photographed slab, and the points that touch the cortex are counted.
where Vcortex indicates the total volume of the cortex, t indicates the slab thickness (1 mm), a(p) indicates the area associated with each grid point (0.06 mm2), and ∑Pcortex indicates the total number of grid points that hit the cerebral cortex for each rat.
Cortex sampling
After the total volume of the cortex was measured, the slabs were randomly sampled every 3 slabs, and the first slab was randomly sampled in the first 3 slabs. Equidistant light points were randomly projected on the occipital surface of the sampled slabs. Tissue blocks of approximately 1 mm3 were cut from the cortex where the points on the plastic sheet hit the cortex, and 5–6 blocks were sampled per rat. This systematic random sampling ensures a uniformly random distribution of the cortex samples and provides an equal sampling probability for all parts of the cortex.
The randomly selected slabs of the hemisphere were postfixed in 4% paraformaldehyde for more than 2 h. The samples were subsequently embedded in agar with the caudal surface face down. The embedded samples were subjected to the isector technique to obtain the isotropic, uniform random (IUR) sections [25,26]. After the IUR surface was obtained, 1 section with a thickness of 5 μm was cut from each block along the direction parallel to the IUR surface (hereafter referred to as the IUR sections). The isector technique ensures that the capillaries have the same probability of being sampled in each direction of 3-dimensional space.
Immunohistochemistry
The sections were immersed in citrate buffer (0.01 M, pH 6.0) and then microwaved for 15 min for antigen retrieval. After cooling, the sections were washed twice in PBS (0.01 M, pH 7.4) and then soaked in 3% hydrogen peroxide for 10 min at room temperature to inactivate endogenous peroxides. Next, the sections were washed 3 times in PBS for 5 min prior to incubation with 5% normal goat serum for 20 min at room temperature to exclude nonspecific staining. Then, the sections were incubated at 4°C overnight, followed by incubation at 37°C for 1 h with rabbit polyclonal anti-collagen IV primary antibody as the primary antibody (ab6586; Abcam, Cambridge, UK) at a dilution of 1:200 in PBS. Next, the sections were washed 3 times in PBS for 5 min each. The sections were transferred to a solution containing biotinylated goat-anti-rabbit immunoglobulin G as the secondary antibody and incubated for 20 min at 37°C. The specimens were incubated with S-A/HRP at 37°C for 20 min, followed by 3 washes in PBS for 5 min. The sections were then transferred to a diaminobenzidine (DAB) solution (DAB, ZLL-9032, ZSGB, Beijing, China) as a chromogen for approximately 10 min. The sections were dehydrated in gradient ethanol and xylene by sequential immersion, cover-slipped, and viewed under light microscopy (Olympus, Tokyo, Japan). From each section, 4–6 fields of view were randomly captured from the cortex under a 100× oil objective lens. The vessels with a luminal diameter of less than 10 μm were defined as capillary net components [17,27,28].
Estimation of the total length, total volume and total surface area of the cortical capillaries
An unbiased counting frame was randomly superimposed on each captured photograph (Figure 3A). The length density of the capillaries in the cerebral cortex was estimated with a previously described stereological method [17,28]:
Figure 3.
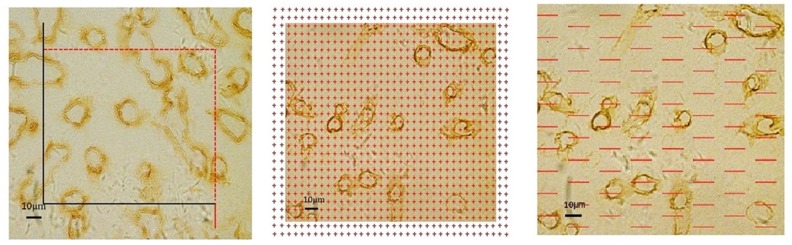
(A) An unbiased counting frame is placed on the randomly captured image. The capillary profiles are counted if they are completely inside the counting frame or partly inside it but only touching the counting lines (dotted lines). The capillary profiles are excluded if they touch the exclusion lines (solid lines). Scale bar=10 μm. (B) The points that hit the cortex and the points hitting on that hit the capillary profiles are counted. Scale bar=10 μm. (C) The intersection points between the test lines and capillary luminal surface are counted. Scale bar=10 μm.
where ∑Q (cap) denotes the total number of the capillary profiles counted per rat cortex, and ∑A (frame) is the total area of counting frames used per rat. The total length of the cortical capillaries equaled the cortex volume, Vcortex, multiplied by the length density of the cortical capillaries, Lv(cap/cortex).
A transparent point grid was randomly placed on the photographs. The points that hit the capillaries, ∑P (cap), and the points that hit the cortex, ∑P (C), were counted (Figure 3B). The volume density of the cortical capillaries, Vv(cap/cortex), was calculated using the following equation [17,28]:
The volume density of the cortical capillaries, Vv(cap/cortex), multiplied by the cortex volume (Vcortex) was the total volume of the cortical capillaries [17,28].
Test lines were randomly placed on each photograph. The intersection points between the test lines and the capillary luminal surface, ∑PI (cap), and the total length of the lines that hit the cortex, ∑L(C), were recorded (Figure 3C). The surface area density of the cortical capillaries, Sv (cap/cortex), was estimated using the following equation [17,28]:
The surface area density of the capillaries, Sv (cap/cortex), multiplied by the cortex volume (Vcortex) was the total surface area of the cortical capillaries [17,28].
Statistical analysis
All data are expressed as the means ± standard deviation (SD), and analyses were performed with SPSS 17.0. The statistical significance of the change in the spatial learning ability over days was analyzed using a repeated measures analysis of variance (ANOVA). The statistical significance of the morphological data was assessed using a Mann-Whitney non-parametric test. Significant differences were defined as p < 0.05.
Results
Effects of long-term exercise on spatial learning ability
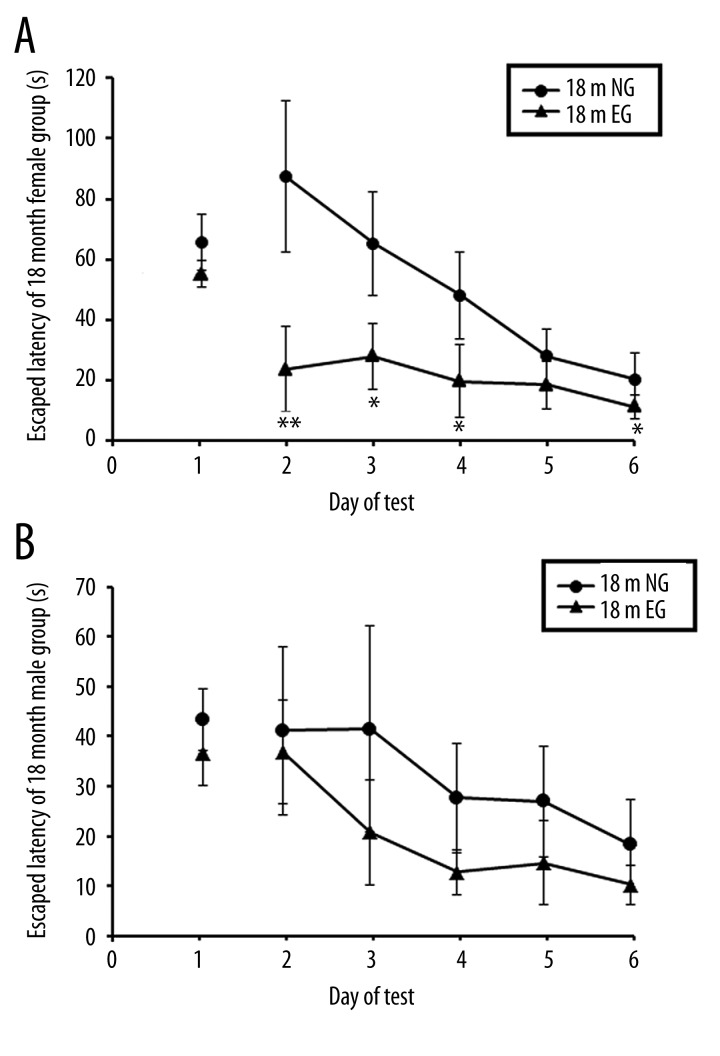
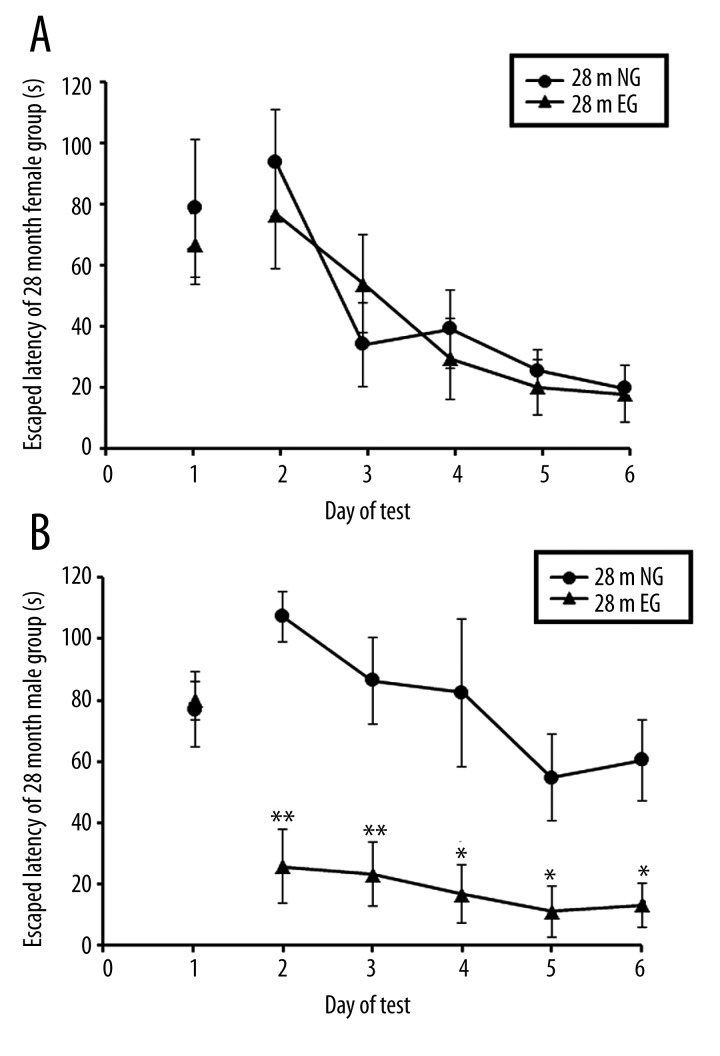
On the first day, with the visible-platform test, there were no significant differences in the escape latency between the EG and NG in 18-month-old (18 m) and 28-month-old (28 m) male and female rats, respectively (p>0.05, Figures 4A, 4B; 5A, 5B). In the hidden-platform test from the second day to sixth day, comparisons of the escape latencies from the Morris water maze demonstrated that after 4 months of treadmill exercise, the spatial learning and memory abilities of the 18 m EG were significantly improved compared with the NG in female rats (p<0.05, Figure 4A), whereas the spatial learning and memory abilities of the 18 m EG were not significantly different from those of the 18 m NG in the male rats (p > 0.05, Figure 4B). After 14 months of treadmill exercise, comparisons of the escape latencies in the Morris water maze indicated that the spatial learning and memory abilities of the 28 m EG were significantly improved compared with the 28 m NG in the male rats (p<0.05; Figure 5B), whereas the spatial learning and memory abilities of the 28 m EG were not significantly different from the 28 m NG in the female rats (p>0.05; Figure 5A).
Figure 4.
(A) Comparison of the escape latencies of female rats between the 18-month-old EG and NG. (B) Comparison of the escape latencies of male rats between the 18-month-old EG and NG. ** indicates p<0.01, * indicates p<0.05. NG: non-exercised group, EG: exercised group.
Figure 5.
(A) Comparison of the escape latencies of female rats between the 28-month-old EG and NG. (B) Comparison of the escape latencies of male rats between the 28-month-old EG and NG. ** indicates p<0.01, * indicates p<0.05. NG: non-exercised group, EG: exercised group.
Cortex volume
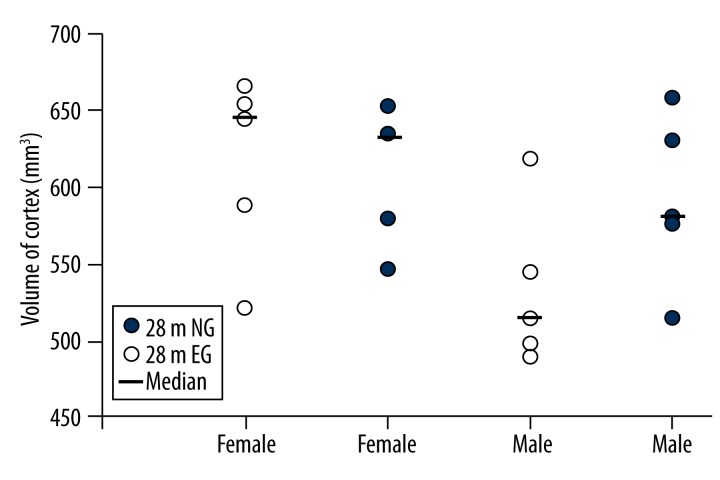
The mean cerebral cortex volume in the 28 m female rats was 608.20±44.44 mm3 in the EG and 614.20±60.07 mm3 in the NG, which was not significantly different (p>0.05; Figure 6). In the 28 m male rats, the mean cerebral cortex volume was 591.40±55.24 mm3 in the EG and 532.90±52.18 mm3 in the NG; the cortex volume in the EG increased by 12.89% compared with the NG, but this difference was also not significant (p>0.05; Figure 6).
Figure 6.
Comparisons of the cortex volumes between the 28-month-old EG and NG. NG: non-exercised group, EG: exercised group. Median: median value.
Cortical capillaries
Immunohistochemistry of the cortical capillaries
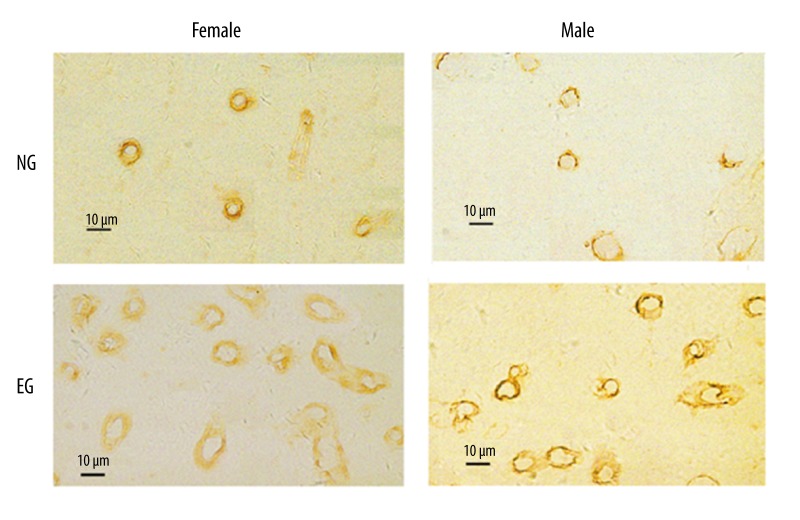
The representative immunohistochemical staining for the cortical capillaries of 28 m NG and EG rats of male and female groups is presented in Figure 7.
Figure 7.
Representative immunohistochemical staining for the cortical capillaries between 28-month-old NG and EG. The ring structure is the cortical capillary. NG: non-exercised group, EG: exercised group. Scale bar=10 μm.
Stereological results for the cortical capillaries
Length of the cortical capillaries
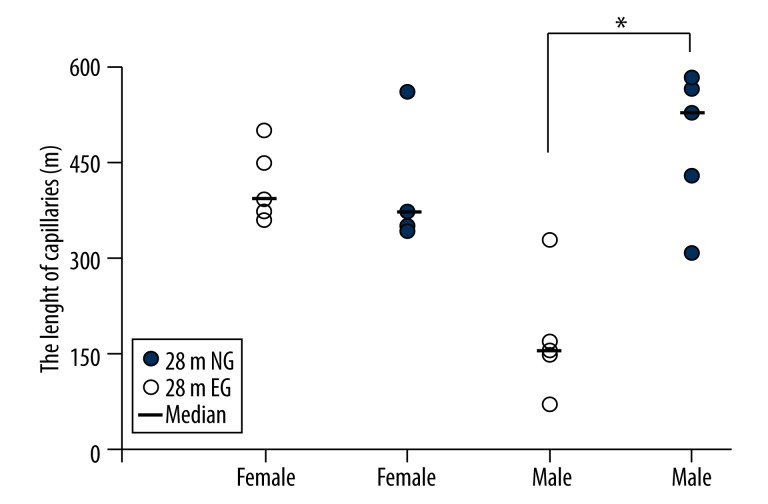
The total length of the cortical capillaries in the 28 m female rats was 439.91±115.28 m in the EG and 418.92 ± 60.22 m in the NG, which was not significantly different (p>0.05; Figure 8). The total length of the cortical capillaries in the 28 m male rats was 484.96±116.74 m in the EG, a significant increase compared with the 175.10±95.63 m in the NG (p<0.05; Figure 8).
Figure 8.
Comparisons of the total lengths of the cortical capillaries between the 28-month-old EG and NG. * indicates p<0.05. NG: non-exercised group, EG: exercised group. Median: median value.
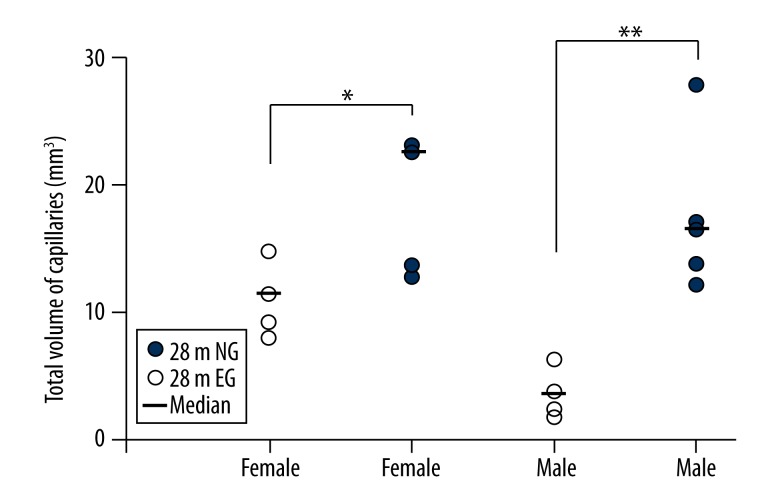
Volume of the cortical capillaries
The volume of the cortical capillaries in the 28 m female rats was 18.97±9.25 mm3 in the EG, a significant increase compared with the 10.84±2.37 mm3 in the NG (p<0.05; Figure 9). The volume of the cortical capillaries in the 28 m male rats was 17.46±6.11 mm3 in the EG, a significant increase compared with the 3.61±1.71 mm3 in the NG (p<0.01; Figure 9).
Figure 9.
Comparisons of the total volumes of the cortical capillaries between the 28-month-old EG and NG. ** indicates p<0.01, * indicates p<0.05. NG: non-exercised group, EG: exercised group. Median: median value.
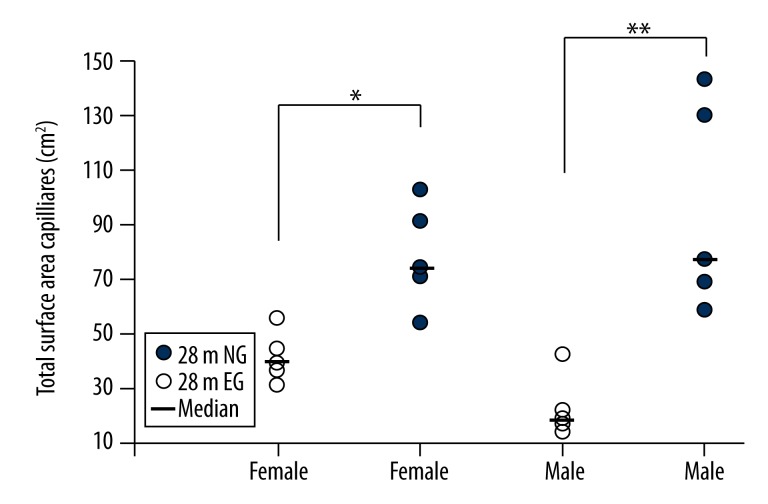
Surface area of the cortical capillaries
The surface area of the cortical capillaries in the 28 m female rats was 79.17±18.90 cm2 in the EG, a significant increase compared with the 41.62±9.02 cm2 in the NG (p<0.05; Figure 10). The surface area of the cortical capillaries in the 28 m male rats was 97.53±38.29 cm2 in the EG, a significant increase compared with the 23.55±10.97 cm2 in the NG (p<0.01; Figure 10).
Figure 10.
Comparisons of the total surface areas of the cortical capillaries between the 28-month-old EG and NG. ** indicates p<0.01, * indicates p<0.05. NG: non-exercised group, EG: exercised group. Median: median value.
Discussion
The current study investigated the effects of long-term aerobic exercise on spatial learning, memory, and physiological characteristics of the cortical capillaries in aged male and female rats. Aerobic treadmill running was used as the exercise model. Because the effects of an exercise program depend on the intensity and duration of the exercise, the magnitude and quality of the response depend on the exercise program [20]. When treadmill aerobic running exercise was previously used as a training method, it had strong concurrent validity and test-retest reliability [29], and we could quantitatively control the running intensity. However, using other forms of training exercise, such as wheel running and swimming, we could not quantitatively control the running intensity [30].
Previous studies have suggested that learning and memory gradually decline with advancing age in humans and rats [31,32]. In recent years, many studies have reported that aerobic exercise may delay the cognitive decline of the aged brain [33–36]. However, Asl et al. (2008) demonstrated that regular aerobic running could induce improvements in the spatial learning and memory capacities of young rats, whereas regular running exercise could not improve the spatial learning and memory capacities of aged rats [37]. In the current study, we demonstrated that after 4 months of treadmill exercise, the spatial learning abilities of the 18 m male EG rats were not significantly different from the 18 m male NG rats. However, after 14 months of treadmill exercise, the escape latencies in the Morris water maze indicated that the spatial learning abilities of the 28 m male EG rats were significantly improved compared with the 28 m male NG rats. In the female rats, after 4 months of treadmill exercise, the spatial learning and memory abilities of the 18 m EG rats were significantly improved compared with the NG rats, but the escape latencies were not significantly different between the 28 m female EG and NG rats. These results suggested that long-term exercise that was initiated in middle age could affect the spatial learning and memory abilities of both male and female EG rats.
Moreover, we found that the exercise-induced improvement of behavioral performance in the aged male rats needed much longer exercise time than for the female rats and that the benefits of exercise in the male rats mainly manifested in the aged rats. In the female rats, the exercise-induced benefit mainly appeared in the middle-aged rats, and there was no significant exercise-induced improvement of the behavioral performance in the aged female rats.
Long-term aerobic running may provide an easy method for sustaining and promoting brain function. What are the structural bases for the positive effects of exercise on brain function? Previous studies have demonstrated that exercise increases the volumes of various brain structures in humans and other animals [2–4]. After long-term exercise, capillaries were increased in the motor cortex [14,38], cerebellum [13,39] and striatum [40]. The increase in the capillaries in the brain may be closely related to improvements in brain function after long-term running. However, previous studies used only a capillary density measurement, which may not reflect changes in the total number of capillaries. Moreover, because the cortical capillaries are not arranged isotropically, it has been difficult for previous researchers to accurately quantify the cortical capillaries. In the current research, the volume, length, and surface area of cortical capillaries were evaluated using modern stereological methods. These techniques avoided the potential biases associated with density calculations. Moreover, in the present study, isotropic, uniform random (IUR) sections were obtained using the isector technique to ensure that all cortical capillaries had an equivalent possibility of being sampled in all orientations [28].
van Pragg et al. (2005) reported that after 45 days of running, exercise increased the perimeter and surface area of the blood vessels in the dentate gyrus of young mice but not aged mice [7]. In our previous study, Huang et al. (2013), we used stereological methods to demonstrate that after 4 months of running, the volume, length and surface area of capillaries in the cortex of middle-aged female EG rats were significantly increased compared with those in middle-aged female NG rats. However, the differences in the cortical capillaries were not significantly different between the middle-aged male EG and NG rats [17]. In the present study, we extended the running time to 14 months to better mimic a long-term exercise lifestyle in humans. We then investigated the effects of long-term running on the cortical capillaries in aged rats and found no significant differences in the cortex volume in EG aged rats compared with NG aged rats in both the male groups and the female groups. Additionally, our results indicated that the volume, length and surface area of the cortical capillaries in the EG were significantly enhanced compared with the NG in the female rats, although the difference in the lengths of the cortical capillaries was not significantly different between the female EG and NG rats. For the male rats, the volume, length and surface area of the cortical capillaries in the EG rats were significantly enhanced compared with the NG rats.
We found that in both male and female aged rats, 14 months of long-term running exercise had positive effects on the cortical capillaries, but there was a sex difference in the effects of long-term aerobic running exercise on the cortical capillaries in aged rats. The total volume, the total surface area and the total length of the capillaries in the cortex of male EG rats were significantly increased compared with those of male NG rats. However, in female rats, only the total volume and the total surface area of the cortical capillaries in EG rats were increased compared to those of NG rats. The slight discrepancy in the effects of exercise on the capillaries of the cortex in male and female rats was not surprising because most vessels formed postnatally are short capillaries that contribute substantially to branching and less to length [41]. The proliferation and sprouting of new capillaries significantly contributed to the increase in the total volume and the total surface area but not the total length in female EG rats compared with female NG rats. We speculate that the increase in the cortical capillaries in the aged rats might be an important structural basis for the effects of long-term aerobic running on brain function in aged rats. The cognitive functions of aged rats are affected by age-related morphological changes such as the changes in nerve cells, synapses and nerve fibers and other factors that occur during normal aging [42–44]. These changes might partly explain why the 28-month-old female rats showed an exercise-induced increase in the cortical capillary parameters but not an exercise-related improvement in the behavioral performance. However, the exact relationship between the exercise-induced changes in the parameters of the cortical capillaries and the exercise-induced changes in the behavioral performance requires further investigation.
What are the possible reasons for the differences between the current and previous studies and for the differences between the sexes? We speculate that differences in the animal species and brain regions examined, the quantitative methods used and the duration of the exercise paradigm might explain the differences in the results between the current and previous studies. The differences in the effects of exercise on male and female animals between Huang’s study and the present study might be related to the differences in estrogen levels. The previous study demonstrated that aerobic exercise could strengthen the effects of estrogen on brain health, plasticity and general well-being, and females were more sensitive to exercise interference because of increased estrogen levels [45]. In aged female rats after a long-term experiment, estrogen levels declined with aging, and the protective effect of estrogen on brain function may decline compared with that in middle-aged animals [46]. This result may explain why the treadmill exercise could improve the brain function of female rats in middle age but not in old age, and it may also explain why the effects of treadmill exercise on cortical capillaries of aged male and female rats were similar despite the significant differences in middle-aged male and female rats. However, the exact reasons for these differences remain unknown and require further investigation.
In summary, long-term exercise had protective effects on the cortical capillaries in aged male and female rats, and these effects might be an important structural basis for the effects of exercise on spatial learning and memory abilities in aged rats. The present results may provide an important foundation for future research into developing advanced methods to delay or prevent the decline in brain function with aging.
Conclusions
Our results show that long-term exercise had positive effects on spatial learning and memory capacity in aged female rats and aged male rats. Long-term exercise increased the total volume, total length and total surface area of cortical capillaries in aged male EG rats. Additionally, long-term exercise increased the total volume and total surface area of cortical capillaries in aged female EG rats. The present results may provide an important basis for the identification of better strategies for delaying or preventing the age-related decline in brain function.
Footnotes
Statement
The funders had no role in the study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
All authors declare that they have no conflicts of interest.
Source of support: This study was supported by the National Natural Science Foundation of China (NSFC, 81171238, 31271288, 31300985) and the Research Foundation for 100 Academic and Discipline Talented Leaders of Chongqing P. R. China (2012)
References
- 1.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–70. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 2.Tyndall AV, Davenport MH, Wilson BJ, et al. The brain-in-motion study: effect of a 6-month aerobic exercise intervention on cerebrovascular regulation and cognitive function in older adults. BMC Geriatr. 2013;13:21. doi: 10.1186/1471-2318-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 4.Raichlen DA, Polk JD. Linking brains and brawn: exercise and the evolution of human neurobiology. Proc Biol Sci. 2013;280:20122250. doi: 10.1098/rspb.2012.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–11. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 6.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–85. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kronenberg G, Bick-Sander A, Bunk E, et al. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–13. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Lee IM, Hennekens CH, Berger K, et al. Exercise and risk of stroke in male physicians. Stroke. 1999;30:1–6. doi: 10.1161/01.str.30.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Ainslie PN, Cotter JD, George KP, et al. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586:4005–10. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gertz K, Priller J, Kronenberg G, et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99:1132–40. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- 12.Van der Borght K, Kobor-Nyakas DE, Klauke K, et al. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19:928–36. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- 13.Isaacs KR, Anderson BJ, Alcantara AA, et al. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12:110–19. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- 14.Swain RA, Harris AB, Wiener EC, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–46. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 15.Diaz Brinton R. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology. 2012;153:3571–78. doi: 10.1210/en.2012-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller RA, Harrison DE, Astle CM, et al. An Aging Interventions Testing Program: study design and interim report. Aging Cell. 2007;6:565–75. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 17.Huang CX, Qiu X, Wang S, et al. Exercise-induced changes of the capillaries in the cortex of middle-aged rats. Neuroscience. 2013;233:139–45. doi: 10.1016/j.neuroscience.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 18.Chen YC, Lei JL, Chen QS, Wang SL. Effect of physical training on the age-related changes of acetylcholinesterase-positive fibers in the hippocampal formation and parietal cortex in the C57BL/6J mouse. Mech Ageing Dev. 1998;102:81–93. doi: 10.1016/s0047-6374(98)00026-8. [DOI] [PubMed] [Google Scholar]
- 19.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 20.Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–22. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kucukatay V, Balkan S, Yaras N, et al. The effect of pergolide on cognitive performance of young and middle-aged rats. Int J Neurosci. 2002;112:1027–36. doi: 10.1080/00207450290026021. [DOI] [PubMed] [Google Scholar]
- 22.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neuroscience Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 25.Tang Y, Nyengaard JR. A stereological method for estimating the total length and size of myelin fibers in human brain white matter. J Neurosci Methods. 1997;73:193–200. doi: 10.1016/s0165-0270(97)02228-0. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Yang S, Chen L, et al. Stereological methods for estimating the myelin sheaths of the myelinated fibers in white matter. Anat Rec. 2009;292:1648–55. doi: 10.1002/ar.20959. [DOI] [PubMed] [Google Scholar]
- 27.Alba C, Vidal L, Diaz F, et al. Ultrastructural and quantitative age-related changes in capillaries of the dorsal lateral geniculate nucleus. Brain Res Bull. 2004;64:145–53. doi: 10.1016/j.brainresbull.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Shao WH, Li C, Chen L, et al. Stereological investigation of age-related changes of the capillaries in white matter. Anat Rec. 2010;293:1400–7. doi: 10.1002/ar.21184. [DOI] [PubMed] [Google Scholar]
- 29.Lee M, Song C, Lee K, et al. Agreement between the spatio-temporal gait parameters from treadmill-based photoelectric cell and the instrumented treadmill system in healthy young adults and stroke patients. Med Sci Monit. 2014;20:1210–19. doi: 10.12659/MSM.890658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moraska A, Deak T, Spencer RL, et al. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol. 2000;279:R1321–29. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- 31.Albert M. Neuropsychological and neurophysiological changes in healthy adult humans across the age range. Neurobiol Aging. 1993;14:623–25. doi: 10.1016/0197-4580(93)90049-h. [DOI] [PubMed] [Google Scholar]
- 32.Wyss JM, Chambless BD, Kadish Ivan Groen T. Age-related decline in water maze learning and memory in rats: strain differences. Neurobiol Aging. 2000;21:671–81. doi: 10.1016/s0197-4580(00)00132-9. [DOI] [PubMed] [Google Scholar]
- 33.Weuve J, Kang JH, Manson JE, et al. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–61. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 34.van Gelder BM, Tijhuis MA, Kalmijn S, et al. Physical activity in relation to cognitive decline in elderly men: the FINE Study. Neurology. 2004;63:2316–21. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- 35.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 36.Chang M, Jonsson PV, Snaedal J, et al. The effect of midlife physical activity on cognitive function among older adults: AGES – Reykjavik Study. J Gerontol A Biol Sci Med Sci. 2010;65:1369–74. doi: 10.1093/gerona/glq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asl NA, Sheikhzade F, Torchi M, et al. Long-term regular exercise promotes memory and learning in young but not in older rats. Pathophysiology. 2008;15:9–12. doi: 10.1016/j.pathophys.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Kleim JA, Cooper NR, VandenBerg PM. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;934:1–6. doi: 10.1016/s0006-8993(02)02239-4. [DOI] [PubMed] [Google Scholar]
- 39.Black JE, Isaacs KR, Anderson BJ, et al. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA. 1990;87:5568–72. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding YH, Luan XD, Li J, et al. Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke. Curr Neurovasc Res. 2004;1:411–20. doi: 10.2174/1567202043361875. [DOI] [PubMed] [Google Scholar]
- 41.Harb R, Whiteus C, Freitas C, Grutzendler J. In vivo imaging of cerebral microvascular plasticity from birth to death. J Cereb Blood Flow Metab. 2013;33:146–56. doi: 10.1038/jcbfm.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.jell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- 43.Piguet O, Double KL, Kril JJ, et al. White matter loss in healthy ageing: a postmortem analysis. Neurobiol Aging. 2009;30:1288–95. doi: 10.1016/j.neurobiolaging.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Pannese E. Morphological changes in nerve cells during normal aging. Brain Struct Funct. 2011;216:85–89. doi: 10.1007/s00429-011-0308-y. [DOI] [PubMed] [Google Scholar]
- 45.Berchtold NC, Kesslak JP, Pike CJ, et al. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- 46.Marosi K, Felszeghy K, Mehra RD, et al. Are the neuroprotective effects of estradiol and physical exercise comparable during ageing in female rats? Biogerontology. 2012;13:413–27. doi: 10.1007/s10522-012-9386-3. [DOI] [PubMed] [Google Scholar]